Abstract
The interactions between bees that depend on floral oil for their larvae and flowers that offer oil involve an intricate mix of obligate and facultative mutualisms. Using recent phylogenies, new data on oil-offering Cucurbitaceae, and molecular-dating, we ask when and how often oil-offering flowers and oil-foraging bees evolved, and how frequently these traits were lost in the cause of evolution. Local phylogenies and an angiosperm-wide tree show that oil flowers evolved at least 28 times and that floral oil was lost at least 36–40 times. The oldest oil flower systems evolved shortly after the K/T boundary independently in American Malpighiaceae, tropical African Cucurbitaceae and Laurasian Lysimachia (Myrsinaceae); the ages of the South African oil flower/oil bee systems are less clear. Youngest oil flower clades include Calceolaria (Calceolariaceae), Iridaceae, Krameria (Krameriaceae) and numerous Orchidaceae, many just a few million years old. In bees, oil foraging evolved minimally seven times and dates back to at least 56 Ma (Ctenoplectra) and 53 Ma (Macropis). The co-occurrence of older and younger oil-offering clades in three of the four geographical regions (but not the Holarctic) implies that oil-foraging bees acquired additional oil hosts over evolutionary time. Such niche-broadening probably started with exploratory visits to flowers resembling oil hosts in scent or colour, as suggested by several cases of Muellerian or Batesian mimicry involving oil flowers.
Keywords: oil-offering flowers, oil-foraging bees, molecular clock dating, evolutionary gain, evolutionary loss, oil biochemistry
1. Introduction
The single most striking change in the study of plant/animal interactions over the past 15 years has been the increasing role of phylogenies and the comparative approach in the study of mutual adaptation and coevolution. Work on flower/pollinator interactions, especially, has benefited from the ability of molecular phylogenies to shed light on the relative and absolute sequence of the evolution of traits and on the degree of temporal or geographic congruence in the evolutionary histories of interacting partners. Most flower/pollinator mutualisms are facultative (Bronstein 1994; Thompson 1999). Obligate mutualisms between plant species and their pollinators by contrast are rare. Nevertheless, they offer the opportunity to examine reciprocal adaptation in detail and have become models for understanding how mutualisms sometimes lead to coevolution (e.g. Bogler et al. 1995; Pellmyr et al. 1996; Pellmyr & Leebens-Mack 2000; Whittall & Hodges 2007). Here we focus on a bee/flower mutualism that comprises a fascinating mix of obligate and facultative dependencies, with numerous reciprocal morphological, behavioural and chemical adaptations. This is the mutualism between oil-offering flowers and bees that depend on floral oil for larval provisioning and cell lining, a mutualism discovered 40 years ago (Vogel 1969, 1974, 1981a,b, 1984, 1990).
Oil flower/oil bee mutualisms involve highly specialized bees belonging to a few genera in the Melittidae and Apidae (including the former Anthophoridae and Ctenoplectridae; Michener 2007). Some of these bees use floral lipids with, or in place of nectar to be mixed with pollen for larval provisioning. Others use the oils not only as larval foodstuffs but also for water-resistant cell linings (Cane et al. 1983; Vogel 1988; Alves dos Santos et al. 2002; Melo & Gaglianone 2005). Earlier reviews of the oil flower syndrome (Neff & Simpson 1981; Buchmann 1987; Rasmussen & Olesen 2000; Machado 2004) provide excellent summaries of the geographic distribution and adaptations of the relevant flowers and their bees. Machado (2004) also includes species-level lists of interacting partners. New oil bee/oil flower interactions continue to come to light (Manning & Goldblatt 2002; H. Schaefer 2008, unpublished observations for African and Asian Cucurbitaceae), especially in tropical Orchidaceae, where they are scattered widely and floral biology is poorly known (Reis et al. 2006; Singer et al. 2006; Stpiczynska et al. 2007; Pansarin et al. 2008, 2009). Some plants also produce oil-rich and semi-liquid pollenkitt, for example, the Zingiberaceae Caulokaempferia coenobialis (Wang et al. 2004, 2005) and a few species of the Myrtaceae Verticordia (Houston et al. 1993). We consider such pollenkitt a reward distinct from the floral oils that are the focus of this paper because it is produced by the inner anther wall (the tapetum), not outer epithelia or trichomes, and is taken up with the glossae, not hairs or brushes on legs and abdomens (as are floral oils).
None of the earlier reviews of oil flower/oil bee mutualisms were able to take advantage of large phylogenies and dated chronograms for the relevant bees and plant clades. Yet, date phylogenies can help answer the question to what extent oil offering in flowers and oil foraging in bees evolved at similar geological times and hence how much evolutionary host broadening or switching may have occurred between pre-existing plant or pollinator clades. Also, when Buchmann (1987) wrote his review of the ecology of oil flowers and bees, no reliable fossils were known of any oil bee. This has changed with the description of an oil-collecting bee from the Eocene (53 Myr) of France (Michez et al. 2007), and there is also an Oligocene (34 Myr) oil gland-bearing malpigh flower from Tennessee (Taylor & Crepet 1987; confirmation of phylogenetic assignment in Davis et al. 2004).
Based on published phylogenies, our own work on oil-offering Cucurbitaceae and their bees (Schaefer & Renner 2008, 2009), and sequence matrices assembled for this study to carry out molecular dating, we here address the following questions: (i) how often and during which geological times did oil-offering angiosperm clades evolve; (ii) at which times did the relevant oil-foraging bees originate; (iii) how often and under which conditions were oil offering and oil foraging lost? The answers to these questions then form the basis for a discussion of likely evolutionary pressures underlying the gain and loss of oil rewards.
2. Methods
(a). Phylogenetics of oil-producing angiosperms and oil-foraging bees
We tabulated all known oil-offering angiosperms with their family placement according to the system proposed by the Angiosperm Phylogeny Group (2003), geographic occurrence, associated oil bees and key references on the interaction. To infer global relationships and genetic distances from close relatives, we downloaded 624 rbcL sequences from GenBank. For Nierembergia and Monttea, we produced new rbcL sequences (GenBank accession numbers FJ911661–FJ911662). We similarly tabulated all known oil-foraging bees, with their phylogenetic placement (Michener 2007; Schaefer & Renner 2008; Michez et al. 2009b; S. Cardinal 2009, Cornell University, personal communication), documented or inferred geological age, and relevant references.
(b). DNA data generation, phylogeny estimation and evolutionary rate analyses
Techniques for DNA extraction, sequencing and alignment follow Schaefer et al. (2009). Maximum-likelihood (ML) tree searches and ML bootstrap searches were performed using RAxML v. 7.0.4 (Stamatakis et al. 2008; available at http://phylobench.vital-it.ch/raxml-bb/). RAxML searches relied on the GTR + G model (six general time-reversible substitution rates, assuming gamma rate heterogeneity, with 25 gamma rate categories), and model parameters were estimated over the duration of specified runs.
For divergence time estimates, sequence subsets for asterids, Ericales, rosids and Lamiales were compiled from GenBank and the sequences aligned by eye using MacClade v. 4.08 (Maddison & Maddison 2003). Bayesian uncorrelated-rates clock estimations used the approach of Drummond et al. (2006), with alignments first imported in BEAUti v. 1.4.8 (part of the BEAST package; Drummond & Rambaut 2007) to generate BEAST input files. In BEAST, we used the GTR + G model with six gamma rate categories. The ages of the following most recent common ancestors were constrained based on fossils or in agreement with Wikström et al. (2001), all with normal prior distributions: The Ericales stem to 110±4 Myr, the Zygophyllales stem to 98±3 Myr, the Fagales stem to 84 Myr, the Eucommia stem to 59–55 Myr, the Icacinaceae crown to 70±5 Myr, the Stilbaceae stem to 49±3 Myr and the Plantago-Antirrhinum split to 48±3 Myr. Eucommiaceae fossils are first known in the Late Cretaceous of Europe, but are most common in Eocene to Oligocene sediments (Call & Dilcher 1997); the oldest fruits of Icacinaceae (tribe Iodeae) come from the Late Paleocene of western North America (Pigg et al. 2008). The earliest Fagales fossils may be ca 84 Myr old (Herendeen et al. 1995), although the Normapolles genus Caryanthus, which belongs to crown group Fagales, is known from the Cenomanian and onwards (Friis et al. 2006). The performance of the BEAST runs was checked using Tracer v. 1.4 (Rambaut & Drummond 2007). The resulting trees were combined using TreeAnnotator v. 1.4.8 (part of the BEAST package), with a burnin of 1000 trees. Final trees were checked and edited in FigTree v. 1.2 (Rambaut 2006–2008).
3. Results
(a). Number of independent gains and losses of floral oil
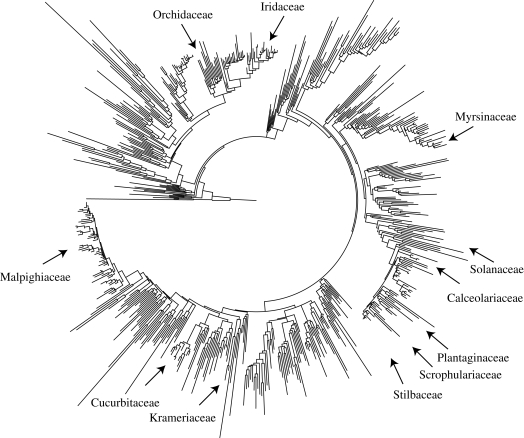
Table 1 shows 28 independent origins of oil-offering flowers indicated by current phylogenies. An angiosperm-wide rbcL phylogeny (figure 1) provides a visual overview of the distribution of oil as a reward in flowering plants. Oil-offering flowers are known from 11 families that occur mainly in the tropics and subtropics. In most of these families oil glands evolved only once, but in Iridaceae and Orchidaceae oil offering evolved multiple times (Goldblatt et al. 2008; Chase et al. 2009; M. Whitten 2009, personal communication; our table 1 and figure 1). For the latter family, phylogenetic understanding is too incomplete to infer the numbers of gains (or losses) in detail. It is safe to assume, however, that orchids harbour many more than the 12 independent origins of oil flowers listed in table 1.
Table 1.
The 28 independent origins of oil-offering flowers indicated by current phylogenies. An angiosperm-wide rbcL phylogeny (figure 1) provides a visual overview of the distribution of oil as a reward in flowering plants.
| independent origins of oil-offering flowers; genera with species numbers and geographical range | age of clade (stem or crown as indicated)a | characteristic oil-foraging bees | selected references on: pollination interaction/plant clade age |
|---|---|---|---|
| America | |||
| Calceolariaceae: Calceolaria (260, Andes to Chile), at least 49 of ca 260 species lack oil | Jovellana/Calceolaria split: 19 Myr1; 15 (27–4) Myr2Calceolaria crown group: 5 (6–1) Myr2 | Centris, Chalepogenus | Vogel (1974) and Sérsic (2004). Phylogeny see Andersson (2006)/1Datson et al. (2008), 2this study |
| Iridaceae I: Sisyrinchium (80–110, N. and S. America), only the 35 S. American species oil-offering | stem lineage: ca 22 Myr | Chalepogenus, Lanthanomelissa | Vogel (1974), Roig-Alsina (1997) and Cocucci & Vogel (2001)/Goldblatt et al. (2008) |
| Iridaceae II: Tigridieae + Trimezieae (Texas to S. America): Alophia (5), Cypella (30), Ennealophus (5), Gelasine (6), Tigridia (55); Trimezia (12) | split btw. tribes: <35 Myr, Tigridieae crown group: <15 Myr | Centris, Paratetrapedia Tetrapedia | Vogel (1974), Lee (1994)/Goldblatt et al. (2008), (Reis et al. 2000, 2003): Trimezieae and Tigridieae are sister taxa, and the plesiomorphic state for the clade is oil secretion (P. Goldblatt 2009, personal communication) |
| Krameriaceae: Krameria (18, N. and S. America) | Krameria crown group: 12 (34–5) Myr | Centris, | Vogel (1974), Simpson et al. (1979); Machado et al. (1997)/this study |
| Malpighiaceae (1250 total, mostly neotropical, the 250 African and few Asian species lack oil glands) | family crown: 75–64 Myr | Centris, Epicharis Paratetrapedia, Tetrapedia | Vogel (1974, 1988, 1990), Anderson (1979), Raw (1979) and Sazima & Sazima (1989)/Davis et al. (2004): oil glands ancestral in family, at least six losses in African clades |
| Orchids I: Cranichideae, Cranichidinae: Ponthieva (40), at least P. racemosa, P. tunguraguae and P. maculata offer oil | ? | ? | Dressler (1993) and G. Gerlach 2009, personal communication. Phylogeny see Salazar et al. (2009) |
| Orchids II: Cymbidieae; Cyrtopodiinae: Grobya (5, at least G. galeata with oil) | ? | Paratetrapedia | Pansarin et al. (2009) |
| Orchids III: Maxillarieae, Bifrenariinae: Rudolfiella (2, Neotropics) | ? | ? | Singer et al. (2006), Stpiczynska & Davies (2008) and G. Gerlach 2009, personal communication |
| Orchids IV: Maxillarieae, Oncidiinae, precise phylogenetic placement unknown, probably several independent origins: Caluera (2), Centroglossa (5), Cyrtochilum (1?), Dunstervillea (1), Platyrhiza (1), Rauhiella (3), Thysanoglossa (2) | ? | Centris, Paratetrapedia, Tetrapedia | Vogel (1974, 1983), Bustos Singer & Cocucci (1999) and Pansarin et al. (2009). Phylogenetic information for these genera from Whitten 2009, personal communication |
| Orchids V: Maxillarieae, Oncidiinae: Chytroglossa (3), Hintonella (1), Ornithocephalus (46) unknown how many offer oil | ? | Paratetrapedia on species of Ornithocephalus | Dressler (1993), van der Cingel (2001) and Silveria (2002) |
| Orchids VI: Maxillarieae, Oncidiinae: Eloyella (7, unknown how many offer oil) | ? | ? | Dressler (1993) |
| Orchids VII: Maxillarieae, Oncidiinae: Oncidium (incl. Sigmatostalix p.p.), Gomesa (1), additional clades likely also offer oil | ? | Paratetrapedia | Reis et al. (2000) (for 1 sp.) and Stpiczynska et al. (2007) (for 2 spp.), Aliscioni et al. (2009), Chase et al. (2009) |
| Orchids VIII: Maxillarieae, Oncidiinae: Trichocentrum, at least T. stipitatum offers oil | ? | Centris | van der Cingel (2001) and Silveria (2002) |
| Orchids IX: Maxillarieae, Oncidiinae: Phymatidium (10, unknown how many offer oil) | ? | ? | Reis et al. (2006) |
| Orchids X: Maxillarieae, Oncidiinae: Zygostates (ca 20, unknown how many offer oil) | ? | ? | Singer et al. (2006) |
| Plantaginaceae: Angelonia (25), Basistemon (8), Monttea (3), the three genera form a clade that includes the oil-less Melosperma (Oxelman et al. 2005). Monopera (2 spp.) probably also belongs in this clade | split btw. Angelonia and Monttea: 13 (34–7) Myr | Centris, Caenonomada, Paratetrapedia Tapinotaspis | Vogel & Machado (1991), Machado et al. (2002) (Angelonia), Sérsic & Cocucci (1999) (Monttea), Vogel & Cocucci (1995) (Basistemon) and Aguiar & Melo (2009) (Monopera)/this study |
| Solanaceae: Nierembergia (21). Oil lost in several species | split btw. Nierembergia and Petunia: 12 (38–10) Myr | Centris, Chalepogenus Tapinotapsis | Neff & Simpson (1981) and Cocucci (1991). Phylogeny see Tate et al. 2009/this study |
| Holarctic region | |||
| Myrsinaceae: Lysimachia (190, Holarctic, 75 with oil; oil lost several times, e.g. Hawaii endemic subgenus Lysimachiopsis) | crown group: 31 (41–8) Myr | Macropis | Vogel (1976, 1986, 1988). Phylogeny see Hao et al. (2004) and Anderberg et al. (2007)/this study |
| Africa | |||
| Cucurbitaceae: Momordica (50, Africa and trop. Asia), oil lost in at least two clades; Siraitia (3–4, Asia); Telfairia (3, Asia); Thladiantha (25, Asia) Baijiania (incl. Sinobaijiania) (5, Asia) | stem age: 46 (55–40) Myr, crown group: 22 (34–12) Myr | Ctenoplectra | Vogel (1981a,b) and H. Schaefer 2008, personal observation. Tanzania (2005), Yunnan (2005), Nigeria (2006) and Australia (2007)/Schaefer et al. (2009) |
| Iridaceae III: Tritoniopsideae: Tritoniopsis (24, S. Africa), only T. parviflora oil-offering | stem age: ca 22 Myr | Redivia | Manning & Goldblatt (2002)/Goldblatt et al. (2008) |
| Orchids XI: Diseae, Coryciinae I (all S. Africa): Ceratandra (6), Corycium (14), Evotella (1), Pterygodium (19) | ? | Rediviva | Pauw (2006) and Whitehead et al. (2008). Phylogeny see Waterman et al. (2009) |
| Orchids XII: Diseae, Coryciinae II: Disperis (74 Africa, few species in Asia). The Asian spp. and some others without oil | ? | Rediviva | Steiner (1989a), Pauw (2006) and Waterman et al. (2009) |
| Orchids XIII: Diseae, Satyriinae: Satyrium (91, S. Africa, Madagascar, 4 in Asia; only S. rhynchanthum oil-offering) | stem group: 28–31 Myr | Rediviva | Johnson (1997), Whitehead & Steiner (2001)/Verboom et al. (2009) |
| Scrophulariaceae I: Alonsoa (16 Neotropic, 2 in Africa). Only the African species with oil glands | split btw. Alonsoa and Nemesia: 47.5–42 Myr1 or 28 (40–17) Myr2 | Rediviva | Steiner (1989b)/1Datson et al. (2008) and 2this study |
| Scrophulariaceae II: Colpias (1, S. Africa, C. mollis) | ? | Rediviva | Steiner & Whitehead (2002) |
| Scrophulariaceae III: Diascia (48, S. Africa) | Nemesia split from Diascia: 32–26 Myr or 15 (24–4) Myr | Rediviva | Vogel (1984), Steiner & Whitehead (1988, 1990, 1991), Pauw (2006)/1Datson et al. (2008), 2this study |
| Scrophulariaceae IV: Hemimeris (4, S. Africa) | Hemimeris + Diclis are sister to Alonsoa | Rediviva | Whitehead & Steiner (1985) and Pauw (2005). Phylogeny see Oxelman et al. (2005) |
| Stilbaceae (all S. Africa): Anastrabe (30), Bowkeria (5), Ixianthes (1) | Stilbaceae stem age: 48 Myr1, split btw. Ixianthes and Nuxia: 17 (18–3) Myr2 | Rediviva | Steiner & Whitehead (1990, 1991) (Bowkeria), Steiner (1993), Steiner & Whitehead (1996) (Ixianthes), Whitehead & Steiner (1992) (Anastrabe)/ 1Wikström et al. (2001) and 2this study |
aSuperscript numerals 1 and 2 refer to references 1 and 2 cited in column 4.
Figure 1.
Maximum-likelihood phylogeny obtained from 626 rbcL sequences representing 440 families of angiosperms (Angiosperm Phylogeny Group 2003), with an over-sampling of oil-offering species. The Iridaceae Sisyrinchium montanum is not known to offer oil (Cocucci & Vogel 2001), but this species is the only Sisyrinchium for which an rbcL sequence was available. Of known oil-offering taxa, the phylogeny lacks the Plantaginaceae Basistemon and Monopera, the Scrophulariaceae Colpias, the Stilbaceae Anastrabe and Bowkeria, and numerous oil-offering orchids (listed in table 1). The tree therefore does not reflect the true number of independent origins of oil in the angiosperms (detailed version provided as figure 1_large in the electronic supplementary material).
Most of the clades producing floral oil subsequently lost this trait in one or several lineages, with the possible exception of Krameriaceae. We know of the following 36–40 independent losses of oil: (i) In Calceolaria, at least 49 of ca 260 species lack oil in their flowers (Sérsic 2004). Not all of these have been included in molecular phylogenies, but in a tree that includes 103 species, 22 lack elaiophores, implying six independent losses (A. Cocucci 2009, Universidad Nacional de Córdoba, Argentina, personal communication). These six losses include one in the ancestors of Stemotria, a group that is nested in Calceolaria. The sister clade of Calceolaria is the non-oil-offering genus Jovellana, with a few species in South America and two in New Zealand. (ii) In Iridaceae, Trimezieae and Tigridieae are sister taxa, and based on the topology (Goldblatt et al. 2008), the most parsimonious explanation for the distribution of oil glands is that they evolved once in the common ancestor of these tribes and that oil secretion was lost at least six times. Even within Tigridia there have been multiple losses in groups that switched to bird pollination or buzz pollination by pollen-collecting bees (Goldblatt et al. 2008; P. Goldblatt 2009, personal communication). (iii) A phylogeny of the neotropical Iridaceae Sisyrinchium implies that glandular trichomes evolved three times in this genus: once in an early diverging group with few species and twice in somewhat larger clades, followed by a few losses. Inferring their precise number requires a more resolved phylogeny (O. Chauveau 2009, personal communication). (iv) In the Malpighiaceae, for which oil glands are a synapomorphy (Anderson 1979, 1990), oil was lost minimally six times in the ancestors of today's African clades, which together comprise about 250 species (Davis et al. 2004; C. Davis 2009, personal communication). Several neotropical Malpighiaceae have also lost floral oil glands (Anderson 1979; Vogel 1990), and oil gland presence can vary even at the population level (Sazima & Sazima 1989). (v) In the Myrsinaceae Lysimachia, Vogel (1986, 1988) studied 105 of 189 species, finding evidence for floral oil in 75 of them. Of the 189 species, a molecular phylogeny by Hao et al. (2004) includes 57, another by Anderberg et al. (2007) includes 86. Based on this sampling and the tree topologies, a minimum of six losses or, alternatively, four gains and two losses can be inferred, depending on whether oil glands are ancestral in Lysimachia or not. Examples of the loss of oil in Lysimachia include the Hawaiian subgenus Lysimachiopsis (Vogel 1986) and species in the South African subgenus Palladia, which have switched to bird pollination (Sérsic & Cocucci 1996). (vi) In the Plantaginaceae, the oil-offering clade (Angelonia, Basistemon, Monopera and Monttea) also includes the oil-less Melosperma and a few oil-less species of Basistemon (Barringer 1985; Oxelman et al. 2005), which implies at least two losses of oil glands. Monopera has not yet been sequenced, but morphologically resembles Angelonia (Barringer 1983; Aguiar & Melo 2009). (vii) In the Solanaceae Nierembergia, oil was lost at least four times (Tate et al. 2009; A. Cocucci 2009, personal communication). (viii) In the Cucurbitaceae, oil glands evolved probably only once and were then lost at least six times (H. Schaefer & S. S. Renner 2008, unpublished data).
Table 2 shows species numbers in sister clades with and without floral oil. Unfortunately, a statistical sign test is not yet possible because of insufficient phylogenetic knowledge; a particular problem is that all larger clades (Calceolaria, Malpighiaceae, Lysimachia, Nierembergia, etc.) are variable for the trait of interest (floral oil; see the previous paragraph on the losses of oil in these clades). So far, there is no obvious trend for oil flower clades to be particularly species rich or species poor.
Table 2.
Species numbers in clades with and without floral oil. The comparisons for Calceolaria, Malpighiaceae, Lysimachia, Nierembergia and possibly others are not strictly valid since these clades vary for the trait of interest (floral oil). The correct comparisons would be between the subclades that lost floral oil and their sister clades.
| clade with floral oil (species number) | clade without floral oil (species number) | data supporting sister relationships |
|---|---|---|
| Calceolaria (260), but at least 49 species lack oil | Jovellana (2–4) | Andersson (2006) |
| Krameria (18) | Zygophyllaceae (285) | Soltis et al. (1998) and Savolainen et al. (2000) |
| Malpighiaceae (1250), but a clade of 250 species lacks oil | Elatinaceae (35) | Davis & Chase (2004) |
| Lysimachia (190), but only 75 with oil, and oil then lost several times | sister unclear, perhaps Trientalis (5–6?) | Anderberg et al. (2007) |
| Angelonia (25), Basistemon (8), Monttea (3), the three genera form a clade that includes the oil-less Melosperma. Monopera (2) probably also belongs in this clade | Ourisia? (30–40) (but very poor sampling, so relationship not finally resolved) | Oxelman et al. (2005) |
| Nierembergia (21), but oil lost in several species | Bouchetia (3)+Hunzikeria (3) | Tate et al. (2009) |
| Telfairia (3) | Ampelosicyos clade (5) | Schaefer et al. (2009) |
| Colpias (1) | Hemimeris (8) + Diclis (9) + Alonsoa (14) | Oxelman et al. (2005) |
| Diascia (48) | Nemesia (65) | Oxelman et al. (2005) |
| Hemimeris (4) | Diclis (9) | Oxelman et al. (2005) |
| Stilbaceae: Anastrabe (30), Bowkeria (5), Ixianthes (1) | Nuxia (15) + Stilbe (8) + Retzia (5) + Euthystachys (1) + Campylostachys (2) | Oxelman et al. (2005) |
(b). Times of origin of floral oil offering
As shown in table 1, the evolution of oil production in flowers has occurred from the K/T boundary onwards, from the oldest inferred gains in the Malpighiaceae (75–64 Myr) and Cucurbitaceae (49 Myr (confidence interval 57–42 Myr)) to the youngest in Calceolariaceae, Iridaceae, Orchidaceae at 12 to 1 Myr. The age of 41 Myr (52–28 Myr) for the Lysimachia stem lineage (table 1) fits with a possible coevolution between Palaeomacropis and Lysimachia (§4).
(c). Times of origin of oil foraging in bees
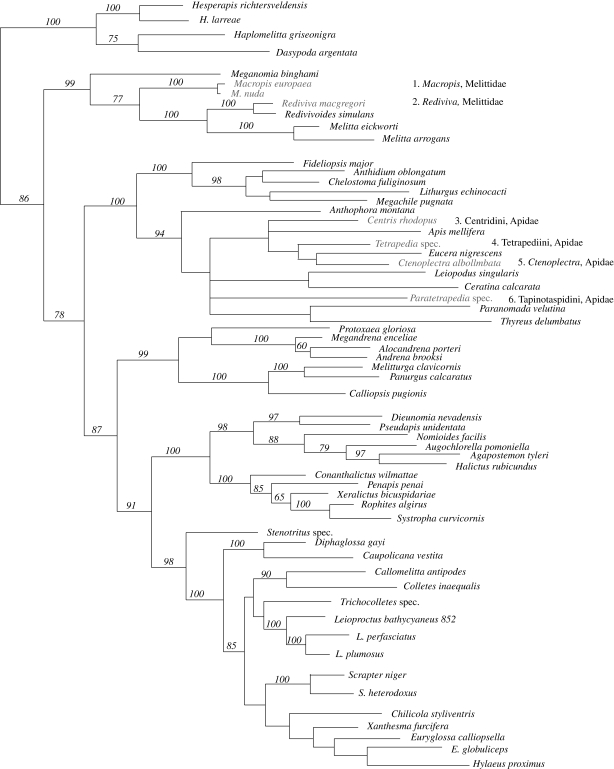
Table 3 lists all known oil-foraging bee genera, with their geographic distribution, number of species and fossil-documented or molecular-clock inferred ages. Overall, some 360–370 species of bees collect oil (table 3), and the behaviour evolved both in the long-tongued and in the short-tongued bees, with the geologically earliest known oil foragers occurring in the Ctenoplectrini and Melittini (figure 2 and table 3). A recent phylogeny of the Melittidae (Michez et al. 2009b) suggests the relationships (Macropidini (Melittini [Rediviva, Melitta])), with Redivivoides embedded in Rediviva. Assuming that gains and losses of oil-collecting behaviour are equally probable, Michez and coworkers prefer a scenario of independent origins of oil-collecting structures in Rediviva and Macropidini over a scenario involving a single origin of oil collecting followed by multiple losses (Steiner & Cruz (2006) prefer the second scenario). They also infer that Macropidini are the sister lineage to Palaeomacropis eocenicus, a 53 Myr old bee from France with the typical setae that help Macropis take up and transport floral oil (Michez et al. 2007). Oil foraging was clearly lost at least once, in Redivivoides.
Table 3.
A list of all known oil-foraging bees with their geographic distribution, number of species and fossil-documented or molecular-clock inferred ages.
| independent origins of oil foraging; genera with species numbers and geographical range | oldest fossils or molecular clock-based age (CI) | selected references on bee behaviour, phylogeny and clade ages |
|---|---|---|
| New World | ||
| Apidae, Centridini: Centris (230, 30 of these not foraging on oil) | expected to be as old as Malpighiaceae | Vogel (1974), Neff & Simpson (1981) and Sazima & Sazima (1989). For molecular phylogeny: S. Cardinal et al. Cornell University, ongoing |
| Apidae, Centridini: Epicharis (14–23) | expected to be as old as Malpighiaceae | Vogel (1974), Neff & Simpson (1981) and Sazima & Sazima (1989). For phylogeny: see Centris |
| Apidae, Tapinotaspidini: Arhysoceble (5), Caenonomada (3), Chalepogenus (21), Lanthanomelissa (5), Monoeca (8–9), Paratetrapedia (30), Tapinotaspis (3), Tapinotaspoides (4), Trigonopedia (4) | monophyly? | Vogel (1974), Neff & Simpson (1981) and Sazima & Sazima (1989). For molecular phylogeny: Antonio Aguiar, Museu de Zoologia de São Paulo, Tapinotaspidini, ongoing |
| Apidae, Tetrapediini: Tetrapedia (13) | ? | Vogel (1974, 1988), Neff & Simpson (1981) and Alves dos Santos et al. (2002) |
| Old World or Holarctic | ||
| Apidae, Ctenoplectrini: Ctenoplectra (16) | stem age Ctenoplectra: 56 (67–44) Myr | Vogel (1981a,b) and Schaefer & Renner (2008) |
| Melittidae, Macropidini: Macropis (16, Holarctic) | Palaeomacropis, 53 Myr | fossil: Michez et al. (2007). For molecular phylogeny see Michez et al. (2009b) |
| Melittidae, Melittini: Rediviva (24) | ? | Vogel (1984), Vogel & Michener (1985) and Steiner & Cruz (2009). For molecular phylogeny see Steiner & Cruz (2006) |
Figure 2.
Maximum parsimony tree showing the relationships of the most important bee groups (modified after Danforth et al. 2006, based on five nuclear DNA regions plus morphological data). Eucerini, Tapinotaspidini and Tetrapediini were not sampled in the original study, but are here added based on the results of Schaefer & Renner (2008). Oil foraging lineages in grey.
Oil foraging evolved a third time in the paleotropic Ctenoplectrini (figure 2), with two genera that comprise 9 species in tropical Africa and at least 10 in Asia and Australia. All species of Ctenoplectra collect floral oil, pollen and nectar from Cucurbitaceae, while their sister clade, Ctenoplectrina with three species, is kleptoparasitic and either lost the oil-foraging behaviour or never had it. Tree topology and molecular dating together suggest that Ctenoplectrini originated in Africa in the Early Eocene and that Ctenoplectra dispersed twice from Africa to Asia, sometime in the Late Eocene, 40 to 30 Myr, from where one species reached the Australian continent via Indonesia and New Guinea in the mid-Miocene, ca 13 Myr (Schaefer & Renner 2008).
The fourth, fifth and sixth origins of oil foraging occurred in Centridini (Centris, Epicharis), Tapinotaspidini (Arhysoceble, Caenonomada, Chalepogenus (including Lanthanomelissa), Monoeca, Paratetrapedia, Tapinotaspis, Tapinotaspoides and Trigonopedia), and Tetrapediini (Tetrapedia; Michener 2007; our figure 2 and table 3). Tetrapediini include one other genus, Coelioxoides, which consists of three species that are kleptoparasitic on Tetrapedia (Michener 2007), and perhaps this genus lost oil foraging similar to the situation of Ctenoplectra and Ctenoplectrina (above). It now appears that Centridini are not monophyletic (S. Cardinal 2009, personal communication), implying a possible seventh origin of oil foraging or, alternatively, losses of the behaviour (reliable interpretation requires a densely sampled phylogeny for Centris). Whether Tapinotaspidini and Tetrapediini are sister groups or not, thus representing one or two origins of oil foraging, is unclear; a molecular phylogeny that included one representative of most tribes in the subfamily Apinae (Schaefer & Renner 2008) found them to be distantly related, but denser sampling is necessary to test this. Michener (1944: 233 cited in Vogel 1974) assumed that Centris, Epicharis and the Exomalopsini (now reclassified partly in Tapinotaspidini) originated in the Upper Cretaceous, and given that they all interact with Malpighiaceae, in which oil glands are a synapomorphy, we expect that the stem lineages of these bees go back to at least 64 Myr (table 1).
4. Discussion
(a). Ages of oil-offering plant clades and oil-foraging bees, and their implications for gradual host niche broadening
Oil as a pollinator reward evolved at least 28 times independently, with the respective clades currently classified in 11 families and comprising 1500–1800 oil-offering species (table 1). The initial studies on floral oil (Vogel 1969, 1974, 1981a,b, 1988, 1990) and subsequent reviews (Neff & Simpson 1981; Buchmann 1987; Endress 1994; Rasmussen & Olesen 2000; Machado 2004) have all stressed that oil rewards evolved many times independently, as evident from the different types of epithelial and trichomatic oil glands on the sepals, petals, tepals and stamens of the various oil-offering flowers. This is the first review, however, to provide a temporal context and to show that oil was lost more often than it was gained. Estimates for the times of initial divergence are available for two-thirds of the plant and bee clades involved (tables 1 and 3). As predicted by Vogel (1974), the Malpighiaceae are among the oldest clades to have acquired oil glands, and their explosive diversification must have played a key role in the evolution of oil-foraging behaviours in Paleocene and Eocene American bees. From the malpigh fossil record it is evident that the family was widespread in Eocene and Oligocene North America and probably also Europe (Taylor & Crepet 1987; Davis et al. 2004 for a summary), but it is not clear whether European malpighs were pollinated by oil-collecting bees.
The Holarctic (Laurasian) Lysimachia/Macropis system is roughly as old as the malpigh/Centris system. This is clear both from the 53 Myr old fossil oil-collecting bee Palaeomacropis eocenicus from France, one of the oldest fossils of bees so far discovered (Michez et al. 2009a) and the molecular clock-based stem age of Lysimachia (41 (28 to 52) Myr). Based on this temporal coincidence it seems plausible that Macropis and Lysimachia coevolved from the onset. Today, Macropis is strictly dependent on Lysimachia from which it obtains not only oil but also pollen, while taking nectar from a range of flowers (Vogel 1986; Michez & Patiny 2005; Michez et al. 2008). The latter two works include maps showing geographic distributions and detailed host–plant associations of Macropis and Lysimachia.
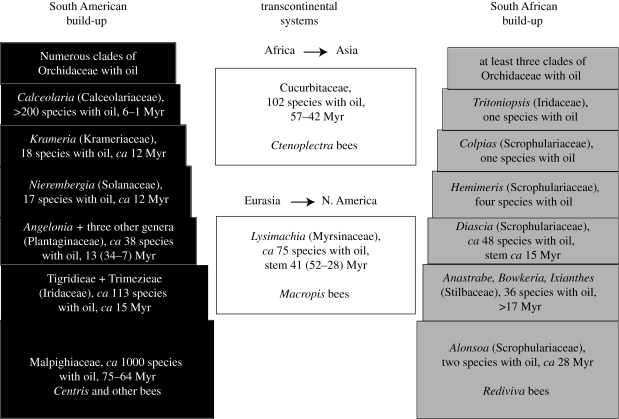
The ages inferred for the crown groups of Calceolaria (8 to 1 Myr), Krameriaceae (12 Myr) and various South African orchids (Satyrium, 31 to 28 Myr), Iridaceae (e.g. Tritoniopsis, 22 Myr), Scrophulariaceae and Stilbaceae (table 1; this also shows confidence intervals around estimates) are relatively young, and individual oil-offering species, of course, are even younger. Older oil-foraging bee lineages therefore must have acquired additional oil hosts over evolutionary time, and figure 3 illustrates how this gradual build-up of oil flower/oil bee interactions could have occurred (as inferred from the molecular clock estimates). In two of the world's four regions with oil flowers, host shifts occurred between plant families (figure 3). In the two others, the Eurasian Macropis/Lysimachia system and the African/Asian Ctenoplectra/Cucurbitaceae system, host shifts did not leave these plant clades, although the latter system involves several finer scale host expansions (H. Schaefer & S. S. Renner 2008, unpublished data).
Figure 3.
Scheme showing the gradual build-up of oil flower/oil bee interactions in two of the World's four regional oil flower systems. The Eurasian Macropis/Lysimachia system and the African/Asian Ctenoplectra/Cucurbitaceae system did not involve switches to hosts outside these plant clades. The latter system, however, involved the repeated acquisition of new cucurbit hosts through time, a finer scale host expansion not shown here.
Most likely host broadening started with occasional exploratory visits to flowers that resembled the oil hosts in colour or scent. This is plausible because oil bees generally interact with several, rather than a single, flower species (Vogel 1974; Steiner & Whitehead 1988; Machado 2004, and numerous studies cited therein). Among the few one-to-one species interactions may be those between Rediviva and some of its hosts (e.g. Steiner & Cruz 2009). Mimicry of Malpighiaceae oil flowers by Orchidaceae is well documented (Silvera 2002; Reis et al. 2007; Pansarin et al. 2008; Aliscioni et al. 2009; Carmona-Díaz & García-Franco 2009) and may occasionally lead to new oil-offering orchids. Indeed, convergence on a stereotypical syndrome of floral traits, associated with pollination by oil-collecting bees, has been so strong that genera, such as Oncidium, which were defined by floral characters, turn out to be grossly polyphyletic (Chase et al. 2009). In some Oncidium-type flowers, oil is produced in vaguely defined areas and may serve more in mimicking the spectral reflection of malpigh flowers, than being an actual reward (Chase et al. 2009). Deciding whether such initially Muellerian, later probably Batesian, mimicry situations explain most evolutionary acquisitions of new oil hosts, however, will require further data on oil bee behaviour. Some oil bees have chemoreceptors on their tarsi for the detection of oil, and oil-collection behaviour is triggered only when the receptors come in contact with an oily surface (Dötterl & Schäffler 2007).
A scenario of gradual evolutionary host niche broadening (indistinguishable from host switching, when prior hosts became extinct), as proposed above for American oil bees (and visually presented in figure 3), also fits the decidedly asymmetric tropical African oil bee/oil flower system, namely that between Ctenoplectra and oil-offering Cucurbitaceae. Ctenoplectra originated in the Early Tertiary, apparently in tropical Africa (Schaefer & Renner 2008) and all species are obligate on Cucurbitaceae from which they obtain nectar, pollen and oil (Vogel 1981a,b, 1990; H. Schaefer 2008, unpublished observations for African and Asian Cucurbitaceae). Its sister clade, Eucerini, does not collect oil. Over the course of evolution, Ctenoplectra broadened its host spectrum from the Momordica clade to the Thladiantha, Siraitia and Telfairia clades (Schaefer et al. (2009) includes a phylogeny showing their distant phylogenetic relationships). We know of no loss of oil foraging within Ctenoplectra, although its kleptoparasitic sister clade, Centoplectrina, may have lost oil foraging (Schaefer & Renner 2008).
(b). Why did oil-flowers evolve and persist in very few plant clades?
Some 28 angiosperm lineages offer oil in their flowers, and from phylogenetic relationships it is possible to infer at least 36–40 losses of floral oil (§3, table 1). This raises several questions. Does the dependence on oil-collecting bees for pollination limit the ability of a plant to expand into new habitats or geographic regions, favouring the loss of oil flowers in the most actively diversifying and expanding clades? Our compilation of sister groups with and without floral oil (table 2) failed to yield an answer to this question because sufficiently detailed phylogenies are not yet available. A second question raised by the few origins, but numerous losses, of oil is how evolutionarily ‘difficult’ it is for a plant to produce oil in floral epithelia or trichomes. What are the biochemical or energetic constraints on floral oils? The chemical composition of floral oils has been analysed in only a few species; it ranges from mainly free fatty acids (Buchmann 1987; Vinson et al. 1997) to acetyl-glycerol derivatives with β-acetoxy-fatty acids (Reis et al. 2000) to β-acetate-substituted free fatty acids and mono-, di- or triglycerides (Vogel 1974; Simpson et al. 1979; Vinson et al. 1997; Silvera 2002; Seibold et al. 2004; Dumri et al. 2008). A study of the floral lipids from Calceolaria (Calceolariaceae) and Krameria (Krameriaceae) revealed a C16–C20 β-acetoxy-substituted free fatty acids, and an unusual 3-hydroxy fatty acid (Seigler et al. 1978). Buchmann's (1987) analyses of the caloric content of various floral oils revealed considerable variation, with some oils containing fewer calories than fatty pollen. Whether the numerous instances of loss of floral oils relate to energy constraints is therefore unclear. What is clear, however, is that oil glands were often lost with the occupation of new habitats in which oil bees were rare or lacking (e.g. spread to the Hawaiian archipelago, spread into Africa by neotropical malpighs, spread into South America by African Momordica). That the presence and absence of oil glands can vary even within species (Sazima & Sazima 1989) illustrates that pollen- or nectar-foraging visitors can take over pollination services from oil bees at ecological as well as evolutionary time scales.
A profound answer to the question of why oil flower/oil bee systems have remained relatively limited evolutionary experiments (with the exception of neotropical Malpighiaceae) will require a much better understanding of the costs and benefits of oil-collecting for the bees. Most bees (bee larvae) obtain their fat from pollenkitt, which is collected at the same time as pollen grains. Collecting floral oil instead requires dedicated foraging bouts and behaviours that must come at great costs for females. Perhaps this explains why only 360–370 of the 16 000 species of bees collect floral oil.
Acknowledgements
We dedicate this paper to Stefan Vogel on the 40th anniversary of his discovery of oil flowers (Vogel 1969).
We thank A. Aguiar, S. Cardinal, O. Chauveau, A. Cocucci, C. Davis, G. Gerlach, P. Goldblatt, T. van der Niet, S. Patiny and M. Whitten for information on phylogenetic relationships or literature suggestions, and P. Endress, E. M. Friis and P. Crane for their comments on the manuscript. The project was supported by DFG Grant RE603/3-1.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Aguiar J. C. A., Melo G. A. R.2009Notes on oil sources for the bee genus Caenonomada (Hymenoptera, Apidae, Tapinotaspidini). Rev. Bras. Entomol. 53, 154–156 [Google Scholar]
- Aliscioni S. S., Torretta J. P., Bello M. E., Galati G. B.2009Elaiophores in Gomesa bifolia (Sims) M.W. Chase & N.H. Williams (Oncidiinae: Cymbidieae: Orchidaceae): structure and oil secretion. Ann. Bot. 104, 1141–1149 (doi:10.1093/aob/mcp199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves dos Santos I., Melo G. A. R., Rozen G. R., Jr2002Biology and immature stages of the bee tribe Tetrapediini (Hymenoptera: Apidae). Am. Museum Nov. 3377, 1–45 (doi:10.1206/0003-0082(2002)377<0001:BAISOT>2.0.CO;2) [Google Scholar]
- Anderberg A. A., Manns U., Källersjö M.2007Phylogeny and floral evolution of the Lysimachieae (Ericales, Myrsinaceae): evidence from ndhF sequence data. Willdenowia 37, 407–421 (doi:10.3372/wi.37.37202) [Google Scholar]
- Anderson W. R.1979Floral conservatism in Neotropical Malpighiaceae. Biotropica 11, 219–223 (doi:10.2307/2388042) [Google Scholar]
- Anderson W. R.1990The origin of the Malpighiaceae: the evidence from morphology. Mem. NY Bot. Gard. 64, 210–224 [Google Scholar]
- Andersson S.2006On the phylogeny of the genus Calceolaria (Calceolariaceae) as inferred from ITS and plastid matK sequences. Taxon 55, 125–137 [Google Scholar]
- Angiosperm Phylogeny Group 2003An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 141, 399–436 (doi:10.1046/j.1095-8339.2003.t01-1-00158.x) [Google Scholar]
- Barringer K.1983Monopera, a new genus of Scrophulariaceae from South America. Brittonia 25, 11–114 [Google Scholar]
- Barringer K.1985Revision of Basistemon (Scrophulariaceae). Syst. Bot. 10, 125–133 (doi:10.2307/2418338) [Google Scholar]
- Bogler D. J., Neff J. L., Simpson B. B.1995Multiple origins of the yucca-yucca moth association. Proc. Natl Acad. Sci. USA 92, 6864–6867 (doi:10.1073/pnas.92.15.6864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein J. L.1994Our current understanding of mutualism. Quart. Rev. Biol. 69, 31–51 [Google Scholar]
- Buchmann S. L.1987The ecology of oil flowers and their bees. Ann. Rev. Ecol. Syst. 18, 343–369 (doi:10.1146/annurev.es.18.110187.002015) [Google Scholar]
- Bustos Singer R., Cocucci A. A.1999Pollination mechanisms in four sympatric Southern Brazilian epidendroid orchids. Lindleyana 14, 47–56 [Google Scholar]
- Call V. B., Dilcher D. L.1997The fossil record of Eucommia (Eucommiaceae) in North America. Am. J. Bot. 84, 798–814 (doi:10.2307/2445816) [PubMed] [Google Scholar]
- Cane J. H., Eickwort G. C., Wesley F. R., Spielholz J.1983Foraging, grooming and mate-seeking behaviors of Macropis nuda (Hymenoptera, Melittidae) and use of Lysimachia ciliata (Primulaceae) oils in larval provisions and cell linings. Am. Midl. Nat. 110, 257–264 (doi:10.2307/2425267) [Google Scholar]
- Carmona-Díaz G., García-Franco J. G.2009Reproductive success in the Mexican rewardless Oncidium cosymbephorum (Orchidaceae) facilitated by the oil-rewarding Malpighia glabra (Malpighiaceae). Plant Ecol. 203, 253–261 (doi:10.1007/s11258-008-9543-6) [Google Scholar]
- Chase M. W., Williams N. H., de Faria A. D., Neubig K. M., Amaral M. C. E., Whitten W. M.2009Floral convergence in Oncidiinae (Cymbidieae; Orchidaceae): an expanded concept of Gomesa and a new genus Nohawilliamsia. Ann. Bot 104, 387–402 (doi:10.1093/aob/mcp067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci A. A.1991The floral biology of Nierembergia (Solanaceae). Plant Syst. Evol. 174, 17–35 (doi:10.1007/BF00937691) [Google Scholar]
- Cocucci A. A., Vogel S.2001Oil-producing flowers of Sisyrinchium species (Iridaceae) and their pollinators in southern South America. Flora 196, 26–46 [Google Scholar]
- Danforth B. N., Sipes S., Fang J., Brady S. G.2006The history of early bee diversification based on five genes plus morphology. Proc. Natl Acad. Sci. USA 103, 15 118–15 123 (doi:10.1073/pnas.0604033103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson P. M., Murray B. G., Steiner K. E.2008Climate and the evolution of annual/perennial life-histories in Nemesia (Scrophulariaceae). Plant Syst. Evol. 270, 39–57 (doi:10.1007/s00606-007-0612-4) [Google Scholar]
- Davis C. C., Chase M. W.2004Elatinaceae are sister to Malpighiaceae; Peridiscaceae belong to Saxifragales. Am. J. Bot. 91, 262–273 (doi:10.3732/ajb.91.2.262) [DOI] [PubMed] [Google Scholar]
- Davis C. C., Fritsch P. W., Bell C. D., Mathews S.2004High latitude Tertiary migrations of an exclusively tropical clade: evidence from Malpighiaceae. Int. J. Plant Sci. 165, S107–S121 (doi:10.1086/383337) [Google Scholar]
- Dötterl S., Schäffler I.2007Flower scent of floral oil-producing Lysimachia punctata as attractant for the oil-bee Macropis fulvipes. J. Chem. Ecol. 33, 441–445 (doi:10.1007/s10886-006-9237-2) [DOI] [PubMed] [Google Scholar]
- Dressler R. L.1993Phylogeny and classification of the orchid family Cambridge, UK: Cambridge University Press [Google Scholar]
- Drummond A. J., Rambaut A.2007BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (doi:10.1186/1471-2148-7-214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. J., Ho S. Y. W., Phillips M. J., Rambaut A.2006Relaxed phylogenetics and dating with confidence. PLoS Biol. 4, e88 (doi:10.1371/journal.pbio.0040088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumri K., Seipold L., Schmidt J., Gerlach G., Dötterl S., Ellis A. G., Wessjohann L. A.2008Non-volatile floral oils of Diascia spp. (Scrophulariaceae). Phytochemistry 69, 1372–1383 (doi:10.1016/j.phytochem.2007.12.012) [DOI] [PubMed] [Google Scholar]
- Endress P. K.1994Diversity and evolutionary biology of tropical flowers Cambridge, UK: Cambridge University Press [Google Scholar]
- Friis E. M., Pedersen K. R., Schönenberger J.2006Normapolles plants: a prominent component of the Cretaceous rosid diversification. Plant Syst. Evol. 260, 107–140 [Google Scholar]
- Goldblatt P., Rodriguez A., Powell M. P., Davies T. J., Manning J. C., van der Bank M., Savolainen S.2008Iridaceae ‘out of Australia’? Phylogeny, biogeography, and divergence time based on plastid DNA sequences. Syst. Bot. 33, 495–508 (doi:10.1600/036364408785679806) [Google Scholar]
- Hao G., Yuan Y.-M., Hu C.-M., Ge X.-J., Zhaoa X.2004Molecular phylogeny of Lysimachia (Myrsinaceae) based on chloroplast trnL–F and nuclear ribosomal ITS sequences. Mol. Phyl. Evol. 31, 323–339 (doi:10.1016/S1055-7903(03)00286-0) [DOI] [PubMed] [Google Scholar]
- Herendeen P. S., Crane P. R., Drinnan A. N.1995Fagaceous flowers, fruits, and capsules from the Campanian (Late Cretaceous) of central Georgia, USA. Int. J. Plant Sci. 156, 93–116 (doi:10.1086/297231) [Google Scholar]
- Houston T. F., Lamot B. B., Radford S., Errington S. G.1993Apparent mutualism between Verticordia nitens and V. aurea (Myrtaceae) and their oil-ingesting bee pollinators (Hymenoptera, Colletidae). Aust. J. Bot. 41, 369–380 (doi:10.1071/BT9930369) [Google Scholar]
- Johnson S. D.1997Insect pollination and floral mechanisms in South African species of Satyrium (Orchidaceae). Plant Syst. Evol. 204, 195–206 (doi:10.1007/BF00989205) [Google Scholar]
- Lee H.1994Oil secretion in Alophia and other Iridaceae. MSc thesis, University of Texas, TX, USA [Google Scholar]
- Machado I. C.2004Oil-collecting bees and related plants: a review of the studies in the last twenty years and case histories of plants occurring in NE Brazil. In Solitary bees conservation, rearing and management for pollination (eds Freitas B. M., Pereira J. O. P.), pp. 225–280 Ceará, Brazil: Federal University of Ceará [Google Scholar]
- Machado I. C., Siqueira-Filho J. A., Lopes A. V., Vogel S.1997Organização e polinização das flores de óleo de Krameria tomentosa (Krameriaceae). In Resumos do XLVIII Congresso Nacional de Botânica, p. 19 Crato: Editora Universitária [Google Scholar]
- Machado I. C., Vogel S., Lopes A. V.2002Pollination of Angelonia cornigera Hook. (Scrophulariaceae) by long-legged, oil-collecting bees in NE Brazil. Plant Biol. 4, 352–359 (doi:10.1055/s-2002-32325) [Google Scholar]
- Maddison D. R., Maddison W. P.2003MacClade 4.0 Sunderland, MA: Sinauer Associates [Google Scholar]
- Manning J., Goldblatt P.2002The pollination of Tritoniopsis parviflora (Iridaceae) by the oil-collecting bee Rediviva gigas (Hymenoptera: Melittidae): the first record of oil-secretion in African Iridaceae. S. Afr. J. Bot. 68, 171–176 [Google Scholar]
- Melo G. A. R., Gaglianone M. C.2005Females of Tapinotaspoides, a genus in the oil-collecting bee tribe Tapinotaspidini, collect secretions from non-floral trichomes (Hymenoptera, Apidae). Rev. Bras. Entomol. 49, 167–168 [Google Scholar]
- Michener C. D.2007The bees of the world, 2nd edn.Baltimore, MD: John Hopkins University Press [Google Scholar]
- Michez D., Patiny S.2005World catalogue, biogeography and floral choices of the oil-collecting bee genus Macropis Panzer 1809 (Hymenoptera, Apoidea, Melittidae). Ann. Soc. Entomol. Fr. 45, 15–28 [Google Scholar]
- Michez D., Nel A., Menier J. J., Rasmont P.2007The oldest fossil of a melittid bee (Hymenoptera: Apiformes) from the early Eocene of Oise (France). Zool. J. Linn. Soc. 150, 701–709 (doi:10.1111/j.1096-3642.2007.00307.x) [Google Scholar]
- Michez D., Patiny S., Rasmont P., Timmermann K., Vereecken N. J.2008Phylogeny and host-plant evolution in Melittidae s.l. (Hymenoptera: Apoidea). Apidologie 39, 146–162 (doi:10.1051/apido:2007048) [Google Scholar]
- Michez D., De Meulemeester T., Rasmont P., Nel A., Patiny S.2009aNew fossil evidence of the early diversification of bees: Paleohabropoda oudardi from the French Paleocene (Hymenoptera, Apidae, Anthophorini). Zool. Scripta 38, 171–181 (doi:10.1111/j.1463-6409.2008.00362.x) [Google Scholar]
- Michez D., Patiny S. M. L., Danforth B. N.2009bPhylogeny of the bee family Melittidae (Hymenoptera: Anthophila) based on combined molecular and morphological data. Syst. Entomol. 34, 574–597 [Google Scholar]
- Neff J. L., Simpson B. B.1981Oil-collecting structures in the Anthophoridae (Hymenoptera): morphology, function and use in systematics. J. Kansas Entomol. Soc. 54, 95–123 [Google Scholar]
- Oxelman B., Kornhall P., Olmstead R. G., Bremer B.2005Further disintegration of Scrophulariaceae. Taxon 54, 411–425 [Google Scholar]
- Pansarin L. M., Pansarin E. R., Sazima M.2008Reproductive biology of Cyrtopodium polyphyllum (Orchidaceae): a Cyrtopodiinae pollinated by deceit. Plant Biol. 10, 650–659 (doi:10.1111/j.1438-8677.2008.00060.x) [DOI] [PubMed] [Google Scholar]
- Pansarin L. M., de M Castro M., Sazima M.2009Osmophore and elaiophores of Grobya amherstiae (Catasetinae, Orchidaceae) and their relation to pollination. Bot. J. Linn. Soc. 159, 408–415 (doi:10.1111/j.1095-8339.2009.00953.x) [Google Scholar]
- Pauw A.2005Inversostyly: a new stylar polymorphism in an oil-secreting plant, Hemimeris racemosa (Scrophulariaceae). Am. J. Bot. 92, 1878–1886 (doi:10.3732/ajb.92.11.1878) [DOI] [PubMed] [Google Scholar]
- Pauw A.2006Floral syndromes accurately predict pollination by a specialized oil-collecting bee (Rediviva peringueyi, Melittidae) in a guild of South African orchids (Coryciinae). Am. J. Bot. 93, 917–926 (doi:10.3732/ajb.93.6.917) [DOI] [PubMed] [Google Scholar]
- Pellmyr O., Leebens-Mack J.2000Reversal of mutualism as a mechanism for adaptive radiation of yucca moths. Am. Nat. 156, 62–76 [DOI] [PubMed] [Google Scholar]
- Pellmyr O., Thompson J. N., Brown J., Harrison R. G.1996Evolution of pollination and mutualism in the yucca moth lineage. Am. Nat. 148, 827–847 (doi:10.1086/285958) [Google Scholar]
- Pigg K. B., Manchester S. R., DeVore M. L.2008Fruits of Icacinaceae (Tribe Iodeae) from the late Paleocene of Western North America. Am. J. Bot. 95, 824–832 (doi:10.3732/ajb.2007340) [DOI] [PubMed] [Google Scholar]
- Rambaut A. 2006–2008 FigTree v. 1.2.3. See http://tree.bio.ed.ac.uk/software/figtree/ [Google Scholar]
- Rambaut A., Drummond A. J.2007Tracer—MCMC trace analysis tool, v. 1.4. See http://beast.bio.ed.ac.uk/Tracer [Google Scholar]
- Rasmussen C., Olesen J. M.2000Oil flowers and oil-collecting bees. Norske Vidensk. Akad. I. Mat. Nat. Kl. Skrifter. Ny Serie 39, 23–31 [Google Scholar]
- Raw A.1979Centris dirrhoda (Anthophoridae), the bee visiting West Indian cherry flowers (Malpighia punicifolia). Rev. Biol. Trop. 27, 203–205 [Google Scholar]
- Reis M. G., de Faria A. D., Bittrich V., Amaral M. C., Marsaioli A. J.2000The chemistry of flower rewards—Oncidium (Orchidaceae). J. Brazil. Chem. Soc. 11, 600–608 [Google Scholar]
- Reis M. G., de Faria A. D., Amaral M. C., Marsaioli A. J.2003Oncidinol—a novel diacylglycerol from Ornithophora radicans Barb. Rodr. (Orchidaceae) floral oil. Tetrahedron Lett. 44, 8519–8523 [Google Scholar]
- Reis M. G., Singer R. B., Gonçalves R., Marsaioli A. J.2006The chemical composition of the floral oils of Phymatidium delicatulum and Phymatidium tillandsioides (Orchidaceae). Nat. Prod. Commun. 1, 757–761 [Google Scholar]
- Reis M. G., de Faria A. D., Alves dos Santos I., Amaral M. C. E., Marsaioli A. J.2007Byrsonic acid—the clue to floral mimicry involving oil-producing flowers and oil-collecting bees. J. Chem. Ecol. 33, 1421–1429 (doi:10.1007/s10886-007-9309-y) [DOI] [PubMed] [Google Scholar]
- Roig-Alsina A.1997A generic study of the bees of the tribe Tapinotaspidini, with notes on the evolution of their oil-collecting structures. Mitt. Münchner Entomol. Ges. 87, 3–21 [Google Scholar]
- Salazar G. A., Cabrera L. I., Madriñán S., Chase M. W.2009Phylogenetic relationships of Cranichidinae and Prescottiinae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences. Ann. Bot. 104, 403–416 (doi:10.1093/aob/mcn257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen V., et al. 2000Phylogenetics of flowering plants based on combined analysis of plastid atpB and rbcL sequences. Syst. Biol. 49, 306–362 (doi:10.1093/sysbio/49.2.306) [DOI] [PubMed] [Google Scholar]
- Sazima M., Sazima I.1989Oil-gathering bees visit flowers of eglandular morphs of the oil-producing Malpighiaceae. Bot. Acta 102, 106–111 [Google Scholar]
- Schaefer H., Renner S. S.2008A phylogeny of the oil bee tribe Ctenoplectrini (Hymenoptera: Anthophila) based on mitochondrial and nuclear data: evidence for Early Eocene divergence and repeated out-of-Africa dispersal. Mol. Phyl. Evol. 47, 799–811 (doi:10.1016/j.ympev.2008.01.030) [DOI] [PubMed] [Google Scholar]
- Schaefer H., Renner S. S.2009A three-genome phylogeny of Momordica (Cucurbitaceae) suggests seven returns from dioecy to monoecy and recent long-distance dispersal to Asia. Mol. Phylogenet. Evol. (doi:10.1016/j.ympev.2009.08.006) [DOI] [PubMed] [Google Scholar]
- Schaefer H., Heibl C., Renner S. S.2009Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proc. R. Soc. B 276, 843–851 (doi:10.1098/rspb.2008.1447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold L., Gerlach G., Wessjohann L.2004A new type of floral oil from Malpighia coccigera (Malpighiaceae) and chemical considerations on the evolution of oil flowers. Chem. Biodiv. 1, 1519–1528 [DOI] [PubMed] [Google Scholar]
- Seigler D. S., Simpson B. B., Martin C., Neff J. L.1978Free 3-acetoxy fatty acids in floral glands of Krameria species. Phytochemistry 17, 995–996 (doi:10.1016/S0031-9422(00)88666-5) [Google Scholar]
- Sérsic A. N.2004Pollination biology in the genus Calceolaria L. (Calceolariaceae). Stapfia 82, 1–121 [Google Scholar]
- Sérsic A. N., Cocucci A. A.1996A remarkable case of ornithophily in Calceolaria: food bodies as rewards for a non-nectarivorous bird. Bot. Acta 109, 172–176 [Google Scholar]
- Sérsic A. N., Cocucci A. A.1999An unusual kind of nectary in the oil flowers of Monttea: its structure and function. Flora 194, 393–404 [Google Scholar]
- Silvera K.2002Adaptive radiation of oil-reward compounds among neotropical orchid species (Oncidiinae). PhD thesis, University of Florida, FL, USA [Google Scholar]
- Simpson B. B., Siegler D. S., Neff J. L.1979Lipids from the floral glands of Krameria. Biochem. Syst. Evol. 7, 193–194 (doi:10.1016/0305-1978(79)90049-8) [Google Scholar]
- Singer R. B., Marsaioli A. J., Flach A., Reis M. G.2006The ecology and chemistry of pollination in Brazilian orchids: recent advances. In Floriculture, ornamental and plant biotechnology, vol. IV (ed. Teixeira da Silva J.), pp. 569–582 Isleworth, Middlesex: Global Science Books [Google Scholar]
- Soltis D. E., Soltis P. S., Mort M. E., Chase M. W., Savolainen V., Hoot S. B., Morton C. M.1998Inferring complex phylogenies using parsimony: an empirical approach using three large DNA data sets for angiosperms. Syst. Biol. 47, 32–42 [DOI] [PubMed] [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J.2008A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 75, 758–771 [DOI] [PubMed] [Google Scholar]
- Steiner K. E.1989aThe pollination of Disperis (Orchidaceae) by oil-collecting bees in southern Africa. Lindleyana 4, 164–183 [Google Scholar]
- Steiner K. E.1989bA second species of the Amphi-Atlantic genus Alonsoa (Scrophulariaceae) in South Africa. Ann. Mo. Bot. Gard. 76, 1152–1159 (doi:10.2307/2399701) [Google Scholar]
- Steiner K. E.1993Has Ixianthes (Scrophulariaceae) lost its special bee? Plant Syst. Evol. 185, 7–16 (doi:10.1007/BF00937717) [Google Scholar]
- Steiner K. E., Cruz C. B.2006. The evolution of oil collection and oil-collecting structures in the Melittidae. Abstract. International Union for the Study of Social Insects, 31 July 2006, Washington, DC. See http://www.ulb.ac.be/sciences/ecoevol/docs/Michez_IUSSI_06.pdf [Google Scholar]
- Steiner K. E., Cruz C. B.2009Hybridization between two oil-secreting orchids in South Africa. Plant Syst. Evol. 277, 233–243 (doi:10.1007/s00606-008-0119-7) [Google Scholar]
- Steiner K. E., Whitehead V. B.1988The association between oil-producing flowers and oil-collecting bees in the Drakensberg of southern Africa. Monogr. Syst. Bot. Mo. Bot. Gard. 25, 259–277 [Google Scholar]
- Steiner K. E., Whitehead V. B.1990Pollinator adaptation to oil-secreting flowers—Rediviva and Diascia. Evolution 44, 1701–1707 (doi:10.2307/2409348) [DOI] [PubMed] [Google Scholar]
- Steiner K. E., Whitehead V. B.1991Oil flowers and oil bees: further evidence for pollinator adaptation. Evolution 45, 1493–1501 (doi:10.2307/2409895) [DOI] [PubMed] [Google Scholar]
- Steiner K. E., Whitehead V. B.1996The consequence of specialization for pollination in a rare South African shrub, Ixianthes retzioides (Scrophulariaceae). Plant Syst. Evol. 201, 131–138 (doi:10.1007/BF00989056) [Google Scholar]
- Steiner K. E., Whitehead V. B.2002Oil secretion and the pollination of Colpias mollis (Scrophulariaceae). Plant Syst. Evol. 235, 53–66 (doi:10.1007/s00606-002-0216-y) [Google Scholar]
- Stpiczynska M., Davies K. L.2008Elaiophore structure and oil secretion in flowers of Oncidium trulliferum Lindl. and Ornithophora radicans (Rchb.f.) Garay & Pabst (Oncidiinae: Orchidaceae). Ann. Bot. 101, 375–384 (doi:10.1093/aob/mcm297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stpiczynska M., Davies K. L., Gregg A.2007Elaiophore diversity in three contrasting members of Oncidiinae (Orchidaceae). Bot. J. Linn. Soc. 155, 135–148 (doi:10.1111/j.1095-8339.2007.00681.x) [Google Scholar]
- Tate J. A., Acosta M. C., McDill J., Moscone E. A., Simpson B. B., Cocucci A. A.2009Phylogeny and character evolution in Nierembergia (Solanaceae): Molecular, morphological, and cytogenetic evidence. Syst. Bot. 34, 198–206 [Google Scholar]
- Taylor D. W., Crepet W. L.1987Fossil floral evidence of Malpighiaceae and an early plant-pollinator relationship. Am. J. Bot. 74, 274–286 (doi:10.2307/2444030) [Google Scholar]
- Thompson J. N.1999The evolution of species interactions. Science 284, 2116–2118 (doi:10.1126/science.284.5423.2116) [DOI] [PubMed] [Google Scholar]
- van der Cingel N. A.2001An atlas of orchid pollination: America, Africa, Asia and Australia Rotterdam, The Netherlands: A. A. Balkema [Google Scholar]
- Verboom G. A., et al. 2009Origin and diversification of the Greater Cape flora: ancient species repository, hot-bed of recent radiation, or both? Mol. Phyl. Evol. 51, 44–53 (doi:10.1016/j.ympev.2008.01.037) [DOI] [PubMed] [Google Scholar]
- Vinson S. B., Williams H. J., Frankie G. W., Shrum G.1997Floral lipid chemistry of Byrsonima crassifolia (Malpigheaceae) and a use of floral lipids by Centris bees (Hymenoptera: Apidae). Biotropica 29, 76–83 [Google Scholar]
- Vogel S.1969Flowers offering fatty oil instead of nectar. Abstracts, XI. Int. Bot. Congress, Seattle, 1969, p. 229 [Google Scholar]
- Vogel S.1974Ölblumen und ölsammelnde Bienen. Trop. u. Subtrop. Pflanzenwelt 7, 1–267 [Google Scholar]
- Vogel S.1976Lysimachia: Ölblumen der Holarktis. Naturwissenschaften 63, 441250427 [Google Scholar]
- Vogel S.1981aAbdominal oil-mopping—a new type of foraging in bees. Naturwissenschaften 68, 627–628 (doi:10.1007/BF00398624) [Google Scholar]
- Vogel S.1981bTrichomatische Blütennektarien bei Cucurbitaceen. Beitr. Biol. Pflanzen 55, 325–353 [Google Scholar]
- Vogel S.1983Ecophysiology of zoophilic pollination. In Encyclopedia of plant physiology, new series 12C: physiological plant ecology III (eds Lange O. L., et al.), pp. 559–624 Berlin, Germany: Springer [Google Scholar]
- Vogel S.1984The Diascia flower and its bee: an oil-based symbiosis in Southern Africa. Acta Bot. Neerl. 33, 509–518 [Google Scholar]
- Vogel S.1986Ölblumen und ölsammelnde Bienen. Zweite Folge. Lysimachia und Macropis. Trop. u. Subtrop. Pflanzenwelt 54, 1–168 [Google Scholar]
- Vogel S.1988Die Ölblumensymbiosen: Parallelismus und andere Aspekte ihrer Entwicklung in Raum und Zeit. Z. Zool. Syst. Evol.-Forsch. 26, 341–362 [Google Scholar]
- Vogel S.1990Ölblumen und ölsammelnde Bienen. Dritte Folge. Momordica, Thladiantha und die Ctenoplectridae. Trop. u. Subtrop. Pflanzenwelt 73, 1–186 [Google Scholar]
- Vogel S., Cocucci A. A.1995Pollination of Basistemon (Scrophulariaceae) by oil-collecting bees in Argentina. Flora 190, 353–363 [Google Scholar]
- Vogel S., Machado I. C.1991Pollination of four sympatric species of Angelonia (Scrophulariaceae), by oil-collecting bees of NE Brazil. Plant Syst. Evol. 178, 153–178 [Google Scholar]
- Vogel S., Michener C. D.1985Long bee legs and oil-producing floral spurs, and a new Rediviva. J. Kansas Entomol. Soc 58, 359–364 [Google Scholar]
- Wang Y., Zhang D., Renner S. S., Chen Z.2004A new self-pollination mechanism. Nature 431, 39–40 (doi:10.1038/431039b) [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Renner S. S., Chen Z.2005Self-pollination by sliding pollen in Caulokaempferia coenobialis (Zingiberaceae). Int. J. Plant Sci. 166, 753–759 (doi:10.1086/431803) [Google Scholar]
- Waterman R. J., Pauw A., Barraclough T. G., Savolainen V.2009Pollinators underestimated: a molecular phylogeny reveals widespread floral convergence in oil-secreting orchids (sub-tribe Coryciinae) of the Cape of South Africa. Mol. Phyl. Evol. 51, 100–110 (doi:10.1016/j.ympev.2008.05.020) [DOI] [PubMed] [Google Scholar]
- Whitehead V. B., Steiner K. E.1985Oil-collecting bees in South Africa. Afr. Wildlife 39, 144–147 [Google Scholar]
- Whitehead V. B., Steiner K. E.1992Two new species of oil-collecting bees of the genus Rediviva from the summer rainfall region of South Africa (Hymenoptera, Apoidea, Melittidae). Ann. S. Afr. Mus. 102, 143–164 [Google Scholar]
- Whitehead V. B., Steiner K. E.2001Oil-collecting bees of the winter rainfall area of South Africa (Melittidae, Rediviva). Ann. S. Afr. Mus. 108, 143–277 [Google Scholar]
- Whitehead V. B., Steiner K. E., Eardley C. D.2008Oil collecting bees mostly of the summer rainfall area of southern Africa (Hymenoptera: Melittidae: Rediviva). J. Kansas Entomol. Soc. 81, 122–141 (doi:10.2317/JKES-703.12.1) [Google Scholar]
- Whittall J. B., Hodges S. A.2007Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–709 (doi:10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- Wikström N., Savolainen V., Chase M. W.2001Evolution of the angiosperms: calibrating the family tree. Proc. R. Soc. Lond. B 268, 211–222 (doi:10.1098/rspb.2001.1782) [DOI] [PMC free article] [PubMed] [Google Scholar]