Abstract
The Southwest Australian Biodiversity Hotspot contains an exceptionally diverse flora on an ancient, low-relief but edaphically diverse landscape. Since European colonization, the primary threat to the flora has been habitat clearance, though climate change is an impending threat. Here, we review (i) the ecology of nectarivores and biotic pollination systems in the region, (ii) the evidence that trends in pollination strategies are a consequence of characteristics of the landscape, and (iii) based on these discussions, provide predictions to be tested on the impacts of environmental change on pollination systems. The flora of southwestern Australia has an exceptionally high level of vertebrate pollination, providing the advantage of highly mobile, generalist pollinators. Nectarivorous invertebrates are primarily generalist foragers, though an increasing number of colletid bees are being recognized as being specialized at the level of plant family or more rarely genus. While generalist pollination strategies dominate among insect-pollinated plants, there are some cases of extreme specialization, most notably the multiple evolutions of sexual deception in the Orchidaceae. Preliminary data suggest that bird pollination confers an advantage of greater pollen movement and may represent a mechanism for minimizing inbreeding in naturally fragmented populations. The effects of future environmental change are predicted to result from a combination of the resilience of pollination guilds and changes in their foraging and dispersal behaviour.
Keywords: pollination, climate change, evolution, conservation, honeyeater, specialization
1. Introduction
‘Dull and uninteresting’ was the impression that the south coast of Western Australia and/or the society of the infant settlement of King George Sound left with Charles Darwin after an inauspicious autumn visit on the Beagle voyage (Nicholas & Nicholas 2002). Had he arrived in spring he would have witnessed a remarkable flora containing some of the world's most intriguing orchids, a family he dedicated an entire volume to, and an exceptional diversity of species (Hopper & Gioia 2004; Brown et al. 2008).
The Southwest Australian Floristic Region (SWAFR, sensu Hopper & Gioia 2004) forms the mesic and semiarid southwest corner of the Australian continent with predominantly winter rainfall ranging between 300 and 1500 mm yr−1 (Hopper & Gioia 2004). The SWAFR was recognized as one of the world's 25 global biodiversity hotspots (Myers et al. 2000) because it contains many endemic species under threat. The highest diversity of species in the SWAFR lies in the medium-sized shrubs on nutrient poor soils, particularly in the Myrtaceae, Proteaceae and Ericaceae (Hopper & Gioia 2004). Southern Western Australia is dominated by old, climatically-buffered infertile landscapes (OCBILs, sensu Hopper 2009), which have important consequences for the ecology, evolution and conservation of its biota and inhabitants (Hopper 2009). It is predicted that the flora of the ancient landscapes of the SWAFR should exhibit a trend towards elevated persistence of lineages, long-lived individuals, high number of localized endemics and strongly differentiated populations (Hopper 2009). Further, plants may have evolved pollination and genetic systems that maintain reproductive success and avoid inbreeding in the small, fragmented populations that characterize many species in the SWAFR (Hopper 2009).
Mechanisms of pollination have profound implications for the evolution, ecology and conservation of plants (Proctor et al. 1996; Kearns et al. 1998; Johnson & Steiner 2000). Pollination has been the subject of increasing research interest in the SWAFR following recognition that the adaptive basis for prevalent pollination strategies remains unresolved and that pollination ecology may prove critical to predicting or avoiding detrimental effects of anthropogenic modifications to the landscape (previously reviewed by Keighery 1980; Hopper & Burbidge 1986; Brown et al. 1997). As for most regions, many members of the SWAFR flora appear to rely on generalist pollination strategies (Waser et al. 1996; Brown et al. 1997), although few quantitative studies are available as yet to affirm this. However, the region is notable for (i) its exceptional levels of vertebrate pollination, representing approximately 15 per cent of the flora, the highest recorded in the world (Keighery 1980; figure 1); (ii) many bird-pollinated plants being also visited by the SWAFR endemic honey possum Tarsipes rostratus (Tarsipedidae) and the Western Pygmy-possum Cercartetus concinnus (Burramyidae; Hopper 1980; Wooller et al. 1983; figure 1); and (iii) the large number of Orchidaceae pollinated by sexual deception of male hymenopterans (Stoutamire 1983; Brown et al. 2008; Phillips et al. 2009; figure 1). Alternatively, the pollination biology of many common plant families has received little or no attention, particularly among insect-pollinated species (Brown et al. 1997), and the study of pollination in a conservation context is only beginning to gain momentum.
Figure 1.
Examples of pollination systems in southwestern Australia. (a) Banksia grandis (Proteaceae) is pollinated by a range of honeyeater species and the honey possum, Tarsipes rostratus (Tarsipedidae), (b) Corymbia ficifolia (Myrtaceae) is pollinated by a variety of insects and honeyeaters (Meliphagidae), including the New Holland Honeyeater Phylidonyris novaehollandiae, and (c) Caladenia falcata (Orchidaceae) attracts a single species of thynnine wasp (an undescribed species of Thynnoides) through sexual deception. Photos (a) and (b) by Stephen Hopper and photo (c) by Bert and Babs Wells.
The largest environmental change that has occurred since European settlement of the SWAFR is the massive removal of native vegetation and resultant degradation of the remaining vegetation (e.g. Abensperg-traun et al. 1996), with approximately 70 per cent of the vegetation already removed (Beard 1999). Land clearance for agriculture and urbanization are the primary cause of range contraction and habitat loss of Australian plants (Burgman et al. 2007). More recently, the SWAFR has undergone a marked decrease in rainfall, with May–October rainfall in the last 25 years undergoing a 10–15% decrease from the preceding 50-year average (Indian Ocean Climate Initiative Panel 2002). Decreases in rainfall and subsequent declines in groundwater levels have been attributed to declines already occurring in some communities (Groom et al. 2000; Horwitz et al. 2008). Fragmentation of habitat is likely to greatly reduce the ability of plants to cope with climate change by reducing population sizes, hindering their ability to migrate across the landscape and reducing the availability of pollinators.
The focus of pollination research in the SWAFR has primarily been natural history studies, though molecular techniques have facilitated direct tests of pollen movement. Here, we review aspects of pollination systems that will potentially affect the ability of plant species to persist under environmental change, such as pollinator dispersal ability (e.g. Thomas et al. 2004), level of pollinator specialization (Ashworth et al. 2004), plant mating and compatibility systems (Aguilar et al. 2006) and specialization of pollination strategies (Dixon 2009). In this paper, we refer to specialization of plant pollination systems as the use of one or few species of pollinators (Oleson & Jordano 2002). However, we also highlight examples of plants that use a variety of pollinator species but are specialized at the level of functional group (e.g. vertebrate pollination; see Ollerton et al. 2006).
In the second part of the paper, we address the role of characteristics of the SWAFR landscape on trends in the pollination ecology of the flora. Given the prevalence of small, naturally fragmented populations that are subject to long periods of stability (Cowling et al. 1996; Hopper 2009), it is predicted that plants will evolve pollination strategies that maximize outcrossing but will also have the opportunity for extreme specialization (Hopper 2009). In particular, it has been proposed that bird pollination may have evolved to maximize outcrossing in isolated populations (Hopper 2009). In this review, we examine the levels of specialization exhibited by plants and pollinators and whether this plays a role in the maintenance of species boundaries, and test if the prevalent bird pollination strategy does result in higher pollen movements or greater outcrossing. Given the interaction between the evolutionary history of a region and its organisms, responses to a changing environment (Hopper 2009), we conclude by reviewing pollination ecology in the SWAFR in terms of both the susceptibility to environmental change and possible adaptations to persistence in the SWAFR landscape.
2. Pollination strategies
(a). Vertebrate pollination
(i). Ecology of nectarivorous birds in the SWAFR
Nectarivorous birds are diverse and abundant members of the avian community in all terrestrial habitats in the SWAFR (Higgins et al. 2001). While the Purple-crowned Lorikeet Glossopsitta porphyrocephala (Psittacidae) and Silvereye Zosterops lateralis (Zosteropidae) are widespread pollinators in the region (Higgins 1999; Higgins et al. 2006), the majority of vertebrate pollen vectors are honeyeaters (Meliphagidae, with 17 species recorded breeding in the SWAFR; Higgins et al. 2001). While all honeyeaters consume nectar, the shorter beaked species, such as those from the genera Melithreptus and Lichenostomus, feed chiefly on insects (Ford & Paton 1977).
The nectarivorous birds of the SWAFR are generalist foragers that consume nectar from a wide variety of plant families. The most frequently visited plant families are the Myrtaceae and Proteaceae and also commonly some members of the Haemodoraceae, Ericaceae, Rutaceae, Loranthaceae, Myoporaceae and Fabaceae (Brown et al. 1997; Higgins et al. 2001). The Purple-crowned Lorikeet feeds primarily on Eucalyptus (Myrtaceae) flowers in the SWAFR, whereas it has broader feeding preferences in southeastern Australia (Paton & Ford 1977; Higgins et al. 2006). There is extensive overlap in the nectar sources used by different species of honeyeaters within sites (Hopper 1980, 1981; Hopper & Moran 1981; Wooller et al. 1983), though they can show preferences for certain species (Hopper & Burbidge 1978, 1986; Hopper 1993).
Some genera of honeyeater undertake extensive movements to feed on spatially and temporally patchy nectar resources. Such movements have mostly been inferred from repeated surveys that have detected marked seasonal fluctuation of pollinator populations in concert with nectar sources (e.g. Keast 1968; Collins et al. 1984a). The nature of these movements varies regionally, probably reflecting biogeographic variation in the nectar-producing community (Keast 1968). Records from the Australian Bird and Bat Banding Scheme (ABBBS) showed that, for the honeyeaters that occur in the SWAFR, 95–100% of recaptures occur within 10 km of the banding site (Higgins et al. 2001), though this may be a result of the distribution of banding sites. However, for seven species the maximum recapture distance was in excess of 145 km (Higgins et al. 2001). Alternatively, in the ABBBS, honeyeaters of the genus Lichenostomus were never recovered more than 2–3 km from their banding site. As such, while some members of the family are capable of extensive movements, there is pronounced variation in the movements between species and genera. Purple-crowned Lorikeet and Silvereye show extensive movements, with Silvereyes recorded moving over 1900 km from the western seaboard to eastern Australia (Higgins 1999; Higgins et al. 2006).
Small-scale movements of honeyeaters are determined by a combination of resource access, foraging behaviour and social interactions. Most studies of the foraging behaviour of honeyeaters at nectar-producing plants have shown that, unless interrupted, they forage primarily by moving between inflorescences within plants or between neighbouring plants (Hopper & Burbidge 1978; Pyke 1981; Day et al. 1997; Yates et al. 2007a). However, larger, behaviourally dominant honeyeaters such as Red Wattlebirds Anthochaera carunculata can aggressively exclude smaller species, leading to changes in both the foraging movements of smaller species and species composition within patches of habitat (Hopper 1993; MacNally & Timewell 2005). Similarly, the extensive interspecific aggression and territorial behaviour shown by many species (Pyke et al. 1996), particularly during periods of higher nectar abundance (Armstrong 1991; MacNally & Timewell 2005), will change the pattern of foraging. As such, the presence of other species of honeyeaters and higher levels of nectar could lead to greater interplant movements of foraging honeyeaters through frequent disruption of optimal foraging patterns.
(ii). Ecology of nectarivorous mammals in the SWAFR
The SWAFR endemic honey possum and the Western Pygmy-possum are the only mammals that are confirmed to regularly act as pollen vectors in the SWAFR (Hopper 1980; Brown et al. 1997). These small, scansorial marsupials primarily feed upon nectar and pollen from bird-pollinated members of the Proteaceae and Myrtaceae, but occasionally visit Ericaceae and Haemodoraceae (Hopper 1980; Wooller et al. 1983; Brown et al. 1997). There is no evidence of any plant species relying entirely upon mammals for pollination in the SWAFR, but some Banksia species have a number of attributes that favour marsupials more than honeyeaters (Wooller et al. 1983). The abundance of honey possums fluctuates in response to the availability of nectar from Myrtaceae and Proteaceae (Wooller et al. 1998; Bradshaw et al. 2007). Honey possums are capable of moving short distances on a nightly basis (up to 370 m; Bradshaw et al. 2007), though distances vary considerably based on patchiness of resources and potential mates (Garavanta et al. 2000; Bradshaw et al. 2007).
(iii). Pollination in vertebrate-pollinated plants in the SWAFR
Vertebrates are believed to be the primary or exclusive pollinators of several genera in the Haemodoraceae, Proteaceae, Myrtaceae plus single genera in many other families (Keighery 1980; Hopper & Burbidge 1986). These plants are specialized at the level of functional group but are generally pollinated by multiple species of honeyeater (e.g. Hopper 1980, 1981; Collins et al. 1984a; Brown et al. 1997; Yates et al. 2007a) and often the honey possum (Hopper 1980; Wooller et al. 1983). However, in habitats with low honeyeater diversity, such as the understorey of the southern Jarrah Eucalyptus marginata forest and sandplains after fire, plants may be pollinated primarily by a single honeyeater species and exhibit a level of specificity approaching that exhibited by neotropical bird-pollinated plants visited by hummingbirds (Stiles 1981; Hopper 1993). Within the SWAFR, intrinsically rare plants exhibit a higher incidence of bird pollination than the flora in general (40% compared with 15%; Hopper et al. 1990). It is unknown if this relationship is caused by ecological characteristics of bird pollination, a tendency for rare species to evolve bird pollination or a correlation between bird pollination and other causes of rarity.
Molecular phylogenetic studies indicate that vertebrate pollination has evolved repeatedly within and among genera in the SWAFR flora. For example, in Haemodoraceae, genera such as Anigozanthos, Macropidia and Blancoa diverged from their insect-pollinated sisters ca. 30 and 20 Ma, respectively, with additional independent origins of vertebrate-pollinated species within the ancestrally bee-pollinated Conostylis occurring through to the early Pleistocene at ca. 1 Ma (Hopper et al. 2009). Within bird-pollinated plants, one of the most pronounced evolutionary developments in the SWAFR is divergence of congeners from taller growth forms requiring perch feeding to those that are prostrate or low-growing, enabling access to nectar by birds standing on the ground. Such divergence is evident across a range of families, including Haemodoraceae (Anigozanthos), Proteaceae (Banksia, Grevillea and Hakea), Myrtaceae (Verticordia, Darwinia, Balaustion and Cheyniana), Xanthorrhoeaceae (Xanthorrhoea) and Fabaceae (Kennedya, Leptosema and Brachysema) (Hopper & Burbidge 1978, 1986; Hopper 1993; Brown et al. 1997; Rye 2009). Evolution of flowering at ground level may have arisen to take advantage of increased visitation by mammals and Tawny-crowned Honeyeaters, the latter of which spend more time feeding on the ground than other honeyeaters (Hopper 1993). Interestingly, in Anigozanthos short stature is correlated with mass flowering post-fire (Hopper 1993), which may represent a strategy to increase fruit set through minimizing competition for the services of honeyeaters.
Detailed field studies have shown that insects routinely visit some species that conform to the bird pollination syndrome. Insects have been reported visiting the putatively bird-pollinated Calothamnus (Myrtaceae; Houston 1983; Collins et al. 1984b), Banksia (Proteaceae; Whelan & Burbidge 1980; Lewis & Bell 1981; Ramsey 1988; Day et al. 1997) and Verticordia staminosa (Myrtaceae; Yates & Ladd 2004). Caging experiments and examination of pollen loads generally have demonstrated that birds play the dominant role in pollination (Whelan & Burbidge 1980; Collins et al. 1984b; Ramsey 1988; Day et al. 1997). However, in Banksia attenuata seed set is similar with and without birds, suggesting that insects play a dominant role in this species (Whelan & Burbidge 1980). This demonstrates that caution needs to be exercised when making assumptions on the identity of pollinator species based on pollination syndromes.
Mating systems have important implications for outcrossing rates and potentially the ability to reproduce in small populations (e.g. Coates et al. 2007). Within Grevillea, Banksia and Eucalyptus there is considerable interspecific variation in mating system with species varying from complete selfing to complete outcrossing (Ramsey & Vaughton 1991; Ellis & Sedgeley 1992; Day et al. 1997; Kennington & James 1997; Hermanutz et al. 1998; Heliyanto et al. 2005). Studies of wild populations have shown that bird-pollinated plants in several genera tend to be primarily outcrossing (Hopper & Moran 1981; Day et al. 1997; Coates et al. 2007). However, levels of outcrossing are affected by local environmental conditions. In a review of several SWAFR species, Coates et al. (2007) found that small populations in disturbed sites tended to have lower levels of outcrossing presumably due to shifts in pollinator behaviour in areas with lower resource availability.
Paternity analysis studies using molecular markers have demonstrated the capacity for honeyeaters to move pollen extensively between some plant populations. In a study of fragmented populations of Calothamnus quadrifidus in the wheatbelt of the SWAFR, pollen was regularly dispersed by honeyeaters between fragments over 5 km away (Byrne et al. 2007). At more local scales, the movement of honeyeaters has shown considerable variation between study systems. Observations of honeyeaters feeding on Anigozanthos manglesii, A. humilis (Haemodoraceae) and Eucalyptus stoatei (Myrtaceae) revealed that birds usually moved between plants within 4 m (Hopper & Burbidge 1978; Hopper & Moran 1981). Extensive movements between inflorescences within flowering individuals have been documented for Calothamnus quadrifidus (Yates et al. 2007a). Alternatively, using amplified fragment length polymorphisms (AFLPs) to assign paternity in a population of Banksia hookeriana, Krauss et al. (2009) showed an average pollen flow distance of 29.9 m (maximum = 80 m). This demonstrates that due to the variety of plant families, bird species and plant communities involved, it is difficult to predict the effects of bird pollination on pollen movements for a species.
(b). Insect pollination
(i). Ecology of nectarivorous insects in the SWAFR
The dominant families of insects recorded pollinating plants in the SWAFR are Colletidae, Halictidae, Thynninidae (Hymenoptera), Buprestidae (Coleoptera) and Bombyliidae (Diptera) (Brown et al. 1997; Houston 2000). Other less prominent families involved in pollination in the SWAFR include Megachilidae, Stenotritidae, Anthophoridae (Hymenoptera), Scarabaeidae (Coleoptera) and Lycaenidae (Lepidoptera) (Brown et al. 1997; Houston 2000). The introduced honeybee Apis mellifera (Apidae) is the most abundant insect pollinator in the SWAFR. For most families, information on plant visitation comes from collection details in papers on insect taxonomy (e.g. Houston 1989) and the Western Australian Museum database of bees visiting flowers (Houston 2000). This literature has demonstrated that for most well-collected species a variety of plant families are visited, often with a bias towards Myrtaceae (Brown et al. 1997; Houston 2000).
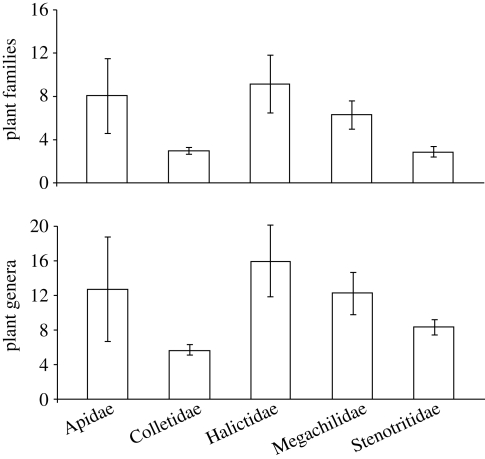
Interrogation of the Western Australian Museum database of bees visiting flowers (Houston 2000) has revealed pronounced variation between bee families in their level of foraging specificity. Using this database, we have quantified the specificity of bee foraging preferences at the level of plant family and genus. In the present review, only species known to occur in the SWAFR and represented by 20 or more individuals in the database were included in the analysis, giving a total of 93 species. It is not specified in the database whether bees were foraging for nectar or pollen. Bees of the families Colletidae and Stenotritidae tend to visit a lower number of plant families and genera than members of the Apidae, Halictidae and Megachilidae (figure 2). Colletidae is the only family where species have been recorded using a single plant family or genus, with 10 and 26 species recorded from a single plant genus or family, respectively. In almost all cases, specialization on a single family involves members of the Myrtaceae and Proteaceae with rare examples involving the Papilionaceae and Haemodoraceae (Houston 2000). Specialization by a bee species on a single genus of food plants has been recorded from a range of plant genera, which show considerable intergeneric variation in floral structure. These genera include Conospermum (Proteaceae), Conostylis (Haemodoraceae), Calothamnus (Myrtaceae), Pileanthus (Myrtaceae) and Verticordia (Myrtaceae) (Houston 2000). More thorough investigations of bee visitation to flowers in the SWAFR may well yield many more cases of specialization and concurrent morphological adaptation in the bee fauna (Houston 2000). For example, Euryglossa tubulifera possesses enormously enlarged maxillary palpi that cohere to form a slender tube up to 80 per cent the length of the head and body, which enables sucking of nectar from Calothamnus flowers (Houston 1983).
Figure 2.
Comparative levels of food source specificity for families of bee in the SWAFR. Analysis is based on the Western Australian Museum database of bees visiting flowers (Houston 2000). Only bee species represented by more than 20 specimens in the Western Australian Museum collection have been included. Number of species: Apidae, 4; Colletidae, 67; Halictidae, 8; Megachilidae, 8 and Stenotritidae, 6. Histograms show mean±standard errors.
There is presently no information on the long distance movements of nectarivorous insects in the SWAFR other than observations of vagrant butterflies well outside of their normal distribution (Braby 2004). At the more local scale of within bushland fragments, the only detailed information comes from studies of the Orchidaceae and overseas studies of A. mellifera. Movements of food-foraging pollinators between individuals averaged less than 6 m (maximum = 21) for both Prasophyllum fimbria and Cyanicula gemmata (Orchidaceae; Peakall 1987, 1989). Alternatively, in Drakaea glyptodon, which is pollinated by sexual deception of the thynnine wasp Zaspilothynnus trilobatus, mean pollinator movement in a capture–recapture study was 32 m (maximum = 132; Peakall 1990). In the Northern Hemisphere, A. mellifera regularly forage as far as 2 km from their hive (Visscher & Seeley 1982; Steffan-Dewenter & Tscharntke 2000). This illustrates the potential for major differences in pollinator movements depending on pollination system, life history strategy and foraging behaviour. Numerous studies from other continents have demonstrated that body size and potential flight distances are poor correlates for the realized distances moved by insect pollinators (e.g. Janzen 1971; Nason et al. 1998; Pasquet et al. 2008).
(ii). Pollination in insect-pollinated plants in the SWAFR
Flowers of a number of the largest and most abundant genera in the SWAFR are visited by a wide range of insect orders and families. Insect-pollinated Eucalyptus, Melaleuca (Myrtaceae), Acacia (Mimosaceae), Leucopogon (Ericaceae) and Hakea (Proteaceae) are usually pollinated by a range of bee, wasp, fly and beetle families (e.g. Bernhardt 1987; Keighery 1996; Brown et al. 1997; Yates et al. 2005). In the most detailed study, Yates et al. (2005) recorded 83 species of insect from 63 genera in 38 families visiting a jarrah tree, Eucalyptus marginata (Myrtaceae), in an urban remnant in less than a month.
Species that are generalists, but utilize only a specific order of insects or a restricted subset of genera, also appear to be common in the flora. For example, most species of Hibbertia (Dilleniaceae) attract beetles, colletid bees and halictid bees with a nectar reward (Bernhardt 1986; Schatral 1996). The number of pollinators at any one location is small, but different pollinators are used in different regions depending on local availability (Schatral 1996). Similar examples have been recorded in beetles visiting Cyanicula (Orchidaceae; Peakall 1987), Leioproctus bees (Halictidae) visiting Comospermum (Proteaceae; Houston 1989) and Lepidopterans visiting Pimelea (Thymelaeaceae; Keighery 1975). With further observations, species in genera such as Conostylis and Pileanthus may also conform to this pattern (Houston 2000). Interpreting levels of pollinator specificity in these species requires some caution. While a spatially restricted plant species may be visited by a single pollinator, there may be other species capable of effecting pollination outside the plant's present distribution. However, if only one species is functioning as a pollinator in a plant's present distribution, the plant must be considered a specialist in terms of its ability to cope with local environmental changes.
Extreme specialization in the use of pollinators is phylogenetically very restricted in the SWAFR. The only recorded cases among the woody flora of the SWAFR are Verticordia nitens and V. aurea (Myrtaceae), which are both pollinated by single species of colletid bee foraging on pollen kit (Houston et al. 1993). In the Orchidaceae, the large numbers of species that use sexual deception to attract pollinators are each believed to be pollinated by a single species of hymenopteran, with minimal sharing of pollinators between orchid species (Hopper & Brown 2007; Phillips et al. 2009). This strategy is exceptionally well represented in the SWAFR, being used by seven orchid genera: Caladenia, Drakaea, Paracaleana, Spiculaea (thynnine wasps; Thynninidae), Calochilus (scoliid wasps; Thynninidae), Cryptostylis (ichneumonid wasps; Ichneumonidae) and Leporella (ants, Myrmecia urens, Formicidae) (Brown et al. 2008). Investigations of pollination ecology in Caladenia and Drakaea have shown that this pollination strategy results in high pollen movement distances but potentially lower fruit set than in con-generics (Peakall 1990; Phillips et al. 2009). In the numerous species pollinated by thynnine wasps, the complexity of the wasp life cycle and the specificity of the plant–pollinator relationship may make them particularly susceptible to environmental change (figure 3).
Figure 3.
The life cycle of Drakaea livida (Orchidaceae). Drakaea livida has a highly specific relationship with a Tulasnella (Tulasnellaceae) mycorrhizal fungus, which it requires for germination and annual growth. Drakaea livida is pollinated solely through sexual deception of Zaspilothynnus nigripes (Thynnidae), where it mimics the calling female. Completion of the wasp life cycle requires appropriate nectar plants, where the male feeds the female in copula, and suitable scarab beetle larvae in which the female lays her eggs. The specific nature of the orchid–pollinator relationship and the number of interacting organisms makes this one of the most highly vulnerable pollination systems in southwestern Australia. Artwork by Martin Thompson.
3. The swafr landscape and the evolution of pollination systems
(a). Incidence of specialized pollination systems
While the continuum between generalist versus specialist pollination systems has yet to be investigated adequately, several factors favouring the evolution of specialized pollination systems have been proposed. The absence of extreme environments, the absence of large year-to-year variation in the growing climate (Armbruster 2006) and an abundance of long-lived or clonal plant species with many reproductive episodes (Waser et al. 1996) are all factors proposed to favour the evolution of specialized pollination systems in a flora. The SWAFR conforms to these criteria in terms of the environment and some of the flora, which comprises many long-lived woody shrubs, trees and herbs. In regards to the pollinator fauna, plant specialization is more likely to arise when pollinator populations are subject to minimal fluctuations and there is variation in the availability and effectiveness of pollinators (Armbruster 2006). While there is little information available on the fluctuation of pollinator populations in the SWAFR, many plants in the SWAFR exhibit differences in pollen load between pollinator species (e.g. Collins et al. 1984b; Ramsey 1988; Schatral 1996). Given these observations, the age of the landscape and the levels of specialization observed in the ecologically similar Cape Floristic Region (Johnson & Steiner 2000), it is predicted that the SWAFR will contain a relatively high level of specialized pollination systems.
Globally, extreme specialization for pollinators is largely confined to species that provide alternative rewards to nectar or pollen or attract pollinators using deceit (Gomez & Zamora 2006). Observations in the SWAFR support this trend, with the most extreme cases of specialization lying within the multiple evolutions of sexual deception in the Orchidaceae and the specialization of some Verticordia on oil-foraging bees (Houston et al. 1993; Brown et al. 2008). Specialization at the level of functional groups is evident in numerous bird-pollinated species (Hopper & Burbidge 1986) and some lepidopteran (Keighery 1975) and bee-pollinated species (Houston 2000), most of which are long-lived trees and shrubs or clonal herbs. As such, while much of the flora is visited by a wide range of pollinator species, there is evidence of a trend towards accentuated levels of pollinator specificity in some families in the SWAFR. However, much work is required, particularly on insect-pollinated species, to better resolve levels of plant–pollinator specificity in the SWAFR and the conditions favouring evolution of specialized pollinator relationships. Only once community-wide studies of pollinator visitation have been conducted will it be possible to make quantitative comparisons with other floras in the incidence of specialization in the use of pollinators.
(b). Role of pollinators in maintaining species boundaries
In those elements of the flora employing generalist pollination strategies, pollinators probably play a minor role in maintaining reproductive isolation between species (e.g. Hopper 1981; Lewis & Bell 1981; Collins et al. 1984b; Houston 2000). However, given the trend towards specialization in some groups, pollinators may play a role in the maintenance of species boundaries in some families. One of the best-documented examples of almost completely pollinator maintained reproductive isolation occurs in sexually deceptive orchids. In both Caladenia and Drakaea congeners regularly occur in sympatry but avoid hybridization through use of different thynnine wasp species (Hopper & Brown 2007; Phillips et al. 2009). Further research into genera that are specialized at the level of functional group and use only a small number of pollinator species may reveal cases where isolation is maintained through differences in pollinator preference or efficacy (Hopper & Burbidge 1986).
Stylidium (Stylidiaceae), which are pollinated by a range of nectar-seeking solitary bees and bombyllid flies (Armbruster et al. 1994; Brown et al. 1997), are unique in the SWAFR in using differential pollen placement on the bodies of insects to avoid the potentially detrimental effects of hybridization and pollen wastage (Armbruster et al. 1994; Armbruster 2006). Pollen is deposited onto the body of the insect using a mobile column that is rapidly triggered by the contact of the insect at the base of petals. A combination of variation in nectar-tube length and positioning of the column results in each species at a site having a unique combination of pollinator species and pollen position on the body of the animal. Armbruster et al. (1994) suggested that this partitioning within communities had arisen through character displacement. In cases of both ethological and mechanical pollinator-mediated isolation, studies addressing the causes of initial reproductive isolation rather than studies confirming mechanisms responsible for current isolation are yet to be undertaken.
(c). Effects of vertebrate pollination on pollen movements
It has been predicted that in ancient landscapes strategies will have evolved to maximize outcrossing and pollen dispersal in small and/or isolated populations (Hopper 2009). Owing to differences in size and behaviour, it is expected that pollination by birds rather than insects will result in higher outcrossing rates and pollen being dispersed greater distances. As such, the prevalence of bird pollination in the SWAFR may represent repeated adaptation to a prolonged history of small or isolated populations (Hopper 2009). Studies elsewhere directly tracking pollen and using molecular markers to assign pollen to parental plants have revealed that there is no simple dichotomy between bird pollination and insect pollination in terms of pollen movement, with several groups of insects moving pollen considerable distances (Nason et al. 1998; Hanson et al. 2008; Pasquet et al. 2008). However, those species where small pollen movement distances have been reported are predominantly insect-pollinated herbs (Kropf & Renner 2008; Llaurens et al. 2008).
The spatial distribution of a plant population is also critical to pollen movement distances, with shorter distances recorded in continuous populations and communities rather than fragmented environments (Hanson et al. 2008). As such, research comparing the efficacy of birds and insects at dispersing pollen will need to address both the spatial distribution of the plants and the specific groups of pollinators involved. A simple method for doing this would be to express pollinator movement distances as the ratio of pollinator movement distance to nearest neighbour of the plants (the Pollinator Movement Index). This provides a measure of the degree to which pollinators exceed the minimum requirements to reach another flowering plant of the same species. Estimates in the SWAFR for bird-pollinated plants are: 2.2—Banksia hookeriana (Proteaceae; Krauss et al. 2009) and 4.4—Eucalyptus stoatei (Myrtaceae; Hopper & Moran 1981). However, such estimates are not available yet for insect-pollinated species in the SWAFR. The few similar studies in other floras suggest that insect-pollinated species have lower pollinator movement distances than the estimates for bird-pollinated shrubs: Asclepias exaltata—0.3 (Apocynaceae; Broyles & Wyatt 1991) and Disa cooperi—1.6 (Orchidaceae; Johnson et al. 2005). Studies using pollen tracking and paternity assignment (e.g. Krauss 1994; Kropf & Renner 2008) are not directly comparable with those based on pollinator observation due to the prevalence of pollen carryover (Broyles & Wyatt 1991). However, use of these techniques will provide the most direct test of the effectiveness of a pollinator at dispersing genes, assuming that the pollinator responsible for each pollen movement can be isolated.
Comparison of pollen movements and the foraging behaviour of nectarivorous animals suggest that in the SWAFR there is a trend towards bird pollination leading to greater pollen movement distances (Hopper & Moran 1981; Peakall 1989; Byrne et al. 2007; Krauss et al. 2009). Additionally, in the eastern Australian Grevillea macleayana (Proteaceae), outcrossing rates were higher in plants where birds were allowed access to flowers in addition to insects (England et al. 2001). These lines of evidence support the hypothesis that bird pollination may evolve through increased outcrossing or pollen movements. As such, the prevalence of bird pollination in the SWAFR compared with other regions may have arisen through strong selection for outbreeding in small, isolated populations (Byrne & Hopper 2008; Hopper 2009). Alternatively, the use of bird pollinators may be driven by the low nutrient and moisture levels of the old landscapes found in the SWAFR (Hopper 2009). Under these conditions, pollinator visitation and pollen transfer may greatly exceed the amount of seed a plant is capable of producing (Stock et al. 1989; Groom et al. 2000). As a result, selection may favour pollinators that result in greater outcrossing and more genetically fit offspring rather than the highest quantity of fruits. Similarly, low resource conditions may favour the evolution of early acting, post-zygotic lethal genes so that scarce resources are not dedicated to the production of low-quality fruit. These hypotheses are not mutually exclusive. In small populations, the cost of inbreeding may be higher and as such selection to favour genetically fit offspring rather than large numbers of fruit may be highest in small and/or isolated populations.
4. Predictions of the impact of a changing environment on pollination in the swafr
While this review of the pollination ecology in the SWAFR has highlighted some areas of potential resilience to environmental change such as the broad foraging preferences and extensive movements of honeyeaters, it has also highlighted some examples of specialization for pollinators, particularly at the level of functional group. Further, evidence suggests that fitness advantages in pollination strategies in this old landscape may be a consequence of differences in foraging behaviour between pollinator functional groups. In light of the findings presented here, we make the following predictions to be tested on the effects of continuing environmental change on pollination ecology in the SWAFR. Given the increase in unpredictability of rainfall experienced in the SWAFR, particularly around its dry margins, we may already be in the position to begin testing some of these predictions.
While the extinction risks to invertebrates are unknown, birds are able to feed on insects and spatially patchy nectar resources (e.g. Collins et al. 1984a; Byrne et al. 2007). As such, species relying on bird pollination are unlikely to suffer permanent losses of pollinator species through climate change alone.
Limits to the ability to sustain seed set imposed by a drying climate will favour strategies that increase seed quality. This may favour specialization on pollinators that maximize outcrossing events or the evolution of early acting incompatibility.
Pollinator loss from fragmented environments will be accentuated through reduced flowering supporting lower populations of nectarivores (e.g. Bradshaw et al. 2007). The impact will be greatest for specialists and those generalist pollinators that only use a small subset of the available pollinator community at each site.
Owing to the probable generalist nature of the majority of the pollination systems in the flora, in most cases the availability of pollinators should not place a limit on the migratory ability of plants. Limits to migration will arise through edaphic and physical barriers, especially in a region dominated by older, fragmented edaphic environments (Hopper 2009).
There has been, and will continue to be, a long-term shift towards nectarivores that are capable of persisting in a drier, fragmented environment.
Distances of pollen movements will change. How these movements change will depend on (a) the animals available to act as pollinators (e.g. England et al. 2001), (b) plant population size and density (e.g. Yates et al. 2007b), (c) the abundance of the animals and the subsequent interspecific and intraspecific interations (e.g. MacNally & Timewell 2005) and (d) pollinator fidelity (e.g. Hopper & Burbidge 1978).
5. Future directions
Many areas of pollination biology remain poorly studied in the SWAFR. Levels of specialization and the pollination vectors involved have been well resolved for some vertebrate-pollinated species, while the majority of the insect-pollinated flora has received no attention. Mechanisms driving the evolution of pollination strategies and reproductive isolation remain poorly understood. In particular, comparative experiments need to be conducted to confirm the role of vertebrate pollination in high pollen movement distances, the fitness consequences for changes in patterns of pollen movement and ultimately the reasons for the repeated evolution of vertebrate pollination. The few instances of highly specific plant–pollinator relationships suggest that pollination may have played a relatively minor role in speciation in most groups. However, further work is required on insect-pollinated species and to test if bird-pollinated plants are able to achieve a degree of reproductive isolation through assortative pollination (where similar phenotypes are more likely to reproduce with each other) resulting from differing placement of inflorescences and modification in floral structure.
Predicted climatic changes have the potential to act in concert with existing environmental alterations to have serious impacts on plant and pollinator networks. Studies of honeyeater communities in the SWAFR have suggested that this group and the species that they pollinate will exhibit some resilience to environmental change, albeit with potentially detrimental local changes to fruit set and pollen movement. Alternatively, the paucity of information on insect pollination is of particular concern, especially for herbaceous species and local endemics that may be pollinated by a small number of species. In most cases, the taxonomy of insects in the SWAFR is poorly resolved and little is known of their life cycles and resilience to drought, fire or anthropogenic landscape modification. With the exception of some studies on honeyeaters, it is unknown if communities in small fragments of bushland suffer loss of pollinators, if this is accentuated by drought and if pollinators move through fragmented landscapes. A promising area of future research will be to determine if the presence or enhancement through restoration activities of keystone plant species that provide nectar for a large number of insects is capable of supporting pollinator communities that will function to maintain the remainder of the plant community (Saffer et al. 2000; Dixon 2009).
Acknowledgements
This review was undertaken while R.D.P. was supported by an Australian Postgraduate Award. R.D.P. gratefully acknowledges the generous support of the Australian Orchid Foundation, the Holsworth Wildlife Research Endowment and the School of Plant Biology at The University of Western Australia, which has enabled three enjoyable years of pollination research in southern Western Australia. S.D.H. is grateful to many collaborators who have assisted directly or in discussions about pollination, evolution and conservation in the Southwest Australian Floristic Region over more than three decades of research on this topic. This research was enabled by institutional support from The University of Western Australia, Department of Fisheries and Wildlife, Department of Conservation and Land Management and Kings Park and Botanic Garden. Bill Chaloner and an anonymous reviewer provided comments that improved the final manuscript.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Abensperg-Traun M., Smith G. T., Arnold G. W., Steven D. E.1996The effects of habitat fragmentation and livestock grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. I Arthropods. J. Appl. Ecol. 33, 1281–1301 (doi:10.2307/2404770) [Google Scholar]
- Aguilar R., Ashworth L., Galetto L., Aizen M. A.2006Plant reproductive susceptibility to habitat fragmentation: review and syntheses through a meta-analysis. Ecol. Lett. 9, 968–980 (doi:10.1111/j.1461-0248.2006.00927.x) [DOI] [PubMed] [Google Scholar]
- Armbruster W. S.2006Evolutionary and ecological aspects of specialized pollination: views from the arctic to the tropics. In Plant-pollinator interactions: from specialization to generalization (eds Waser N. M., Ollerton J.). Chicago, IL: University of Chicago Press [Google Scholar]
- Armbruster W. S., Edwards M. E., Debevec E. M.1994Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidum). Ecology 75, 315–329 (doi:10.2307/1939537) [Google Scholar]
- Armstrong D. P.1991Aggression of breeding territorial honeyeaters corresponds to seasonal changes in nectar availability. Behav. Ecol. Sociobiol. 29, 103–111 (doi:10.1007/BF00166484) [Google Scholar]
- Ashworth L., Aguilar R., Galetto L., Aizen M. A.2004Why do pollination generalists and specialist plant species show similar susceptibility to habitat fragmentation? J. Ecol. 92, 717–719 (doi:10.1111/j.0022-0477.2004.00910.x) [Google Scholar]
- Beard J. S.1999Southwest Australia. In Hotspots: Earth's biologically richest and most endangered terrestrial ecoregions (eds Mittermeier R. A., Myers N., Gil P. R., Mittermeier C. G.). Mexico City, Mexico: CEMEX [Google Scholar]
- Bernhardt P.1986Bee-pollination in Hibbertia fasciculata (Dilleniaceae). Plant Syst. Evol. 152, 231–241 (doi:10.1007/BF00989430) [Google Scholar]
- Bernhardt P.1987A comparison of the diversity, density, and foraging behavior of bees and wasps on Australian Acacia. Ann. Mo. Bot. Gard. 74, 42–50 (doi:10.2307/2399260) [Google Scholar]
- Braby M. F.2004The complete field guide to butterflies of Australia Melbourne, VIC: CSIRO Publishing [Google Scholar]
- Bradshaw S. D., Phillips R. D., Tomlinson S., Holley R. J., Jennings S., Bradshaw F. J.2007Ecology of the honey possum, Tarsipes rostratus, in Scott National Park, Western Australia. Aust. Mammal. 29, 25–38 [Google Scholar]
- Brown E. M., Burbridge A. H., Dell J., Edinger D., Hopper S. D., Wills R. T.1997Pollination in Western Australia: a database of animals visiting flowers Handbook No. 15 Perth, WA: WA Naturalists' Club [Google Scholar]
- Brown A., Dundas P., Dixon K., Hopper S.2008Orchids of Western Australia Perth, WA: University of Western Australian Press [Google Scholar]
- Broyles S. B., Wyatt R.1991Effective pollen dispersal in a natural population of Asclepias exaltata: the influence of pollinator behaviour, genetic similarity and mating success. Am. Nat. 138, 1239–1249 (doi:10.1086/285280) [Google Scholar]
- Burgman M. A., Keith D., Hopper S. D., Widyatmoko D., Drill C.2007Threat syndromes and conservation of the Australian flora. Biol. Conserv. 134, 73–82 (doi:10.1016/j.biocon.2006.08.005) [Google Scholar]
- Byrne M., Hopper S. D.2008Granite outcrops as ancient islands in old landscapes: evidence from the phylogeography and population genetics of Eucalyptus caesia (Myrtaceae) in Western Australia. Biol. J. Linn. Soc. 93, 177–188 [Google Scholar]
- Byrne M., Elliott C. P., Yates C., Coates D. J.2007Extensive pollen dispersal in a bird-pollinated shrub, Calothamnus quadrifidus, in a fragmented landscape. Mol. Ecol. 16, 1303–1314 (doi:10.1111/j.1365-294X.2006.03204.x) [DOI] [PubMed] [Google Scholar]
- Coates D. J., Sampson J. F., Yates C. J.2007Plant mating systems and assessing population persistence in fragmented landscapes. Aust. J. Bot. 55, 239–249 (doi:10.1071/BT06142) [Google Scholar]
- Collins B. G., Briffa P., Newland C.1984aTemporal changes in abundance and resource utilization by honeyeaters at Wongamine Nature Reserve. Emu 84, 159–166 [Google Scholar]
- Collins B. G., Newland C., Briffa P.1984bNectar utilization and pollination by Australian honeyeaters and insects visiting Calothamnus quadrifidus (Myrtaceae). Aust. J. Ecol. 9, 353–365 (doi:10.1111/j.1442-9993.1984.tb01373.x) [Google Scholar]
- Cowling R. M., Rundel P. W., Lamont B. B., Arroyo M. K., Arianoutsou M.1996Plant diversity in mediterranean-climate regions. Trends Ecol. Evol. 11, 362–366 (doi:10.1016/0169-5347(96)10044-6) [DOI] [PubMed] [Google Scholar]
- Day D. A., Collins B. G., Rees R. G.1997Reproductive biology of the rare and endangered Banksia brownii Baxter ex R. Br. (Proteaceae). Aust. J. Ecol. 22, 307–315 (doi:10.1111/j.1442-9993.1997.tb00676.x) [Google Scholar]
- Dixon K. W.2009Pollination and restoration. Science 325, 571–573 (doi:10.1126/science.1176295) [DOI] [PubMed] [Google Scholar]
- Ellis M. F., Sedgeley M.1992Floral morphology and breeding system of three species of Eucalyptus, Section Bisectaria (Myrtaceae). Aust. J. Bot. 40, 249–262 (doi:10.1071/BT9920249) [Google Scholar]
- England P. R., Beynon F., Ayre D. J., Whelan R. J.2001A molecular genetic assessment of mating-system variation in a naturally bird-pollinated shrub: contributions from birds and introduced honeybees. Conserv. Biol. 15, 1645–1655 (doi:10.1046/j.1523-1739.2001.00236.x) [Google Scholar]
- Ford H. A., Paton D. C.1977The comparative ecology of ten species of honeyeaters in South Australia. Aust. J. Ecol. 2, 399–407 [Google Scholar]
- Garavanta C. A. M., Wooller R. D., Richardson K. C.2000Movement patterns of honey possums, Tarsipes rostratus, in the Fitzgerald River National Park. Wildl. Res. 27, 179–183 (doi:10.1071/WR98088) [Google Scholar]
- Gomez J. M., Zamora R.2006Ecological factors that promote the evolution of generalization in pollination systems. In Plant-pollinator interactions from specialisation to generalisation (eds Waser N. M., Ollerton J.). London, UK: The University of Chicago Press [Google Scholar]
- Groom P. K., Froend R. H., Mattiske E. M., Koch B.2000Myrtaceous shrub species respond to long-term decreasing groundwater levels on the Gnangarra mound, northern Swan Coastal Plain. J. R. Soc. West. Aust. 83, 75–82 [Google Scholar]
- Hanson T. R., Brunsfeld S. J., Finegan B., Waits L. P.2008Pollen dispersal and genetic structure of the tropical tree Dipteryx panamensis in a fragmented Costa Rican landscape. Mol. Ecol. 17, 2060–2073 (doi:10.1111/j.1365-294X.2008.03726.x) [DOI] [PubMed] [Google Scholar]
- Heliyanto B., Veneklaas E. J., Lambers H., Krauss S. L.2005Preferential outcrossing in Banksia ilicifolia (Proteaceae). Aust. J. Bot. 53, 163–170 (doi:10.1071/BT04011) [DOI] [PubMed] [Google Scholar]
- Hermanutz L., Innes D., Denham A., Whelan R.1998Very low fruit : flower ratios in Grevillea (Proteaceae) are independent of breeding system. Aust. J. Bot. 46, 465–478 (doi:10.1071/BT97046) [Google Scholar]
- Higgins P. J.1999Handbook of Australian, New Zealand and Antarctic birds, volume 4: parrots to Dollarbird Melbourne, VIC: Oxford University Press [Google Scholar]
- Higgins P. J., Peter J. M., Steele W. K.2001Handbook of Australian, New Zealand and Antarctic birds, volume 5: tyrant-flycatchers to chats Melbourne, VIC: Oxford University Press [Google Scholar]
- Higgins P. J., Peter J. M., Cowling S. J.2006Handbook of Australian, New Zealand and Antarctic birds, volume 7: boatbills to starlings Melbourne, VIC: Oxford University Press [Google Scholar]
- Hopper S. D.1980Bird and mammal pollen vectors in Banksia communities at Cheyne Beach, Western Australia. Aust. J. Bot. 28, 61–75 (doi:10.1071/BT9800061) [Google Scholar]
- Hopper S. D.1981Honeyeaters and their winter food plants on granite rocks in the central wheatbelt of Western Australia. Aust. Wildl. Res. 8, 187–197 (doi:10.1071/WR9810187) [Google Scholar]
- Hopper S. D.1993Kangaroo paws and catspaws: a natural history and field guide Perth, WA: Department of Conservation and Land Management [Google Scholar]
- Hopper S. D.2009OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322, 49–86 (doi:10.1007/s11104-009-0068-0) [Google Scholar]
- Hopper S. D., Brown A. P.2007A revision of Australia's hammer orchids (Drakaea: Orchidaceae), with some field data on species-specific sexually deceived wasp pollinators. Aust. Syst. Bot. 20, 252–285 (doi:10.1071/SB06033) [Google Scholar]
- Hopper S. D., Burbidge A. H.1978Assortative pollination by Red Wattlebirds in a hybrid population of Anigozanthos Labill (Haemodoraceae). Aust. J. Bot. 26, 335–350 (doi:10.1071/BT9780335) [Google Scholar]
- Hopper S. D., Burbidge A. H.1986Speciation of bird-pollinated plants in south-western Australia. In The dynamic partnership: bird and plants in Southern Australia (eds Ford H. A., Paton D. C.). Adelaide, SA: Government Printer [Google Scholar]
- Hopper S. D., Gioia P.2004The Southwest Australian Floristic Region: evolution and conservation of a global diversity hotspot. Annu. Rev. Ecol. Evol. Syst. 35, 623–650 (doi:10.1146/annurev.ecolsys.35.112202.130201) [Google Scholar]
- Hopper S. D., Moran G. F.1981Bird pollination and the mating system of Eucalyptus stoatei. Aust. J. Bot. 29, 625–638 (doi:10.1071/BT9810625) [Google Scholar]
- Hopper S. D., van Leeuwin S., Brown A. P., Patrick S. J.1990Western Australia's endangered flora and other plants under consideration for declaration Perth, WA: Department of Environment and Conservation [Google Scholar]
- Hopper S. D., Smith R. J., Fay M. F., Manning J. C., Chase M. W.2009Molecular phylogenetics of Haemodoraceae in the Greater Cape and Southwest Australian Floristic Regions. Mol. Phylogenet. Evol. 51, 19–30 (doi:10.1016/j.ympev.2008.11.015) [DOI] [PubMed] [Google Scholar]
- Horwitz P., Bradshaw D., Hopper S., Davies P., Froend R., Bradshaw F.2008Hydrological change escalates the risk of ecosystem stress in Australia's threatened biodiversity hotspot. J. R. Soc. West. Aust. 91, 1–11 [Google Scholar]
- Houston T. F.1983An extraordinary new bee and adaptation of palpi for nectar-feeding in some Australian Colletidae and Pergidae (Hymenoptera). J. Aust. Entomol. Soc. 22, 263–270 (doi:10.1111/j.1440-6055.1983.tb01894.x) [Google Scholar]
- Houston T. F.1989Leioproctus bees associated with Western Australian smoke bushes (Conospermum spp.) and their adaptations for foraging and concealment (Hymenoptera: Colletidae: Paracolletini). Rec. West. Aust. Mus. 14, 275–292 [Google Scholar]
- Houston T. F.2000Native bees on wildflowers in Western Australia Perth, WA: Western Australian Insect Study Society Inc [Google Scholar]
- Houston T. F., Lamont B. B., Radford S., Errington S. G.1993Apparent mutualism between Verticordia nitens and V. aurea (Myrtaceae) and their oil-ingesting bee pollinators (Hymenoptera: Colletidae). Aust. J. Bot. 41, 369–380 (doi:10.1071/BT9930369) [Google Scholar]
- Indian Ocean Climate Initiative Panel 2002Climate variability and change in south west Western Australia Perth, WA: Indian Ocean Climate Initiative Panel [Google Scholar]
- Janzen D. H.1971Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205 (doi:10.1126/science.171.3967.203) [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Steiner K. E.2000Generalisation versus specialization in plant pollination systems. Trends Ecol. Evol. 15, 140–143 (doi:10.1016/S0169-5347(99)01811-X) [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Neal P. R., Harder L. D.2005Pollen fates and the limits on male reproductive success in an orchid population. Biol. J. Linn. Soc. 86, 175–190 (doi:10.1111/j.1095-8312.2005.00541.x) [Google Scholar]
- Kearns C. A., Inouye D. W., Waser N. M.1998Endangered mutualisms: the conservation of plant–pollinator interactions. Annu. Rev. Ecol. Syst. 29, 83–112 (doi:10.1146/annurev.ecolsys.29.1.83) [Google Scholar]
- Keast A.1968Seasonal movements in the Australian honeyeaters (Meliphagidae) and their ecological significance. Emu 67, 159–210 [Google Scholar]
- Keighery G. J.1975Parallel evolution of floral structures in Darwinia (Myrtaceae) and Pimelea (Thymelaeaceae). West. Aust. Nat. 13, 46–50 [Google Scholar]
- Keighery G. J.1980Bird pollination in South Western Australia: A checklist. Plant Syst. Evol. 135, 171–176 (doi:10.1007/BF00983185) [Google Scholar]
- Keighery G. J.1996Phytogeography, biology and conservation of Western Australian Epacridaceae. Ann. Bot. 77, 347–355 (doi:10.1006/anbo.1996.0042) [Google Scholar]
- Kennington W. J., James S. H.1997The effect of small population size on the mating system of a rare clonal mallee, Eucalyptus argutifolia. Heredity 78, 252–260 (doi:10.1038/hdy.1997.39) [Google Scholar]
- Krauss S. L.1994Restricted gene flow within the morphologically complex species Persoonia mollis (Proteaceae): contrasting evidence from the mating system and pollen dispersal. Heredity 73, 142–154 (doi:10.1038/hdy.1994.113) [Google Scholar]
- Krauss S. L., He T., Barrett L. G., Lamont B. B., Enright N. J., Miller B. P., Hanley M. E.2009Contrasting impacts of pollen and seed dispersal on spatial genetic structure in the bird-pollinated Banksia hookeriana. Heredity 102, 274–285 (doi:10.1038/hdy.2008.118) [DOI] [PubMed] [Google Scholar]
- Kropf M., Renner S. S.2008Pollinator-mediated selfing in two deceptive orchids and a review of pollinium tracking studies addressing geitonogamy. Oecologia 155, 497–508 (doi:10.1007/s00442-007-0919-4) [DOI] [PubMed] [Google Scholar]
- Lewis J., Bell D. T.1981Reproductive isolation of co-occuring Banksia species at the Yule Brook Botany Reserve, Western Australia. Aust. J. Bot. 29, 665–674 (doi:10.1071/BT9810665) [Google Scholar]
- Llaurens V., Castric V., Austerlitz F., Vekemans X.2008High paternal diversity in the self-incompatible herb Arabidposis halleri despite clonal reproduction and spatially restricted pollen dispersal. Mol. Ecol. 17, 1577–1588 (doi:10.1111/j.1365-294X.2007.03683.x) [DOI] [PubMed] [Google Scholar]
- MacNally R., Timewell C. A. R.2005Resource availability controls bird-assemblage composition through interspecific aggression. Auk 122, 1097–1111 (doi:10.1642/0004-8038(2005)122[1097:RACBCT]2.0.CO;2) [Google Scholar]
- Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J.2000Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- Nason J. D., Herre E. A., Hamrick J. L.1998The breeding structure of a tropical keystone plant resource. Nature 391, 685–687 (doi:10.1038/35607) [Google Scholar]
- Nicholas F. W., Nicholas J. M.2002Charles Darwin in Australia Cambridge, UK: Cambridge University Press [Google Scholar]
- Oleson J. M., Jordano P.2002Geographic patterns in plant–pollinator mutualistic networks. Ecology 83, 2416–2424 [Google Scholar]
- Ollerton J., Johnson S. D., Hingston A. B.2006Geographical variation in diversity and specificity of pollination systems. In Plant–pollinator interactions from specialisation to generalisation (eds Waser N. M., Ollerton J.). London, UK: The University of Chicago Press [Google Scholar]
- Pasquet R. S., Peltier A., Hufford M. B., Oudin E., Saulnier J., Paul L., Knudsen J. T., Herren H. R., Gepts P.2008Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl Acad. Sci. 105, 13 456–13 461 (doi:10.1073/pnas.0806040105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton D. C., Ford H. A.1977Pollination by birds of native plants in South Australia. Emu 77, 73–85 [Google Scholar]
- Peakall R.1987Genetic systems of Australian terrestrial orchids. Unpublished PhD thesis, The University of Western Australia
- Peakall R.1989A new technique for monitoring pollen flow in orchids. Oecologia 79, 361–365 (doi:10.1007/BF00384315) [DOI] [PubMed] [Google Scholar]
- Peakall R.1990Responses of male Zaspilothynnus trilobatus Turner wasps to females and the sexually deceptive orchid it pollinates. Funct. Ecol. 4, 159–167 (doi:10.2307/2389335) [Google Scholar]
- Phillips R. D., Faast R., Bower C. C., Brown G. R., Peakall R.2009Implications of pollination by food and sexual deception on pollinator specificity, fruit set, population genetics and conservation of Caladenia. Aust. J. Bot. 57, 259–275 (doi:10.1071/BT08157) [Google Scholar]
- Proctor M., Yeo P., Lack A.1996The natural history of pollination Portland, OR: Timber Press [Google Scholar]
- Pyke G. H.1981Honeyeater foraging: a test of optimal foraging theory. Anim. Behav. 29, 878–888 (doi:10.1016/S0003-3472(81)80024-3) [Google Scholar]
- Pyke G. H., Christy M., Major R. E.1996Territoriality in honeyeaters: reviewing the concept and evaluating available information. Aust. J. Zool. 44, 297–317 (doi:10.1071/ZO9960297) [Google Scholar]
- Ramsey M. W.1988Differences in pollinator effectiveness of birds and insects visiting Banksia menziesii (Proteaceae). Oecologia 76, 119–124 [DOI] [PubMed] [Google Scholar]
- Ramsey M., Vaughton G.1991Self-incompatibility, protandry, pollen production and pollen longevity in Banksia menziesii. Aust. J. Bot. 39, 497–504 (doi:10.1071/BT9910497) [Google Scholar]
- Rye B. L.2009A reduced circumscription of Balaustion and description of the new genus Cheyniana (Myrtaceae: Chamelaucieae). Nuytsia 19, 129–148 [Google Scholar]
- Saffer V. M., Brown E. M., Hopper S. D., Wills R. T., Burbidge A. H., Majer J. D.2000Pollination and revegetation in the south west of Western Australia. West. Aust. Nat. 22, 221–279 [Google Scholar]
- Schatral A.1996Floral predators, pollinators and seed set in Western Australian species of the genus Hibbertia (Dilleniaceae). In Gondwanan heritage: past, present and future of the Western Australian biota (eds Hopper S. D., Chappill J. A., Harvey M. S., George A. S.). Sydney, NSW: Surrey Beatty & Sons [Google Scholar]
- Steffan-Dewenter I., Tscharntke T.2000Resource overlap and possible competition between honey bees and wild bees in central Europe. Oecologia 122, 288–296 (doi:10.1007/s004420050034) [DOI] [PubMed] [Google Scholar]
- Stiles F. G.1981Geographical aspects of bird–flower coevolution, with particular reference to Central America. Ann. Mo. Bot. Gard. 68, 323–351 (doi:10.2307/2398801) [Google Scholar]
- Stock W. D., Pate J. S., Kuo J., Hansen A. P.1989Resource control of seed set in Banksia laricina C. Gardner (Proteaceae). Funct. Ecol. 3, 453–460 (doi:10.2307/2389619) [Google Scholar]
- Stoutamire W. P.1983Wasp-pollinated species of Caladenia (Orchidaceae) in South-western Australia. Aust. J. Bot. 31, 383–394 (doi:10.1071/BT9830383) [Google Scholar]
- Thomas C. D., et al. 2004Extinction risk from climate change. Nature 427, 145–148 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- Visscher P. K., Seeley T. D.1982Foraging strategy of honeybee colonies in a temperate deciduous forest. Ecology 63, 1790–1801 (doi:10.2307/1940121) [Google Scholar]
- Waser N. M., Chittka L., Price M. V., Williams N. M., Ollerton J.1996Generalisation in pollination systems, and why it matters. Ecology 77, 1043–1060 (doi:10.2307/2265575) [Google Scholar]
- Whelan R. J., Burbidge A. H.1980Flowering phenology, seed set and bird pollination of five Western Australian Banksia species. Aust. J. Ecol. 5, 1–7 (doi:10.1111/j.1442-9993.1980.tb01225.x) [Google Scholar]
- Wooller R. D., Russell E. M., Renfree M. B., Towers P. A.1983A comparison of seasonal changes in the pollen loads of nectarivorous marsupials and birds. Aust. Wildl. Res. 10, 311–317 (doi:10.1071/WR9830311) [Google Scholar]
- Wooller R. D., Richardson K. C., Garavanta C. A. N., Saffer V. M., Anthony C., Wooller S. J.1998The influence of annual rainfall upon capture rates of a nectar-dependent marsupial. Wildl. Res. 25, 165–169 (doi:10.1071/WR97089) [Google Scholar]
- Yates C. J., Ladd P. G.2004Breeding system, pollination and demography in the rare granite endemic shrub Verticordia staminosa ssp. staminosa in south-west Western Australia. Aust. Ecol. 29, 189–200 (doi:10.1111/j.1442-9993.2004.01336.x) [Google Scholar]
- Yates C. J., Hopper S. D., Taplin R. H.2005Native insect flower visitor diversity and feral honeybees on Jarrah (Eucalyptus marginata) in Kings Park, an urban bushland remnant. J. R. Soc. West. Aust. 88, 147–153 [Google Scholar]
- Yates C. J., Coates D. J., Elliott C., Byrne M.2007aComposition of the pollinator community, pollination and the mating system for a shrub in fragments of species rich kwongan in south-west Western Australia. Biodivers. Conserv. 16, 1379–1395 (doi:10.1007/s10531-006-6736-y) [Google Scholar]
- Yates C. J., Elliott C., Byrne M., Coates D. J., Fairman R.2007bSeed production, germinability and seedling growth for a bird pollinated shrub in fragments of kwongan in south-west Australia. Biol. Conserv. 136, 306–314 (doi:10.1016/j.biocon.2006.12.003) [Google Scholar]