Abstract
A major innovation in angiosperms is the recruitment of animal pollinators as a means to enhance the efficiency and specificity of pollen transfer. The implementation of this reproductive strategy involved the rapid and presumably coordinated evolution of multiple floral traits. A major question concerns the molecular identity of the genetic polymorphisms that specify the phenotypic differences between distinct pollination syndromes. Here, we report on our work with Petunia, an attractive model system for quantitative plant genetics and genomics. From interspecific crosses, we obtained F2 plants that differed in the length of the floral tube or the size of the limb. We used these plants to study the behaviour of the hawkmoth pollinator, Manduca sexta. Plants with larger limbs were preferentially visited, consistent with the notion that flower size affects visibility under low light conditions. The moths also displayed an innate preference for shorter tubes. However, in those cases that flowers with long tubes were chosen, the animals fed for equal time. Thus, the perception of tube length may help the moths, early on, to avoid those plants that are more difficult to handle.
Keywords: Petunia, hawkmoth, floral morphology, pollination syndrome, QTL
1. Introduction
Reproductive isolation of plants often involves processes that occur before fertilization or even before pollen deposition. Such barriers can be overcome by artificial crosses leading to viable offspring from parents that differ vastly in developmental, morphological and physiological characters. For that reason, the genetic basis of natural variation can be studied in a much wider variety of traits in plants than is possible in animal systems. Of particular interest is the rapid divergence in floral characteristics present in the angiosperms. Pollination syndromes are defined as ensembles of floral traits that are optimized to attract specific pollinators (Proctor et al. 1996). They include floral characteristics such as petal colour, scent, nectar reward and morphology. For instance, flowers pollinated by nocturnal hawkmoths tend to be white, UV absorbing, strongly fragrant and to have long and narrow tubes. In contrast, flowers visited by hummingbirds are often red, non-fragrant, with reflexed petals and an exserted stigma.
Pollination syndromes may differ in some or all of the traits mentioned above and the difference in each of these traits is likely to be caused by polymorphisms in multiple genes. Thus, the transitions between pollination syndromes are genetically complex. Yet, shifts in pollination syndromes have occurred frequently. In the Solanaceae, for instance, bird-type pollination syndromes evolved at least 10 times (Knapp 2010). What is the genetic basis of such transitions, how many genes are involved and what is their identity? Are the genetic changes mostly in cis- or trans-acting regulatory factors, or also in structural genes?
Unfortunately, the major plant model species, Arabidopsis, maize, rice, Antirrhinum, are self-fertilizing, wind-pollinated or belong to taxa with a single pollinator. Recent advances in high throughput DNA sequencing and quantitative genetics offer the prospect to study the molecular basis of well-defined pollination syndromes in non-model species. We have selected the solanaceous genus Petunia (2n = 14) for our studies on the genetics of pollination syndromes. Petunia is native to South America and has been divided into up to 16 species, but the phylogeny of the genus has not yet been rigorously resolved (Ando et al. 2005; Kulcheski et al. 2006; Lorenz-Lemke et al. 2006, Stehmann et al. 2009). Most members of the genus present characteristics of bee-pollinated species (typical representatives: P. integrifolia or P. inflata). The recently discovered P. exserta displays a typical hummingbird syndrome. P. axillaris has been divided into three subspecies: P. axillaris axillaris, P. axillaris parodii and P. axillaris subandina (Ando & Hashimoto 1996). All three subspecies are pollinated by hawkmoths and have white flowers with long tubes, produce large amount of nectar and are heavily scented at night (Ando et al. 1995a,b, 2000). Thus, the genus Petunia includes species with well-differentiated pollination syndromes.
Petunia has a long history as a genetic model system (Gerats & Vandenbussche 2005). Genetic maps, transposon mutagenesis, transgenic technology and transcriptome data are available (Vandenbussche et al. 2008). Garden petunias have been intensely studied for many horticultural traits. This is especially apparent in the area of flower colour. All the enzymes of anthocyanin biosynthesis have been characterized and a number of regulatory genes are known. In the early 1980s, Wijsman (1982) performed interspecific crosses between bee-pollinated P. integrifolia and moth-pollinated P. axillaris. He identified three loci that conditioned the difference in petal colour. One of them, AN2, was subsequently cloned and shown to encode a myb-type transcription factor (Quattrocchio et al. 1999). Interestingly, all P. axillaris accessions contain AN2 genes with knock-out mutations in their coding region. Within a sample of 35 accessions, five distinct such mutations were found, suggesting that the loss of colour happened multiple times (Galliot et al. 2006; Hoballah et al. 2007). A transgenic P. axillaris with the active AN2 gene from P. integrifolia had a pink corolla indicating reactivation of the anthocyanin pathway. While these plants were indistinguishable from P. axillaris with respect to fragrance, nectar production and flower morphology, the pink colour caused a major shift in pollinator preference, from hawkmoths towards bees. The C-terminus of the AN2 protein contains ancient polymorphisms that separate the two species. Domain swapping and gene transfer studies strongly suggest that these ancient polymorphisms in the C-terminus are functional and that the N-terminal AN2 loss-of-function mutations are not the initial event in the transition from bee to hawkmoth pollination.
Here, we describe the genetic segregation of flower morphology in crosses between P. axillaris axillaris and P. axillaris parodii. We bred plants that differed in either the size of the corolla limb or the length of the corolla tube. Such plants were tested in behavioural assays for their effect on hawkmoth visitation.
2. Material and methods
(a). Plant material
P. axillaris parodii S7 and P. axillaris axillaris N were obtained from the Department of Genetics of the Free University, Amsterdam. These lines were maintained by selfing for many generations. P. axillaris parodii S7 is referred to as P. parodii by Wijsman (1982). Its flowers are white, UV reflecting, have strong nocturnal fragrance caused by benzenoids. It presents didynamous stamens (two long, two medium and one short stamen), a long corolla tube and a small limb width.
Flowers of P. axillaris axillaris N are white, UV absorbing, have strong nocturnal fragrance caused by benzenoids. It has didynamous stamens, a shorter corolla tube and a larger limb than P. axillaris parodii (Galliot et al. 2006).
An F2 mapping population (n = 192) was produced from a single F1 progeny of a cross between P. axillaris parodii S7 (as male) and P. axillaris axillaris N (as female). The reciprocal cross yielded phenotypically indistinguishable F1 plants. Plants were grown in trays, regularly moved around the glasshouse to minimize environmental effects. Individual lines were vegetatively propagated by cutting.
(b). Data capture and trait analysis
Limb surface data were extracted from front view pictures of fully opened (after anthesis) flowers, so the picture taken represents the projection of what the pollinator sees when approaching the flower. Five flowers per plant were sampled. Each image was processed using imageJ (Abramoff et al. 2004). Surface values were extracted from pictures converted into binary files. D1 measurements were taken along the main vein of the dorsal petal of the flowers. Statistical analysis was performed using SPSS (Rel. 11.0.1. 2001. Chicago: SPSS Inc).
(c). QTL analysis
A linkage map was constructed with 45 CAPS and SSR markers. Linkage analysis was performed using QTX (Manly et al. 2001; kosambi mapping function). The seven linkage groups gave a total length of 232, 5 cM.
Composite interval mapping (CIM) was performed for each trait using qGene 4.2.3 (Roby & James 2008), using the implementation of the least square methods (CIM-LS), with a window of 1 cM. The distribution of traits analysed was tested for a normal distribution in SPSS using a Kolmogorov–Smirnov test. Permutation tests of 1000 replicates of CIM-LS were used to estimate significance thresholds for each QTL with a large effect. Percentage of variation explained (PVEs) by the underlying QTL was derived from the R-square values.
(d). Behavioural experiments
Female M. sexta, L. strain ‘Yamamoto’ were ordered from the North Carolina State University Insectary as pupae. They were stored until hatching at 24–26°C, 65 per cent humidity, 16 h light. Once hatched, the naive moths were used within 2–5 days. The moths were released at dusk into a cage (144 × 248 × 368 cm), built inside a greenhouse harbouring numerous petunia plants. The entrance site was varied between moths, to prevent bias. Each moth was allowed to fly for 5 min and the number and duration of feeding events were recorded. A feeding event begins when the proboscis is clearly inserted into the flower tube; it finishes when the insect removes it. As each event was recorded separately, we could also count the first choice made, as a measure of innate preference. We carried out the observations until 30 hawkmoths made a choice. If a hawkmoth never fed on any flower, it was discarded.
For choice experiments, extreme F2 lines were paired with a parent. Pairs were always matched for UV reflectance. The plants were semi-randomly arranged in a rectangular pattern. The mean number of feeding events per moth was then calculated with SPSS. The average time spent feeding and the average duration of one single feeding event were treated the same way. If normality and homogeneity of variance were respected, we used the T-test. If one of these two prerequisites was not respected, a non-parametric Mann–Whitney test was employed. For the first choice, we used the χ-square test. All statistical analyses were carried out in SPSS software.
3. Results
(a). Flower morphology of P. axillaris subspecies and attributes of associated hawkmoth pollinators
The subspecies P. axillaris, subsp. axillaris and subsp. parodii are very similar in all traits relevant for pollinator behaviour except flower morphology. P. axillaris parodii has a longer tube but a smaller surface area of the limb (Ando et al. 1994). P. axillaris parodii and P. axillaris axillaris display typical hawkmoth pollination syndromes, and field observations confirm that both subspecies are indeed pollinated by hawkmoths (Ando et al. 2001). However, it should be noted that P. axillaris axillaris is also visited by non-cognate diurnal pollinators (Hoballah et al. 2005, 2007), in line with the claim that pollination syndromes are often less specific than expected (Ollerton et al. 2009).
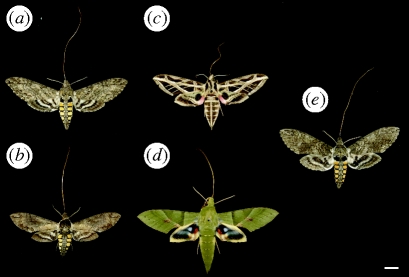
The difference in the length of the tube between the two subspecies suggests that they are visited by hawkmoth species with proboscides of corresponding lengths. We collected data on nocturnal visitors of P. axillaris axillaris in Uruguay and found species with proboscides between 41 and 73 mm (figure 1). Two species from the genus Manduca have a proboscis that is longer than the tube of P. axillaris axillaris, which allows them to pollinate while hovering. Members of the Eumorpha genus had a shorter proboscis, forcing them to land on the flower while drinking nectar. We also observed hawkmoths pollinating P. axillaris parodii, but were unable to collect them. Because they were hovering, we assume that they had proboscides at least as long as the P. axillaris parodii floral tubes. The two Manduca species observed on P. axillaris axillaris (figure 1) have a sufficiently long proboscis to access nectar of wild P. axillaris parodii.
Figure 1.
Hawkmoths species pollinating P. axillaris axillaris. All specimen presented here are natural pollinators of P. axillaris. (a) Manduca sexta, collected in Rivera, Uruguay. Proboscis length: 73 mm. (b) M. diffissa, from Carmelo, Uruguay. Proboscis length: 66 mm. (c) Eumorpha vitis, from Carmelo, Uruguay. Proboscis length: 41 mm. (d) E. labruscae, from Carmelo, Uruguay. Proboscis length: 58 mm. (e) M. sexta, strain from NCSU insectary. Proboscis length: 70 mm; scale bar, 10 mm.
(b). Genetic dissection of morphological characters in P. axillaris
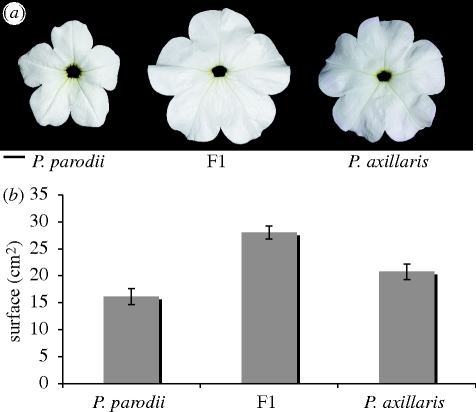
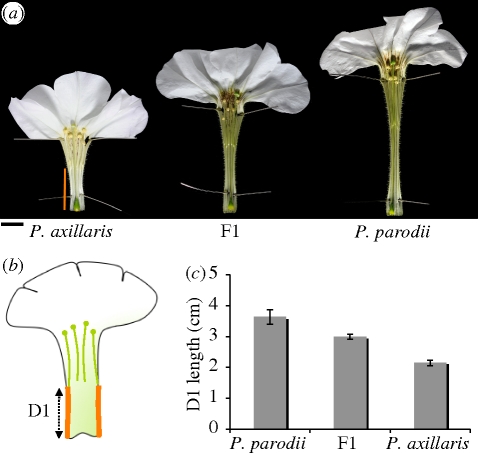
Crosses between P. axillaris axillaris and P. axillaris parodii yielded an F1 generation of homogeneous appearance in all characters tested. The limb area of these plants exceeds the value of the largest parent (P. axillaris axillaris) by 35 per cent (figure 2). By contrast, the plants show a D1 value intermediate to that of parents (figure 3). Thus, genetically, these two morphological traits behave differently.
Figure 2.
Limb surface variation between Petunia species. (a) Front view of flowers of P. axillaris, P. parodii and F1. Scale bar, 1 cm. (b) Surface quantification of limb surface for P. axillaris, P. axillaris parodii and F1. F1 individuals are significantly larger.
Figure 3.
Variation in tube length between Petunia species. (a) Opened flowers of P. axillaris, P. parodii and F1. The orange bar represents D1, as indicated in (b). Scale bar, 1 cm. (b) Drawing of an opened flower. D1 is measured from the bottom of the flower tube up to where the fusion between the petal and the anther filaments stops. (c) Quantification of D1 length for P. axillaris, P. parodii and the F1, showing an intermediate phenotype.
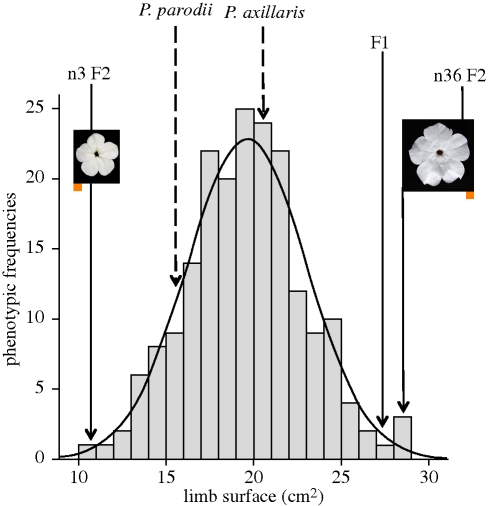
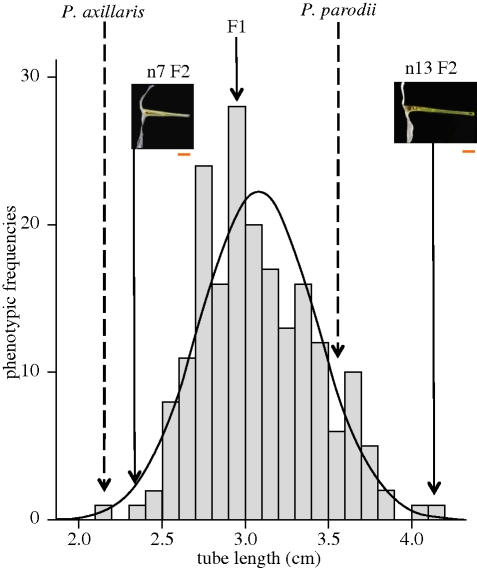
A population of 192 F2 plants was produced by self-fertilization from a single F1 plant. These plants segregated for both tube length (D1) and limb surface area. The segregation of the limb surface trait followed a normal distribution, which suggests the involvement of multiple genetic loci. This trait shows strong transgressive behaviour, with some individuals exhibiting substantially larger or smaller values than the parents (figure 4). Tube length also segregates according to a normal distribution, which similarly suggests the involvement of multiple loci (figure 5). This trait presents modest transgressive behaviour, with a few F2 individuals that exceed the value of the longest parent.
Figure 4.
Segregation of limb surface in the F2 population. Parental phenotypes are P. parodii and P. axillaris. n3 F2 and n36 F2 are, respectively, the smallest and largest phenotypes observed in the F2. The orange square underneath the pictures of n3 F2 and n36 F2 corresponds to 1 cm2. The values fit a normal distribution as represented by the solid line (K–S test Z = 0.494).
Figure 5.
Segregation of the D1 trait. Parental phenotypes are P. axillaris parodii and P. axillaris. n7 F2 and n13 F2 are, respectively, the smallest and the largest phenotypes observed in the F2. The scale bar underneath the pictures of n7 F2 and n13 F2 corresponds to 1 cm. The values fit a normal distribution (K–S test Z = 0.911) as represented by the plain line.
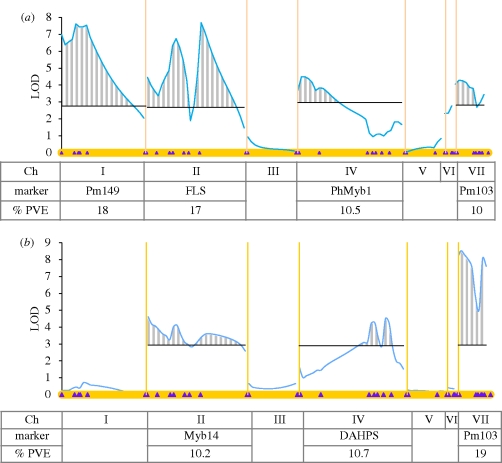
A QTL analysis for limb surface area and D1 length was performed using a genetic map based on a combination of CAPS and SSR markers (figure 6). Four QTL for limb surface were identified on chromosomes I, II, IV and VII with significant effects explaining 18, 17, 10 and 10 per cent of the total parental variation, respectively. The values for the additive effects suggest strong dominance for the axillaris alleles on chromosomes 1 and 4, whereas on chromosomes 2 and 7 the parodii loci are dominant. The data do not provide a convincing explanation for the strong transgression observed for this trait. In the case of D1 tube length, three significant QTL on chromosomes 2, 4 and 7 explain 10, 10 and 20 per cent of the trait variation, respectively. The statistical analysis suggests that the alleles are almost completely codominant.
Figure 6.
QTL analysis (composite interval mapping) of limb surface and D1 length. (a) Limb surface and (b) tube length (D1). The seven linkage group are represented horizontally (as the X axis), and separated by yellow vertical lines. Purple diamonds indicate marker positions along each linkage groups. LOD scores for the position of the QTLs are plotted as the blue line. LOD significance thresholds (α = 0.05) are indicated by single dark horizontal lines. Significant QTLs regions detected by CIM (above LOD thresholds) are represented with a grey stripy pattern. Table underneath indicates chromosome numbers (Ch), name of the marker with the highest percentage of phenotypic variation (%PVE; p < 0.001 for all values).
(c). Pollinator preferences
The QTL analysis (figure 6) indicates that at least three or four loci contribute to the morphological differences between the two accessions. Owing to the complex genetic constitution of these traits, introgression and pyramiding of individual traits to obtain immortalized lines will be a long and arduous process. Fortunately, even in the absence of such genetic material, meaningful behavioural experiments can be performed. Here, we address the question to what extent each of the two characters, limb size and tube length, conditions preference of the hawkmoth pollinator M. sexta. Limb size is likely to influence the visibility of the plant and thus affect visitation rates. By contrast, differences in tube length affect the accessibility of the reward and thus are likely to enhance pollinator specificity. For instance, spurs of Angraecum orchids that were as long as the proboscides of their pollinators prevented swing hovering, a protective behaviour against ambush predators (Wasserthal 1997; Whittall & Hodges 2007). By contrast, Herrera (1993) found no correlation between Viola cazorlensis spur length and seed set after pollination by the diurnal hawkmoth Macroglossum stellatarum (Whittall & Hodges 2007).
We paired selected F2 plants with either parent such that they were essentially identical in all phenotypic traits except either limb surface or tube length. The occurrence of strong transgression, especially in limb surface, made it possible to match plants that exceeded the parental difference for these two traits. Individual plants were propagated by cutting, thus yielding genetically identical material. Such pairs of plants were exposed to naive hawkmoths under controlled conditions in a glasshouse. Each line had the same number of plants and flowers, and moths were allowed to fly freely in the cage for 5 min. Their foraging and feeding behaviours were recorded (see §2).
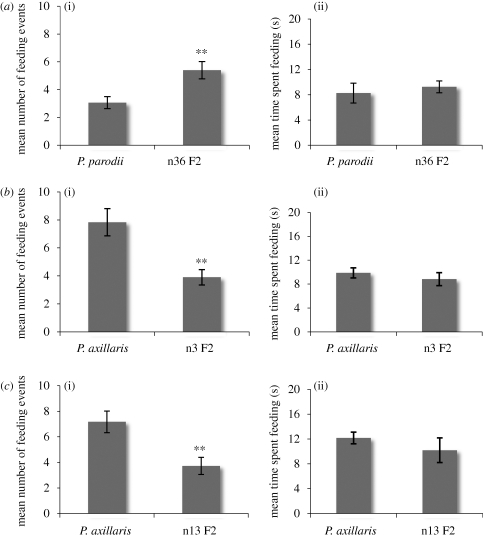
F2 plant n36 has a limb surface area that is 57 per cent larger than that of P. axillaris parodii. When these plants were exposed to M. sexta, a highly significant preference for the plant with the larger projected surface area was observed (figure 7a(i)). By contrast, the moths spent the same time feeding on each flower (figure 7a(ii)). Comparable results were obtained when a plant (n3 F2) with small surface area was compared with the other parent, P. axillaris axillaris (figure 7b). Thus, we conclude that the pollinators displayed a preference for flowers with larger limbs, even though they presumably removed equal amounts of nectar from either flower type.
Figure 7.
Behavioural responses of Manduca sexta to different morphologies of flowers. (a) M. sexta prefer larger flowers. P. parodii compared to the n36 F2 selected for large limb: (i) mean number of feeding events per moth during 5 min (T-test: p = 0.003); (ii) mean duration of a single feeding event in seconds (T-test: p = 0.590). (b) M. sexta prefer larger flowers. P. axillaris compared to the n3 F2 selected for small limb: (i) mean number of feeding events per moth during 5 min (Mann–Whitney U-test: p = 0.003); (ii) mean duration of a single feeding event in seconds (T-test: p = 0.444). (c) M. sexta prefer shorter floral tubes. P. axillaris compared to the n13 F2 selected for long tube: (i) mean number of feeding events per moth during 5 min (T-test: p = 0.002); (ii) mean duration of a single feeding event in seconds (Mann–Whitney U-test: p = 0.099). **, significant at p < 0.005.
Similar experiments were performed with plants that differed in tube length. P. axillaris axillaris (D1 = 2.15 cm, total tube length = 4.0 cm) was matched with F2 plants (n13 F2; D1 = 4.12 cm, total tube length = 7.3 cm). The moths fed significantly more often on P. axillaris axillaris than on the longer-tubed F2 (figure 7c(i)). The first feeding event was significantly in favour of P. axillaris axillaris (χ-square test: χ-square = 4.17, p = 0.041, N = 29; data not shown) suggesting an innate preference for shorter tubes. Thus, it appears that the moths obtain information on tube length while approaching the flower, that is, before they extend their proboscis.
The M. sexta moths used in these experiments had a mean proboscis length of 65.3 mm (s.d. ± 0.5; N = 28; figure 1e), which forced them to land on the limb of the n13 F2 flower, while they hovered during feeding on the parent. Interestingly, the moths spent the same time feeding on each flower (figure 7c(ii)). Thus, once the moths had chosen a flower, they completed the feeding process regardless of tube length. We hypothesize that the early recognition of the length of the tube allows the moths to avoid unfavourable morphologies before expending considerable effort.
4. Conclusion and outlook
Shifts in pollination syndromes have occurred many times. In the Solanaceae alone, bird pollination evolved at least 10 times (Knapp 2010). Such shifts require changes in multiple traits, including flower colour, morphology, scent and nectar production, each change probably involving multiple genes. How many genes needed to change, what type of genes were they, mostly transcription factors or also structural genes? In which order did they change? Were the same genes targeted over and over again in different taxa? How much introgression occurred? QTL analysis can be used to dissect the genetic basis of complex traits and answer such questions (Doebley et al. 2006). In their pioneering work, Schemske & Bradshaw (1999) showed that in Mimulus, the pollinator shift from bees to birds involved a limited number of loci of medium to major effect. Reciprocal introgressions of the YUP locus allowed them to study the effect of colour in an otherwise isogenic background (Bradshaw & Schemske 2003). Similar studies are in progress in other plant taxa. In Petunia, the transcription factor AN2 is active in the purple bee-pollinated P. integrifolia, but has acquired knock-out mutations in all moth-pollinated P. axillaris accessions (Quattrocchio et al. 1999; Hoballah et al. 2007). The phylogeny of the genus Petunia is not well resolved and it is not clear which pollination syndrome is ancestral. It is tempting to use potential speciation genes to infer possible evolutionary relationships between pollination syndromes. Based on the AN2 coding sequences, it seems plausible that moth pollination is derived from bee pollination. As the molecular data also indicate that the loss of AN2 function was not the initial speciation event, one can speculate that the ancestral species were phenotypically similar to P. integrifolia. It then evolved a longer tube, perhaps attracting diurnal moths, and subsequently lost its colour. Obviously, basing such speculations on a single gene is adventurous, but it demonstrates the value of functional polymorphisms to infer phylogenies.
The availability of increasingly detailed data on the biochemistry and genetics of flower colour and fragrance production provides good opportunities to isolate the major genes underlying shifts in these characters. For other traits, such as nectar production and morphology, further advances in high throughput genomics technologies will be essential. Yet, even without previous knowledge of gene identities, information on ecology and evolution of pollination syndromes can be obtained, as was demonstrated for the YUP locus in Mimulus (Bradshaw & Schemske 2003).
In the work presented here, we have dissected the differences in morphology between two closely related and interbreeding P. axillaris accessions. A population of 192 F2 plants was scored for differences in limb surface and tube length. Even though the differences in both traits are genetically complex, we found F2 plants that differed in either limb size or tube length, but were virtually indistinguishable in all other traits. This appears to apply to other characters as well (Bradshaw & Schemske 2003; our unpublished results). If linkage between traits can be broken in a few generations, the genes involved are neither tightly linked nor highly pleiotropic. This suggests that strong selection is required to maintain specific pollination syndromes.
Flower morphology affects the behaviour of pollinators both by advertising for reward and by restricting access. We showed that under controlled greenhouse conditions, M. sexta moths prefer flowers with a larger limb size, which may simply reflect better visibility under low light conditions. The effects of differences in tube length are unexpected and raise interesting questions about the feeding strategy of the insects. Further experiments, especially under field conditions, will be needed to answer such questions. Similar studies with other traits and in different taxonomic groups will help to further our understanding of the genetics, ecology and evolution of pollination syndromes.
Acknowledgements
We thank our colleagues for their valuable contributions and thought-provoking discussions. Christopher Ball and Rebecca Alder took expert care of those thousands of plants. We are grateful to Marc Gremillon for phenotypic measurements and professional photography. Work in the authors’ laboratory was supported by the Swiss National Center of Competence in Research ‘Plant Survival’ and the University of Bern.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Abramoff M. D., Magelhaes P. J., Ram S. J.2004Image processing with ImageJ. Biophoton. Int. 11, 36–42 [Google Scholar]
- Ando T., Hashimoto G.1996A new Brazilian species of Petunia (Solanaceae) from interior Santa Catarina and Rio Grande do Sul, Brazil. Brittonia 48, 217–223 (doi:10.2307/2807818) [Google Scholar]
- Ando T., Iida S., Kokubun H., Ueda Y., Marchesi E.1994Distribution of intraspecific taxa of Petunia axillaris (Solanaceae) in Uruguay as revealed by discriminant analysis. Acta Phytotax. Geobot. 45, 95–109 [Google Scholar]
- Ando T., Iida S., Kokubun H., Ueda Y., Marchesi E.1995aDistribution of Petunia axillaris sensu lato in Uruguay as revealed by discriminant analysis of the live plants. J. Jpn. Soc. Horticul. Sci. 64, 381–391 [Google Scholar]
- Ando T., Kurata M., Sasaki S., Ueda Y., Hashimoto G., Marchesi E.1995bComparative morphological studies on infraspecific taxa of Petunia integrifolia (Hook.) Schinz et Thell. (Solanaceae). J. Jpn. Bot. 70, 205–217 [Google Scholar]
- Ando T., et al. 2000Differences in the floral anthocyanin content of red petunias and Petunia exserta. Phytochemistry 54, 495–501 (doi:10.1016/S0031-9422(00)00113-8) [DOI] [PubMed] [Google Scholar]
- Ando T., Nomura M., Tsukahara J., Watanabe H., Kokubun H., Tsukamoto T., Hashimoto G., Marchesi E., Kitching I. J.2001Reproductive isolation in a native population of Petunia sensu Jussieu (Solanaceae). Ann. Bot. 88, 403–413 (doi:10.1006/anbo.2001.1485) [Google Scholar]
- Ando T., Kokubun H., Watanabe H., Tanaka N., Yukawa T., Hashimoto G., Marchesi E., Suarez E., Basualdo I. L.2005Phylogenetic analysis of Petunia sensu Jussieu (Solanaceae) using chloroplast DNA RFLP. Ann. Bot. 96, 289–297 (doi:10.1093/aob/mci177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw H. D., Schemske D. W.2003Allele substitution at a flower colour locus produces a pollinator shift in monkey flowers. Nature 426, 176–178 (doi:10.1038/nature02106) [DOI] [PubMed] [Google Scholar]
- Doebley J. F., Gaut B. S., Smith B. D.2006The molecular genetics of crop domestication. Cell 127, 1309–1321 (doi:10.1016/j.cell.2006.12.006) [DOI] [PubMed] [Google Scholar]
- Galliot C., Hoballah M. E., Kuhlemeier C., Stuurman J.2006Genetics of flower size and nectar volume in Petunia pollination syndromes. Planta 225, 203–212 (doi:10.1007/s00425-006-0342-9) [DOI] [PubMed] [Google Scholar]
- Gerats T., Vandenbussche M.2005A model system comparative for research: Petunia. Trends Plant Sci. 10, 251–256 (doi:10.1016/j.tplants.2005.03.005) [DOI] [PubMed] [Google Scholar]
- Herrera C. M.1993Selection on floral morphology and environemental determinants of fecundity in an hawk moth pollinated violet. Ecol. Monogr. 63, 251–275 (doi:10.2307/2937101) [Google Scholar]
- Hoballah M. E., Stuurman J., Turlings T. C., Guerin P. M., Connetable S., Kuhlemeier C.2005The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta 222, 141–150 (doi:10.1007/s00425-005-1506-8) [DOI] [PubMed] [Google Scholar]
- Hoballah M. E., Gubitz T., Stuurman J., Broger L., Barone M., Mandel T., Dell'olivo A., Arnold M., Kuhlemeier C.2007Single gene-mediated shift in pollinator attraction in Petunia. Plant Cell 19, 779–790 (doi:10.1105/tpc.106.048694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S.2010On ‘various contrivances’: pollination, phylogeny and flower form in the Solanaceae. Phil. Trans. R. Soc. B 365, 449–460 (doi:10.1098/rstb.2009.0236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulcheski F. R., Muschner V. C., Lorenz-Lemke A. P., Stehmann J. R., Bonatto S. L., Salzano F. M., Freitas L. B.2006Molecular phylogenetic analysis of Petunia Juss. (Solanaceae). Genetica 126, 3–14 (doi:10.1007/s10709-005-1427-2) [DOI] [PubMed] [Google Scholar]
- Lorenz-Lemke A. P., Mader G., Muschner V. C., Stehmann J. R., Bonatto S. L., Salzano F. M., Freitas L. B.2006Diversity and natural hybridization in a highly endemic species of Petunia (Solanaceae): a molecular and ecological analysis. Mol. Ecol. 15, 4487–4497 (doi:10.1111/j.1365-294X.2006.03100.x) [DOI] [PubMed] [Google Scholar]
- Manly K., Cudmore R. H., JR, Meer J. M.2001Map manager QTX, cross-platform software for genetic mapping. Mamm. Genome 12 (doi:10.1007/s00335-001-1016-3) [DOI] [PubMed] [Google Scholar]
- Ollerton J., Alarcon R., Waser N. M., Price M. V., Watts S., Cranmer L., Hingston A., Peter C. I., Rotenberry J.2009A global test of the pollination syndrome hypothesis. Ann. Bot. (Lond.) 103, 1471–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor M., Yeo P., Lack A.(ed.)1996The natural history of pollination London, UK: HarperCollins [Google Scholar]
- Quattrocchio F., Wing J., van der Woude K., Souer E., de Vetten N., Mol J. N. M., Koes R.1999Molecular analysis of the anthocyanin2 gene of Petunia and its role in the evolution of flower color. Plant Cell 11, 1433–1444 (doi:10.2307/3870973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby J., James C. N.2008QGene 4.0, an extensible Java QTL-analysis platform. Bioinformatics 24, 2788–2789 (doi:10.1093/bioinformatics/btn523) [DOI] [PubMed] [Google Scholar]
- Schemske D. W., Bradshaw H. D.1999Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc. Natl Acad. Sci. USA 96, 11 910–11 915 (doi:10.1073/pnas.96.21.11910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehmann J. R., Lorenz-Lemke A. P., Freitas L. B., Semir J.2009The genus Petunia. In Petunia (eds Gerats T., Strommer J.). New York, NY: Springer [Google Scholar]
- Vandenbussche M., et al. 2008Generation of a 3D indexed Petunia insertion database for reverse genetics. Plant J. 54, 1105–1114 (doi:10.1111/j.1365-313X.2008.03482.x) [DOI] [PubMed] [Google Scholar]
- Wasserthal L. T.1997The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Bot. Acta 110, 1–17 [Google Scholar]
- Whittall J. B., Hodges S. A.2007Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–712 (doi:10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- Wijsman H. J. W.1982On the interrelationships of certain species of petunia. 1. Taxonomic notes on the parental species of petunia hybrida. Acta Bot. Neer. 31, 477–490 [Google Scholar]