Abstract
In the second half of the nineteenth century, pioneering discoveries of rich assemblages of fossil plants from the Cretaceous resulted in considerable interest in the first appearance of angiosperms in the geological record. Darwin's famous comment, which labelled the ‘rapid development’ of angiosperms an ‘abominable mystery’, dates from this time. Darwin and his contemporaries were puzzled by the relatively late, seemingly sudden and geographically widespread appearance of modern-looking angiosperms in Late Cretaceous floras. Today, the early diversification of angiosperms seems much less ‘rapid’. Angiosperms were clearly present in the Early Cretaceous, 20–30 Myr before they attained the level of ecological dominance reflected in some mid-Cretaceous floras, and angiosperm leaves and pollen show a distinct pattern of steadily increasing diversity and complexity through this interval. Early angiosperm fossil flowers show a similar orderly diversification and also provide detailed insights into the changing reproductive biology and phylogenetic diversity of angiosperms from the Early Cretaceous. In addition, newly discovered fossil flowers indicate considerable, previously unrecognized, cryptic diversity among the earliest angiosperms known from the fossil record. Lineages that today have an herbaceous or shrubby habit were well represented. Monocotyledons, which have previously been difficult to recognize among assemblages of early fossil angiosperms, were also diverse and prominent in many Early Cretaceous ecosystems.
Keywords: abominable mystery, Darwin, early angiosperms, Early Cretaceous, fossil flowers, monocots
1. Introduction
As improved information about the plant fossil record accumulated during the late nineteenth century, the history of flowering plants stood out as increasingly anomalous. Darwin's famous quote, ‘The rapid development as far as we can judge of all the higher plants within recent geological times is an abominable mystery’, in a letter written to J. D. Hooker 22 July 1879, captures his own reflections on this topic. Not only was the first appearance of angiosperms during the Cretaceous relatively ‘recent’ in terms of the evolution of other groups of plants, but the apparent rapidity with which modern-looking angiosperm leaves entered the fossil record in several different parts of the world appeared to undermine the possibility of gradual evolution from some pre-existing form.
Central to the mid-nineteenth century interest in early angiosperm evolution was the discovery and study of rich Cretaceous leaf floras from North America, Europe and the Arctic. These leaf floras included the flora of the Dakota group in North America (Newberry 1863; Lesquereux 1874), floras from western Greenland and Spitsbergen (e.g. Heer 1868, 1882; Saporta 1877) and floras from different parts of Europe (e.g. Saporta 1878). The composition of these floras, which were usually dominated by flowering plants, contrasted strongly with that of earlier floras in the Cretaceous from which angiosperms were apparently absent. To reconcile these observations Darwin speculated that angiosperms perhaps had a long cryptic history in remote areas, which left no trace in the fossil record (Darwin in letter to J. D. Hooker 22 July 1879; see also Friedman 2009). This idea has been reappraised several times since, and it has been suggested that the rapid appearance of angiosperms in the Cretaceous fossil record reflects a pattern resulting from migration rather than in situ evolution (e.g. Axelrod 1952).
Over the past 120 years, an improved stratigraphic framework combined with detailed studies of Cretaceous angiosperms has removed some of the tensions at the heart of Darwin's concerns. The discovery of rich Early Cretaceous palynological assemblages showed that angiosperms were present in the Early Cretaceous, but missing from the very large numbers of Jurassic and older samples from all over the world (e.g. Scott et al. 1960). It was also recognized that the earliest angiosperm pollen grains were restricted to monocolpate rather than triaperturate forms (Muller 1970; Hughes 1994), which was consistent with phylogenetic interpretations that extant magnoliids were most similar to the common ancestor of all living angiosperms.
In the mid-1970s, studies of fossil angiosperm pollen and leaves in the Early and mid-Cretaceous Potomac Group of eastern North America demonstrated a coordinated increase in the complexity of both types of organs (Brenner 1963; Doyle & Hickey 1976; Hickey & Doyle 1977). The increase in the architectural complexity of angiosperm leaves was also shown to be broadly consistent with interpretations of leaf evolution based on extant plants (Doyle & Hickey 1976; Hickey & Doyle 1977, see also Hickey & Wolfe 1975).
More recently, the discovery of angiosperm mesofossils containing three-dimensionally preserved angiosperm flowers from the Cretaceous has provided a completely new, and previously unimagined, source of information about the pattern of early angiosperm diversification as well as new insights into the reproductive biology and phylogenetic diversity of angiosperms during the first 70 Myr of their evolutionary history (e.g. Friis & Skarby 1981; Friis 1984; Friis et al. 2006a). The more detailed picture that emerges is broadly consistent with the pattern of phylogenetic relationships among extant angiosperms inferred from studies of molecular sequence data (e.g. The Angiosperm Pyhlogeny Group 2003; Soltis et al. 2005). Taken together, several lines of evidence from the fossil record, combined with inferences from extant plants, now provide a strong indication that the first major phylogenetic diversification and ecological radiation of angiosperms took place in the Early Cretaceous over a relatively short interval of some 20–30 Myr (Brenner 1963, 1996; Doyle & Hickey 1976; Hughes 1976, 1994; Crane et al. 1995; Friis et al. 2006a).
Studies of fossil flowers and other angiosperm reproductive organs from many different stratigraphic levels in the Cretaceous continue to add further details to the emerging picture of early angiosperm evolution (e.g. Friis et al. 2006a; Takahashi et al. 2008; Viehofen et al. 2008; von Balthazar et al. 2008; Martinez-Millan et al. 2009). However, in this paper, we focus on fossil assemblages from the Early Cretaceous that contain the oldest structurally and phylogenetically informative angiosperm flowers, fruits and seeds. Information from these floras suggests that much angiosperm diversity prior to the mid-Cretaceous was mainly among lineages with an herbaceous or shrubby habit, and that many of these early angiosperms probably grew in wet to fully aquatic environments. Within these assemblages, the presence of Chloranthaceae, Nymphaeales, Alismatales, Ranunculales, Proteales, Buxales and other groups is now well documented, and there is also evidence of many plants that are not closely related to any extant taxa. However, new results reinforce previous indications that monocotyledons were also diverse in these ancient angiosperm communities and contributed substantially to the angiosperm component of Early Cretaceous ecosystems.
2. Morphological diversity of early angiosperms: evidence from fossil pollen and flowers
(a). Morphological diversity of early pollen
The earliest unequivocal remains of angiosperms are pollen grains from the Early Cretaceous, and some of the most convincing detailed information is from the Early Cretaceous palynofloras described from southern England by Hughes and colleagues (see overview in Hughes 1994). These records range in age from the Hauterivian through to the Aptian and have been studied with both light and scanning electron microscopy. Grains ascribed to angiosperms from Israel, which are of possible Late Valanginian–Early Hauterivian age (Brenner 1996), are more difficult to evaluate because the available illustrations reveal few structural details.
Hughes (1994) distinguished three phases of angiosperm evolution through the Mid-Hauterivian to Early Barremian (phases 0–2) and three phases (phases 3–5) through the Late Barremian to Aptian.
Almost all of the angiosperm grains from the six phases recognized by Hughes (1994) are monoaperturate. Most of the grains have a long straight colpus. More rarely the aperture is expanded and more or less circular. The pollen grains are minute to small and range in size from about 9 µm to about 29 µm in diameter. There is considerable variation in pollen wall structure and ornamentation. This ranges from continuous tectate, with either punctate or spinulate ornamentation, to reticulate semitectate, with muri that are smooth or have various kinds of supratectal ornamentation.
Only two different types of angiosperm pollen are recorded by Hughes (1994) from phase 0: one has a continuous tectum and the other has a reticulate (semitectate) pollen wall. Already by phase 1, there is greater diversity among the reticulate monocolpate grains. Forms that have fine transverse striations on the muri also enter the fossil record at this level and become very common in later Barremian and Aptian palynofloras. Monocolpate and reticulate pollen with spiny ornamentation on the muri first appear in phase 2 and are also common through the later Barremian and Aptian. Crotonoid pollen and pollen with trichotomocolpate apertures occur for the first time in phase 3.
Angiosperm pollen from phases 4 and 5 continues to be small and predominantly monoaperturate. During phase 4, pollen grains with a distinctly graded reticulum are seen in the fossil record for the first time. A significant event in angiosperm evolution is the first appearance of tricolpate pollen grains at around the latest Barremian–Early Aptian. Hughes records a single tricolpate grain in phase 4 and one other tricolpate grain of a different kind in phase 5, which is dated as Early Aptian (Hughes 1994).
Angiosperm pollen is also known in many other parts of the world from the Late Barremian–Early Aptian interval, more or less contemporaneous with phases 4 and 5 of Hughes, and shows the same pattern of diversity as observed for the British sequence. Especially important are sequences of palynofloras from the Potomac Group of eastern North America (e.g. Brenner 1963; Doyle 1969; Doyle & Hickey 1976; Doyle & Robbins 1977), from Egypt (e.g. Penny 1986, 1988b) and from Equatorial Africa (Doyle et al. 1977). The evolutionary signal that emerges from these floras is a considerable expansion of angiosperm pollen diversity, both in terms of aperture configuration and also pollen wall sculpture. Scattered records of tricolpate pollen grains are also known from contemporaneous pollen assemblages from Israel (Brenner 1996), Equatorial Africa (Doyle 1992), Egypt (Penny 1988b) and North America (Doyle 1992).
The first mesofossil floras with angiosperm flowers and pollen in situ are also from this time interval. Mesofossil floras consist of three-dimensionally preserved coalified plant remains in the size range between microfossils and macrofossils. The fragments are typically a few millimetres long and may include whole flowers with all floral organs preserved (for a review, see Friis et al. 2006a). Flowers sometimes have stamens with pollen in situ. Ultrastructural details of the pollen grains in situ studied with scanning electron microscopy also indicate that the number of taxa is higher than would be expected from studies of dispersed grains using light microscopy, which has been standard in most palynostratigraphic studies. Scanning electron microscopy of dispersed grains also provides evidence of cryptic diversity through improved discrimination of different pollen types based on fine details of sculpture. Relatively low pollen production in many early angiosperms may also imply that certain pollen types seen in mesofossils are poorly represented in palynofloras that comprise dispersed pollen.
(b). Morphological diversity of early flowers and other reproductive organs
There are now a large number of mesofossil floras described from the Late Cretaceous that contain angiosperm flowers and other reproductive structures. However, the key mesofossil floras from the Early Cretaceous are all from Portugal and eastern North America. Some of the fossils in these floras are preserved as charcoal formed by vegetational fires and have the three-dimensional structure intact. Others are preserved as lignite formed from chemically altered organic material that may be more or less compressed. These fossil assemblages were discovered in the late 1980s in soft clays, silts and sands, which range in age from the Late Barremian–Early Aptian to around the Albian–Cenomanian boundary (for a summary, see Friis et al. 2006a).
The Torres Vedras flora from Portugal is possibly the oldest of these mesofossil floras (Late Barremian–Early Aptian, approximately corresponding to phases 4 and 5 of Hughes). The Torres Vedras assemblage includes three-dimensionally preserved floral structures as well as fruits and seeds, but also contains diverse isolated stamens with pollen in situ and many coprolites consisting almost exclusively of pollen. Other important floras from Portugal are slightly younger. Among these are the mesofossil floras from Catefica (Late Barremian–Aptian), Famalicão (Late Aptian) and Buarcos, Vila Verde 2, Vale de Agua and several others (Late Aptian–Early Albian). The most diverse mesofossil flora from the Early Cretaceous of North America is from the Puddledock locality (Early–Middle Albian), but Early Cretaceous mesofossil floras from the Potomac Group sequence range from around the Barremian–Aptian boundary (Drewry's Bluff and Dutch Gap localities) to the end of the Early Cretaceous (West Brothers’ locality, latest Albian).
There are also a few other Early Cretaceous floras that contain floral remains of angiosperms from other parts of the world. These include the impression/compression flora from the Yixian Formation (Late Barremian–Early Aptian) from Liaoning Province and adjacent areas in northeastern China (e.g. Sun et al. 2001; Leng et al. 2003) and from the Late Aptian–Early Albian Crato Formation of northeast Brazil (Mohr et al. 2007). Fossils in both these assemblages are important in providing whole plant preservation. Angiosperms are rare in the Yixian Formation, represented by only few taxa and relatively few specimens. Angiosperms are more common in the perhaps slightly younger Crato flora. Further, a petrified floral structure was also recently described from the Late Albian of Australia (Dettmann et al. 2009).
(i). Size
All early angiosperm flowers known from the Late Barremian–Middle Albian are of small to medium size. Flowers from the many Early Cretaceous mesofossil floras range from about 0.5 mm up to about 5 mm, while slightly larger flowers are reported from the Crato flora (up to about 6–7 mm; Mohr & Bernardes-de-Oliveira 2004) and from Western Australia (up to about 16 mm; Dettmann et al. 2009). Many flowers were borne in dense inflorescences, mostly spikes, but there are also a few solitary flowers, both in the mesofossil floras and in the compression/impression Crato flora. Archaefructus from the Yixian Formation (Sun et al. 2002) is relatively large, but if the inflorescence interpretation of Friis et al. (2003) and Endress & Doyle (2009) is correct then the individual flowers of Archaefructus are small, simple and also borne in a spike-like inflorescence.
Early angiosperm fruits and seeds are also typically small. In a preliminary analysis of the Famalicão mesofossil flora (Late Aptian), 37 different kinds of angiosperm fruits and 64 different kinds of seeds were identified (Eriksson et al. 2000b). This flora is broadly representative of the currently known Early Cretaceous mesofossil floras. Fruits range in volume between 0.12 and 8.34 mm3 and seeds vary between 0.02 and 6.86 mm3 (Eriksson et al. 2000b). Fruits and seeds continue to be small compared with their living relatives throughout the Cretaceous (Tiffney 1984; Eriksson et al. 2000a).
(ii). Sex expression
All Early Cretaceous mesofossil floras include both unisexual and bisexual flowers. Unisexual flowers seem slightly more common in the Early Cretaceous than in mid-Cretaceous floras. They are certainly much more common than in Late Cretaceous floras where bisexual forms predominate, even among flowers that are most closely related to extant wind-pollinated taxa with unisexual flowers (Friis et al. 2006b).
(iii). Position of floral organs
Most of the early angiosperm flowers from the Late Barremian–Early Aptian and through to the Early–Middle Albian are hypogynous, but there are also several epigynous forms. This is in contrast to flowers from Late Cretaceous floras. Beginning in the Turonian, or perhaps even earlier in the Late Cenomanian, about half of all flowers in typical mesofossil floras are epigynous or semiepigynous. The difference may reflect initial strategies for protection of the ovules in the face of increased biotic pollination, as well as the high relative diversity of particular groups of angiosperms (probably early core eudicots) at this stage in angiosperm evolution.
(iv). Perianth
A surprisingly high proportion of ancient angiosperm flowers appear to lack a perianth or to have a simple undifferentiated perianth. None of the flowers from the Early Cretaceous have a perianth that is clearly differentiated into calyx and corolla. The number of floral parts is usually also low. Exceptions to this generalization are Virginianthus and Carpestella from the Early–Middle Albian Puddledock flora, Endressinia from the Late Aptian–Early Albian of Brazil and Lovellea from the Albian of Australia, all of which have multipartite flowers with numerous floral organs (Friis et al. 1994; Mohr & Bernardes-de-Oliveira 2004; von Balthazar et al. 2008; Dettmann et al. 2009). In Virginianthus, and also in Teixeiraea lusitanica (von Balthazar et al. 2005), the tepals grade from sepaloid on the outside to petaloid on the inside.
In many Early Cretaceous fossil flowers, the phyllotaxis of the floral organs, including the perianth, is difficult to establish. In some cases this may be because of distortion during fossilization, but in other cases floral phyllotaxis may be irregular. This is in contrast to Late Cretaceous flowers that predominantly have distinct heterochlamydous perianth of sepals (calyx) and petals (corolla) that are clearly arranged in whorls.
(v). Stamens
Evidence from mesofossils shows that there is considerable diversity in the organization of the androecium among the earliest angiosperm flowers. Early Cretaceous floras also include a surprisingly large number of dispersed stamens with pollen in situ.
In the Torres Vedras mesofossil flora (Late Barremian–Early Aptian) stamens typically have simple anthers with only modest development of the connective between the pollen sacs. The filament is not well developed and the stamen base is short. Anthers vary in shape and size from short and sagittate to narrow and elongate. Sometimes there is an apical expansion of the connective, but typically it is small.
On average, anthers in Early Cretaceous floras are relatively large compared with those from Late Cretaceous floras. Many of the Late Aptian–Albian stamens are also bulky with extensive development of the connective, both between the pollen sacs and at the stamen apex. This apical extension may be strongly developed and in some cases it may be as long as the pollen sacs. The lower part of the stamen may be short, or longer and developed into a filament, but it is often flattened and always grades into the anther without a distinct joint. Even though anthers are relatively large, the pollen sacs are often very small compared with the total size of the stamen. Flattened stamens typically have pollen sacs borne on one surface. Anther dehiscence in the earliest known angiosperm stamens (Late Barremian–Early Aptian) was probably by longitudinal slits, but none of the available specimens show the mode of dehiscence very clearly. However, in floras from the Late Aptian–Early Albian, the variety of anthers is greater and many have valvate dehiscence (e.g. Friis et al. 2006a). Most anthers with valvate dehiscence have laterally hinged valves over each theca. These open like shutters to expose the two pollen sacs of each theca.
In general, the form of the androecium in Turonian–Maastrichtian flowers is markedly different from that of flowers from the Early Cretaceous. In the Late Cretaceous, dispersed stamens are much less common and bulky stamen types are rare. Stamen phyllotaxis is mainly whorled and the number of androecial whorls is typically one or two, rarely more. Anthers are mostly dithecate with a distinct band- or thread-shaped filament that is separated from the anther by a distinct joint.
(vi). Gynoecium
Most Early Cretaceous angiosperm flowers apparently had monocarpellate flowers, judging from the many unilocular, often one-seeded fruits in the early floras. However, multicarpellate flowers were also present early in angiosperm history. In the Torres Vedras flora, both apocarpous fruits with many free carpels and syncarpous gynoecia formed from two or three carpels are present. From the contemporaneous Yixian flora, Archaefructus has a gynoecium of one or two free carpels (inflorescence interpretation) and Sinocarpus is syncarpous.
None of the early flowers have a distinct style with an elevated stigma. Instead, the stigmatic surface is often extended or indistinct. This is in strong contrast to flowers from the Late Cretaceous, which normally have distinct styles that elevate the stigma. From the Turonian through to the Maastrichtian, there is also a clear dominance of flowers with syncarpous gynoecia, which are mainly related to groups of core eudicots.
(vii). Fruits and seeds
Fruit types among Early Cretaceous angiosperms are diverse. There are several apocarpous-follicular fruits that have more than one seed per carpel in the Torres Vedras assemblages, but the vast majority of fruits at this level are indehiscent, unilocular nuts or drupes with one or very few seeds. For instance, in the Famalicão mesofossil flora (Late Aptian), seed number could be determined for 18 fruits. Of these, 12 had only a single seed, while the remaining six had two, three or more seeds per fruit. The proportion of fruits with a fleshy outer layer is surprisingly high. In the Famalicão flora, about 25 per cent of the fruits were either drupes or few-seeded berries. This probably indicates the early establishment of animal dispersal among angiosperms (Eriksson et al. 2000b). Because of their small size, the fleshy portion of each fruit was small, but there are several indications that the fruits were borne in dense infructescences. Collectively, the amount of fleshy tissue may have been sufficient for attracting animal dispersers.
Early Cretaceous angiosperms typically have anatropous ovules, but rare orthotropous ovules are also known from the earliest mesofossil floras including the Torres Vedras flora. Campylotropous ovules are known from the Late Aptian flora of Famalicão. Most early angiosperm seeds are apparently formed from bitegmic ovules. Most are exotegmic with a hard outer seed coat (Friis et al. 1999).
3. Phylogenetic diversity of early angiosperms
All angiosperm pollen grains from the earliest phases of the angiosperm diversification described by Hughes (1994) are monocolpate. There are also some records of inaperturate pollen from the Valanginian–Hauterivian of Israel (Brenner 1996), but these need further documentation. Among extant angiosperms, monoaperturate pollen, in which the aperture is generally colpate, is exclusively produced by angiosperms at the ANITA grade (the earliest diverging lineages of extant angiosperms including Amborella, Nymphaeaceae, Illicium, Trimeniaceae and Austrobaileyaceae sensu Qiu (1999)) as well as by Chloranthaceae, monocots and eumagnoliids. Within these groups, some families and genera have very distinctive pollen features and can be identified based on isolated pollen alone. However, despite the very extensive record of dispersed angiosperm pollen from the Early Cretaceous, very few of these grains can be assigned confidently to extant lineages at the order, family or generic level. Recent discoveries of fossil flowers and other angiosperm reproductive organs allow much more detailed evaluation of the phylogenetic relationships of early angiosperms to extant plants. These fossils show that although angiosperms are already diverse by the Barremian–Aptian, this diversity is restricted to early diverging lineages of extant angiosperms, as well as other lineages that appear to be extinct.
(a). ANITA lineages
(i). Nymphaeales
Fossil remains of Nymphaeales are very well represented in Palaeogene and Neogene floras. Nymphaealean seeds are particularly common and easily recognized by their distinct bitegmic, exotestal organization, the presence of a micropylar lid and the often strongly undulating anticlinal walls of the cells on the surface of the exotesta. The Cretaceous fossil record of Nymphaeales is much more meagre. Dispersed Nymphaea-like pollen, described as Zonosulcites scollardensis and Zonosulcites parvus, is known from the Maastrichtian of Canada (Srivastava 1969; Muller 1981) and unequivocal nymphaealean seeds, described as Symphaenale futabensis, are known from the Late Cretaceous (Early Santonian) Gokurakuzawa locality of northeastern Honshu, Japan (Takahashi et al. 2007). The nymphaealean affinity suggested for flowers of Microvictoria, described from the Turonian of New Jersey, USA (Gandolfo et al. 2004), has been questioned (Endress 2008).
Despite their sparse Late Cretaceous record, and even though the extant genera may not have diversified until the Cenozoic, Nymphaeales were clearly present at an early stage in angiosperm evolution. A key early record of Nymphaeales is the flower of Monetianthus mirus, from the Late Aptian–Early Albian flora of Vale de Agua (Friis et al. 2001, 2009b). Monetianthus provides unequivocal evidence of crown group Nymphaeales, even though it cannot be accommodated in any extant genus.
The Monetianthus flower is perigynous, about 3 mm long and 2 mm in diameter, with remains of perianth, androecium and gynoecium. The gynoecium is particularly informative, with a whorl of 12 carpels that are fused for most of their length and free only in the apical part. There is a small central projection of the floral axis between the carpels. Each carpel contains many small ovules, which do not fill out the locules completely. Placentation is laminar. The combined characters of the gynoecium are unique for Nymphaeaceae, and phylogenetic analysis suggests a position of Monetianthus at the root of the Barclaya + Nymphaeoideae clade (Friis et al. 2009b).
Pluricarpellatia peltata from the Early Cretaceous (Late Aptian–Early Albian) of Brazil also provides evidence of Early Cretaceous Nymphaeales. It is a fruiting structure preserved in organic connection with peltate nymphaealean leaves (Mohr et al. 2007). Other Early Cretaceous leaf fossils with distinct nymphaealean features also support the presence and diversity of the group early in angiosperm history. Among these are Brasenia-like leaves from the Early Cretaceous (Late Aptian or Early Albian) flora of Buarcos-para-Tavarede, Portugal, assigned to the fossil genus Braseniopsis (Saporta 1894), and Scutifolium jordanicum from the Early Cretaceous (Albian) of Jordan, which was assigned to the Cabombaceae (Taylor et al. 2008). There are also many seeds with distinct nymphaealean features among the Early Cretaceous mesofossils from Portugal and eastern North America. Their first occurrences are in the Late Barremian–Early Aptian Torres Vedras flora, as well as in the more or less contemporaneous mesofossil flora from Drewry's Bluff, Virginia (Friis et al. 1999, 2006a).
Carpestella lacunata from the Early–Middle Albian Puddledock flora of Virginia, USA (von Balthazar et al. 2008) is another Early Cretaceous flower probably related to ANITA grade angiosperms. The fossil is a small fragmentary gynoecium, about 0.65 mm long and 0.45 mm in diameter, that shows many helically arranged scars from detached floral organs. The gynoecium is syncarpous, consisting of 13 carpels arranged radially around a central column in a star-shaped pattern, as is seen in extant Nymphaeaceae and Illicium (Schisandraceae). The presence of septal slits suggests a link between the fossil and extant Nymphaeaceae, but the precise position of Carpestella cannot be established securely with the information currently available (von Balthazar et al. 2008).
There are other fossils of putative relationship with ANITA grade angiosperms from Early Cretaceous mesofossil floras (Friis et al. 1999), but none can be attributed to an extant family. They probably represent extinct lineages that diverged close to the base of the angiosperm phylogenetic tree.
(b). Chloranthaceae
Asteropollis is a genus of distinctive early angiosperm pollen grains that is closely comparable with pollen of the extant genus Hedyosmum (Chloranthaceae) (Walker & Walker 1984). Asteropollis includes species characterized by their star-shaped distal aperture, reticulate pollen wall and muri that have beaded ornamentation. In addition, some species of Clavatipollenites, which have a monocolpate or trichotomocolpate aperture and reticulate pollen wall with beaded muri, are very similar to grains of extant Ascarina (Chloranthaceae) (Couper 1960; Walker & Walker 1984).
Studies of mesofossils confirm indications from dispersed pollen that Chloranthaceae entered the angiosperm fossil record very early. The family was probably already diverse in the earliest mesofossil floras (Friis et al. 2006a). In the Torres Vedras mesofossil flora (Late Barremian–Early Aptian) inflorescences, fruits, dispersed stamens and coprolites suggest the presence of several different species of Chloranthaceae. Hedyosmum-like staminate inflorescences often associated with Hedyosmum-like fruits are also known from several of the Aptian–Early Albian floras from Portugal (Friis et al. 2006a). Hedyosmum-like fruits with adhering Asteropollis pollen have also been recorded from the Early–Middle Albian Puddledock flora (Friis et al. 1997). Unequivocal androecia of Chloranthus-like plants are known from Late Cretaceous mesofossil floras (Crane et al. 1989; Eklund et al. 1997).
(c). Eumagnoliids
Eumagnoliids are well known in the Cenozoic fossil record where they are represented extensively by wood, leaves, fruits and seeds. There is also a growing record of well-preserved eumagnoliid flowers from the Cretaceous (e.g. Friis et al. 1997). Cenozoic fossils are generally easily assigned to extant genera, but all Cretaceous eumagnoliids appear to represent extinct genera. The Early Cretaceous record consists mainly of taxa with smaller flowers, and lauralean taxa are especially prominent (e.g. Virginianthus, Potomacanthus, see below). Larger, multipartite flowers similar to those of extant Magnoliaceae, which were previously believed to be archaic in angiosperms, first enter the fossil record in the Late Albian at the very end of the Early Cretaceous (Dilcher & Crane 1984).
(i). Magnoliales
Dispersed permanent tetrads of pollen described as Walkeripollis (Doyle et al. 1990) probably represent stem-group Winteraceae. The earliest flower of probable Magnoliales is Endressinia brasiliana from the Crato Formation (Late Aptian–Early Albian) of Brazil (Mohr & Bernardes-de-Oliveira 2004). It is particularly similar to extant Eupomatiaceae and Himantandraceae, but cannot be placed in any extant family and may represent an extinct lineage. Archaeanthus linnenbergeri from latest Albian–earliest Cenomanian Dakota Formation is very similar in floral structure to extant Magnoliaceae, but the fruits differ from all extant taxa in producing many small seeds (Dilcher & Crane 1984). Archaeanthus possibly represents stem-group Magnoliaceae. Flowers of crown group Annonaceae are known from the Early Coniacian of Japan (Takahashi et al. 2008).
(ii). Laurales
Laurales have an extensive Cenozoic fossil record and are becoming increasingly well documented from the Cretaceous based on numerous remains of flowers and inflorescences (e.g. Drinnan et al. 1990; Eklund 2000; Takahashi et al. 2001). The first of these fossils to be described was Mauldinia mirabilis from the Mauldin Mountain flora of Maryland (Early Cenomanian) (Drinnan et al. 1990). Mauldinia and similar genera have since been recognized from several fossil floras in Europe and Central Asia (Eklund & Kvaček 1998; Frumin et al. 2004; Viehofen et al. 2008), and Mauldinia currently includes four distinct species.
In the Early Cretaceous, lauralean flowers are known only from the Puddledock flora (Early–Middle Albian) of Virginia, USA. Laurales known from this flora are the calycanthaceous flower Virginianthus (Friis et al. 1994), the lauraceous flower Potomacanthus lobatus (von Balthazar et al. 2007) and several other undescribed forms. The fossil flower Lovellea wintonensis, described by Dettmann et al. (2009) from the mid-Cretaceous of Western Australia, is another Early Cretaceous lauralean element. Its closest similarities are to extant Gomortegaceae and Monimiaceae, but its precise position among Laurales remains to be established (Dettmann et al. 2009).
(iii). Piperales
Several dispersed pollen grains from the Early Cretaceous, such as species assigned to the pollen genera Tucanopollis and Transitoripollis, are monoaperturate with a continuous tectum. In these features they are very similar to pollen grains of extant Piperales. This kind of pollen has been found on fruits (Friis et al. 1995) from the Puddledock flora (Early–Middle Albian). The same kind of pollen has also been found on fruits as well as in stamens and in coprolites from the Torres Vedras locality (Late Barremian–Early Aptian) (Friis et al. 2006a). The combined fruit, seed and pollen characters suggest a relationship to extant Piperales, but other features of the plants are unknown and the putative relationship to Piperales remains to be established more securely.
(d). Eudicots
The earliest record of triaperturate pollen is a single grain from Zone 4 of Hughes (Late Barremian–Early Aptian; Hughes & McDougall 1990; Hughes 1994). Above this level in the Wealden succession of southern England, triaperturate pollen becomes more common. In palynofloras from other areas tricolpate pollen also enters the fossil record around the Barremian–Aptian boundary. Tricolpate grains remain rare until later in the Aptian (e.g. Doyle et al. 1977; Penny 1991) and first dominate palynofloras in the Late Albian where they include tricolpate as well as tricolporoidate forms (Doyle & Hickey 1976). Tricolpate pollen grains are also known from flowers and dispersed stamens in many Early Cretaceous mesofossil floras. In the Late Barremian–Early Aptian Torres Vedras flora, tricolpate pollen has not so far been found in flowers or on the surface of carpels or fruits. Nor has it been observed in the dispersed palynoflora from the same samples. However, two different kinds of tricolpate pollen were found in a coprolite and in a stamen. Similarly, in the Late Aptian flora of Famalicão, no tricolpate pollen grains have been observed in situ. In the younger Early–Middle Albian Puddledock flora, a rough estimate of distribution of angiosperm pollen types in situ shows that about one-third are triaperturate.
All Early Cretaceous floral structures with tricolpate pollen in situ that have been placed systematically have relationships to groups of eudicots that diverged very early from the lineage that gave rise to the bulk of eudicot diversity. Currently, Early Cretaceous floral structures related to Ranunculales, Buxales and Proteales have been identified. Probable Ranunculales are represented by a single staminate flower, Teixeiraea lusitanicum, from the Vale de Agua flora (Late Aptian–Early Albian) (von Balthazar et al. 2005). Also from the Vale de Agua flora are four different kinds of pistillate structures (Aguacarpus hirsutus, Lusicarpus planatus, Silucarpus camptostylus, Valecarpus petiolatus) and one kind of staminate flower (Lusistemon striatus) that are related to the Buxales (Pedersen et al. 2007). Unequivocal buxalean flowers and inflorescence fragments are also known from the Potomac Group. Spanomera marylandensis is from the Late Albian West Brothers locality and Spanomera mauldinensis is from the Early Cenomanian Mauldin Mountain locality (Drinnan et al. 1991), both in Maryland, USA.
Proteales are known from several Potomac Group mesofossil floras. Especially common are a variety of flowers and inflorescences of platanoid affinity. These include pistillate flowers and inflorescences of Friisicarpus brookensis and the associated staminate material of Aquia brookensis from the Early–Middle Albian Bank near Brooke locality in Virginia (Crane et al. 1993), as well as pistillate Friisicarpus marylandensis and associated staminate Platananthus potomacensis from the Late Albian West Brothers locality (Friis et al. 1988).
The presence of Proteales in the Early Cretaceous is further supported by the occurrence of Nelumbo-like leaves described as Nelumbites from the Bank near Brooke (Early–Middle Albian) and the Quantico localities (Late Albian) (Doyle & Hickey 1976; Upchurch et al. 1994), both from Virginia, USA.
(e). Monocotyledons
Fossil remains of monocotyledons are common in Cenozoic floras where they are represented by many different organs, including stems, leaves, rhizomes, flowers, fruits, seeds, pollen and phytoliths. There are also reliable monocot fossils from the Late Cretaceous, including stems, fruits, seeds and pollen (e.g. Herendeen & Crane 1995). However, the record of monocotyledons is much less extensive in the Cretaceous than in the Cenozoic. It is also interesting that some of the key monocotyledons of present day wetland communities, which are well represented in the Cenozoic, are absent in the Cretaceous. For example, Cyperaceae have fruits that fossilize well, are easily recognizable and that are extremely common in Palaeogene and Neogene fossil assemblages, but they have not yet been documented from the Cretaceous.
Until now, the Early Cretaceous record of monocots has been especially meagre and, according to Gandolfo et al. (2000), all Early Cretaceous records of monocotyledons are ambiguous.
Recognizing the pollen of monocots among the monocolpate grains of early angiosperms has been a particular challenge because the morphological and structural features that potentially distinguish monocot pollen from that of other angiosperms are relatively subtle. Walker & Walker (1984) listed seven features of potential importance for assigning dispersed monocolpate grains to monocots. These include: (i) reticulate sculpturing differentiated into coarse and fine areas (graded reticulum), (ii) smooth muri, (iii) lumina of different sizes, (iv) ‘frilled’ muri caused by laterally extended columellae, (v) regular and clearly polygonal lumina, (vi) a thin pollen wall combined with a very thin non-apertural nexine and (vii) absence of endexine.
While there are many exceptions to these generalizations, among extant angiosperms monoaperturate pollen with a graded reticulum is known only for monocots. The reticulum may have lumina decreasing in size either towards the polar areas (e.g. some palms) or towards restricted areas of the equatorial region (e.g. some Liliales and Asparagales), but there are also other variations. Based on these features, Walker & Walker (1984) suggested that many of the dispersed pollen grains from the Early Cretaceous that are assigned to extinct pollen genera, such as Retimonocolpites and Liliacidites, were produced by monocots. Support for a monocot affinity of some Liliacidites grains was also inferred by Doyle et al. (2008) from a recent phylogenetic analysis.
Another character that, as far as we are aware, is restricted to monocot pollen is acolumellate-reticulate wall structure, in which the reticulum is only loosely attached to the foot layer and where the infratectal layer is reduced to an ultrathin granular layer. This kind of wall structure is particularly common among extant Alismatales (Grayum 1990). It may reflect an evolutionary transition to the predominantly atectate condition seen in extant aroid and other monocots. In the fossil record, transitional states between columellate and acolumellate grains reported for Pennistemon-type pollen (Penny 1988a) have also been observed for various Retimonocolpites-type pollen grains.
(i). Alismatales–Araceae
Mayoa portugallica is a small fragment of lignitized plant tissue with masses of striate (polyplicate) pollen adhering to its irregular surface (Friis et al. 2004). The pollen grains are inaperturate and elliptical in outline. They have a finely striate tectum composed of closely spaced, straight ribs that cross each other in a characteristic way. The tectum is supported by a very thin granular or weakly columellate infratectal layer and the endexine is extremely thin and granular. These distinctive pollen grains are closely similar to those of extant Holochlamys in every respect. Although other information on the Mayoa plant has not yet been discovered, the unique pollen morphology combined with the unusual wall ultrastructure strongly suggests a close relationship of the fossil to the subfamily Monsteroideae, and this supports the presence of crown group Araceae in the Early Cretaceous. Flowers of extant Monsteroideae are bisexual and have a perianth.
In contrast to Mayoa, several new fossils from a new Early Cretaceous locality in Portugal (Vila Verde 2, northeast of Figueira da Foz, Figueira da Foz Formation, Late Aptian–Early Albian) are known from much more complete material. These fossils, which comprise inflorescence axes (spadices) and flowers with pollen in situ, can also be assigned to Araceae.
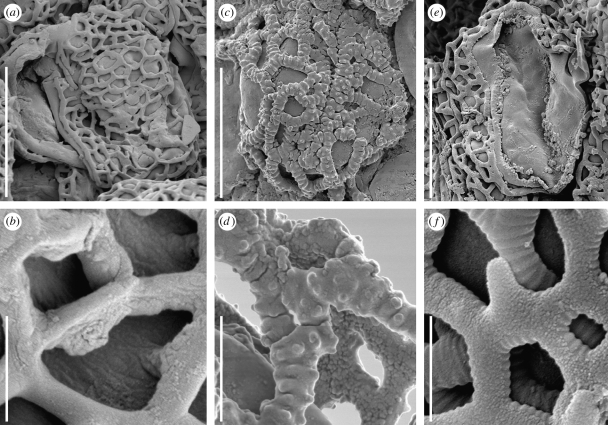
One of these araceous fossils, here informally referred to as ‘Araceae fossil sp. A’ (figures 1a–f and 2a,b) is known from many small inflorescence fragments bearing simple flowers that are densely packed in a low spiral. Most of the specimens are charcoalified and have retained their original three-dimensional shape. The flowers are unisexual (staminate) and lack a perianth (in aroid terminology: aperigonate). Each consists of one or two free stamens. The anthers are tetrasporangiate, basifixed and almost sessile, with a short, inconspicuous filament. Anthers are also bulky, broad and almost square apically. They appear to have been fleshy with a very extensively thickened connective between the four small and widely spaced pollen sacs. The thecae are placed opposite each other and the pollen sacs placed along the four edges of the anther. The surface of the anthers usually has distinctive bulges, indicating the presence of densely spaced secretory cells. In many specimens, the apical part of the connective shows the remains of an abundant secretion, and we infer that the anther connective was odour producing (osmophoric). Pollen grains found in the stamens are clustered in elongated strands. Pollen is monocolpate with a long extended colpus and a semitectate-reticulate pollen wall. The reticulum is coarse with dimorphic lumina. Muri are psilate towards the outside and almost granular towards the inside. The reticulum is supported only by a few columellae. The columellae are uneven in length and shape. They detach easily from the main body of the grain, leaving scattered scars on the outer surface of the foot layer.
Figure 1.
Scanning electron micrographs of fossil inflorescences of Araceae from the Early Cretaceous (Late Aptian–Early Albian of the Vila Verde 2 locality (sample Vila Verde 2 439), Portugal. (a–f) Araceae fossil sp. A, and in situ pollen. (a) Inflorescence fragment with densely spaced and spirally arranged staminate flowers on the central axis; S165015. (b) Inflorescence fragment with many spirally arranged staminate flowers; S165005. (c) Single inflorescence section with 10 stamens; S165002. (d) Inflorescence fragment with several flowers, central axis missing; S165001. (e,f) Pollen grains from staminate flower in inflorescence fragment shown in figure 1c; S165005. (g–i) Araceae fossil sp. B, with densely spaced and spirally arranged flowers and periporate pollen; S165007. (g) Internal view of inflorescence showing axis and attached flowers. (h) External view of inflorescence. (i) Periporate pollen grain from anther; arrows show three of several pores. Scale bars: a = 2 mm; c = 250 µm; b, d, g, h = 1 mm; e, f = 10 µm; i = 20 µm. All specimens housed in the palaeobotanical collections at the Swedish Museum of Natural History (S).
Figure 2.
Scanning electron micrographs of in situ pollen from Early Cretaceous mesofossil floras from Portugal. (a,b) Pollen from Araceae fossil sp. A shown in figure 1b; S165005 (sample Vila Verde 2 439). (a) Pollen showing long open colpus and coarse, loose reticulum supported by scattered columellae. (b) Detail of wall showing muri without supratectal ornamentation, but with a granular structure on the internal surface and on the columellae. (c,d) Pennipollis-type pollen from the Late Aptian–Early Albian Vale de Agua locality (sample Vale de Agua 139); S105603. (c) Pollen showing long colpus and very coarse, loose reticulum with spiny supratectal ornamentation. (d) Detail of reticulum showing spiny supratectal ornamentation externally (above) and granular internal surface of muri (below). (e,f) ‘Retimoncolpites’-type pollen from Torres Vedras (sample Torres Vedras 44). (e) Pollen showing long open colpus and coarse, loose reticulum with transverse supratectal striations. (f) Detail of reticulum from two different grains. The upper reticulum is loosed from the main body of the pollen grain and shows the granular inner surface of the muri; the reticulum of the grain below shows the striate supratectal ornamentation of the outside of the muri. Scale bars: a = 10 µm; b = 1 µm; c, e = 12 µm; d = 3 µm; f = 1.2 µm. All specimens housed in the palaeobotanical collections at the Swedish Museum of Natural History (S).
An isolated stamen from the Catefica locality (Late Barremian–Aptian) with very bulky anthers and a connective that was probably also osmophoric may belong to the same taxon as the Villa Verde aroid, indicating a wider occurrence of this kind of plant. Dispersed pollen grains very similar to the pollen grains in situ within the stamens from Villa Verde 2 are common in Early Cretaceous palynofloras. They are typically assigned to Retimonocolpites or occasionally to Liliacidites. Both of these genera include a variety of different dispersed grains that vary in colpus configuration as well as in the pattern of the reticulum and the ornamentation of the muri. We infer that these other forms of Retimonocolpites and Liliacidites are probably also monocotyledons as suggested previously by Walker & Walker (1984), although they most likely belong to different taxa at generic or higher levels.
All characters of the new fossil strongly support a close relationship with the true aroids, subfamily Aroideae, which are characterized by their unisexual, perianth-less flowers (Cabrera et al. 2008). They therefore support earlier inferences based on Mayoa and provide further evidence of crown group Araceae in the Early Cretaceous. Most extant Aroideae have inaperturate and atectate pollen. However, Calla, which under some analyses is the sister genus to all other true aroids (Cabrera et al. 2008), has aperturate, tectate pollen. Tectate–semitectate pollen, sometimes with extended colpi, is also a characteristic of the closely related Lasioideaeae and Zamioculcadoideae and is most likely plesiomorphic within the family. Reduction of columellae as seen in the new fossil may be a step towards loss of tectum characterizing most aroids.
Another araceous fossil from the Vila Verde 2 locality, here informally referred to as ‘Araceae fossil sp. B’ (figure 1g–i), consists of a single inflorescence with numerous, densely spaced flowers that are borne in a spiral arrangement. The fossil is lignitic and slightly compressed, which impedes a full understanding of the floral organization. Nevertheless, it is clear that the flowers have a perianth (in aroid terminology, perigonate). Scars left by the flowers, and the organization of the surface of the inflorescence, show that there were four tepals. It is unclear whether they were in opposite pairs or in a whorl. The flowers appear bisexual, with a massive central gynoecium surrounded by free stamens. The stamens consist of a long anther and a short, indistinct filament. The apical, exposed surface of the gynoecium is rhomboidal.
Pollen grains preserved in situ are periporate and semitectate–reticulate. The reticulum is coarse with homogeneous lumina. Muri are smooth with a sharp triangular profile. The tectum is supported by scattered, long columellae. The pores are scattered over the surface of the pollen and the aperture membranes are covered with densely spaced granules. We are not aware that similar grains have been described from dispersed Early Cretaceous palynofloras. However, from the Famalicão locality (Late Aptian), Portugal, similar periporate grains have been observed in situ in a tetrasporangiate anther (Friis et al. 1999). This pollen differs in having more densely spaced columellae.
The characters of this fossil indicate a phylogenetic relationship to subfamily Pothoideae. It thus provides further evidence of crown group Araceae in the Early Cretaceous. Extant Pothoideae is the only extant group of Araceae that has perigonate, bisexual flowers with periporate semitectate–reticulate pollen.
A further Early Cretaceous fossil that may also belong to Pothoideae is a small inflorescence fragment from the Vale de Agua locality (Late Aptian–Early Albian) of Portugal. The fossil is strongly compressed and the organization of the flowers difficult to establish, but the flowers appear very similar to the Araceae fossil sp. B. The in situ pollen are similarly periporate and semitectate–reticulate, but the reticulum is much more dense with very small lumina, and muri have a supratectal ornamentation of minute spinules.
There are also several fruiting structures in the early mesofossil floras from Portugal that may be linked to Araceae. Some have many fruits borne in a dense spiral along a central inflorescence axis, sometimes with rhombic apical faces, others are isolated fruits with morphological similarities to extant fruits of Araceae and have Retimonocolpites-type pollen attached. All these need to be studied in further detail to confirm their systematic affinity.
(ii). Other Alismatales
A general relationship to Alismatales has been suggested for the Pennistemon/Pennicarpus/Pennipollis plant (Friis et al. 2000). In contrast, an affinity with Chloranthaceae has been suggested by Hesse & Zetter (2007), Doyle et al. (2008) and Doyle (2009). Acolumellate pollen with an extremely loose reticulum is widespread in Alismatales and other basal monocotyledons (Grayum 1992), but is not known among extant Chloranthaceae. The finely granular inner lining of the muri seen in pollen of the Pennistemon/Pennicarpus/Pennipollis plant (figure 2c,d) is very similar to that seen in the in situ pollen grains from the new Araceae fossil sp. A (figure 2a,b), which supports the possible alismatalean affinity for these fossils. This feature also occurs in many other Retimonocolpites-type pollen grains found in situ in dispersed stamens that range in age from Late Barremian to Middle Albian (figure 2e,f).
Dispersed pollen grains of Pennipollis are diverse and abundant in Early Cretaceous palynological assemblages from southern Laurasia and northern Gondwana, indicating that the Pennistemon/Pennicarpus/Pennipollis plants were widespread and probably common in the Barremian–Albian vegetation (Friis et al. 2000).
Several other floral structures that are probably related to extant Alismatales are known from the Early Cretaceous mesofossil floras of Portugal. One is a fragmentary bisexual flower with remains of stamens around an apocarpous gynoecium of three or four free follicular carpels. Pollen grains found in situ are periporate and tectate with echinate tectum ornamentation and clusters of small spines covering the apertures. These pollen grains are closely similar to grains of Alismataceae, and a systematic position close to Alismataceae is supported also by the general organization of the flower. We have not seen similar pollen from dispersed palynological assemblages, but comparable periporate, tectate and echinate grains occur in coprolites from the Torres Vedras flora (Late Barremian–Early Aptian).
(iii). Extinct groups of Early Cretaceous angiosperms
Notwithstanding the clear presence of chloranthoid, eumagnoliid, eudicot, monocot, nymphaealean and winteroid lineages during the Early Cretaceous, as well as possible Piperales, most of the angiosperm pollen grains, flowers and fruits at this level cannot be linked convincingly to any particular extant angiosperm lineage. From the Famalicão flora for instance, more than 100 taxa of angiosperms are identified, but only a few have currently been placed in extant lineages. The degree to which such fossils reflect the presence of extinct lineages that are relatively independent of extant taxa at this level of angiosperm evolution remains to be determined. However, the relatively few fossil angiosperms in Early Cretaceous floras that can be assigned to extant taxa are strongly suggestive of considerable extinction. Especially, clear cases are those where very distinctive Early Cretaceous pollen types have no counterpart among any group of extant angiosperm (e.g. Stellatopollis). Such examples provide unambiguous evidence of extinction, probably at a high taxonomic level.
4. Conclusions
The extensive and highly informative record of charcoalified and lignitized angiosperm remains from the Cretaceous accumulated over the past 30 years has provided an unexpected picture of the diversity of angiosperms in Early Cretaceous plant communities (e.g. Friis et al. 2006a). Flowers, fruits and seeds are mainly small. It has been suggested that Early Cretaceous angiosperms were probably herbaceous on the basis of phylogenetic inferences (Taylor & Hickey 1992) or riparian weeds inferred from leaf architecture (Doyle & Hickey 1976). The identification of diverse monocot flowers in the Early Cretaceous mesofossil floras together with other fossils closely related to extant lineages that comprise predominantly herbaceous or small shrubby plants (Nymphaeaceae, Piperales, Chloranthaceae, Ranunculales, Buxales) supports these ideas. It is also interesting that many of the mesofossil and impression/compression fossils such as Monetianthus mirus, Pluricarpellatia peltata, the three species of Archaefructus and many isolated leaves suggest an aquatic habitat. The colonization of the freshwater bodies by angiosperms may also have had a considerable impact on aquatic life.
Based on the presence of monoaperturate pollen, both dispersed in palynofloras and in situ within floral structures, it is clear that there is a great diversity of forms related to early lineages of angiosperms in the Early Cretaceous. Eudicots do not appear until after the diversification of lineages with monoaperturate pollen is well underway. In the earliest (pre-Albian) phases of the angiosperm radiation, both palynological and floral evidence show that the diversity of non-eudicot angiosperms (ANITA lineages and Chloranthaceae, eumagnoliids, monocots and other apparently extinct forms) greatly exceeded that of eudicots, but these proportions changed dramatically through the Late Cretaceous as eudicots quickly came to dominate both mesofossil and macrofossil floras as well as most palynofloras.
An important new conclusion from the information presented here is that some of the diversity of angiosperms during the Early Cretaceous included key lineages of early monocotyledons. This had been suspected previously, but until now the early diversity of monocots has been surprisingly cryptic. More monocots may now be recognized as the search image is adjusted in light of these new discoveries. Recognition of Early Cretaceous Araceae raises interesting new questions about the timing of diversification in this family and in monocotyledons as a whole. In addition, the presence of araceous flowers of two very different kinds, together with the considerable diversity of floral form seen in other ancient angiosperms from the Early Cretaceous, highlights the extent of developmental versatility among angiosperm flowers at this very early stage in the evolution of the group.
Improved knowledge of fossil angiosperms from the Early Cretaceous has made some aspects of early angiosperm evolution much less mysterious, but a key remaining issue is the relationship of angiosperms to other seed plants. While this is still very uncertain, new fossils are constantly being added to the fossil record of angiosperms and potentially related seed plants (Friis et al. 2009a). These discoveries may ultimately help to clarify the phylogenetic position of angiosperms in the broader context of seed plant evolution. They may also help illuminate how the key features of the angiosperm flower came together, and provide the information needed to better understand the history of pollination and dispersal biology among angiosperms and their relatives.
Acknowledgements
We thank W. G. Chaloner, London, and an anonymous reviewer for valuable suggestion to this paper. This research was supported by the Swedish Natural Science Research Council (EMF), the Carlsberg Foundation, Denmark (KRP) and the National Science Foundation (PRC).
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Axelrod D. I.1952A theory of angiosperm evolution. Evolution 6, 29–60 (doi:10.2307/2405502) [Google Scholar]
- Brenner G. J.1963The spores and pollen of the Potomac Group of Maryland. Md. Dep. Geol. Mines Water Resour. Bull. 27, 1–215 [Google Scholar]
- Brenner G. J.1996Evidence for the earliest stage of angiosperm pollen evolution: a paleoequatorial section from Israel. In Flowering plant origin, evolution and phylogeny (eds Taylor D. W., Hickey L. J.), pp. 91–115 New York, NY: Chapman & Hall [Google Scholar]
- Cabrera L. I., Salazar G. A., Chase M. W., Mayo S. J., Bogner J., Davila P.2008Phylogenetic relationships of aroids and duckweeds (Araceae) inferred from coding and noncoding plastid DNA. Am. J. Bot. 95, 1153–1165 (doi:10.3732/ajb.0800073) [DOI] [PubMed] [Google Scholar]
- Couper R. A.1960New Zealand Mesozoic and Cainozoic plant microfossils. N. Z. Geol. Surv. Paleontol. Bull. 32, 1–87 [Google Scholar]
- Crane P. R., Friis E. M., Pedersen K. R.1989Reproductive structure and function in Cretaceous Chloranthaceae. Plant Syst. Evol. 165, 211–226 (doi:10.1007/BF00936003) [Google Scholar]
- Crane P. R., Pedersen K. R., Friis E. M., Drinnan A. N.1993Early Cretaceous (Early to Middle Albian) platanoid inflorescences associated with Sapindopsis leaves from the Potomac Group of Eastern North America. Syst. Bot. 18, 328–344 (doi:10.2307/2419407) [Google Scholar]
- Crane P. R., Friis E. M., Pedersen K. R.1995The origin and early diversification of angiosperms. Nature 374, 27–33 (doi:10.1038/374027a0) [Google Scholar]
- Dettmann M. E., Clifford H. T., Peters M.2009Lovellea wintonensis gen. et sp. nov.—Early Cretaceous (late Albian), anatomically preserved, angiosperm flowers and fruits from the Winton Formation, western Queensland. Cretaceous Res. 30, 339–355 (doi:10.1016/j.cretres.2008.07.015) [Google Scholar]
- Dilcher D. L., Crane P. R.1984Archaeanthus: an early angiosperm from the Cenomanian of the Western Interior of North America. Ann. Mo. Bot. Gard. 71, 351–383 (doi:10.2307/2399030) [Google Scholar]
- Doyle J. A.1969Cretaceous angiosperm pollen of the Atlantic Coastal Plain and its evolutionary significance. J. Arnold Arb. 50, 1–35 [Google Scholar]
- Doyle J. A.1992Revised palynological correlations of the lower Potomac Group (USA) and the Cocobeach sequence of Gabon (Barremian–Aptian). Cretaceous Res. 13, 337–349 (doi:10.1016/0195-6671(92)90039-S) [Google Scholar]
- Doyle J. A.2009Evolutionary significance of granular exine structure in the light of phylogenetic analyses. Rev. Palaeobot. Palynol. 156, 198–210 (doi:10.1016/j.revpalbo.2008.08.001) [Google Scholar]
- Doyle J. A., Hickey L. J.1976Pollen and leaves from the Mid-Cretaceous Potomac Group and their bearing on early angiosperm evolution. In Origin and early evolution of angiosperms (ed. Beck C. B.), pp. 139–206 New York, NY: Columbia University Press [Google Scholar]
- Doyle J. A., Robbins E. I.1977Angiosperm pollen zonation of the continental Cretaceous of the Atlantic Coastal Plain and its application to deep wells in the Salisbury Embayment. Palynology 1, 43–78 [Google Scholar]
- Doyle J. A., Biens P., Doerenkamp A., Jardiné S.1977Angiosperm pollen from the pre-Albian Lower Cretaceous of Equatorial Africa. Bull. Cent. Rech. Explor. Prod. Elf-Aquitaine 1, 451–473 [Google Scholar]
- Doyle J. A., Hotton C. L., Ward J. V.1990Early Cretaceous tetrads, zonasulculate pollen, and Winteraceae. I. Taxonomy, morphology, and ultrastructure. Am. J. Bot. 77, 1544–1557 (doi:10.2307/2444487) [Google Scholar]
- Doyle J. A., Endress P. K., Upchurch G. R.2008Early Cretaceous monocots: a phylogenetic evaluation. Sborník Národního muzea v Praze, B 64, 59–87 [Google Scholar]
- Drinnan A. N., Crane P. R., Friis E. M., Pedersen K. R.1990Lauraceous flowers from the Potomac Group (Mid-Cretaceous) of eastern North America. Bot. Gaz. 151, 370–384 (doi:10.1086/337838) [Google Scholar]
- Drinnan A. N., Crane P. R., Pedersen K. R., Friis E. M.1991Angiosperm flowers and tricolpate pollen of buxaceous affinity from the Potomac Group (Mid-Cretaceous) of eastern North America. Am. J. Bot. 78, 153–176 (doi:10.2307/2445239) [Google Scholar]
- Eklund H.2000Lauraceous flowers from the Late Cretaceous of North Carolina, USA. Bot. J. Linn. Soc. 132, 397–428 (doi:10.1111/j.1095-8339.2000.tb01220.x) [Google Scholar]
- Eklund H., Kvaček J.1998Lauraceous inflorescences and flowers from the Cenomanian of Bohemia (Czech Republic, Central Europe). Int. J. Plant Sci. 159, 668–686 (doi:10.1086/297585) [Google Scholar]
- Eklund H., Friis E. M., Pedersen K. R.1997Chloranthaceous floral structures from the Late Cretaceous of Sweden. Plant Syst. Evol. 207, 13–42 (doi:10.1007/BF00985207) [Google Scholar]
- Endress P. K.2008Perianth biology in the basal grade of extant angiosperms. Int. J. Plant Sci. 169, 844–862 (doi:10.1086/589691) [Google Scholar]
- Endress P. K., Doyle J. A.2009Reconstructing the ancestral angiosperm flower and its initial specializations. Am. J. Bot. 96, 22–66 (doi:10.3732/ajb.0800047) [DOI] [PubMed] [Google Scholar]
- Eriksson O., Friis E. M., Löfgren P.2000aSeed size, fruit size and dispersal spectra in angiosperms from the Early Cretaceous to the Late Tertiary. Am. Nat. 156, 47–58 (doi:10.1086/303367) [DOI] [PubMed] [Google Scholar]
- Eriksson O., Friis E. M., Pedersen K. R., Crane P. R.2000bSeed size and dispersal systems of Early Cretaceous angiosperms from Famalicão, Portugal. Int. J. Plant Sci. 161, 319–329 (doi:10.1086/314248) [DOI] [PubMed] [Google Scholar]
- Friedman W. E.2009The meaning of Darwin's ‘abominable mystery’. Am. J. Bot. 96, 5–21 (doi:10.3732/ajb.0800150) [DOI] [PubMed] [Google Scholar]
- Friis E. M.1984Preliminary report on Upper Cretaceous angiosperm reproductive organs from Sweden and their level of organization. Ann. Mo. Bot. Gard. 71, 403–418 (doi:10.2307/2399032) [Google Scholar]
- Friis E. M., Skarby A.1981Structurally preserved angiosperm flowers from the Upper Cretaceous of southern Sweden. Nature 291, 485–486 (doi:10.1038/29148490) [Google Scholar]
- Friis E. M., Crane P. R., Pedersen K. R.1988Reproductive structures of Cretaceous Platanaceae. K. Danske Vidensk. Selsk. Biol. Skr. 31, 1–55 [Google Scholar]
- Friis E. M., Eklund H., Pedersen K. R., Crane P. R.1994Virginianthus calycanthoides gen. et sp. nov.—a calycanthaceous flower from the Potomac Group (Early Cretaceous) of eastern North America. Int. J. Plant Sci. 155, 772–785 (doi:10.1086/297217) [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.1995Appomattoxia ancistrophora gen. et sp. nov., a new Early Cretaceous plant with similarities to Circaeaster and extant Magnoliidae. Am. J. Bot. 82, 933–943 (doi:10.2307/2445980) [Google Scholar]
- Friis E. M., Crane P. R., Pedersen K. R.1997Fossil history of magnoliid angiosperms. In Evolution and diversification of land plants (eds Iwatsuki K., Raven P. H.), pp. 121–156 Tokyo, Japan: Springer Verlag [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.1999Early angiosperm diversification: the diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. Ann. Mo. Bot. Gard. 86, 259–296 (doi:10.2307/2666179) [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.2000Fossil floral structures of a basal angiosperm with monocolpate, reticulate-acolumellate pollen from the Early Cretaceous of Portugal. Grana 39, 226–245 [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.2001Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature 410, 357–360 (doi:10.1038/35066557) [DOI] [PubMed] [Google Scholar]
- Friis E. M., Doyle J. A., Endress P. K., Leng Q.2003Archaefructus—angiosperm precursor or specialized early angiosperm? Trends Plant Sci. 8, 369–373 (doi:10.1016/S1360-1385(03)00161-4) [DOI] [PubMed] [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.2004Araceae from the Early Cretaceous of Portugal: evidence on the emergence of monocotyledons. Proc. Natl Acad. Sci. USA 101, 16 565–16 570 (doi:10.1073/pnas.0407174101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.2006aCretaceous angiosperm flowers: innovation and evolution in plant reproduction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 251–293 (doi:10.1016/j.palaeo.2005.07.006) [Google Scholar]
- Friis E. M., Pedersen K. R., Schönenberger J.2006bNormapolles plants: a complex of extinct fagalean lineages. Plant Syst. Evol. 260, 107–140 [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.2009aEarly Cretaceous mesofossils from Portugal and eastern North America related to the Bennettitales–Erdtmanithecales–Gnetales group. Am. J. Bot. 96, 252–283 (doi:10.3732/ajb.0800113) [DOI] [PubMed] [Google Scholar]
- Friis E. M., Pedersen K. R., von Balthazar M., Grimm G. W., Crane P. R.2009bMonetianthus mirus gen. et sp. nov., a nymphaealean flower from the Early Cretaceous of Portugal. Int. J. Plant Sci. 170, 1086–1101 (doi:10.1086/605120) [Google Scholar]
- Frumin S., Eklund H., Friis E. M.2004Mauldinia hirsuta sp. nov., a new member of the extinct genus Mauldinia (Lauraceae) from the Late Cretaceous (Cenomanian–Turonian) of Kazakhstan. Int. J. Plant Sci. 165, 883–895 (doi:10.1086/421479) [Google Scholar]
- Gandolfo M. A., Nixon K. C., Crepet W. L.2000Monocotyledons: a review of their Early Cretaceous record. In Monocots: systematics and evolution (eds Wilson K. I., Morrison D. A.), pp. 44–51 Melbourne, Australia: CSIRO [Google Scholar]
- Gandolfo M. A., Nixon K. C., Crepet W. L.2004Cretaceous flowers of Nymphaeaceae and implications for complex insect entrapment pollination mechanisms in early angiosperms. Proc. Natl Acad. Sci. USA 101, 8056–8060 (doi:10.1073/pnas.0402473101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayum M. H.1990Evolution and phylogeny of the Araceae. Ann. Mo. Bot. Gard. 77, 628–697 (doi:10.2307/2399668) [Google Scholar]
- Grayum M. H.1992Comparative external pollen ultrastructure of the Araceae and putative related taxa. Monogr. Syst. Bot. Mo. Bot. Gard. 43, 1–167 [Google Scholar]
- Heer O.1868Flora Fossilis Arctica. I. Die fossile Flora der polarländer Zurich, Switzerland: J. Wurster & Comp [Google Scholar]
- Heer O.1882Flora fossilis Grönlandica. Die fossile flora Grönlands. I. Flora fossilis arctica. Die fossile Flora der Polarländer Zurich, Switzerland: J. Wurster & Comp [Google Scholar]
- Herendeen P. S., Crane P. R.1995The fossil history of the monocotyledons. In Monocotyledons: systematics and evolution (eds Rudall P. J., Cribb P. J., Cutler D. F., Humphries C. J.), pp. 1–21 Kew, Victoria: Royal Botanic Gardens [Google Scholar]
- Hesse M., Zetter R.2007The fossil pollen record of Araceae. Plant Syst. Evol. 263, 93–115 (doi:10.1007/s00606-006-0468-z) [Google Scholar]
- Hickey L. J., Doyle J. A.1977Early Cretaceous fossil evidence for angiosperm evolution. Bot. Rev. 43, 2–104 [Google Scholar]
- Hickey L. J., Wolfe J. A.1975The base of angiosperm phylogeny: vegetative morphology. Ann. Mo. Bot. Gard. 62, 538–589 (doi:10.2307/2395267) [Google Scholar]
- Hughes N. F.1976Palaeobiology of angiosperm origins Cambridge, UK: Cambridge University Press [Google Scholar]
- Hughes N. F.1994The enigma of angiosperm origins Cambridge, UK: Cambridge University Press [Google Scholar]
- Hughes N. F., McDougall A. B.1990Barremian–Aptian angiospermid pollen records from southern England. Rev. Palaeobot. Palynol. 65, 145–151 (doi:10.1016/0034-6667(90)90065-Q) [Google Scholar]
- Leng Q., Wu S., Friis E. M.2003Angiosperms. In The Jehol biota (eds Chang M., Chen P., Wang Y., Wang Y., Miao D.), pp. 178–185 Shanghai, China: Shanghai Scientific and Technical Publishers [Google Scholar]
- Lesquereux L.1874. Contributions to the fossil flora of the Western territories. Part I. The Cretaceous flora. Report of the United States Geological Survey of the Territories, Department of the Interior, Washington [Google Scholar]
- Martinez-Millan M., Crepet W. L., Nixon K. C.2009Pentapetalum trifasciculandricus gen. et sp. nov., a thealean fossil flower from the Raritan Formation, New Jersey, USA (Turonian, Late Cretaceous). Am. J. Bot. 96, 933–949 (doi:10.3732/ajb.0800347) [DOI] [PubMed] [Google Scholar]
- Mohr B. A. R., Bernardes-de-Oliveira M. E. C.2004Endressinia brasiliana, a Magnolialean angiosperm from the Lower Cretaceous Crato Formation (Brazil). Int. J. Plant Sci. 165, 1121–1133 (doi:10.1086/423879) [Google Scholar]
- Mohr B. A. R., Bernardes-de-Oliveira M. E. C., Loveridge R. F.2007The macrophyte flora of the Crato Formation. In The Crato fossil beds of Brazil window into an ancient world (eds Martill D. M., Bechly G., Loveridge R. F.), pp. 537–565 Cambridge, UK: Cambridge University Press [Google Scholar]
- Muller J.1970Palynological evidence on early differentiation of angiosperms. Biol. Rev. 45, 417–450 (doi:10.1111/j.1469-185X.1970.tb01649.x) [Google Scholar]
- Muller J.1981Fossil pollen records of extant angiosperms. Bot. Rev. 47, 1–142 (doi:10.1007/BF02860537) [Google Scholar]
- Newberry J. S.1863Description of the fossil plants collected by Mr George Gibbs, Geologist to the United States Northwest Boundary Commission, under Mr. Archibald Campbell, United States Commissioner. Boston J. Nat. Hist. 7, 506–524 [Google Scholar]
- Pedersen K. R., von Balthazar M., Crane P. R., Friis E. M.2007Early Cretaceous floral structures and in situ tricolpate-striate pollen: new early eudicots from Portugal. Grana 46, 176–196 (doi:10.1080/00173130701526507) [Google Scholar]
- Penny J. H. J.1986An Early Cretaceous angiosperm pollen assemblage from Egypt. Special Papers Palaeontol. 35, 121–134 [Google Scholar]
- Penny J. H. J.1988aEarly Cretaceous acolumellate semitectate pollen from Egypt. Palaeontology 31, 373–418 [Google Scholar]
- Penny J. H. J.1988bEarly Cretaceous striate pollen from the Borehole Mersa Matruh 1, North West Desert, Egypt. J. Micropalaeontol. 7, 201–215 [Google Scholar]
- Penny J. H. J.1991Early Cretaceous angiosperm pollen from the borehole Mersa Matruh 1, North West desert, Egypt. Palaeontographica B 222, 31–88 [Google Scholar]
- Qiu Y. L.1999The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402, 404–407 (doi:10.1038/46536) [DOI] [PubMed] [Google Scholar]
- Saporta G. de.1877L'ancienne végétation polaire d'après les travaux de M. le professeur Heer et les dernière découvertes des explorateurs suédois. Comptes-rendue du Congrès International des Sciences Géographiques, Paris, pp. 1–47 [Google Scholar]
- Saporta G. de.1878Les anciens climats de l'Europe et le développement de la végétation Conférence donnée au Congrès de l'association Française pour l'Avancement des Sciences, Tenu au Hâvre en août 1877. Aix-en-Province: Marius Illy [Google Scholar]
- Saporta G. de.1894Flore fossile du Portugal. Nouvelles contributions à la flore Mésozoique. Accompagnées d'une notice stratigraphique par Paul Choffat Lisbon, Portugal: Imprimerie de l Academie Royale des Sciences [Google Scholar]
- Scott R. A., Barghoorn E. S., Leopold E.1960How old are the angiosperms? Am. J. Sci. 258-A, 284–299 [Google Scholar]
- Soltis D. E., Soltis P. S., Endress P. K., Chase M. W.2005Phylogeny and evolution of angiosperms Sunderland, MA: Sinauer Associates, Inc. Publishers [Google Scholar]
- Srivastava S. K.1969Assorted angiosperm pollen from the Edmonton Formation (Maastrichtian), Alberta, Canada. Can. J. Bot. 47, 975–989 (doi:10.1139/b69-138) [Google Scholar]
- Sun G., Zheng S., Dilcher D. L., Wang Y., Mei S.2001Early angiosperms and their associated plants from western Liaoning, China Shanghai, China: Shanghai Scientific and Technological Education Publishing House [Google Scholar]
- Sun G., Ji Q., Dilcher D. L., Zheng S., Nixon K. C., Wang X.2002Archaefructaceae, a new basal angiosperm family. Science 296, 899–904 (doi:10.1126/science.1069439) [DOI] [PubMed] [Google Scholar]
- Takahashi M., Herendeen P. S., Crane P. R.2001Lauraceous flowers from the Kamikitaba locality (Lower Coniacian; Upper Cretaceous) of Northeast Japan. J Plant Res. 112, 187–206 (doi:10.1007/PL00013872) [Google Scholar]
- Takahashi M., Crane P. R., Friis E. M.2007Fossil seeds of Nymphaeales from the Tamayama Formation (Futaba Group), Upper Cretaceous (Early Santonian) of northeastern Honshu, Japan. Int. J. Plant Sci. 168, 341–350 (doi:10.1086/510414) [Google Scholar]
- Takahashi M., Friis E. M., Uesugi K., Suzuki Y., Crane P. R.2008Floral evidence of Annonaceae from the Late Cretaceous of Japan. Int. J. Plant Sci. 169, 890–898 [Google Scholar]
- Taylor D. W., Hickey L. J.1992Phylogenetic evidence for the herbaceous origin of angiosperms. Plant Syst. Evol. 180, 137–156 (doi:10.1007/BF00941148) [Google Scholar]
- Taylor D. W., Brenner G. J., Basha S. H.2008Scutifolium jordanicum gen. et sp. nov. (Cabombaceae), an aquatic fossil plant from the Lower Cretaceous of Jordan, and the relationships of related leaf fossils to living genera. Am. J. Bot. 95, 340–352 (doi:10.3732/ajb.95.3.340) [DOI] [PubMed] [Google Scholar]
- Tiffney B. H.1984Seed size, dispersal syndromes, and the rise of the angiosperms: evidence and hypothesis. Ann. Mo. Bot. Gard. 71, 551–576 (doi:10.2307/2399037) [Google Scholar]
- The Angiosperm Pyhlogeny Group 2003An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APGII. Bot. J. Linn. Soc. 141, 399–436 (doi:10.1046/j.1095-8339.2003.t01-1-00158.x) [Google Scholar]
- Upchurch G. R., Crane P. R., Drinnan A. N.1994The megaflora from the Quantico locality (Upper Albian), Lower Cretaceous Potomac Group of Virginia Martinsville, VA: Virginia Museum of Natural History [Google Scholar]
- Viehofen A., Hartkopf-Fröder C., Friis E. M.2008Inflorescences and flowers of Mauldinia angustiloba sp. nov. (Lauraceae) from Mid-Cretaceous karst infillings in the Rhenish Massif, Germany. Int. J. Plant Sci. 169, 871–889 (doi:10.1086/589975) [Google Scholar]
- von Balthazar M., Pedersen K. R., Friis E. M.2005Teixeiraea lusitanica gen. et sp. nov., a Ranunculalean flower from the Early Cretaceous of Portugal. Plant Syst. Evol. 255, 55–75 [Google Scholar]
- von Balthazar M., Pedersen K. R., Crane P. R., Friis E. M.2007Potomacanthus lobatus gen. et sp. nov., a new flower of probable Lauraceae from the Early Cretaceous (Early to Middle Albian) of eastern North America. Am. J. Bot. 94, 2041–2053 (doi:10.3732/ajb.94.12.2041) [DOI] [PubMed] [Google Scholar]
- von Balthazar M., Pedersen K. R., Crane P. R., Friis E. M.2008Carpestella lacunata gen. et sp. nov., a new basal angiosperm flower from the Early Cretaceous (Early to Middle Albian) of eastern North America. Int. J. Plant Sci. 169, 890–898 (doi:10.1086/589692) [Google Scholar]
- Walker J. W., Walker A. G.1984Ultrastructure of Lower Cretaceous angiosperm pollen and the origin and early evolution of flowering plants. Ann. Mo. Bot. Gard. 71, 464–521 (doi:10.2307/2399035) [Google Scholar]