Abstract
Pollen limitation (PL) of seed production creates unique conditions for reproductive adaptation by angiosperms, in part because, unlike under ovule or resource limitation, floral interactions with pollen vectors can contribute to variation in female success. Although the ecological and conservation consequences of PL have received considerable attention in recent times, its evolutionary implications are poorly appreciated. To identify general influences of PL on reproductive adaptation compared with those under other seed-production limits and their implications for evolution in altered environments, we derive a model that incorporates pollination and post-pollination aspects of PL. Because PL always favours increased ovule fertilization, even when population dynamics are not seed limited, it should pervasively influence selection on reproductive traits. Significantly, under PL the intensity of inbreeding does not determine whether outcrossing or autonomous selfing can evolve, although it can affect which response is most likely. Because the causes of PL are multifaceted in both natural and anthropogenically altered environments, the possible outcrossing solutions are diverse and context dependent, which may contribute to the extensive variety of angiosperm reproductive characteristics. Finally, the increased adaptive options available under PL may be responsible for positive global associations between it and angiosperm diversity.
Keywords: competition for pollination, habitat fragmentation, inbreeding depression, mate limitation, pollen limitation, seed production
1. Introduction
… if humble-bees were to become rare in any country, it might be a great advantage to the red clover to have a shorter or more deeply divided tube to its corolla, so that the hive-bee could visit its flowers.
Darwin (1859, pp. 94–95)
Pollination biology was effectively born as an evolutionary science because Darwin (1859, 1862, 1876) was the first to appreciate fully the role of flowers as outcrossing mechanisms and he used floral ‘contrivances’ as compelling evidence of adaptation by natural selection. As the preceding quotation illustrates, Darwin appreciated the dependence of many species on pollen vectors for reproduction and the ecological and evolutionary implications of the absence or inadequacy of their service. In particular, the results of his extensive experiments on what is now known as inbreeding depression led Darwin (1876) to his well-known claim that ‘nature abhors perpetual self-fertilization’, and yet he acknowledged ‘the self-evident proposition that the propagation of the species, whether by self-fertilization or by cross-fertilization, or asexually by buds, stolons, etc., is of paramount importance’ (p. 8). Thus, Darwin recognized that failure of cross-fertilization could precipitate evolution that otherwise seems at odds with his general interpretation of floral traits as outcrossing mechanisms.
A century and a half later, pollen limitation (PL) is seen as an important ecological factor determining the quantity and quality of seed output (Ashman et al. 2004). Although PL is often considered primarily as a quantitative problem resulting from insufficient pollination caused intrinsically by a plant's attractiveness or extrinsically by scarcity of pollen vectors (Ashman et al. 2004), it can also occur or be intensified by poor-quality pollination (Aizen & Harder 2007). Through its effects on plant fecundity, PL has the capacity to limit plant recruitment and so can influence population dynamics and even viability (Morgan et al. 2005; Price et al. 2008). As a result, PL is a subject of considerable attention in conservation biology (Allen-Wardell et al. 1998; Kearns et al. 1998), especially as human activity increasingly and persistently disturbs natural environments (e.g. Aguilar et al. 2006).
Owing to its potential to influence plant fitness, PL can also act as a key influence on selection on reproductive traits. As Bateman (1948) and Trivers (1972) clarified, the limits on reproductive capacity can differ between the sex roles. For species with some parental care, such as seed provisioning, a male's mating potential depends on the reproductive capacity of all the females with which he can mate, whereas a female's opportunities depend on the lesser of her own egg production or the number of offspring that she can mature given the available resources. Thus, although average female and male outcrossing success must be equal in a closed population, outcrossing potential through male function greatly exceeds that through female function, so that selection on mating traits, especially floral and inflorescence characteristics, can differ between the sex roles. However, this asymmetry is reduced or eliminated when insufficient pollen dispersal and/or embryo failure produce fewer viable embryos than maternal resource capacity to develop seeds. Under PL, a female's success is limited by mating opportunities, like male success, rather than by her capacity to produce seeds, so that the pollination environment determines the nature of selection on floral traits. As a result, PL theoretically has diverse evolutionary consequences, such as reducing the threshold inbreeding depression that allows the evolution of selfing, restricting the conditions necessary for initial stages in the evolution of heterostyly and increasing the equilibrium frequency of hermaphrodites in gynodioecious and subdioecious populations (Lloyd 1974, 1992; Lloyd & Webb 1992; Maurice & Fleming 1995; Morgan & Wilson 2005; Morgan et al. 2005). According to this view, selection through female function for traits that increase pollen deposition and post-pollination pollen success should vary positively with the intensity of PL, an expectation confirmed for traits involved in pollinator attraction (Ashman & Morgan 2004).
Despite recognition of PL as an ecologically and evolutionarily significant process, misconceptions exist concerning its scope and implications. Importantly, PL is often oversimplified as a consequence of inadequate pollinator attraction, implying that evolutionary options are limited to increased autonomous selfing or increased attractiveness (e.g. Haig & Westoby 1988; Ashman & Morgan 2004; Burd 2008); however, this characterization overlooks many relevant aspects of plant reproduction. Therefore, in the spirit of the Discussion Meeting on ‘Darwin and the evolution of flowers’, we will review current understanding of possible causes of PL and speculate on their implications for floral evolution, both in the absence and presence of anthropogenic disturbance. We begin by clarifying the requirements for PL and the other possible constraints on seed production before briefly summarizing evidence concerning the incidence of PL. To establish a context for considering the evolutionary implications of PL, we then derive simplified models of selection on floral traits and the mating system under contrasting seed-production limits. In this light, we then explore some micro- and macro-evolutionary consequences of PL. Finally, we briefly consider possible effects of human-modified environments for the occurrence of PL and reproductive evolution.
2. What is pollen limitation?
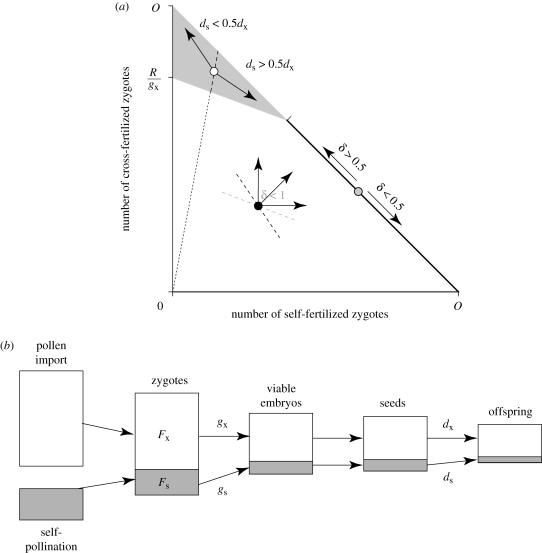
Seed production requires pollen, ovules and resources, so it will be limited by whichever of these components is in shortest supply (Harder & Routley 2006). As figure 1a illustrates, resource limitation occurs whenever the number of embryos exceeds the number of seeds that can be developed given the maternal resources (figure 1a, grey area), regardless of whether all ovules are fertilized. If too few embryos have been formed to consume maternal resources, then seed production is ovule limited if all ovules are fertilized (figure 1a, heavy black line), but it is pollen limited when fertilization is incomplete (figure 1a, white area below diagonal). Therefore, PL of seed production requires two conditions: (i) a plant receives too few pollen grains to fertilize all of its ovules and (ii) it has sufficient resources to develop all genetically viable embryos. Accordingly, PL involves both quantity and quality components because incomplete fertilization can result from poor pollination and/or poor survival of male gametophytes after pollination (Aizen & Harder 2007). Pollen quality can also affect the genetic viability of embryos, although this influence is better considered as a component of offspring quality when an embryo's viability depends on its diploid genotype (e.g. expression of paternal recessive lethal allele), rather than its paternal haplotype. Finally, although the preceding definition focuses on a plant's seed production, PL within a population requires poor siring success overall, as we demonstrate below. Furthermore, from the male perspective opportunities for improvement of pollen dispersal and post-pollination performance exist regardless of whether seed production is pollen limited.
Figure 1.
Graphical model of (a) the limits on total seed production and (b) the reproductive transitions considered in the model of selection on reproductive traits (which also considers outcross siring success). Panel a illustrates combinations of cross-fertilization (Fx) and self-fertilization (Fs) that result in PL (white area below diagonal), ovule limitation (heavy diagonal line) and resource limitation (grey area) of seed production (areas above the diagonal are not possible) given that 30 and 80% of selfed and outcrossed zygotes survive genetic death to become embryos (i.e. gs = 0.3, gx = 0.8), maternal plants have sufficient resources to mature 60% of their ovules into seeds (R/O = 0.6) and the probability that selfed seeds establish reproductive offspring is 70% that for outcrossed seeds (ds/dx = 0.7). The arrows projecting from the black, grey and white symbols illustrate examples of possible directions of evolution under pollen, ovule and resource limitation, respectively, and the associated equations relate necessary conditions for inbreeding depression. The slope of the dashed line projecting from the origin is Fx/Fs, which is a selection threshold under resource limitation (see equation (4.3)). The black dashed line through the pollen-limited (i.e. black) point is its associated fitness isocline: selection can move the mating system only to the right of this isocline. The grey dashed line through the same point is the seed-production isocline. In panel (b) the height of each block depicts the number of entities (pollen, zygotes, etc.) during each reproductive stage, with white and grey areas representing outcrossed and selfed fractions, respectively.
PL of seed production arises for diverse reasons (table 1). Most obviously, stigmas can receive few pollen grains because of few pollinator visits, insufficient pollen availability and/or inefficient pollen dispersal. Visit limitation can occur because either pollen vectors are rare in the environment (e.g. the calm air in forest understories compromises wind pollination), or animal pollinators visit a plant's flowers infrequently because they are less attractive than available alternatives (Mitchell et al. 2009). Such mechanisms seem to be especially common causes of PL for species that produce pollen aggregated in pollinia, for which pollen removed by pollinators has a high chance of reaching stigmas (Harder & Johnson 2008). The second cause of insufficient pollen import, low pollen availability, can arise because the environment includes few compatible pollen donors when stigmas are receptive (Aizen 2001; Busch & Schoen 2008; Jakobsson et al. 2009); some flower-visiting animals act as pollen thieves, rather than pollinators (Hargreaves et al. 2009) or rain or wind dislodges pollen before removal (Aizen 2003; Hase et al. 2006). Finally, inefficient pollen transfer can arise because pollen falls during transport, often assisted by pollinator grooming (Thomson 1986; Rademaker et al. 1997), it is deposited on stigmas of other species (Morales & Traveset 2008; Mitchell et al. 2009) or carried out of the population (Cresswell et al. 2002). Together, reduced pollen availability and inefficient pollen transfer must commonly contribute to PL of species with granular pollen because less than 1 per cent of the pollen removed from such species reaches conspecific stigmas (Harder & Johnson 2008). PL can also occur despite abundant pollen import if fewer male gametophytes access ovules than are necessary for complete fertilization, because of limited pollen-grain germination or pollen-tube attrition owing to self-incompatibility, gametophyte competition, cool temperatures or physical limits imposed by the cross-sectional area of stylar transmitting tissue (Hormaza & Herrero 1996; Hedhly et al. 2005). Finally, zygote death due to the expression of lethal genes can reduce the number of embryos below a plant's capacity to mature seeds, given the available resources (Charlesworth 1989).
Table 1.
A summary of factors that could cause PL and associated adaptive responses. References were selected primarily to illustrate the adaptive solution, so some do not consider its association with PL.
| cause of PL | possible adaptive solutions | sample references |
|---|---|---|
| visit limitation | ||
| pollen vector rare | autonomous selfing | Armbruster et al. (2002), Barrett et al. (2009) |
| different pollen vector | Bernardello et al. (2001), Johnson (2006), Friedman & Barrett (2009) | |
| different flowering period | Armbruster & Muchhala (2009) | |
| unattractive, either absolutely or relative to co-flowering speciesa | autonomous selfing | Fishman & Wyatt (1999) |
| increased floral signals and/or rewards, more flowers open simultaneously | Armbruster & Muchhala (2009) | |
| different pollen vector | Johnson (2006), Friedman & Barrett (2009) | |
| floral mimicry of attractive species | Peter & Johnson (2008) | |
| expanded spectrum of pollen vectors | Armbruster & Baldwin (1998) | |
| increased floral longevity | Ashman (2004) | |
| limited pollen availability | ||
| mate limitation | increased compatibility of mating classesb | Busch & Schoen (2008) |
| different sexual systemc | Ehlers & Bataillon (2007), Sakai & Wright (2008) | |
| pollen theft | flowering when thieves are inactive | Hargreaves et al. (2009) |
| hidden pollen | Hargreaves et al. (2009) | |
| deterrent pollen | Hargreaves et al. (2009) | |
| pollinator shift converting thieves to pollinators | Hargreaves et al. (2009) | |
| pollen displaced (rain, wind) | enclosed sex organs | |
| pendant flowers | Aizen (2003) | |
| flower closure during inclement periods | Hase et al. (2006) | |
| inefficient pollen transfer | ||
| transport loss | increased precision of pollen exchange with pollinatorsa | Armbruster & Muchhala (2009) |
| fewer flowers displayed simultaneously, reducing pollen discounting caused by geitonogamy | ||
| shift to more efficient vector | Thomson & Wilson (2008), Armbruster & Muchhala (2009), Friedman & Barrett (2009) | |
| interspecific transfer | enhanced attractivenessa | Morales & Traveset (2008) |
| shifted flowering period | Armbruster & Muchhala (2009) | |
| carried out of population | stickier stigma | |
| low pollen-tube survival | ||
| self-incompatibility | self-compatibility | Busch & Schoen (2008), Igic et al. (2008) |
| limited transmitting tissue | altered pistil characteristics | |
| zygote death | purging of deleterious alleles | Husband & Schemske (1996), Crnokrak & Barrett (2002) |
aApplicable only to animal-pollinated species.
bApplicable only to species with self- or heteromorphic incompatibility.
cPrimarily applies to heterostylous or (gyno)dioecious species.
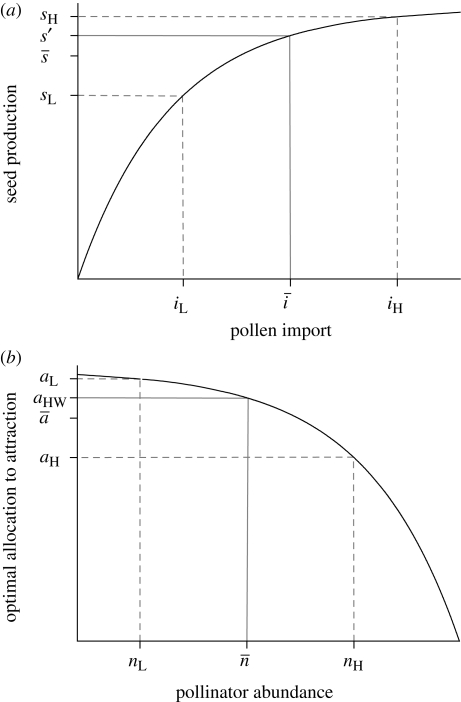
Our definition of PL focuses on individual plants, rather than individual flowers or the entire population, as this perspective is most relevant from an evolutionary perspective. Importantly, PL can occur even if it affects only some of a plant's fruits. Indeed, because of extensive variation in pollen import among its flowers, a plant (or population) could experience PL, even though the average flower received enough pollen for complete fertilization (Richards et al. 2009; figure 2a). Such ‘variance limitation’ can be a significant component of PL, even for species with uniovulate flowers (Richards et al. 2009).
Figure 2.
Graphical models of the effects of (a) variation in pollen import (i) on mean seed production (s; based on Richards et al. 2009) and (b) variation in pollinator abundance (n) on the expected optimal allocation to pollinator attraction (a; inspired by Burd 2008). The black curve in each panel depicts the relation of the dependent to the independent variable. The solid grey line maps the mean independent variable into the corresponding value of the dependent variable and the dashed grey lines map low (L) and high (H) values of the independent variable that are equally spaced below and above the mean independent variable. Because seed production increases nonlinearly with pollen import, mean seed production for variable pollen import (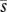 ) is less than the fecundity expected given the mean pollen import (s′), both of which are lower than the maximum possible seed production, indicating PL. Richards et al. (2009) referred to the difference between s′ and
) is less than the fecundity expected given the mean pollen import (s′), both of which are lower than the maximum possible seed production, indicating PL. Richards et al. (2009) referred to the difference between s′ and 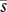 as variance limitation. Similarly, because the optimal allocation to pollinator attraction declines nonlinearly with pollinator abundance, the average optimal allocation to attraction (
as variance limitation. Similarly, because the optimal allocation to pollinator attraction declines nonlinearly with pollinator abundance, the average optimal allocation to attraction (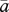 ) is less than the optimum expected for average pollinator abundance (aHW), which is equivalent to the balance between pollen and resource limitation predicted by Haig & Westoby (1988), resulting in chronic PL.
) is less than the optimum expected for average pollinator abundance (aHW), which is equivalent to the balance between pollen and resource limitation predicted by Haig & Westoby (1988), resulting in chronic PL.
3. How common is pollen limitation and when does it matter?
The general ecological and evolutionary relevance of PL depends on both its frequency and consequences. Unfortunately, the typical incidence of PL remains unclear and the reported frequency probably represents a maximum. PL is usually assessed by supplementing the pollen received by flowers with cross-pollen and comparing subsequent fruit or, preferably, seed production with that of naturally pollinated plants. Such experiments focus on quantitative aspects of pollen import, but they overestimate this component if the cross-pollen used for supplementation has higher average genetic quality than the mixture of self- and cross-pollen that many flowers receive naturally (Aizen & Harder 2007). Furthermore, the common supplementation of only a fraction of a plant's flowers often causes a stronger response than supplementation of all flowers because partial supplementation allows reallocation of resources to fruits with more and/or better embryos (Zimmerman & Pyke 1988; Knight et al. 2006). Even when supplementation indicates PL during a single flowering season, iteroparous species may not suffer lifetime limitation, which is most relevant for population dynamics and natural selection, because elevated reproductive effort stimulated by supplementation during one flowering season typically reduces a plant's seed production during subsequent seasons (Knight et al. 2006). Finally, the populations and species for which published results of pollen supplementation experiments are available may not be representative because of unequal geographic and taxonomic sampling (Vamosi et al. 2006) and a publication bias favouring statistically significant responses (Knight et al. 2006).
Given the preceding problems, the finding that roughly 60 per cent of pollen supplementation experiments significantly elevate seed production (Larson & Barrett 2000; Ashman et al. 2004; Knight et al. 2005a) must overestimate the general incidence of PL; however, it still suggests that incomplete ovule fertilization compromises seed production by many plants. Both non-adaptive and adaptive causes probably contribute to the incidence of PL, although their relative importance is unknown. One non-adaptive explanation proposes that PL has recently increased in frequency because of anthropogenic disturbance of plant–pollinator interactions (Ashman et al. 2004)—we address this possibility and its possible evolutionary consequences in more detail below (§6).
A second proposed non-adaptive explanation suggests that PL often persists because it is not subject to selection to eliminate it (Calvo 1993; Bond 1994) if seed production does not limit recruitment. Although Kelly et al. (2007) and Price et al. (2008) recently demonstrated both pollen and seed limitation for two species, supporting this assumption, about half of the seed-addition experiments on 90 species reviewed by Turnbull et al. (2000) found no evidence of seed limitation, especially in established communities, indicating that other processes govern abundance in many plant populations. In such cases, PL is of no ecological (or conservation) consequence. However, seed limitation does not alter the evolutionary relevance of PL (e.g. Morgan & Wilson 2005; Morgan et al. 2005) if plants that are less pollen limited and produce more seeds are represented by a larger fraction of offspring in the next generation than more pollen-limited individuals. Thus, the occurrence of seed limitation cannot explain the long-term persistence of PL.
To date, the only adaptive explanation for persistent PL (Burd 2008) recognizes that pollination varies extensively among plants and among reproductive seasons for individual plants (Herrera 2002; Kilkenny & Galloway 2008; Jakobsson et al. 2009). Burd (2008) considered a stochastic variant of Haig & Westoby's (1988) model of the consequences of a resource trade-off between investment in pollinator attraction (counteracting PL) and investment in seed development (counteracting resource limitation). Haig and Westoby predicted that selection poised seed production at the transition between pollen and resource limitation. In contrast, because increased attractiveness returns more benefit when pollinators are rare than when they are common, the optimal attractiveness with variable pollinator abundance (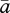 in figure 2b) is lower than Haig and Westoby's prediction (aHW in figure 2b) and so would involve adaptive PL. Burd's explanation has much to commend it, as animal pollination is highly variable (Richards et al. 2009). However, this explanation cannot easily account for the extreme PL experienced by some species, such as many deceitful orchids (Tremblay et al. 2005), and it is not directly applicable to abiotically pollinated species, which cannot manipulate the frequency of interaction with their pollen vector. Thus, a complete adaptive explanation for the incidence of PL remains elusive. Perhaps more importantly, whether the PL seen in many populations represents an equilibrium maintained by selection or a non-equilibrium state that is either transitory as populations evolve to a non-pollen-limited state after a historical perturbation or persists because of ongoing stochastic perturbation is unclear.
in figure 2b) is lower than Haig and Westoby's prediction (aHW in figure 2b) and so would involve adaptive PL. Burd's explanation has much to commend it, as animal pollination is highly variable (Richards et al. 2009). However, this explanation cannot easily account for the extreme PL experienced by some species, such as many deceitful orchids (Tremblay et al. 2005), and it is not directly applicable to abiotically pollinated species, which cannot manipulate the frequency of interaction with their pollen vector. Thus, a complete adaptive explanation for the incidence of PL remains elusive. Perhaps more importantly, whether the PL seen in many populations represents an equilibrium maintained by selection or a non-equilibrium state that is either transitory as populations evolve to a non-pollen-limited state after a historical perturbation or persists because of ongoing stochastic perturbation is unclear.
4. Model of selection under contrasting seed-production limits
(a). Derivation and results
To illustrate the micro-evolutionary consequences of constraints on seed production, especially PL, we now present a simplified model of selection on floral traits and the mating system (summarized in figure 1b). This model examines the fate of a variant individual in a population that otherwise includes only resident individuals with floral characteristics that result in cross-fertilization of Fx ovules, self-fertilization of Fs ovules and siring of E embryos on other plants (for the residents Fx = E because every zygote has a mother and a father; however it is useful to keep these components separate). We assume that pollen used in self-pollination does not reduce opportunities for siring seeds on other plants (see Harder et al. 2008 for the effects of such pollen discounting). Fertilization is ovule limited if all O ovules are fertilized (Fs + Fx = O; figure 1a, diagonal line), or pollen limited otherwise (Fs + Fx < O; figure 1a, below diagonal line). We assume that some zygotes die immediately after fertilization, because of lethal genetic traits, so selfed and outcrossed zygotes survive to become embryos with probabilities gs and gx, respectively, where gs < gx because of the higher homozygosity of selfed zygotes. If the number of resulting embryos is less than the R seeds that a plant can produce given its available resources (i.e. Fsgs + Fxgx ≤ R), then all embryos develop and seed production is either pollen limited (figure 1a, white area) or ovule limited (figure 1a, heavy portion of diagonal line) depending on which component constrained ovule fertilization. Otherwise (i.e. Fsgs + Fxgx > R), seed production is resource-limited (figure 1a, grey area), and we assume that selfed and outcrossed embryos compete for maternal resources in proportion to their abundance, resulting in FsgsR/(Fxgx + Fsgs) and FxgxR/(Fxgx + Fsgs) selfed and outcrossed seeds, respectively (unequal competition does not affect the results, so we do not consider it explicitly here). If outcrossed and selfed seeds disperse and survive to become reproductive adults with probabilities dx and ds, lifetime inbreeding depression equals
| 4.1 |
Outcrossed offspring possess one maternal haplotype compared with two for selfed offspring, so the latter are twice as valuable as fitness contributions if they survive. Selection on reproductive traits occurs when the variant's fitness, w′, exceeds that of resident individuals, w, or
| 4.2 |
We present the detailed fitness expressions and invasion criteria in the appendix.
First consider ovule limitation (i.e. Fs = O − Fx and Fsgs + Fxgx ≤ R: invasion criterion equation (A 1)), which leads to familiar results because this condition is implicit in many influential models of mating-system evolution (e.g. Lloyd 1979; Lande & Schemske 1985). In this case, the variant can invade with no change in siring success either by producing more outcrossed seeds if inbreeding depression is strong, δ > 0.5, or by producing more selfed seeds if it is weak, δ < 0.5 (e.g. figure 1a, grey point). The variant can also invade with no change in its own seed production if its outcrossed siring exceeds that of resident individuals, regardless of inbreeding depression, because outcrossed siring uses ovules on other plants, displacing both self- and cross-fertilization by competing resident individuals. The benefit of increased pollen export is universal, as it also applies under resource and PL, so that constraints on seed production qualitatively modify only the evolution of traits that influence female function. We will not consider the evolution of traits that affect only male success further.
Resource competition among developing embryos alters the invasion conditions (equation (A 2)). Now, floral traits that promote a plant's production of outcrossed seeds evolve if
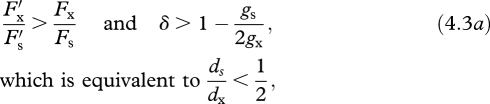 |
whereas traits that promote selfing evolve if
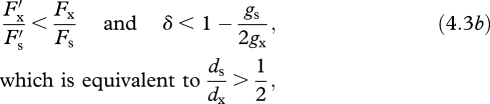 |
(e.g. figure 1a, white point). Thus, selection for enhanced outcrossing requires that the ratio of the variant's cross- to self-fertilization ability exceeds that of residents (represented by the slope of the dashed line in figure 1a), with the opposite condition for the evolution of increased selfing. Because gs < gx, the inbreeding-depression threshold for the evolution of increased outcrossing under resource limitation is more stringent than under ovule limitation, whereas the condition for the evolution of selfing is less rigorous. Specifically, selection depends on the relative survival of selfed and outcrossed offspring only after seed dispersal (ds/dx), rather than total lifetime inbreeding depression after fertilization because, according to our model, the selfed embryos that compete for maternal resources have already survived early-acting inbreeding depression.
Finally, PL leads to a simple, yet profound, result: PL allows for selection of traits for either increased cross-fertilization (Fx′ > Fx) and/or increased self-fertilization (Fs′ > Fs), regardless of inbreeding depression (invasion criterion equation (A 1)). Indeed, combinations of increased selfing and reduced outcrossing (and vice versa) can evolve as long as they increase maternal fitness (e.g. any direction from the black point in figure 1a to the right of the dashed black fitness isocline). Notice that this fitness increment need not be gained by increased seed production, which are not equivalent owing to the relatively lower survival of selfed seeds coupled with their potential two-fold fitness benefit (compare black fitness isocline and grey seed-production isocline in figure 1a). Because PL eliminates key constraints that otherwise fetter floral and mating-system evolution under ovule and resource limitation, it releases the adaptive potential of selection and creates novel evolutionary opportunities. Which of these opportunities are explored depends on ecological circumstance, the additive genetic variance and covariance of relevant floral and inflorescence traits (see Fishman & Willis 2008) and the evolutionary history of the population (see §4b).
(b). Specific evolutionary responses to pollen limitation
Despite simplifying many aspects of plant reproduction, our model reveals several general conclusions about the nature of PL and its evolutionary consequences. Of underlying importance is the recognition of a third constraint on seed production, so that alleviation of PL need not cause resource limitation, as is generally assumed (e.g. Haig & Westoby 1988; Zimmerman & Pyke 1988; Ashman et al. 2004; Burd 2008; Ornelas & Lara 2009), but it could alternatively lead to ovule limitation, whereby increased ovule production during flower development would elevate seed production. When gs ≤ R/O ≤ gx (as in figure 1a; see Harder et al. (2008) for other conditions), these alternatives create the possibility of three adaptive mating systems, each associated with particular limits on seed production: exclusive outcrossing coupled with resource limitation when ds/dx < 0.5 (upper vertex in figure 1a); exclusive selfing coupled with ovule limitation when ds/dx > 0.5 and δ < 0.5 (right vertex in figure 1a); or mixed mating at the intersection between ovule and resource limitation when ds/dx > 0.5 and δ > 0.5 (upper end of the solid black diagonal in figure 1a). None of these options involve PL, so that it is less likely to prompt the evolution of the best possible mating system. Instead, under PL, the evolution of the optimal mating system becomes somewhat secondary to simply resolving the limit on seed production. For this reason, selfing that provides reproductive assurance is generally beneficial under PL (Lloyd 1992; Eckert et al. 2006), even though it seldom produces the best combination of selfed and outcrossed offspring (Harder et al. 2008). Of more central importance is the general conclusion that PL allows complete latitude for the selection of increased selfing and the autonomy from pollen vectors that it can provide and/or various means of improving outcrossing (also see Eckert et al. in press). Notice that because of the diversity of pollination and post-pollination influences on PL (table 1), the variety of opportunities for increased outcrossing under PL includes options other than increased pollinator attraction, most of which would be applicable to both biotically and abiotically pollinated species. We now consider some of the possible alternative adaptive solutions to PL.
The universal possibility of increased selfing under PL supports Darwin's (1877) intuition that ‘it would manifestly be more advantageous to a plant to produce self-fertilized seeds rather than none at all or extremely few seeds’ (p. 292), as it provides a reliable means of mitigating PL despite inbreeding depression (Eckert et al. 2006). Thus, the evolution of autonomous selfing mechanisms in response to PL is especially likely when pollen vectors rarely deliver pollen (e.g. Moeller & Geber 2005; Fishman & Willis 2008) and alternative vectors are not available. In principle, a shortage of mates could also promote increased selfing; however, if pollen vectors are sufficiently abundant, this effect should be ephemeral, unless population density is severely seed limited, because as it succeeds and increases mate number the original problem disappears (although see Fishman 2000). Models that contrast alternate selfing modes predict that self-pollination prior to outcrossing should evolve more easily than delayed selfing in pollen-limited environments, especially in annual species, even when inbreeding depression is relatively strong (Morgan & Wilson 2005; Morgan et al. 2005). Prior selfing is facilitated by the evolution of smaller flowers, close proximity of stigmas and anthers, simultaneous anther dehiscence and stigma receptivity and complete self-compatibility (Lloyd 1965; Ritland & Ritland 1989; Armbruster et al. 2002; Moeller & Geber 2005; Vallejo-Marín & Barrett 2009). A corollary of the reproductive-assurance hypothesis proposes that a capacity for selfing can pre-adapt species to occupy new environments with few pollinators and/or potential mates (Baker 1955, 1967). This expectation is supported by recent comparisons of species that have naturalized beyond their native ranges with congeneric species that have not naturalized (van Kleunen et al. 2008; also van Kleunen & Johnson 2007).
Note that despite the elimination of inbreeding depression as a constraint on whether selfing can evolve under PL, inbreeding depression can still influence mating-system evolution in a population with appreciable genetic load for two reasons. First, inbreeding depression influences how alternate changes in selfing and outcrossing alter fitness, so that small increases in selfing associated with large reductions in outcrossing are unlikely (e.g. shifts from the black point in figure 1a to the right and below the black fitness isocline). Second, initial stages during the evolution of increased selfing would be fraught by the impact of increased expression of deleterious recessive traits on fecundity, establishment and survival as homozygosity increases, which could reduce population size and intensify mate limitation. If the population survives, the resulting selection should purge lethal alleles, but it may lead to the fixation of less deleterious traits (Crnokrak & Barrett 2002; Keller & Waller 2002), so that autonomy from outcrossing is realized initially at the expense of lower survival (e.g. Busch 2006), perhaps decreasing population size sufficiently to cause either extinction or a genetic bottleneck that reduces genetic diversity (Foxe et al. 2009). Given these viability problems with strong initial inbreeding depression, outcrossing options may offer a less resistant evolutionary trajectory, so that autonomous selfing evolves most often in this situation when no alternatives are readily accessible. In contrast, when inbreeding depression is initially low, no such resistance exists and autonomous selfing should evolve readily, given its twofold advantage of genetic transmission.
Despite the benefit of autonomous selfing when pollinators and/or mates are rare, outcrossing solutions to PL may also evolve more commonly because they are relevant in more diverse ecological contexts (table 1). The evolution of autonomous selfing has been recognized as one of the most common transitions during angiosperm history (Stebbins 1974; Barrett et al. 2009) and approximately 15 per cent of angiosperms self-fertilize almost exclusively (Igic & Kohn 2006, fig. 2c). However, this statistic implies that approximately 85 per cent of angiosperms have not followed the path to autonomous selfing, even though PL seems to be common. Floras of oceanic islands provide particularly relevant case studies because isolated islands often have few pollinators. For example, the Juan Fernández Islands support such a limited fauna, but few plant species are obligate selfers (Bernardello et al. 2001). Instead, species in 30 per cent of genera on these islands have a different outcrossing pollination system than those of their putative ancestors and 47 per cent of species are apparently wind-pollinated. Indeed, oceanic island floras generally have disproportionate frequencies of wind pollination, gynodioecy and dioecy (Barrett 1996), which have often evolved in situ (Sakai et al. 1995; Bernardello et al. 2001). As table 1 illustrates, specific outcrossing remedies to PL probably evolve under particular conditions, depending on the underlying cause of the limitation. Furthermore, because multiple factors can contribute simultaneously to PL, the evolutionary response may involve a variety of traits. For example, shifts between cross-pollination systems (e.g. bee- to bird-pollination) could arise when a species' typical pollinator(s) becomes rare or attracted by a new member of the local plant assemblage. However, alternate pollen vectors are likely to disperse pollen inefficiently initially because of a mismatch between floral and inflorescence characteristics, on one hand, and pollinator morphology and behaviour, on the other. This combination of problems may lead to species- or even population-specific evolutionary responses, as exemplified by pollination ecotypes (Johnson 2006). Thus, the array of causes of PL creates a broad palette of opportunities for reproductive diversification.
Nevertheless, some evolutionary options for mitigating PL may be precluded by past events in a lineage's evolutionary history. For example, recent phylogenetic evidence suggests that transitions from pollination by short-tongued pollinators to those with longer tongues, such as from bee to hummingbird, may often be irreversible (Whittall & Hodges 2007; Thomson & Wilson 2008; but see Tripp & Manos 2008). Similarly, the evolution of dioecy probably reduces the possibility for selfing solutions to pollinator rarity and/or mate limitation, instead favouring the evolution of wind pollination (Friedman & Barrett 2009). Such historical constraints may even contribute to the limited evolutionary success of some reproductive systems. For example, dioecy is inherently susceptible to PL because subdivision of the population into females and males increases the chance of mate limitation. This feature coupled with the limited evolutionary flexibility of dioecy probably contributes to the relatively high extinction rates of dioecious lineages (Vamosi & Otto 2002; Vamosi & Vamosi 2005). Thus, although PL generally expands evolutionary options, it need not do so in specific cases, depending on historical constraints.
5. Pollen limitation and plant diversification
Given the increased latitude for selection on floral traits afforded by PL, it should create fertile ground for reproductive diversification within clades. This potential may not be realized when obligate selfing evolves for, as Darwin (1877, p. 292) suspected, selfing lineages are relatively ephemeral, perhaps because of increased extinction rates associated with reduced genetic diversity and accumulation of deleterious mutations (Takebayashi & Morrell 2001; Bartkowska & Johnston 2009). Furthermore, obligate selfing is an absorbing state from which outcrossing forms cannot evolve because no outcrossing benefit can counteract the twofold transmission advantage of selfed offspring (Takebayashi & Morrell 2001). Thus, the evolution of obligate selfing may be an expedient solution to prevailing PL that has a limited evolutionary future. In contrast, outcrossing solutions to PL have the capacity to generate considerable diversity, especially when the nature of the constraint differs among populations, resulting in contrasting adaptations to local pollination environments (e.g. Totland 2001; Moeller 2004). Local shifts between pollen vectors or sexual systems or in flowering phenology would be particularly effective in this context because they could intrinsically impose pre-zygotic reproductive isolation should diverged populations subsequently come in contact (Armbruster et al. 2002; Harder & Johnson 2009).
Current diversity is a product of past events, so direct evidence of a generative role of PL in angiosperm diversity will be elusive; however, geographical associations between contemporary PL and species diversity may provide indirect evidence. In this context, Vamosi et al. (2006) found that global ‘hotspots’ of angiosperm diversity also tend to be areas of common PL. They interpreted this association as an ecological consequence of heightened competition for pollinators (see also Armbruster & Muchhala 2009). However, as table 1 illustrates, such competition represents only a fraction of the various causes of PL, and the influences of the remaining causes in regions of lower diversity should tend to weaken such an ecological association. Furthermore, despite the intrinsic appeal of competition for pollinators as an explanation of ecological patterns, convincing evidence supporting its widespread occurrence is rather limited (Mitchell et al. 2009) and is offset by many examples of facilitation among co-flowering species, especially those subject to PL (e.g. Moeller 2004; Peter & Johnson 2008). Alternatively, high biodiversity may be an evolutionary outcome of common PL in particular regions (Johnson 1996). Such extreme diversification requires the appropriate conditions, such as a diversity of functionally different pollen vectors, which may not exist in some areas, such as the arctic and alpine. A corollary of this hypothesis emerges from the tendency for self-incompatible species to experience more PL than self-compatible species (Larson & Barrett 2000; Knight et al. 2005a). Specifically, Igic et al. (2004, 2008) noted that because self-incompatibility seems not to evolve from self-compatibility, its prevalence despite common transitions in the opposite direction implies greater diversification among self-incompatible species. Clearly, the hypothesis that PL stimulates diversification is speculative; however, it does arise logically from the micro-evolutionary effects of PL discussed above, for which more evidence exists.
6. Evolutionary implications of anthropogenic pollen limitation
I have very little doubt that if the whole genus of humble-bees became extinct or very rare in England, the heartsease and red clover would become very rare, or wholly disappear. The number of humble-bees in any district depends in a great degree on the number of field-mice, which destroy their combs and nests … . Now the number of mice is largely dependent, as every one knows, on the number of cats … . Hence it is quite credible that the presence of a feline animal in large numbers in a district might determine, through the intervention first of mice and then of bees, the frequency of certain flowers in that district!
Darwin (1859, pp. 73–74)
The preceding quotation illustrates Darwin's recognition of the role of pollination as an ecological linkage and the possible consequences of its disruption (see Knight et al. (2005b) for a contemporary example). Because of this ecosystem function, the increasing degradation of most natural or seminatural terrestrial biomes by human activity and the resulting expansion of highly disturbed anthropogenic habitats (Sala et al. 2000) raises concerns about the possibility of globally increasing PL and impairment of plant reproduction (Kearns et al. 1998; Knight et al. 2005a; Vamosi & Vamosi 2005; Aguilar et al. 2006). The ecological effects of human disturbance on a population will alter the relations of traits to fitness and so can modify the nature of selection. Therefore, we now consider evidence of increasing pollination limitation under disturbance and assess the relative importance of selection for increased selfing versus outcrossing in disturbed environments, recognizing that these long-term effects are secondary, from a conservation perspective, to the more immediate ecological impacts (e.g. Pauw 2007).
The evolutionary response to aggravated PL caused by anthropogenic disturbance will depend on the nature and severity of the disturbance, although most effects are likely to involve pollination, rather than post-pollination, components of PL. Aizen & Vázquez's (2006) survey of the ecological effects of human disturbance on pollination indicate diverse impacts (see also Knight et al. 2005a; Eckert et al. in press). Habitat destruction could affect reproduction directly by reducing plant density and imposing mate limitation, whereas indirect effects through reduction or loss of pollinator species could cause visit limitation. The introduction of non-native plant species could intensify PL by increasing competition for pollinators and/or interspecific pollen transfer, or it could alleviate this seed-production limit if native and introduced species facilitate each other's pollination. In contrast, the introduction of non-native floral visitors could reduce pollen availability if they act as pollen thieves, reduce pollen-transfer efficiency if they pollinate poorly or reduce PL if they pollinate efficiently. Given that these different causes of PL stimulate different selection responses (table 1), human activity probably has diverse effects on floral evolution. As a more detailed illustration of possible evolutionary effects of human disturbance, we now consider possible impacts of habitat fragmentation.
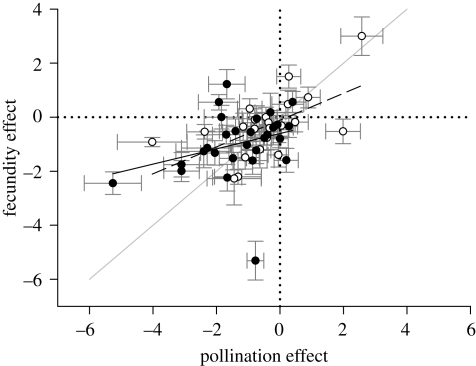
Evidence that habitat fragmentation intensifies PL was provided recently by Aguilar et al.'s (2006) meta-analysis of its effects on pollination (visit frequency, stigmatic pollen loads or pollen tubes in styles) and female fecundity (fruit or seed production). They found that fecundity varied positively with pollination among species, and that both performance measures were lower overall for plants inhabiting habitat fragments (figure 3), suggesting that fragmentation generally aggravates PL. Overall, the slope of the relation between the effects of fragmentation on fecundity and on pollination was <1 (b ± s.e. = 0.495 ± 0.176, t46 = 2.87, p < 0.01; figure 3), indicating a weaker influence on fecundity, probably because seed production increases asymptotically with pollen import (Aizen & Harder 2007), but also perhaps because post-pollination processes, such as relaxation of self-incompatibility with flower age (e.g. Stephenson et al. 2003), buffer the effects of fragmentation. In general, self-incompatible species experienced stronger effects of fragmentation than self-compatible species. This difference is unlikely to reflect reproductive assurance for the latter group because it involved both pollination and fecundity, rather than fecundity alone. Figure 3 also illustrates that some species, such as the New Zealand mistletoe Peraxilla tetrapetala (Burgess et al. 2006), benefit from fragmentation (upper-right quadrant), suggesting that human-modified habitats can sometimes be pollinator-rich. Indeed, moderately disturbed habitats and fine-grained habitat mosaics may sustain high abundance and diversity of pollinators (Winfree et al. 2009). In such environments, mate availability, rather than pollinator availability, may primarily determine the severe pollination limitation frequently observed in outcrossing species under anthropogenic disturbance (Aizen & Feinsinger 2003). In extreme conditions, such mate limitation could lead to extinction (Vamosi & Vamosi 2005).
Figure 3.
Relation between the mean (±s.e.) effects of habitat fragmentation on pollination and female fecundity for 23 self-compatible (dashed line) and 27 self-incompatible (solid line) species (modified from Aguilar et al. 2006). Effect size was measured by Hedge's d. Reanalysis by ANCOVA detected a positive relation of fecundity to pollination success (F1, 46 = 7.95, p < 0.01), but this effect did not differ significantly between self-compatible and self-incompatible species (compatibility class, F1, 46 = 1.60, p > 0.2; interaction, F1, 46 = 0.76, p > 0.3). Open circle, self compatible; filled circle, self-incompatible. The grey diagonal line indicates equal effect sizes for pollination and fecundity.
Is increased selfing or outcrossing the primary adaptive response to habitat disturbance? This question cannot be answered unequivocally because direct effects may be too recent to have caused detectable change, given the lack of a relevant historical record, and studies of phenotypic selection on floral traits have yet to contrast populations that differ in anthropogenic disturbance. Nevertheless, we sought relevant evidence by considering recent evolutionary transitions in sexual system associated with the occupation of disturbed habitats in Bertin's (1993) compilation of floral, pollination and breeding traits for more than 4000 plant species. We specifically searched for genera of terrestrial species with some species inhabiting disturbed environments (Bertin's ‘disturbed ground’) and congeners from any of the eight other terrestrial habitats that Bertin recognized. For each genus, we considered three traits often associated with the selfing–outcrossing gradient (Lloyd 1965; Ritland & Ritland 1989; Armbruster et al. 2002): compatibility system (self-compatible = 0, variable = 0.5, self-incompatible = 1), autonomous autogamy (no = 0, variable = 0.5, yes = 1) and flower size (continuous, back-transformed from log values), and used averaged data for multiple congeners in the same habitat class. For each trait, we assessed whether the selfing category was represented by species from disturbed or more natural habitats (for flower size, a 25% difference was required to establish a trend). Table 2 summarizes the results for the 35 genera in 20 families with suitable data (26, 19 and 14 genera for compatibility system, autogamy and flower size, respectively).
Table 2.
Evidence for the evolution of enhanced selfing in ‘disturbed’ habitats compared with congeneric species from ‘undisturbed’ habitats based on the categorization and data compiled by Bertin (1993). We evaluated the change in three characteristics associated with selfing: self-compatibility, spontaneous autogamous and small flowers. For each trait we recorded ‘yes’ if species in disturbed habitats exhibited greater self-compatibility (SC), more autonomous autogamy and/or decreased flower size, ‘no’ if the trend suggests the opposite, ‘=’ if all surveyed species of the genus shared the same state (listed parenthetically for compatibility) and ‘ … ’ if the trait was not recorded for the genus.
| genus | family | pollination mode | increased SC? | increased autogamy? | decreased flower size?b |
|---|---|---|---|---|---|
| Arenaria | Caryophyllaceae | autogamy/insect | =(SC) | … | yes |
| Cerastium | Caryophyllaceae | autogamy/insect | =(SC) | = | yes |
| Sagina | Caryophyllaceae | autogamy/insect | … | = | … |
| Stellaria | Caryophyllaceae | autogamy/insect | =(SC) | yes | yes |
| Geranium | Geraniaceae | autogamy/insect | =(SC) | yes | yes |
| Stachys | Lamiaceae | autogamy/insect | =(SC) | = | … |
| Malva | Malvaceae | autogamy/insect | … | … | yes |
| Veronica | Plantaginaceae | autogamy/insect | =(SC) | = | yes |
| Epilobium | Onagraceae | autogamy/insecta | =(SC) | no | no |
| Chrysanthemum | Asteraceae | insect | =(SI) | = | … |
| Cirsium | Asteraceae | insect | no | … | … |
| Pyrrhopappus | Asteraceae | insect | =(SC) | = | … |
| Senecio | Asteraceae | insect | … | yes | … |
| Solidago | Asteraceae | insect | … | = | … |
| Erysimum | Brassicaceae | insect | yes | … | … |
| Thlaspi | Brassicaceae | insect | … | yes | … |
| Silene | Caryophyllaceae | insect | =(SC) | … | no |
| Dalechampia | Euphorbiaceae | insect | =(SC) | … | … |
| Spiranthes | Orchidaceae | insect | =(SC) | … | … |
| Melampyrum | Orobanchaceae | insect | =(SC) | = | … |
| Portulaca | Portulacaceae | insect | =(SC) | = | … |
| Ranunculus | Ranunculaceae | insect | no | no | = |
| Agrimonia | Rosaceae | insect | =(SC) | … | … |
| Potentilla | Rosaceae | insect | … | … | = |
| Asperula | Rubiaceae | insect | … | … | = |
| Galium | Rubiaceae | insect | … | … | yes |
| Lobelia | Campanulaceae | insect/bird | =(SC) | yes | … |
| Lopezia | Onagraceae | insect/bird | … | … | = |
| Passiflora | Passifloriaceae | insect/bird | yes | = | … |
| Atriplex | Chenopodiaceae | wind | =(SC) | … | … |
| Plantago | Plantaginaceae | wind | yes | … | yes |
| Alopecurus | Poaceae | wind | no | … | … |
| Bromus | Poaceae | wind | yes | yes | … |
| Lolium | Poaceae | wind | yes | … | … |
| Polygonum | Polygonaceae | wind | =(SC) | yes | … |
aSome Epilobium spp. are bird-pollinated but they are not included in this comparison.
bAn arbitrary 25% difference in flower size between habitat types was required to be recognized as a change.
Of the 59 possible genus–trait contrasts, 32 were uninformative because the species in a genus shared the same category, but 20 of the remaining 27 contrasts are consistent with more selfing by species that occupy disturbed habitats and seven suggested the opposite (binomial test of equal probability, p = 0.019). This pattern persists after counting common responses for multiple traits only once to guard against pseudoreplication, resulting in 16 versus five contrasts in favour of more selfing in disturbed habitats (p = 0.027). These results could reflect an ecological, rather than evolutionary association, whereby species capable of reproductive assurance are better suited to occupy disturbed sites (Baker 1955, 1967). However, for three of the eight genera for which the occurrence of self-incompatibility differed between the two disturbance categories, disturbed habitats are occupied by self-incompatible species (p = 0.22), which is inconsistent with this explanation (evolution of self-incompatibility from self-compatibility in response to disturbance seems unlikely, given the lack of evidence for this transition among angiosperms as a whole; Igic et al. 2008). In this context, our results imply that increased selfing may evolve about three to four times more often than increased outcrossing in response to anthropogenic disturbance, at least for short-lived herbs, which are represented disproportionately in table 2 (see also Eckert et al. in press). In contrast, for long-lived species, which bear relatively high genetic loads (Klekowski 1988), the evolutionary trajectory of least resistance may lead to outcrossing adaptation to human disturbance. In particular, mechanisms that promote outcrossing may be common for trees, as habitat fragmentation, and disturbance in general, can promote long-distance pollen dispersal (Young et al. 1996).
We conclude this extended example by considering whether anthropogenic habitat disturbance primarily causes visit or mate limitation. Not surprisingly, the results in table 2 provide mixed evidence that either factor dominates. For instance, several contrasts in table 2 suggest a shift towards increased autogamy from an insect-pollinated ancestor (table 2). All the species in genera with mixtures of autogamous and insect-pollinated species are self-compatible, indicating that self-compatibility per se does not provide enough reproductive assurance in habitats that are pollinator-poor. On the other hand, the 3 : 1 ratio in favour of increased selfing for species that occupy disturbed habitats in the entire dataset is also evident for the smaller subset of wind-pollinated taxa (table 2), which represent most of the cases of self-incompatibility rather than self-compatibility in disturbed habitats, suggesting an influence of mate limitation. Often, visit and mate limitation may cause PL synergistically because the restriction of pollen dispersal caused by pollinator rarity will exacerbate the effect of few mates. However, as humans generate more persistent and widespread disturbed environments, depauperate pollinator faunas also become more common (Ricketts et al. 2008; Winfree et al. 2009), so the relative importance of visit limitation may be increasing. Clearly, better understanding of the varied causes of PL and their evolutionary consequences will be required to predict, manage and mitigate human influence on the evolution of plant reproduction.
7. Concluding comments
Left unchecked, the selection on reproductive traits stimulated by PL should eventually improve reproduction sufficiently that it becomes either ovule- or resource-limited, so the commonness of PL is enigmatic. As mentioned above (§3; figure 2b), human disturbance and stochasticity in pollination may contribute to persistent PL. Our analysis identifies some additional aspects of selection and diversification under PL that may also contribute, so we close by considering their possible effects.
The implementation of delayed self-pollination to assure reproduction when ovule fertilization is incomplete (Eckert et al. 2006) may complicate selection against PL. Although delayed self-pollination may fertilize all ovules, the vector-mediated component of fertilization is pollen limited. This limitation will be (partially) detected by pollen-supplementation experiments to the extent that cross-pollen applied during supplementation is genetically superior to the self-pollen involved in reproductive assurance (Aizen & Harder 2007). To appreciate the evolutionary consequence of reproductive assurance, consider a plant represented by the black point in figure 1a. Fitness increases fastest from this point perpendicular to the fitness isocline (dashed black line), which should be the evolutionary trajectory, baring genetic and historical constraints. In contrast, reproductive assurance shifts the realized seed production parallel to the abscissa. Thus, if the optimal mating system (and associated floral traits) involves increased outcrossing, reproductive assurance would tend to slow selection towards the optimum, so the population may persist in a maladapted state, probably involving PL of outcrossing, longer than would be the case in the absence of reproductive assurance.
As selection against PL drives the population towards ovule or resource limitation, the population will begin to include plants that are pollen limited and others that are not. Correspondingly, the conditions that control the evolution of selfing and/or outcrossing traits will also differ among plants (figure 1a), possibly resulting in a heterogeneous selection, which may confound the elimination of PL. For populations approaching ovule limitation, this effect could be compounded by a weakening of selection on mildly pollen-limited plants because of the asymptotic improvement in ovule fertilization and subsequent fruit and seed production with increasing pollen import (Mitchell 1997; Aizen & Harder 2007).
The historical constraints that arise during some transitions between pollination and/or sexual systems (§4b) may also be responsible for some cases of persistent PL. For example, suppose that long-tubed flowers evolve in association with a pollinator shift prompted by PL. If this shift is irreversible and the current long-tongued pollinator subsequently becomes rare, the species may be left with no available evolutionary solution, resulting in chronic PL. If so, species pollinated by long-tongued pollinators might be especially susceptible to effects of human disturbance on their pollinators.
Whatever the reason for its prevalence, PL has unique and significant consequences for the evolution and diversification of angiosperm reproduction. Given these consequences and the ecological and conservation implications of PL, its true incidence both within and among populations deserves focused attention (see also Aizen & Harder 2007). In addition, explicit recognition of the diverse causes of PL (table 1) will facilitate implementation of management policies to mitigate anthropogenic PL, understanding of its ecological effects in natural populations and its role in the past, current and future diversity of floral and inflorescence traits, mating systems and sexual systems.
Acknowledgements
We thank Peter Crane, Else Marie Friis and William Chaloner for the opportunity to participate in the Discussion Meeting on Darwin and the evolution of flowers, Ramiro Aguilar for contributing data for figure 3 and discussion, Steven D. Johnson for discussion and comments on the manuscript and the Natural Sciences and Engineering Research Council of Canada (L.D.H.), the Argentina National Council for Research (PIP 5066) (M.A.A.) and the National University of Comahue (B126/04) (M.A.A.) for research funding.
Appendix A: Modelled fitness expressions and invasion criteria
Under ovule limitation, the fitnesses of resident and variant individuals are
where the first two terms represent a plant's own production of outcrossed and selfed offspring, respectively, and the third term represents its success siring offspring with other plants. After simplification, the invasion criterion (equation (4.2)) becomes
| A 1 |
Under resource limitation (i.e. Fsgs+Fxgx > R), the fitnesses of resident and variant individuals are
and
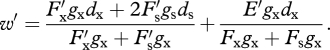 |
Note that because the variant is so rare, its pollen has little impact on the female outcrossing success of resident plants, on average, and the variant sires seeds only in pistils of resident plants. The invasion criterion (equation (4.2)) now is
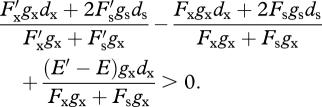 |
A 2 |
Finally, under PL, the fitnesses of resident and variant individuals are
and
and the invasion criterion (equation (4.2)) is
| A 3 |
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Aguilar R., Ashworth L., Galetto L., Aizen M. A.2006Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol. Lett. 9, 968–980 (doi:10.1111/j.1461-0248.2006.00927.x) [DOI] [PubMed] [Google Scholar]
- Aizen M. A.2001Flower sex ratio, pollinator abundance, and the seasonal pollination dynamics of a protandrous plant. Ecology 82, 127–144 (doi:10.1890/0012-9658(2001)082[0127:FSRPAA]2.0.CO;2) [Google Scholar]
- Aizen M. A.2003Down-facing flowers, hummingbirds and rain. Taxon 52, 675–680 (doi:10.2307/3647342) [Google Scholar]
- Aizen M. A., Feinsinger P.2003Bees not to be? Responses of insect pollinator faunas and flower pollination to habitat fragmentation. In How landscapes change: human disturbance and ecosystem disruptions in the Americas (eds Bradshaw G. A., Marquet P. A.), pp. 111–129 Berlin, Germany: Springer-Verlag [Google Scholar]
- Aizen M. A., Harder L. D.2007Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271–281 (doi:10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- Aizen M. A., Vázquez D. P.2006Flower performance in human-altered habitats. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 159–179 Oxford, UK: Oxford University Press [Google Scholar]
- Allen-Wardell G., et al. 1998The potential consequences of pollinator declines on the conservation of biodiversity and stability of food crop yields. Conserv. Biol. 12, 8–17 (doi:10.1111/j.1523-1739.1998.97154.x) [Google Scholar]
- Armbruster W. S., Baldwin B. G.1998Switch from specialized to generalized pollination. Nature 394, 632 (doi:10.1038/29210) [Google Scholar]
- Armbruster W. S., Muchhala N.2009Associations between floral specialization and species diversity: cause, effect, or correlation? Evol. Ecol. 23, 159–179 (doi:10.1007/s10682-008-9259-z) [Google Scholar]
- Armbruster W. S., Mulder C. P. H., Baldwin B. G., Kalisz S., Wessa B., Nute H.2002Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae s.l.). Am. J. Bot. 89, 37–49 (doi:10.3732/ajb.89.1.37) [DOI] [PubMed] [Google Scholar]
- Ashman L.2004Flower longevity. In Cell death in plants (ed. Nooden L. D.), pp. 349–362 London, UK: Elsevier [Google Scholar]
- Ashman T.-L., Morgan M. T.2004Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proc. R. Soc. Lond. B 271, 553–559 (doi:10.1098/rspb.2003.2642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T. L., et al. 2004Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–2421 (doi:10.1890/03-8024) [Google Scholar]
- Baker H. G.1955Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution 9, 347–349 (doi:10.2307/2405656) [Google Scholar]
- Baker H. G.1967Support for Baker's law—as a rule. Evolution 21, 853–856 (doi:10.2307/2406780) [DOI] [PubMed] [Google Scholar]
- Barrett S. C. H.1996The reproductive biology and genetics of island plants. Phil. Trans. R. Soc. Lond. B 351, 725–733 (doi:10.1098/rstb.1996.0067) [Google Scholar]
- Barrett S. C. H., Ness R. W., Vallejo-Marín M.2009Evolutionary pathways to self-fertilization in a tristylous plant species. New Phyt. 183, 546–556 (doi:10.1111/j.1469-8137.2009.02937.x) [DOI] [PubMed] [Google Scholar]
- Bartkowska M. P., Johnston M. O.2009Quantitative genetic variation in populations of Amsinckia spectabilis that differ in rate of self-fertilization. Evolution 63, 1103–1117 (doi:10.1111/j.1558-5646.2008.00607.x) [DOI] [PubMed] [Google Scholar]
- Bateman A. J.1948Intra-sexual selection in Drosophila. Heredity 23, 349–368 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- Bernardello G., Anderson G. J., Stuessy T. F., Crawford D. J.2001A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernández Islands (Chile). Bot. Rev. 67, 255–308 (doi:10.1007/BF02858097) [Google Scholar]
- Bertin R. I.1993Incidence of monoecy and dichogamy in relation to self-fertilization in angiosperms. Am. J. Bot. 80, 557–560 (doi:10.2307/2445372) [DOI] [PubMed] [Google Scholar]
- Bond W. J.1994Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Phil. Trans. R. Soc. Lond. B 344, 83–90 (doi:10.1098/rstb.1994.0055) [Google Scholar]
- Burd M.2008The Haig-Westoby model revisited. Am. Nat. 171, 400–404 (doi:10.1086/527499) [DOI] [PubMed] [Google Scholar]
- Burgess V. J., Kelly D., Robertson A. W., Ladley J. J.2006Positive effects of forest edges on plant reproduction: literature review and a case study of bee visitation to flowers of Peraxilla tetrapetala (Loranthaceae). NZ J. Ecol. 30, 179–190 [Google Scholar]
- Busch J. W.2006Heterosis in an isolated, effectively small, and self-fertilizing population of the flowering plant Leavenworthia alabamica. Evolution 60, 184–191 (doi:10.1111/j.0014-3820.2006.tb01092.x) [PubMed] [Google Scholar]
- Busch J. W., Schoen D. J.2008The evolution of self-incompatibility when mates are limiting. Trends Plant Sci. 13, 128–136 (doi:10.1016/i.tplants.2008.01.002) [DOI] [PubMed] [Google Scholar]
- Calvo R. N.1993Evolutionary demography of orchids: intensity and frequency of pollination and the cost of fruiting. Ecology 74, 1033–1042 (doi:10.2307/1940473) [Google Scholar]
- Charlesworth D.1989Evolution of low female fertility in plants: pollen limitation, resource allocation and genetic load. Trends Ecol. Evol. 4, 289–292 (doi:10.1016/0169-5347(89)90023-2) [DOI] [PubMed] [Google Scholar]
- Cresswell J. E., Osborne J. L., Bell S. A.2002A model of pollinator-mediated gene flow between plant populations with numerical solutions for bumblebees pollinating oilseed rape. Oikos 98, 375–384 (doi:10.1034/j.1600-0706.2002.980302.x) [Google Scholar]
- Crnokrak P., Barrett S. C. H.2002Purging the genetic load: a review of the experimental evidence. Evolution 56, 2347–2358 (doi:10.1111/j.0014-3820.2002.tb00160.x) [DOI] [PubMed] [Google Scholar]
- Darwin C. R.1859On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Darwin C. R.1862On the various contrivances by which British and foreign orchids are fertilised by insects London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Darwin C. R.1876The effects of cross and self-fertilisation in the vegetable kingdom London, UK: John Murray [Google Scholar]
- Darwin C. R.1877The various contrivances by which orchids are fertilised by insects, 2nd edn London, UK: John Murray [Google Scholar]
- Eckert C. G., Samis K. E., Dart S.2006Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 183–203 Oxford, UK: Oxford University Press [Google Scholar]
- Eckert C. G., et al. In press Plant mating systems in a changing world. Trends Ecol. Evol. (doi:10.1016/j.tree.2009.06.013) [DOI] [PubMed] [Google Scholar]
- Ehlers B. K., Bataillon T.2007‘Inconstant males’ and the maintenance of labile sex expression in subdioecious plants. New Phytol. 174, 194–211 (doi:10.1111/j.1469-8137.2007.01975.x) [DOI] [PubMed] [Google Scholar]
- Fishman L.2000Pollen discounting and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution 54, 1558–1565 (doi:10.1111/j.0014-3820.2000.tb00701.x) [DOI] [PubMed] [Google Scholar]
- Fishman L., Willis J. H.2008Pollen limitation and natural selection on floral characters in the yellow monkeyflower, Mimulus guttatus. New Phytol. 177, 802–810 (doi:10.1111/j.1469-8137.2007.02265.x) [DOI] [PubMed] [Google Scholar]
- Fishman L., Wyatt R.1999Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae). Evolution 53, 1723–1733 (doi:10.2307/2640435) [DOI] [PubMed] [Google Scholar]
- Foxe J. P., Slotte T., Stahl E. A., Neuffer B., Hurka H., Wright S. I.2009Recent speciation associated with the evolution of selfing in Capsella. Proc. Natl Acad. Sci. USA 106, 5241–5245 (doi:10.1073/pnas.0807679106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Barrett S. C. H.2009Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515–1527 (doi:10.1093/aob/mcp035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D., Westoby M.1988On limits to seed production. Am. Nat. 131, 757–759 (doi:10.1086/284817) [Google Scholar]
- Harder L. D., Johnson S. D.2008Function and evolution of aggregated pollen in angiosperms. Int. J. Plant Sci. 169, 59–78 (doi:10.1086/523364) [Google Scholar]
- Harder L. D., Johnson S. D.2009Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol. 183, 530–545 (doi:10.1111/j.1469-8137.2009.02914.x) [DOI] [PubMed] [Google Scholar]
- Harder L. D., Routley M. B.2006Pollen and ovule fates and reproductive performance by flowering plants. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 61–80 Oxford, UK: Oxford University Press [Google Scholar]
- Harder L. D., Richards S. A., Routley M. B.2008Effects of reproductive compensation, gamete discounting and reproductive assurance on mating-system diversity in hermaphrodites. Evolution 62, 157–172 (doi:10.1111/j.1558-5646.2007.00272.x) [DOI] [PubMed] [Google Scholar]
- Hargreaves A. L., Harder L. D., Johnson S. D.2009Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biol. Rev. 84, 259–276 (doi:10.1111/j.1469-185X.2008.00074.x) [DOI] [PubMed] [Google Scholar]
- Hase A. V., Cowling R. M., Ellis A. G.2006Petal movement in Cape wildflowers protects pollen from exposure to moisture. Plant Ecol. 184, 75–87 (doi:10.1007/s11258-005-9053-8) [Google Scholar]
- Hedhly A., Hormaza J. I., Herrero M.2005The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biol. 7, 476–483 (doi:10.1055/s-2005-865850) [DOI] [PubMed] [Google Scholar]
- Herrera C. M.2002Censusing natural microgametophyte populations: variable spatial mosaics and extreme fine-graininess in winter-flowering Helleborus foetidus (Ranunculaceae). Am. J. Bot. 89, 1570–1578 (doi:10.3732/ajb.89.10.1570) [DOI] [PubMed] [Google Scholar]
- Hormaza J. I., Herrero M.1996Dynamics of pollen tube growth under different competition regimes. Sex. Plant Reprod. 9, 153–160 (doi:10.1007/BF02221395) [Google Scholar]
- Husband B. C., Schemske D. W.1996Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50, 54–70 (doi:10.2307/2410780) [DOI] [PubMed] [Google Scholar]
- Igic B., Kohn J. R.2006The distribution of plant mating systems: study bias against obligately outcrossing species. Evolution 60, 1098–1103 (doi:10.1111/j.0014-3820.2006.tb01186.x) [PubMed] [Google Scholar]
- Igic B., Bohs L., Kohn J. R.2004Historical inferences from the self-incompatibility locus. New Phytol. 161, 97–105 (doi:10.1046/j.1469-8137.2003.00952.x) [Google Scholar]
- Igic B., Lande R., Kohn J. R.2008Loss of self-incompatibility and its evolutionary consequences. Int. J. Plant Sci. 169, 93–104 (doi:10.1086/523362) [Google Scholar]
- Jakobsson A., Lázaro A., Totland Ø.2009Relationships between the floral neighborhood and individual pollen limitation in two self-incompatible herbs. Oecologia 160, 707–719 (doi:10.1007/s00442-009-1346-5) [DOI] [PubMed] [Google Scholar]
- Johnson S. D.1996Pollination, adaptation and speciation models in the Cape flora of South Africa. Taxon 45, 59–66 (doi:10.2307/1222585) [Google Scholar]
- Johnson S. D.2006Pollinator-driven speciation in plants. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 295–310 Oxford, UK: Oxford University Press [Google Scholar]
- Kearns C. A., Inouye D. W., Waser N. M.1998Endangered mutualisms: the conservation of plant–pollinator interactions. Annu. Rev. Ecol. Syst. 29, 83–112 (doi:10.1146/annurev.ecolsys.29.1.83) [Google Scholar]
- Keller L. F., Waller D. M.2002Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 (doi:10.1016/S0169-5347(02)02489-8) [Google Scholar]
- Kelly D., Ladley J. J., Robertson A. W.2007Is the pollen-limited mistletoe Peraxilla tetrapetala (Loranthaceae) also seed limited? Austral Ecol. 32, 850–857 (doi:10.1111/j.1442-9993.2007.01765.x) [Google Scholar]
- Kilkenny F. F., Galloway L. F.2008Reproductive success in varying light environments: direct and indirect effects of light on plants and pollinators. Oecologia 155, 247–255 (doi:10.1007/s00442-007-0903-z) [DOI] [PubMed] [Google Scholar]
- Klekowski E. J.1988Genetic load and its causes in long-lived plants. Trees 2, 195–203 (doi:10.1007/BF00202374) [Google Scholar]
- Knight T. M., et al. 2005aPollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 36, 467–497 (doi:10.1146/annurev.ecolsys.36.102403.115320) [Google Scholar]
- Knight T. M., McCoy M. W., Chase J. M., McCoy K. A., Holt R. D.2005bTrophic cascades across ecosystems. Nature 437, 880–883 (doi:10.1038/nature03962) [DOI] [PubMed] [Google Scholar]
- Knight T. M., Steets J. A., Ashman L.2006A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am. J. Bot. 93, 271–277 (doi:10.3732/ajb.93.2.271) [DOI] [PubMed] [Google Scholar]
- Lande R., Schemske D. W.1985The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution 39, 24–40 (doi:10.2307/2408514) [DOI] [PubMed] [Google Scholar]
- Larson B. M. H., Barrett S. C. H.2000A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 69, 503–520 (doi:10.1111/j.1095-8312.2000.tb01221.x) [Google Scholar]
- Lloyd D. G.1965Evolution of self-compatibility and racial differentiation in Leavenworthia (Cruciferae). Cont. Gray Herb. Harvard Univ. 195, 3–134 [Google Scholar]
- Lloyd D. G.1974Theoretical sex ratios of dioecious and gynodioecious angiosperms. Heredity 32, 11–34 (doi:10.1038/hdy.1974.2) [Google Scholar]
- Lloyd D. G.1979Some reproductive factors affecting the selection of self-fertilization in plants. Am. Nat. 113, 67–79 (doi:10.1086/283365) [Google Scholar]
- Lloyd D. G.1992Self-fertilization and cross-fertilization in plants. II. The selection of self-fertilization. Int. J. Plant Sci. 153, 370–380 (doi:10.1086/297041) [Google Scholar]
- Lloyd D. G., Webb C. J.1992The selection of heterostyly. In Evolution and function of heterostyly (ed. Barrett S. C. H.), pp. 179–207 Berlin, Germany: Springer-Verlag [Google Scholar]
- Maurice S., Fleming T. H.1995The effect of pollen limitation on plant reproductive systems and the maintenance of sexual polymorphisms. Oikos 74, 55–60 (doi:10.2307/3545674) [Google Scholar]
- Mitchell R. J.1997Effects of pollination intensity on Lesquerella fendleri seed set: variation among plants. Oecologia 109, 382–388 (doi:10.1007/s004420050097) [DOI] [PubMed] [Google Scholar]
- Mitchell R. J., Flanagan R. J., Brown B. J., Waser N. M., Karron J. D.2009New frontiers in competition for pollination. Ann. Bot 103, 1403–1414 (doi:10.1093/aob/mcp062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller D. A.2004Facilitative interactions among plants via shared pollinators. Ecology 85, 3289–3301 (doi:10.1890/03-0810) [Google Scholar]
- Moeller D. A., Geber M. A.2005Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution 59, 786–799 (doi:10.1111/j.0014-3820.2005.tb01753.x) [DOI] [PubMed] [Google Scholar]
- Morales C. L., Traveset A.2008Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Crit. Rev. Plant Sci. 27, 221–238 (doi:10.1080/07352680802205631) [Google Scholar]
- Morgan M. T., Wilson W. G.2005Self-fertilization and the escape from pollen limitation in variable pollination environments. Evolution 59, 1143–1148 (doi:10.1111/j.0014-3820.2005.tb01050.x) [PubMed] [Google Scholar]
- Morgan M. T., Wilson W. G., Knight T. M.2005Plant population dynamics, pollinator foraging, and the selection of self-fertilization. Am. Nat. 166, 169–183 (doi:10.1086/431317) [DOI] [PubMed] [Google Scholar]
- Ornelas J., Lara C.2009Nectar replenishment and pollen receipt interact in their effects on seed production of Penstemon roseus. Oecologia 160, 675–685 (doi:10.1007/s00442-009-1337-6) [DOI] [PubMed] [Google Scholar]
- Pauw A.2007Collapse of a pollination web in small conservation areas. Ecology 88, 1759–1769 (doi:10.1890/06-1383.1) [DOI] [PubMed] [Google Scholar]
- Peter C. I., Johnson S. D.2008Mimics and magnets: the importance of color and ecological facilitation in floral deception. Ecology 89, 1583–1595 (doi:10.1890/07-1098.1) [DOI] [PubMed] [Google Scholar]
- Price M. V., Campbell D. R., Waser N. M., Brody A. K.2008Bridging the generation gap in plants: pollination, parental fecundity, and offspring demography. Ecology 89, 1596–1604 (doi:10.1890/07-0614.1) [DOI] [PubMed] [Google Scholar]
- Rademaker M. C. J., de Jong T. J., Klinkhamer P. G. L.1997Pollen dynamics of bumble-bee visitation on Echium vulgare. Funct. Ecol. 11, 554–563 (doi:10.1046/j.1365-2435.1997.00124.x) [Google Scholar]
- Richards S. A., Williams N. M., Harder L. D.2009Variation in pollination: causes and consequences for plant reproduction. Am. Nat. 174, 382–398 (doi:10.1086/603626) [DOI] [PubMed] [Google Scholar]
- Ricketts T. H., et al. 2008Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 11, 499–515 (doi:10.1111/j.1461-0248.2008.01157.x) [DOI] [PubMed] [Google Scholar]
- Ritland C., Ritland K.1989Variation of sex allocation among eight taxa of the Mimulus guttatus species complex (Scrophulariaceae). Am. J. Bot. 76, 1731–1739 (doi:10.2307/2444472) [Google Scholar]
- Sakai S., Wright S. J.2008Reproductive ecology of 21 coexisting Psychotria species (Rubiaceae): when is heterostyly lost? Biol. J. Linn. Soc. 93, 125–134 (doi:10.1111/j.1095-8312.2007.00890.x) [Google Scholar]
- Sakai A. K., Wagner W. L., Ferguson D. M., Herbst D. R.1995Origins of dioecy in the Hawaiian flora. Ecology 76, 2517–2529 (doi:10.2307/2265825) [Google Scholar]
- Sala O. E., et al. 2000Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- Stebbins G. L.1974Flowering plants: evolution above the species level Cambridge, MA: Belknap [Google Scholar]
- Stephenson A. G., Travers S. E., Mena-Ali J. I., Winsor J. A.2003Pollen performance before and during the autotrophic–heterotrophic transition of pollen tube growth. Phil. Trans. R. Soc. Lond. B 358, 1009–1017 (doi:10.1098/rstb.2003.1290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi N., Morrell P. L.2001Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. Am. J. Bot. 88, 1143–1150 (doi:10.2307/3558325) [PubMed] [Google Scholar]
- Thomson J. D.1986Pollen transport and deposition by bumble bees in Erythronium: influences of floral nectar and bee grooming. J. Ecol. 74, 329–341 [Google Scholar]
- Thomson J. D., Wilson P.2008Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. Int. J. Plant Sci. 169, 23–38 (doi:10.1086/523361) [Google Scholar]
- Totland Ø.2001Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82, 2233–2244 (doi:10.1890/0012-9658(2001)082[2233:EDPLAS]2.0.CO;2) [Google Scholar]
- Tremblay R. L., Ackerman J. D., Zimmerman J. K., Calvo R. N.2005Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol. J. Linn. Soc. 84, 1–54 (doi:10.1111/j.1095-8312.2004.00400.x) [Google Scholar]
- Tripp E. A., Manos P. S.2008Is floral specialization an evolutionary dead-end? Pollination system transitions in Ruellia (Acanthaceae). Evolution 62, 1712–1737 (doi:10.1111/j.1558-5646.2008.00398.x) [DOI] [PubMed] [Google Scholar]
- Trivers R. L.1972Parental investment and sexual selection. In Sexual selection and the descent of man, 1871–1971 (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- Turnbull L. A., Crawley M. A., Rees M.2000Are plant populations seed-limited? A review of seed sowing experiments. Oikos 88, 225–238 (doi:10.1034/j.1600-0706.2000.880201.x) [Google Scholar]
- Vallejo-Marín M., Barrett S. C. H.2009Modification of flower architecture during early stages in the evolution of self-fertilization. Ann. Bot. 103, 951–962 (doi:10.1093/aob/mcp015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamosi J. C., Otto S. P.2002When looks can kill: the evolution of sexually dimorphic floral display and the extinction of dioecious plants. Proc. R. Soc. Lond. B 269, 1187–1194 (doi:10.1098/rspb.2002.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamosi J. C., Vamosi S. M.2005Present day risk of extinction may exacerbate the lower species richness of dioecious clades. Divers. Distrib. 11, 25–32 (doi:10.1111/j.1366-9516.2005.00119.x) [Google Scholar]
- Vamosi J. C., Knight T. M., Steets J. A., Mazer S. J., Burd M., Ashman L.2006Pollination decays in biodiversity hotspots. Proc. Natl Acad. Sci. USA 103, 956–961 (doi:10.1073/pnas.0507165103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleunen M., Johnson S. D.2007Effects of self-compatibility on the distribution range of invasive European plants in North America. Conserv. Biol. 21, 1537–1544 (doi:10.1111/j.1523-1739.2007.00765.x) [DOI] [PubMed] [Google Scholar]
- van Kleunen M., Manning J. C., Pasqualetto V., Johnson S. D.2008Phylogenetically independent associations between autonomous self-fertilization and plant invasiveness. Am. Nat. 171, 195–201 (doi:10.1086/525057) [DOI] [PubMed] [Google Scholar]
- Whittall J. B., Hodges S. A.2007Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–709 (doi:10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., Aizen M. A.2009A meta-analysis of bees' responses to anthropogenic disturbance. Ecology 90, 2068–2076 (doi:10.1890/08-1245.1) [DOI] [PubMed] [Google Scholar]
- Young A., Boyle T., Brown T.1996The population genetic consequences of habitat fragmentation for plants. Trends Ecol. Evol. 11, 413–418 (doi:10.1016/0169-5347(96)10045-8) [DOI] [PubMed] [Google Scholar]
- Zimmerman M., Pyke G. H.1988Reproduction in Polemonium: assessing the factors limiting seed set. Am. Nat. 131, 723–738 (doi:10.1086/284815) [Google Scholar]