Abstract
The flora of southern Africa has exceptional species richness and endemism, making it an ideal system for studying the patterns and processes of evolutionary diversification. Using a wealth of recent case studies, I examine the evidence for pollinator-driven diversification in this flora. Pollination systems, which represent available niches for ecological diversification, are characterized in southern Africa by a high level of ecological and evolutionary specialization on the part of plants, and, in some cases, by pollinators as well. These systems are asymmetric, with entire plant guilds commonly specialized for a particular pollinator species or functional type, resulting in obvious convergent floral evolution among guild members. Identified modes of plant lineage diversification involving adaptation to pollinators in these guilds include (i) shifts between pollination systems, (ii) divergent use of the same pollinator, (iii) coevolution, (iv) trait tracking, and (v) floral mimicry of different model species. Microevolutionary studies confirm that pollinator shifts can be precipitated when a plant species encounters a novel pollinator fauna on its range margin, and macroevolutionary studies confirm frequent pollinator shifts associated with lineage diversification. As Darwin first noted, evolutionary specialization for particular pollinators, when resulting in ecological dependency, may increase the risk of plant extinction. I thus also consider the evidence that disturbance provokes pollination failure in some southern African plants with specialized pollination systems.
Keywords: adaptive radiation, coevolution, mutualism, pollination ecotypes, pollination syndrome, speciation
Research in these fields [pollination and dispersal biology] is going to be the most fruitful approach to understanding evolutionary trends in higher plants.
1. Introduction
The discovery that flowers are shaped principally through natural selection by their pollinators was made by Darwin, who had a lifelong interest in plant pollination systems. The two editions of his book on the pollination of orchid flowers (Darwin 1862, 1877) provided a new adaptationist framework for pollination studies (Harder & Johnson 2009). However, evolutionary studies of flowers in the Victorian era were mostly limited to the description of ‘functional morphology’, usually taken uncritically to be evidence for adaptation. Darwin speculated that pollinators may have been instrumental for the angiosperm radiation (Friedman 2009), but further progress on this topic was stalled for almost a century. It was only with the rise of ecology and ‘biosystematics’ in the 1960s, followed by phylogenetics and comparative biology in the late twentieth century, that interest in pollinator-driven diversification in plants really blossomed.
The development of a conceptual model of pollinator-driven diversification can be attributed to Grant and Stebbins (Grant 1949; Grant & Grant 1965; Stebbins 1970). Grant & Grant (1965) recognized that pollinators are distributed in a geographical mosaic, which they called the ‘pollination climate’, and that selection of flowers would therefore differ across a species' range, in some cases leading to pollinator shifts (utilization of different pollinators through modification of floral traits) which precipitate speciation. Grant's studies of the Polemoniaceae, though hampered by the unavailability of a reliable phylogeny at that time, were among the first to consider the radiation of pollination systems in an entire plant family. In an influential paper, Stebbins (1970) recognized five key principles associated with pollinator-driven diversification, including the most effective pollinator principle, the evolution of character syndromes, selection along lines of least resistance, transfer of function via an intermediate stage of double function and reversals of evolutionary trends.
Despite the tidy logic of the Grant–Stebbins model, there is still very little direct supporting evidence for the idea that pollinator shifts are stimulated by a geographical pollinator mosaic. Stebbins (1970) placed the blame for this situation on the tendency for pollination biologists to conduct studies of a single species at a single locality, rather than using a comparative approach. Macroevolutionary studies in which pollination systems are mapped onto phylogenies (Givnish & Sytsma 1997; Weller & Sakai 1999) give valuable insights into the frequency and direction of pollinator shifts, but extinctions and post-speciation range expansion of taxa in these lineages can mean that insights into the actual process of divergence are often limited. An alternative and complementary approach to testing the Grant–Stebbins process is to focus on ecotypes or newly diverged sister taxa in their geographical context (Herrera et al. 2006), specifically to determine whether these fulfil the requirements of a trait–environment correlation between flowers and pollinators, corresponding selection acting on floral traits and an underlying geographical mosaic of pollinators (Johnson 2006).
Although Grant and Stebbins emphasized pollinator shifts as the primary mode of diversification, there are several other possible processes that could be involved. Stebbins (1970) recognized that many floral radiations involve divergent use of the same pollinator rather than shifts between pollinators. Coevolution (Ehrlich & Raven 1964; Thompson 1994) may apply in cases where reciprocally specialized plants and pollinators influence each other's evolution. Furthermore, non-rewarding species that do not influence the evolution of pollinator traits may nevertheless track changes in pollinators that arise from coevolution with other plant species (Anderson & Johnson 2009). It is thus important to consider the strength of evidence for several alternative modes of diversification (Anderson & Johnson 2009; Whittall & Hodges 2007).
Southern Africa is one of the global hotspots of plant diversity and endemism, and thus an ideal natural laboratory for advancing our understanding of evolutionary diversification in plants (cf. Linder 2003). The two main centres of diversification in this region are the Greater Cape Floristic Region (including the Cape and Succulent Karoo) and the Drakensberg mountains. There is now good macroevolutionary evidence that much of the speciation in this region has been a consequence of ecological shifts (identified as a difference in habitat or pollinator utilization between sister taxa). A recent analysis of 188 sister species pairs representing eight southern African plant clades showed that speciation was associated with ecological shifts in 80 per cent of these pairs (Van der Niet & Johnson 2009). This is a minimum estimate since data were not available for all ecological traits. The frequency of different kinds of ecological shifts did not differ between the species-rich Cape and the rest of southern Africa, suggesting that the main drivers of speciation, e.g. general habitat, pollinators and climate, could be the same across the subcontinent (Van der Niet & Johnson 2009). There are few comparable studies of sister taxa from other floras in the world (but see Price & Wagner 2004), but the picture emerging from these and other lines of evidence is that ecological shifts are the most important driver of diversification in plants at a global level (Schluter 2000; Rieseberg et al. 2002).
Many of the larger southern African clades are characterized by spectacular floral diversity. In particular, these include the following families (ordered by the approximate number of species present in southern Africa): Iridaceae (1050), Ericaceae (804), Apocynaceae (700), Scrophulariaceae s.s. (700), Orchidaceae (470), Hyacinthaceae (410), Asphodelaceae (339), Geraniaceae (290) and Amaryllidaceae (280). Quantitative analyses have confirmed that phenotypic divergence between sister taxa in some of these lineages involved mainly floral traits (Van der Niet & Johnson 2009). Given that lineages that show floral rather than vegetative diversification are likely to have diversified mainly through selection by pollinators, rather than selection from the physical environment or herbivores (Grant 1949; Stebbins 1970), it can be hypothesized that adaptation to pollinators has been a driving force behind the diversification of some of the major elements of the southern African flora (Johnson 1996).
To assess the evidence for pollinator-driven diversification in the southern African flora, I start with an analysis of the diversity and level of specialization in pollination systems in this region. The term pollination system can be ambiguous because it can be used to describe any level within a hierarchy of nested functional levels (e.g. oil-bee pollination is nested within bee pollination, which is nested within insect pollination, which is nested within biotic pollination). In this contribution, I focus on pollination guilds that can be defined as the smallest possible unit of a pollination system, and the ones most relevant for the geographical scale of speciation. Thus, in the example of the oil-bee pollination system, given above, the Cape mountain bee Rediviva gigas, and its oil-producing host plant species (table 1), comprises a distinct pollination guild. I argue that pollination systems, and pollination guilds, in particular, serve as available niches for pollinator-driven diversification, in the same manner that different soil types serve as the environmental template for edaphic-driven diversification.
Table 1.
Specialized pollination guilds documented in southern Africa.
| pollinator(s) of guild | tongue/bill length (mm) | plant species in guild | plant families | habitat, distribution | convergent floral traits | references |
|---|---|---|---|---|---|---|
| tanglewing flies (Nemestrinidae) | ||||||
| Moegistorhynchus longirostris | 40–90 | 20 | Iridaceae, Orchidaceae, Geraniaceae | sandplains, Western Cape lowlands | cream or pink flower colour, nectar guides, very long corolla tube, early summer (September–November) flowering time | Manning & Goldblatt (1997), Johnson & Steiner (1997), Pauw et al. (2009) |
| Stenobasipteron wiedmannii | 19–30 | 19 | Iridaceae, Orchidaceae, Acanthaceae, Balsaminaceae, Gesneriaceae, Lamiaceae | forest patches in eastern region | pale blue-pink flower colour, nectar guides, long corolla tubes, autumn (February–April) flowering time | Goldblatt & Manning (2000), Potgieter & Edwards (2005) |
| Prosoeca ganglbaueri | 20–50 | 20 | Amaryllidaceae, Iridaceae, Orchidaceae, Scrophulariaceae | Drakensberg mountains | pink or cream flower colour, nectar guides, long corolla tube, autumn (January–April) flowering time | Goldblatt & Manning (2000), Anderson & Johnson (2009) |
| Prosoeca peringueyi | 25–40 | 18 | Iridaceae, Geraniaceae | mainly granite soils, central Namaqualand highlands | violet-pink flower colour, nectar guides, long corolla tubes, early spring (July–September) flowering time | Manning & Goldblatt (1996) |
| Prosoeca longipennis | 38–40 | 5 | Iridaceae, Geraniaceae | Southern Cape | autumn (March–April) flowering time | Goldblatt & Manning (2000) |
| Prosoeca sp. | 32–48 | 6 | Iridaceae | dolerite and clay soils, Nieuwoudtville plateau | violet-blue flower colour, spring (August–September) flowering time | Goldblatt & Manning (2000) |
| horseflies (Tabanidae) | ||||||
| Philoliche aethiopica horsefly | 10–25 | 15 | Amaryllidaceae, Iridaceae, Orchidaceae, Scrophulariaceae | eastern region | pink flower colour, long corolla tube, summer (November–March) flowering time | Johnson (2000), Johnson & Morita (2006), S. D. Johnson & S. Morita (2009, unpublished data) |
| Philoliche rostrata | 21–27 | 20 | Iridaceae, Orchidaceae, Geraniaceae | southwestern Cape mountains | pink or cream flower colour, nectar guides, long corolla tube, summer (October–January) flowering time | Goldblatt & Manning (2000) |
| Philoliche gulosa | 18–33 | 13 | Iridaceae, Orchidaceae, Geraniaceae | Western Cape and Namaqualand mountains | pale cream or blue flower colour, nectar guides, long corolla tube, spring (July–November) flowering time | Goldblatt & Manning (2000) |
| bee-flies (Bombyliidae) | ||||||
| Megapalpus capensis | 10 | 6 | Asteraceae, Geraniaceae | Western Cape and Namaqualand | raised dark spots, short corolla tubes | Johnson & Midgley (2001), Ellis & Johnson (2009) |
| oil-bees (Melittidae) | ||||||
| Rediviva peringueyi | N/A | 20 | Orchidaceae, Scrophulariaceae | clay flats, southwestern Cape | greyish green flower colour, open or bisaccate, oil production, no scent or soapy scent, August/September flowering time | K. E. Steiner (2009, unpublished data), Steiner 1989), Pauw (2005, 2006), Whitehead & Steiner (2001), Steiner & Cruz (2009) |
| Rediviva gigas | N/A | 19 | Orchidaceae, Scrophulariaceae | montane, southwestern Cape | yellow to greenish yellow, magenta to pink and white flower colour, open or pouched, oil production, rarely nectar, October–November flowering time | Whitehead & Steiner (2001), Steiner & Cruz (2009), K. E. Steiner (2009, unpublished data), Manning & Goldblatt (2002) |
| Rediviva longimanus | N/A | 8 | Orchidaceae, Scrophulariaceae | Western Cape | greyish magenta, white or cream flower colour, soapy or no scent, August/September | Whitehead & Steiner (2001), K. E. Steiner (2009, unpublished data) |
| Rediviva nitida and R. intermedia | N/A | 11 | Scrophulariaceae | Western and Northern Cape | greyish magenta, pink, yellow, oil production, August/September | Whitehead & Steiner (2001), K. E. Steiner (2009, unpublished data) |
| Rediviva neliana, R. brunnea and R. pallidula | N/A | 29 | Orchidaceae, Scrophulariaceae | Drakensberg mountains | deep floral spurs, oil production, December–March flowering pink to red, white | Steiner & Whitehead (1988), Steiner & Whitehead (1990), Whitehead et al. (2008) |
| Rediviva colorata | N/A | 6 | Orchidaceae | forest patches, Drakensberg mountains | floral sacs or spurs, flower colour white, January–March flowering | Steiner(1989), Whitehead et al. (2008) |
| Rediviva rufocincta | N/A | 5 | Scrophulariaceae | Drakensberg mountains | yellow or white pouched flowers, oil production, December–January flowering | Manning & Brothers (1986), Steiner & Whitehead (1991), Whitehead et al. (2008) |
| spider-hunting wasps (Pompilidae) | ||||||
| Hemipepsis spp. | 10 | 60 | Apocynaceae, Orchidaceae, Hyacinthaceae | summer-rainfall | dull flower colour, open shape, bitter nectar, pungent scent | Ollerton et al. (2003), Shuttleworth & Johnson (2006, 2009a,b) |
| butterflies and moths (Lepidoptera) | ||||||
| mountain pride butterfly (Aeropetes tulbaghia) | 28–35 | 21 | Amaryllidaceae, Iridaceae, Orchidaceae, Crassulaceae, | Cape and Drakensberg mountains | red flower colour, large flowers, narrow corolla tube, late summer flowering time | Johnson & Bond (1994) |
| Convolvulus hawkmoth (Agrius convolvuli) | 90–110 | 25 | Amaryllidaceae, Iridaceae, Orchidaceae, Capparaceae, Rubiaceae | eastern region | pale flowers, very long corolla tube, evening scent | Alexandersson & Johnson (2002), S. D. Johnson & R. Raguso (2009, unpublished data) |
| birds | ||||||
| Cape sugarbird (Promerops cafer) | 30–36 | >20 | Proteaceae | Cape mountains | brush-like inflorescences, unscented, copious dilute nectar | Rebelo et al. (1984) |
| orange-breasted sunbird (Anthobaphes violacea) | 18–23 | >66 | Amaryllidaceae, Ericaceae, Iridaceae | Cape mountains | curved corolla tube, unscented, copious dilute nectar | Rebelo et al. (1984) |
| malachite sunbird (Nectarinia famosa) | 29–34 | >44 | Iridaceae, Amaryllidaceae | Cape mountains | very long curved corolla tube, unscented, copious dilute nectar | Geerts & Pauw (in press) |
| rodents | ||||||
| Acomys and Aethomys rodents | 20 | 30 | Colchicaceae, Hyacinthaceae, Proteaceae | Cape floral region | flower position, scent, anther–stigma distance | Wiens et al. (1983), Johnson et al. (2001), Kleizen et al. (2008) |
In the second part of the paper, I consider the evidence for different modes of pollinator-driven diversification, including pollinator shifts and coevolution, from both micro- and macroevolutionary studies. Finally, I consider the proposition that plants with specialized pollination systems are at risk of extinction owing to pollination failure.
2. Pollination systems as niches
Evolutionary divergence commonly involves an adaptive shift between different ecological niches (Schluter 2000). As pointed out by Stebbins (1970), botanists have historically tended to focus on the physical environment when considering the role of ecology in diversification because of the misconception that fitness can be equated with plant survival. Yet, since lifetime fecundity is often also strongly affected by pollination success, in addition to survival, plants will also adapt to their pollinator environment, resulting in diversification in floral traits (Harder & Johnson 2009). Pollination systems represent ecological niches for diversification, equivalent to soil types and other aspects of the physical environment. However, there is still considerable uncertainty around the application of the niche concept in pollination systems. In one sense, a particular pollinator or functionally equivalent set of pollinators can be considered to comprise a fundamental niche. However, ecological outcomes and selection on floral traits are determined not only by the innate sensory modalities and morphology of particular pollinators, but also by three-way or higher order interactions with the pollinators and other plants. These can result in processes such as conditioned pollinator foraging preferences (cf. Gumbert 2000) and interference in pollen transfer processes (Armbruster et al. 1994) which alter the niche space. The structure of realized niches within a particular community can be observed to some extent from general pollination network studies (Bascompte et al. 2003, 2006; Olesen et al. 2007), but because of logistical constraints these are based mostly on crude observational data linking flowers and their visitors, and thus seldom reveal actual ecological outcomes or the strengths of interactions (Kay & Schemske 2004). Compartment boundaries in these general networks are often fuzzy and contradictive of the apparent specialization in floral traits (Ollerton 1996). Most of our knowledge of pollination system niches has been gained from detailed studies that focus on guilds associated with particular pollinators, rather than the entire network (Feinsinger 1976; Johnson & Bond 1994; Manning & Goldblatt 1996, 1997; Pauw 2007).
Like edaphic niches, pollination system niches are distributed unevenly over space. However, they also have a strong temporal dimension and there are typically many pollination system niches available in a particular habitat. Understanding why evolution in particular plant lineages leads to incorporation into any particular one or more of these niches is one of the great challenges in plant evolutionary biology and has seldom been addressed with the exceptions of the general principles for shifts established by Stebbins (1970), and some recent studies that show that the direction of shifts is strongly determined by the pre-adaptation of existing traits (Armbruster 1993; Whittall & Hodges 2007).
(a). Specialized pollination systems in southern Africa
The existence of extraordinarily specialized pollination systems involving animals such as long-proboscid flies, oil-bees, hawkmoths, butterflies, birds and rodents in southern Africa has been highlighted recently (Johnson & Steiner 2000, 2003). Many of these pollination systems have been characterized in detail down to the level of particular guilds (table 1). These guilds tend to be geographically restricted, often exhibit a short phenophase (typically a few weeks of flowering and pollinator activity) and include many unrelated plants that tend to exhibit convergent floral traits. They are also usually highly asymmetrical with a large number of plants (typically about 20–30) depending on a single pollinator species or small set of pollinators of the same functional type. Thus, the pollinators of these guilds can be considered keystone species. It has been noted that within a given community, there are typically about six guild member species (Goldblatt & Manning 2006), although this number can also vary from a single species to more than a dozen in one community. In long-proboscid fly pollination systems, coexisting guild members usually place pollen on different parts of the body of the shared fly pollinator (Goldblatt & Manning 2000, 2006). Although this phenomenon has been suggested to be an outcome of competitive displacement (Armbruster et al. 1994; Goldblatt & Manning 2000), it can also be explained in some instances by intrinsic differences in floral construction among phylogenetically unrelated guild members. Nevertheless, pollen placement may play a role in determining which species can coexist in the same assemblage (cf. Armbruster et al. 1994). In contradiction to typical niche theory that emphasizes competition, pollination guild members appear to often facilitate each other's reproductive success (Waser & Real 1979; Johnson et al. 2003). This can be due to staggered flowering that supports long-lived pollinators through energetic cross-subsidization (Waser & Real 1979), and the beneficial effects to rare or non-rewarding species when abundant rewarding ‘magnet’ guild members increase the local abundance of pollinators and condition pollinator behaviour (Gumbert & Kunze 2001; Johnson et al. 2003).
There are clear phylogenetic effects on pollination niche occupancy. For example, the large Rediviva oil-bee pollination system in southern Africa is confined to the Scrophulariaceae, Orchidaceae and Iridaceae, while long-proboscid fly pollination systems are mostly concentrated in Iridaceae, Orchidaceae, Geraniaceae and Lamiaceae (table 1). This phylogenetic niche conservatism probably arises because species with particular pollination systems are replicated by non-pollinator-driven diversification and because floral construction in some groups favours particular pollination systems (e.g. presence of spurs in Orchidaceae and long-proboscid fly pollination).
Some plant families in southern Africa tend to be dominated by species with specialized pollination systems. An analysis of existing pollinator data for 114 southern African species of Iridaceae and Orchidaceae revealed that these typically have a single pollinator species (Johnson & Steiner 2003). By contrast, temperate Northern Hemisphere species of Orchidaceae have a median of five pollinator species (Johnson & Steiner 2003). These tend to be in the same clades as the southern African orchids, which suggest that the specialization in this family in southern Africa is not simply a phylogenetic effect, but also reflects the uneven global distribution of certain specialized pollination system niches. In another analysis, Ollerton et al. (2006) calculated a median of two pollinator species for southern African representatives of the Apocynaceae and eight pollinator species for temperate Northern Hemisphere representatives of the same family. Importantly, plant species included in these analyses of Orchidaceae and Apocynaceae were sampled for pollinators with the same effort in southern Africa as elsewhere. In a more recent meta-analysis of studies on 375 species of Iridaceae found in southern Africa, Goldblatt & Manning (2006) concluded that only 2 per cent of species have generalized pollination systems, while 95 per cent of species have specialized ones. For most other plant families in the region there are unfortunately still too few data to assess their levels of pollination system specialization.
(b). Proximate and ultimate causes of specialization
Flowers, even relatively unspecialized ones, are usually visited by only a small subset of the potential flower visitors in a habitat. The proximate (immediate) basis for ecological specialization is easy to understand when flowers have specialized rewards, such as oil, because few insects can use these. In the case of pollination systems involving long-tongued insects or long-billed birds, the main filter to potential flower visitors appears to be accessibility of nectar. That shorter tongued insects often locate and then pierce the corolla of these long-tubed flowers to rob the nectar (Irwin et al. 2001) indicates that floral morphology, rather than advertising traits or reward composition, is the main restriction on the number of legitimate pollinators of these species.
However, cases of plants that have flowers with exposed nectar or pollen rewards and yet have specialized pollination systems are harder to understand. In southern African plants with inconspicuous flowers pollinated solely by spider-hunting wasps, a combination of specific scent signals and bitter-tasting nectar seems to be the proximate basis of specialization (Shuttleworth & Johnson 2009a). Experiments show that even flowers concealed from view attract these wasps by means of scent signals, and that honeybees, which have full access to the nectar, usually reject the nectar on account of its taste (Shuttleworth & Johnson 2009, 2009a). The taste of nectar was also shown to be important for explaining why the open-shaped flowers of a southern African Aloe are pollinated mainly by a suite of short-billed birds (Johnson et al. 2006). Honeybees and sunbirds have full access to the copious nectar produced in an exposed position in flowers of this aloe, but reject it on account of its bitter taste. These studies suggest that floral traits may evolve not only under selection for attractants, but also under selection for filters that inhibit visitation by certain animals.
An alternative proximate explanation for the extreme specialization found in many southern African pollination guilds is simply that there is a lack of pollinator species diversity. Although there is clearly a high diversity of functional pollinator types in southern Africa, the diversity of pollinator species within each type is often very low, and, at the regional level, there may be only one species of a particular functional type available at a particular time of year. A good example is the orange-breasted sunbird that is associated with a large guild of plant species, particularly in the genus Erica, and is the only sunbird that occurs consistently at high altitudes in the Cape mountains (Rebelo et al. 1984). This form of specialization due to a depauperate pollinator species diversity is well known from oceanic islands (McMullen 1990; Olesen & Jordano 2002). While a limited availability of pollinator species diversity within each functional type may be a proximate explanation for some of the specialization observed in southern Africa, an ultimate explanation is still needed for evolutionary specialization to a particular functional type.
Floral specialization is the hallmark of angiosperm diversification, yet we still do not have good theory or data to show why evolution often favours specialized pollination systems over generalized ones. One simple explanation is that adaptation to effective pollinators involves trade-offs. A simple example might be that the evolution of a long corolla tube through adaptation to locally effective long-tongued pollinators incidentally excludes other flower visitors. If these trade-offs are weak, then selection should favour unspecialized pollination systems (Aigner 2001). However, as discussed above, there is now evidence that some floral traits evolve specifically as filters, suggesting that specialization per se may be the target of selection. The most likely explanation for selection for specialization per se is that it improves the efficiency of pollen transfer between conspecific flowers. Pollen transfer is a very wasteful process in which only a small fraction (typically ca 0.1%) of pollen reaches stigmas (Harder & Johnson 2008). Selection for traits that improve the efficiency of this process has been invoked to explain the evolution of floral traits such as pollen packaging (Harder & Johnson 2008). There is evidence that pollinators can influence the efficiency of pollen transfer (Wilson & Thomson 1991; reviewed by Hargreaves et al. 2009), but I am not aware of any study that has tested specifically whether pollen transfer efficiency is enhanced in plants that restrict their number of flower visitors.
(c). Floral syndromes
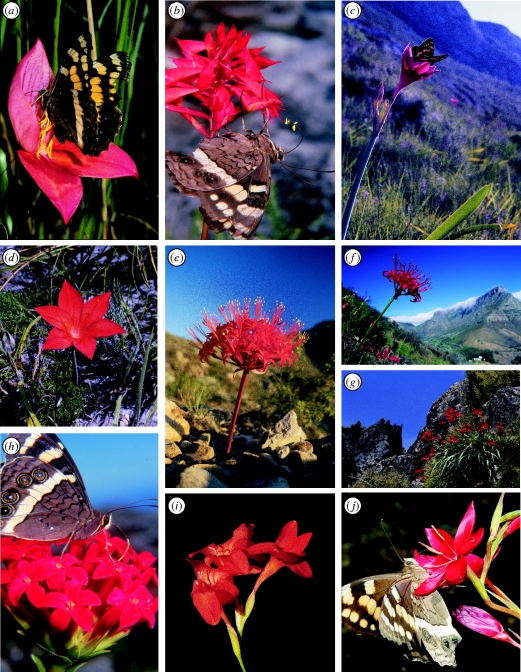
‘Pollination syndromes’, patterns of convergent floral evolution among unrelated plants adapted to the same pollinators (Fenster et al. 2004), were first described for the flora of southern Africa in a classic paper by Vogel (1954). They are most evident among plants that are members of specialized pollination guilds (Johnson & Bond 1994; Manning & Goldblatt 1996, 1997; Pauw 2006). A striking example is the guild of 20 southern African plants representing eight genera and four families that are pollinated by the mountain pride butterfly Aeropetes tulbaghia (figure 1; Johnson & Bond 1997). The large red flowers in this guild can be clearly distinguished from those pollinated by birds by their straight and narrow as opposed to broad and curved corolla tubes, and lack of perches (Johnson & Bond 1994).
Figure 1.
Convergent evolution of large red narrow-tubed flowers in a guild of plants pollinated exclusively by the mountain pride butterfly Aeropetes tulbaghia (after Johnson & Bond 1994). (a) Disa uniflora (Orchidaceae), (b) Disa ferruginea (Orchidaceae), (c) Cyrtanthus elatus (Amaryllidaceae), (d) Cyrtanthus guthrieae (Amaryllidaceae), (e) Brunsvigia marginata (Amaryllidaceae), (f) Nerine sarniensis (Amaryllidaceae), (g) Gladiolus cardinalis (Iridaceae), (h) Crassula coccinea (Crassulaceae), (i) Gladiolus nerinoides (Iridaceae) and (j) Hesperantha coccinea (Iridaceae).
However, floral syndromes are not always clear-cut, especially in plants that have generalist pollination systems or straddle two different pollination systems (Manning & Goldblatt 2005; Shuttleworth & Johnson 2008). Ollerton et al. (2009) recently scored binary traits in flowers in combination with floral visitors in several communities, including one in eastern South Africa, and found that combinations of these traits for individual species seldom coincide with classical floral syndromes. However, this study tested for global patterns at the level of higher pollinator taxa, such as Orders, and this may not be realistic in the case of groups such as the Diptera, which in southern Africa includes groups as functionally divergent as long-proboscid nemestrinids, fungus gnats and carrion-seeking flies. It is much more useful to search for syndromes at the level of functional pollination guilds (table 1), particularly since patterns of convergence within these guilds can be evident right down to the very fine scale of local communities (Anderson & Johnson 2009). A different way of testing whether floral syndromes are uniquely associated with particular pollinators is to use them to make predictions about pollinators and then test these by means of observations or experiments. Using the established syndrome of traits associated with rodent pollination, Johnson et al. (2001) predicted that the geophyte Massonia depressa would be pollinated by rodents, and then confirmed this with observations. They also made further predictions about likely members of the rodent pollination guild, which have since been confirmed with additional observations (Kleizen et al. 2008). Pauw (2006) used a distinctive syndrome of floral traits associated with oil-bee pollination to predict which species will belong to an oil flower guild, although the plants in this guild are related orchids and thus might be similar to some extent because of common descent rather than convergent evolution. In aloes and proteas, species with a syndrome of traits associated with bird pollination have generally been found to be pollinated primarily by birds (Hargreaves et al. 2004; Botes et al. 2009). This suggests that Ollerton's (1996) paradox (the mismatch between observed pollinators and floral traits) may not apply in these specialized pollination systems.
Another approach that can be used to verify whether convergent floral traits could be a signature of pollinator-mediated selection is to test their functional significance. Experiments have established the functional significance of convergent floral traits in several specialized pollination systems in southern Africa. Floral advertising traits, which have been shown by means of manipulative experiments to be functionally important for pollinator attraction, include convergent yellow or orange coloration and dark central markings of flowers pollinated by hopliine beetles (Van Kleunen et al. 2007), dark raised spots typically found on flowers pollinated by Megapalpus bee-flies (Johnson & Midgley 1997), the size and red colour of flowers pollinated by Aeropetes butterflies (Johnson & Bond 1994) and the scent of flowers pollinated by spider-hunting wasps (Shuttleworth & Johnson 2009).
3. Modes of diversification
Floral adaptation results in patterns of convergent evolution among plants that share pollinators, and also diversification within the lineages of these guild members. However, there has been insufficient attention paid to the diversity of adaptive processes that can be involved in generating these patterns. Here, I identify five different modes of pollinator-driven diversification (pollination system shifts, divergent use of the same pollinator, coevolution, trait tracking and mimicry) that appear to have been important in the evolution of elements of the southern African flora. This can be considered a preliminary assessment as it is likely that other modes of diversification will become apparent as our understanding of pollinator-driven evolution in plants expands.
(a). Pollination system shifts
In discussing the role of pollinators in angiosperm diversification, Grant and Stebbins placed much emphasis on shifts between different pollination systems, including shifts between different pollinators and shifts to wind pollination and selfing.
Macroevolutionary evidence confirms that pollination system shifts have occurred frequently during the radiation of many plant lineages globally (Givnish & Sytsma 1997; Weller & Sakai 1999). In southern Africa, Johnson et al. (1998) found that cladogenesis in the orchid genus Disa was almost always associated with pollinator shifts, while Goldblatt & Manning (2006) placed a minimum estimate of a shift for every five to six species in the large genera Gladiolus and Babiana (Iridaceae).
The Grant–Stebbins model shifts posits that pollination system shifts occur in response to a change in the pollinator fauna, either induced by the expansion of a species' range or because of change in pollinator composition over time (Johnson 2006). Darwin (1859, pp. 94–95) first alluded to this mode of diversification when discussing the possibility that clover would adapt morphologically to pollination by honeybees if bumblebees were to become rare in a particular region (Harder & Aizen 2010). Testing the process implicit in the model thus requires a biogeographical or temporal perspective on pollination systems.
There are still very few examples of pollination system shifts, or even mating system shifts, induced by range expansion (Lloyd 1965; Johnson & Steiner 1997; Barrett et al. 2009). One particularly clear-cut case involves the switch in Dalechampia (Euphorbiaceae) from pollination by resin-collecting bees in Africa to pollination by pollen-collecting bees in Madagascar where specialist resin-collecting bees are absent (Armbruster & Baldwin 1998).
There are now a number of published studies of pollinator shifts in southern African plants that have given deeper insights into the geographical process of these shifts and their consequences for floral diversification. In the orchid Satyrium hallackii, a shift between carpenter bee pollination in short-spurred Cape populations and hawkmoth pollination in long-spurred eastern grassland populations could be attributed to the abundance of carpenter bees and virtual absence of hawkmoths in the Cape region and opposite pattern of abundance of these two insect groups in the grassland region (Johnson 1997).
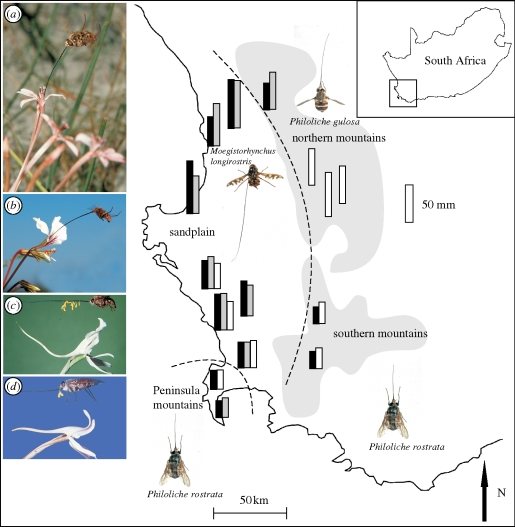
Geographical mosaics arising from the localized distributions of long-proboscid flies in southern Africa have been an important stimulus for plant diversification. In a particularly well-documented system in the southwestern Cape, orchids in the Disa draconis complex as well as irises belonging to the genus Lapeirousia have shifted between short-proboscid Philoliche horseflies that occur only in the mountains and a very long-proboscid tanglewing fly, Moegistorhynchus longirostris, that is endemic to the lowland sandplain (Johnson & Steiner 1997; Pauw et al. 2009; figure 2). This has given rise to local ecotypes of the orchids and irises that differ markedly in the depth of their flowers (figure 2). The adaptive significance of flower depth in these systems has been investigated by means of phenotypic selection experiments. Johnson & Steiner (1997) artificially shortened the very long spurs of lowland forms of D. draconis so that they matched the spur length of mountain populations and found that this led to a drastic reduction in pollination success. Pauw et al. (2009) similarly found a positive relationship between tube length and pollen receipt in emasculated plants of Lapeirousia anceps, one of the members of the lowland M. longirostris pollination guild.
Figure 2.
The geographical context of floral diversification in some Cape plants pollinated by long-proboscid flies. The scale given on the right refers to the bars for fly proboscis length and flower corolla tube length. Lapeirousia anceps (a) and 19 other plant species, including Pelargonium longicaule (b) make up a guild pollinated by the sandplain endemic fly M. longirostris that has an extremely long proboscis (table 1). Covariation in the proboscis length of M. longirostris and guild members on the sandplain probably reflects diffuse coevolution. Populations of L. anceps in the mountains around the sandplain have shorter corolla tubes and are pollinated by horseflies. Parallel shifts have occurred in non-rewarding orchids in the D. draconis complex with long-spurred sandplain forms (c) pollinated by M. longirostris and short-spurred southern mountain forms (d) pollinated by horseflies. In the northern mountains, extremely long-spurred orchids belonging to this complex show frequent pollination failure which may reflect extinction of the (as yet unknown) pollinator to which they are adapted. Modified from Johnson & Steiner (1997) and Pauw et al. (2009). Black bar, long-proboscid fly; grey bar, L. anceps; unfilled bar, D. draconis complex.
Shifts in pollination systems are thought to be precipitated when pollinators are rare and plants experience pollen limitation, favouring traits that attract more effective pollinators in the local habitat. A difficulty with this model is that pollen limitation should also favour shifts to selfing (Morgan & Wilson 2005) or to unspecialized pollination systems (Waser et al. 1996), yet many southern African lineages, including self-compatible orchids well known for their propensity for pollen limitation, exhibit a pattern of repeated shifts between specialized pollination systems (Johnson et al. 1998). One possibility is that selfing evolves often, but represents a dead end that leads to lineage extinction (Takebayashi & Morrell 2001). Another possibility is that pollinator shifts allow plants to escape from pollen limitation without incurring the cost of inbreeding depression (Harder & Aizen 2010).
Extreme levels of pollination system specialization probably inhibit pollination system shifts because of the canalization of floral traits and the reduced probability of attracting different pollinators (Tripp & Manos 2008). The canalization of wind-pollination traits in angiosperms is the best known example. Plant lineages with brood-site mutualisms (those in which plants provide their pollinators with sites for larval development as a reward for pollination) and those with specialized oil or resin rewards also tend to show a high degree of evolutionary conservatism (see Armbruster & Baldwin 1998). At the other extreme, there is probably a different kind of canalization in plants with generalized pollination systems. There is a marked lack of floral diversification at the macroevolutionary level in families with generalized pollination systems such as the Brassicaceae and Apiaceae, despite microevolutionary studies that show strong between-population variation in selection on floral traits in members of these families (Conner 2006; Gomez et al. 2009; Sandring & Agren 2009). This contradiction between micro- and macroevolutionary evidence probably arises because selection in generalist flowers due to fine-scale spatio-temporal variation in the pollinator assemblage is usually not sufficiently consistent over time to drive floral diversification at the species level.
Pollinator shifts appear to be most likely in plants that have pollination systems that are specialized, but not so specialized that they cannot pass through Stebbin's ‘intermediate stage of double-function’. Recent macroevolutionary studies have given good insights into the direction and likelihood of these transitions. For example, in Aquilegia, there tends to be shifts from bee to bird to moth pollination (Whittall & Hodges 2007), while in Ruellia, bird-pollinated flowers most likely give rise to other pollination systems, while bat-pollinated flowers tend to represent an evolutionary dead end (Tripp & Manos 2008). In the southern African flora, there are numerous cases of evolutionary shifts between hawkmoth and long-proboscid fly pollination and evidence from introgressive hybridization that these transitions can involve a stage of double function in which they are visited by both sets of pollinators (Johnson et al. 2002; Archibald et al. 2004; Johnson 2006), thus providing a good example of Stebbins' ‘line of least resistance’.
(b). Divergent use of the same pollinator
Floral diversification does not always involve wholesale shifts between pollinators and can, instead, involve divergent use of the same pollinator. This could involve the exploitation of different behaviours (e.g. pollen versus nectar feeding) or placement of pollen on different parts of the body. For example, Macior (1982) documented a wide variety of floral types associated with different placements of pollen on bumblebees in Pedicularis, and Armbruster et al. (1994) similarly documented variation in pollen placement among sympatric species of Stylidium. Variation in flowers within lineages of wind-pollinated plants, although limited, can be considered a special case of this mode of diversification.
In general, divergent use of the same pollinator is the least well-understood mode of floral diversification in plants. The most common explanation given for divergent use of the same pollinator is competition leading to character displacement (Armbruster & Muchhala 2009), but there is still only limited evidence for the role of competition in shaping selection on floral traits (Caruso 2000).
There are several examples of floral radiations in southern Africa that have involved divergent use of the same pollinator. Recent studies of the genus Pachycarpus (Apocynaceae) in southern Africa have shown that most species are pollinated by spider-hunting wasps in the genus Hemipepsis (Ollerton et al. 2003; Shuttleworth & Johnson 2006, 2009a,c). Mechanisms for placing pollinaria on different parts of the bodies of these wasps form the basis of differences in floral structure between various Pachycarpus species.
Ellis & Johnson (2009) recently demonstrated that the geographically structured evolution of a spectacular variety of floral capitulum forms in the daisy Gorteria diffusa is associated with a single pollinator, the bee-fly Megapalpus capensis. There is some evidence that a basis for different forms could be that they exploit either feeding or mate-seeking behaviour in the flies, but non-adaptive causes for this radiation cannot be ruled out.
(c). Coevolution
Fitness relations in most plant–pollinator systems are strongly asymmetrical, with plants having strong dependencies on particular pollinators, which, in turn, are usually generalist foragers and thus not dependent on any particular plant species. Until recently, many authors have thus considered coevolution between plants and their pollinators to be unlikely, except in the case of obligate brood-site mutualisms, such as those involving figs and fig wasps (Schemske 1983). However, diffuse, and even pairwise, coevolution (Thompson 1994) may be a common feature in southern African pollination guilds (Steiner & Whitehead 1990; Anderson & Johnson 2008, 2009; Pauw et al. 2009).
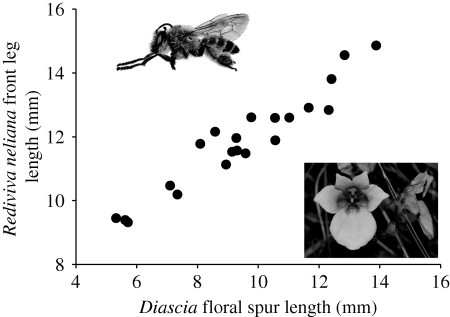
In a study of a specialized guild of Diascia species that depend on the oil-bee Rediviva neliana for pollination, Steiner & Whitehead (1990) observed a strong pattern of geographical covariation between the mean length of the floral spurs and the front legs of the bees across 22 sites in the Drakensberg mountains of South Africa (figure 3). The bee uses its front legs to gather oil from the tip of the floral spurs of Diascia, and the system thus has the potential to conform to the classic Darwinian coevolutionary model, whereby deep flowers are favoured by selection because they force pollinators to make good contact with the floral reproductive organs, and long tongues (or legs, in this case) are alternately favoured because they allow rewards to be extracted from deeper flowers. By controlling for allometric and physical covariates that could explain variation in bee leg length, Steiner and Whitehead convincingly demonstrated that the pattern of bee and plant covariation is consistent with diffuse coevolution.
Figure 3.
The strong positive relationship between the length of the front legs of R. neliana and floral spur length in a guild of Diascia species in the Drakensberg mountains. Modified from Steiner & Whitehead (1990).
Long-proboscid fly pollination systems in southern Africa also appear to be characterized by coevolution. The extraordinary proboscides of these flies clearly represent adaptations for flower feeding and show considerable variation between species and populations. A recent study showed that M. longirostris flies obtain more nectar from flowers when their proboscis is the same length or longer than the corolla than when it is shorter (Pauw et al. 2009). Thus, selection for a longer proboscis length when flies are feeding on long-tubed flowers almost certainly acts via energetic gains. On the other hand, selection on corolla tube length acts via its influence on the pollination success of plants (Nilsson 1988; Alexandersson & Johnson 2002). This has now been demonstrated through phenotypic selection studies in many plants pollinated by long-proboscid flies (Anderson & Johnson 2008, 2009; Pauw et al. 2009). This potential for reciprocal selection sets up the possibility of Darwin's race in the form of escalatory coevolution.
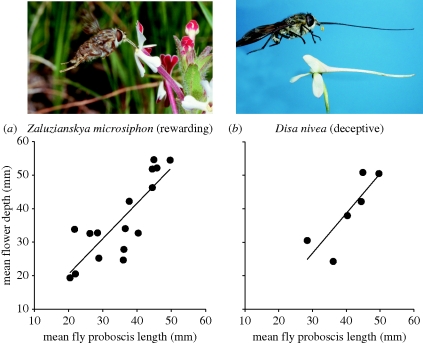
In a study of the long-proboscid fly Prosoeca ganglbaueri and its principal nectar plant Zaluzianskya microsiphon in the Drakensberg mountains, Anderson & Johnson (2008) found strong patterns of geographical covariation between the proboscis length and flower tube length of these strongly interacting species (figure 4a). This is the expected outcome if coevolution operates at the level of local populations (Thompson 1994). Indeed, this relationship remained significant even in models that included other allometric and environmental predictors. The adaptive significance of floral tube length in Z. microsiphon in this system was also confirmed by means of a reciprocal translocation experiment. In a subsequent study, Anderson & Johnson (2009) showed that covariation also applies at the level of the entire plant guild pollinated by P. ganglbaueri, resulting in patterns of divergence among populations of guild members and convergent evolution within a site. Pauw et al. (2009) observed very similar patterns of geographical covariation between fly proboscis length and flower tube length in the M. longirostris pollination guild in the southwestern Cape region (figure 2).
Figure 4.
Covariation of traits in a guild of plants pollinated by the tanglewing fly Prosoeca ganglbaueri in the Drakensberg mountains (table 1). (a) Coevolution probably accounts for the strong relationship across 16 sites between the proboscis length of P. ganglbaueri and the flower depth of Z. microsiphon (Scrophulariaceae) which is pollinated almost exclusively by this fly and also its main source of nectar. (b) On the other hand, a similar relationship involving the orchid Disa nivea must be due to trait-tracking as this species is non-rewarding and thus unlikely to influence the evolution of the fly proboscis. Modified from Anderson et al. (2005) and Anderson & Johnson (2008, 2009).
These studies, together with others dealing with insect proboscis length in antagonistic systems (Toju & Sota 2006), confirm that coevolution can be an important driver of phenotypic divergence among populations of strongly interacting species.
(d). Trait tracking
In pollination guilds that exhibit diffuse coevolution, individual guild members can participate differently in the coevolutionary process (Anderson & Johnson 2009; Pauw et al. 2009). Guild members that are common and/or have large floral rewards will have a strong influence on pollinator evolution, and thus participate in coevolution, while rare or non-rewarding species may have little or no influence. Yet, even non-rewarding species can show geographical covariation with pollinator traits. For example, in a long-proboscid fly pollination guild in the Drakensberg mountains, spur length of a deceptive orchid closely matches that of the fly pollinator, which, in turn, is involved in a coevolutionary relationship with a common nectar plant (figure 4). This can be interpreted as a process of trait tracking whereby rare or non-rewarding species are forced to keep pace with morphological changes in their pollinator that arise from coevolutionary interactions with common rewarding guild members (Anderson & Johnson 2009).
(e). Mimicry of different model flowers
Although innate sensory preferences and morphology of pollinators are believed to be most important for shaping floral traits, there is also a sensory component that is conditioned and based on experience (cf. Gumbert 2000). These conditioned preferences form the basis of floral mimicry systems in which food-seeking insects are deceived by pollinators conditioned by feeding on rewarding model flowers. Selection through this process can shape mimic flowers to be extraordinarily similar to those of their models (Johnson 1994). If a mimic extends its range beyond that of its model, but still within that of its pollinator, selection could favour traits that resemble those of different rewarding plants used by the pollinator. This is the most likely explanation for the evolution of two different forms of the deceptive butterfly-pollinated Cape orchid Disa ferruginea (figure 1b). Flowers of western forms are red and mimic those of an iris, while flowers of eastern forms are orange and mimic those of an asphodel (Johnson 1994). In the orchid genus Disa, diversification of one of the clades (section Stenocarpa) seems to have involved shifts between different floral mimicry systems (S. D. Johnson 2009, unpublished).
Mimicry in the broadest sense could be important for the development of floral syndromes in pollination guilds. New guild members may be under strong selection for traits that confer resemblance of their flowers to those of abundant existing guild members. Brown & Kodric-Brown (1979) suggested that conditioned preferences could explain why guilds of hummingbird pollinated flowers in North America show convergence for traits such as red flower colour. Colour preferences of many insects, such as bumblebees, are strongly affected by conditioning (Gigord et al. 2002). It remains to be determined whether striking colour similarity among flowers in various specialized pollination guilds in southern Africa (table 1) reflects innate foraging preferences of pollinators or the result of selection via conditioned preferences.
4. Diversification and speciation
The relationship between adaptation and speciation remains a contentious issue in evolutionary biology. Darwin's uniformitarian view was that speciation is simply a consequence of profound phenotypic divergence driven by adaptation. However, the focus in speciation research since the new synthesis has been on the role of adaptation in producing isolating barriers, since these are deemed to be the defining characteristic of species. In reality, species are usually characterized by both phenotypic differences and isolating barriers that allow them to coexist with others, and the goal of speciation research must thus be to explain both of these properties (Johnson 2006). In this section, I ask how research on pollinator-driven diversification can contribute to our understanding of the nature of species.
As discussed in the preceding sections, pollinator-mediated selection is the major driver of morphological evolution in flowers, and accounts for many of the traits that are used to characterize species. There is thus a clear link between floral adaptation and phenotypic divergence of species. Following the evolutionary synthesis, Grant and others sought to understand how pollinators could also be involved in the evolution of isolating barriers. In particular, Grant & Grant (1965) placed emphasis on ethological isolation due to ‘mutual exclusiveness’ between forms that had specialized for different pollinators as one of the pathways to speciation in plants. This idea has received considerable support (Fulton & Hodges 1999; Schemske & Bradshaw 1999), but cases where pollinator-mediated ethological or mechanical isolation are responsible for integrity of sympatric species may be exceptions rather than the rule (Ramsey et al. 2003). For most plant species, geographical or habitat differences are probably the main barriers to gene flow (Van der Niet & Johnson 2009). Thus, the role of pollinators in speciation almost certainly has more to do with diversification of phenotype than reproductive isolation.
Several authors have noted that shifts in pollination systems are often associated with soil-type shifts (Patterson & Givnish 2004; Goldblatt & Manning 2006). This raises challenging questions about which of these two forms of diversification is most important during speciation. Johnson argued that shifts in pollination system are probably more important for phenotypic diversification than soil shifts, and thus more likely to lead to recognizably different species. Goldblatt & Manning (2006), on the other hand, argued that speciation in southern African Iridaceae is driven mainly by soils and that pollination systems evolve subsequently under selection through reinforcement. Van der Niet et al. (2006) found support for this view from macroevolutionary data that show that sister taxa that have diverged in soil type and have overlapping ranges are significantly more likely to have also diverged in pollination systems than have sister taxa that have diverged in soil type and have non-overlapping distributions (and thus could not have experienced reinforcement). However, these patterns could also be explained by competitive displacement or species sorting. If pollinator distributions are linked to habitat type (figure 2), then parallel edaphic and pollination system shifts seem the most likely explanation for these patterns. Furthermore, there are serious theoretical objections to reinforcement, the most important of which is that traits that evolve through reinforcement at a contact zone are unlikely to have fitness value outside that zone and are thus unlikely to spread to other populations and become fixed at the species level.
5. Pollination failure and its environmental correlates
Pollen limitation of seed production is pervasive in natural ecosystems (Knight et al. 2005; Harder & Aizen 2010), including those in southern Africa (Johnson & Bond 1997), and is probably an important catalyst for pollinator shifts in evolutionary time. However, plants that receive insufficient pollen cannot always ‘solve’ the problem over evolutionary time by allocating more resources to pollinator attraction, evolving selfing or shifting to more effective pollinators. If unmitigated, pollination failure could conceivably lead to demographic declines of populations (Ehrlén & Eriksson 1995), and even species extinctions.
It is usually challenging to distinguish between natural and anthropogenic pollen limitation (Harder & Aizen 2010). Severe pollen limitation has been recorded for many Cape wildflower species, even in pristine natural habitats. Steiner (1993) noted that the shrub Ixianthes is rarely visited by its specialist oil-bee pollinator R. gigas in the Cape mountains, although it still persists through clonal reproduction and visits by pollen-collecting bees. Extreme pollen limitation, such as the 1000-fold increase in fruit set when flowers in remnant urban populations of the hawkmoth-pollinated southern African tree Oxyanthus pyriformis are hand-pollinated (Johnson et al. 2004), is usually attributed to anthropogenic factors. However, there is often no historical benchmark with which to compare current pollen limitation. Pauw (2004) overcame this problem by comparing rates of pollen removal in current orchid populations with those in preserved century-old herbarium specimens and found historical pollination failure in some populations, and more recent failure in others which he attributed to anthropogenic factors.
More commonly, anthropogenic causes of pollen limitation are identified by comparing populations in different environmental contexts. Habitat fragmentation, population size, population density, population isolation and the presence of co-flowering plants are among the environmental factors that have been investigated (Aguilar et al. 2006). However, many of these studies are limited because they typically focus on just one factor, most commonly habitat fragmentation (cf. Donaldson et al. 2002). Since habitat fragmentation is often associated with changes in population size, density and isolation, multivariate approaches are needed to tease apart their influences. In a study of the southern African lily Brunsvigia radulosa that considered several environmental factors, Ward & Johnson (2005) found that population size, rather than habitat fragmentation or population isolation, best explained variation in pollen limitation among populations. On the other hand, pollination success in other species, such as the hawkmoth-pollinated southern African orchid Satyrium longicauda (Johnson et al. 2009), appears to be relatively resilient to a variety of environmental contexts.
Southern Africa, with a plethora of highly specialized pollination systems, is an ideal place to test for linked extinctions, particularly given the asymmetric structure of guilds, whereby many specialized plants tend to rely on a relatively generalist ‘keystone’ pollinator species or group of functionally equivalent species (table 1). In a detailed study of a guild of oil-producing orchids pollinated by the bee Rediviva peringueyi (table 1), Pauw (2007) found correlated pollination failure across six guild members in habitats where this bee was apparently absent or scarce, suggesting that extinctions could be linked. In particular, R. peringueyi was most likely to be absent from small urban habitat fragments. However, this bee is also naturally absent from many sites, and there the orchids appear to persist through clonal reproduction.
Bond (1994) developed a useful framework for evaluating the risk that failure of a pollination mutualism would lead to extinction. He argued that species with a combination of a specialized pollination system, an outbreeding mating system and demographic dependence on seeds are at highest risk of extinction. However, using southern African pollination guilds (table 1), he also showed that few species fit into this highest risk category because of trade-offs between risk in life-history components. Thus, species with highly specialized pollination systems are often buffered against pollination failure by self-compatibility or clonal reproduction.
6. Future directions
Darwin's evolutionary framework provided pollination biology with the tools needed to explain how and why flowers have diversified. The field has been extraordinarily successful in explaining how the traits of angiosperm flowers (Harder & Johnson 2009), and even gymnosperm cones (Terry et al. 2007), have diversified under selection by animal pollinators. However, there are still few studies that sufficiently explain why flowers have diverged between populations, and ultimately, between species. Answers to these questions are fundamental for understanding why some plant groups have diverged under selection by pollinators, while others have not, and why some regions seem to be hotspots for floral diversification. My view is that answers to these questions will be obtained primarily from studies that use a combination of the microevolutionary approach of studying the process of divergence among ecotypes and recently diverged taxa, and the macroevolutionary approach of studying the pattern and tempo of diversification using phylogenies. In particular, there is a need, not just in southern Africa, but globally, to better understand the geographical context of pollinator-driven diversification. The pollinator environment is a geographical selection mosaic and the structure of this mosaic is determined not only by the distribution patterns of pollinators themselves, but also by their organization into particular pollination guilds that function as niches for further plant diversification. Characterizing the niche spaces for pollinator-driven plant diversification at a regional level, something which was done only crudely in this paper (table 1), should be an important goal for future studies. This outside-in approach of understanding how the environment imposes selection on floral traits is as important as the inside-out approach of understanding how genes give rise to floral traits, yet the two approaches have become balkanized into separate traditions of evolutionary ecology and developmental biology, respectively. Studies that combine ecological and molecular approaches are still rare, but offer the best potential for a complete evolutionary understanding of pollinator-driven diversification (see Kramer & Hodges 2010; Venail et al. 2010).
Unlike much of the developed world, southern Africa still has vast tracts of land that are pristine enough to be considered representative of the actual environment in which plants diversified. However, these environments are being rapidly altered and a necessary component of future research will also have to be devoted to improving our understanding of the role of pollination niches for the maintenance of plant species diversity.
Acknowledgements
I am grateful to Kim Steiner for sharing his extensive knowledge about oil-bee pollination guilds in southern Africa, Anton Pauw for giving advice on the preparation of figure 2, Lawrence Harder for many useful discussions about the role of pollinators in floral evolution and the organizers of this Discussion Meeting for bringing together floral biologists from an unusually diverse range of subdisciplines.
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Aguilar R., Ashworth L., Galetto L., Aizen M. A.2006Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol. Lett. 9, 968–980 (doi:10.1111/j.1461-0248.2006.00927.x) [DOI] [PubMed] [Google Scholar]
- Aigner P. A.2001Optimality modeling and fitness trade-offs: when should plants become pollinator specialists? Oikos 95, 177–184 (doi:10.1034/j.1600-0706.2001.950121.x) [Google Scholar]
- Alexandersson R., Johnson S. D.2002Pollinator-mediated selection on flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae). Proc. R. Soc. Lond. B 269, 631–636 (doi:10.1098/rspb.2001.1928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B., Johnson S. D.2008The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution 62, 220–225 [DOI] [PubMed] [Google Scholar]
- Anderson B., Johnson S. D.2009Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants. New Phytol. 182, 533–540 (doi:10.1111/j.1469-8137.2009.02764.x) [DOI] [PubMed] [Google Scholar]
- Anderson B., Johnson S. D., Carbutt C.2005Exploitation of a specialized mutualism by a deceptive orchid. Am. J. Bot. 92, 1342–1349 (doi:10.3732/ajb.92.8.1342) [DOI] [PubMed] [Google Scholar]
- Archibald J. K., Wolfe A. D., Johnson S. D.2004Hybridization and gene flow between a day- and night-flowering species of Zaluzianskya (Scrophulariaceae SS, tribe Manuleeae). Am. J. Bot. 91, 1333–1344 (doi:10.3732/ajb.91.9.1333) [DOI] [PubMed] [Google Scholar]
- Armbruster W. S.1993Evolution of plant pollination systems: hypotheses and tests with the neotropical vine Dalechampia. Evolution 47, 1480–1505 (doi:10.2307/2410162) [DOI] [PubMed] [Google Scholar]
- Armbruster W. S., Baldwin B. G.1998Switch from specialized to generalized pollination. Nature 394, 621 (doi:10.1038/29210) [Google Scholar]
- Armbruster W. S., Muchhala N.2009Associations between floral specialization and species diversity: cause, effect, or correlation? Evol. Ecol. 23, 159–179 (doi:10.1007/s10682-008-9259-z) [Google Scholar]
- Armbruster W. S., Edwards M. E., Debevec E. M.1994Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium). Ecology 75, 315–329 (doi:10.2307/1939537) [Google Scholar]
- Barrett S. C. H., Ness R. W., Vallejo-Marín M.2009Evolutionary pathways to self-fertilization in a tristylous plant species. New Phytol. 183, 546–556 (doi:10.1111/j.1469-8137.2009.02937.x) [DOI] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Melian C. J., Olesen J. M.2003The nested assembly of plant–animal mutualistic networks. Proc. Natl Acad. Sci. USA 100, 9383–9387 (doi:10.1073/pnas.1633576100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascompte J., Jordano P., Olesen J. M.2006Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433 (doi:10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- Bond W. J.1994Do mutualisms matter? Assessing the impact of pollinator and disperser disruption on plant extinction. Phil. Trans. R. Soc. Lond. B 344, 83–90 (doi:10.1098/rstb.1994.0055) [Google Scholar]
- Botes C., Johnson S. D., Cowling R. M.2009The birds and the bees: using selective exclusion to identify effective pollinators of African tree aloes. Int. J. Plant Sci. 170, 151–156 (doi:10.1086/595291) [Google Scholar]
- Brown J. H., Kodric-Brown A.1979Convergence, competition, and mimicry in a temperate community of hummingbird-pollinated flowers. Ecology 60, 1022–1035 (doi:10.2307/1936870) [Google Scholar]
- Caruso C. M.2000Competition for pollination influences selection on floral traits of Ipomopsis aggregata. Evolution 54, 1546–1557 [DOI] [PubMed] [Google Scholar]
- Conner J. K.2006Ecological genetics of floral evolution. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 260–277 Oxford, UK: Oxford University Press [Google Scholar]
- Darwin C. R.1859On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Darwin C. R.1862On the various contrivances by which British and foreign orchids are fertilized by insects London, UK: John Murray; [PMC free article] [PubMed] [Google Scholar]
- Darwin C. R.1877The various contrivances by which orchids are fertilised by insects London, UK: John Murray [Google Scholar]
- Donaldson J., Nanni I., Zabchariades C., Kemper J.2002Effects of habitat fragmentation on pollinator diversity and plant reproductive success in renosterveld shrublands of South Africa. Conserv. Biol. 16, 1267–1276 (doi:10.1046/j.1523-1739.2002.99515.x) [Google Scholar]
- Ehrlén J., Eriksson O.1995Pollen limitation and population growth in a herbaceous perennial legume. Ecology 76, 652–656 (doi:10.2307/1941223) [Google Scholar]
- Ehrlich P. R., Raven P. H.1964Butterflies and plants: a study in coevolution. Evolution 18, 586–608 (doi:10.2307/2406212) [Google Scholar]
- Ellis A. G., Johnson S. D.2009The evolution of floral variation without pollinator shifts in Gorteria diffusa (Asteraceae). Am. J. Bot. 96, 793–801 (doi:10.3732/ajb.0800222) [DOI] [PubMed] [Google Scholar]
- Feinsinger P.1976Organization of a tropical guild of nectarivorous birds. Ecol. Monogr. 46, 257–291 (doi:10.2307/1942255) [Google Scholar]
- Fenster C. B., Armbruster W. S., Wilson P., Dudash M. R., Thomson J. D.2004Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403 (doi:10.1146/annurev.ecolsys.34.011802.132347) [Google Scholar]
- Friedman W. E.2009The meaning of Darwin's ‘abominable mystery’. Am. J. Bot. 96, 5–21 (doi:10.3732/ajb.0800150) [DOI] [PubMed] [Google Scholar]
- Fulton M., Hodges S. A.1999Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proc. R. Soc. Lond. B 266, 2247–2252 (doi:10.1098/rspb.1999.0915) [Google Scholar]
- Geerts S., Pauw A.In press Hyper-specialization for long-billed bird pollination in a guild of South African plants: the malachite sunbird pollination syndrome. S. Afr. J. Bot [Google Scholar]
- Gigord L. D. B., Macnair M. R., Stritesky M., Smithson A.2002The potential for floral mimicry in rewardless orchids: an experimental study. Proc. R. Soc. Lond. B 269, 1389–1395 (doi:10.1098/rspb.2002.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish T. J., Sytsma A.1997Molecular evolution and adaptive radiation Cambridge, UK: Cambridge University Press [Google Scholar]
- Goldblatt P., Manning J. C.2000The long-proboscid fly pollination system in southern Africa. Ann. Mo. Bot. Gard. 87, 146–170 (doi:10.2307/2666158) [Google Scholar]
- Goldblatt P., Manning J. C.2006Radiation of pollination systems in the Iridaceae of sub-Saharan Africa. Ann. Bot. 97, 317–344 (doi:10.1093/aob/mcj040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J. M., Perfectti F., Bosch J., Camacho J. P. M.2009A geographic selection mosaic in a generalized plant–pollinator–herbivore system. Ecol. Monogr. 79, 245–263 (doi:10.1890/08-0511.1) [Google Scholar]
- Grant V.1949Pollination systems as isolating mechanisms in angiosperms. Evolution 3, 82–97 (doi:10.2307/2405454) [DOI] [PubMed] [Google Scholar]
- Grant V., Grant K. A.1965Flower pollination in the Phlox family New York, NY: Columbia University Press [Google Scholar]
- Gumbert A.2000Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behav. Ecol. Sociobiol. 48, 36–43 (doi:10.1007/s002650000213) [Google Scholar]
- Gumbert A., Kunze J.2001Colour similarity to rewarding model plants affects pollination in a food deceptive orchid, Orchis boryi. Biol. J. Linn. Soc. 72, 419–433 (doi:10.1111/j.1095-8312.2001.tb01328.x) [Google Scholar]
- Harder L., Aizen M.2010Floral adaptation and diversification under pollen limitation. Phil. Trans. R. Soc. B 365, 529–543 (doi:10.1098/rstb.2009.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder L. D., Johnson S. D.2008Function and evolution of aggregated pollen in angiosperms. Int. J. Plant Sci. 169, 59–78 (doi:10.1086/523364) [Google Scholar]
- Harder L. D., Johnson S. D.2009Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol. 183, 530–545 (doi:10.1111/j.1469-8137.2009.02914.x) [DOI] [PubMed] [Google Scholar]
- Hargreaves A. L., Johnson S. D., Nol E.2004Do floral syndromes predict specialization in plant pollination systems? An experimental test in an ‘ornithophilous’ African Protea. Oecologia 140, 295–301 (doi:10.1007/s00442-004-1495-5) [DOI] [PubMed] [Google Scholar]
- Hargreaves A. L., Harder L. D., Johnson S. D.2009Consumptive emasculation: the ecological and evolutionary implications of pollen theft. Biol. Rev. 84, 259–276 (doi:10.1111/j.1469-185X.2008.00074.x) [DOI] [PubMed] [Google Scholar]
- Herrera C. M., Castellanos M. C., Medrano M.2006Geographical context of floral evolution: towards an improved research programme in floral diversification. In Ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 278–294 Oxford, UK: Oxford University Press [Google Scholar]
- Irwin R. E., Brody A. K., Waser N. M.2001The impact of floral larceny on individuals, populations, and communities. Oecologia 129, 161–168 (doi:10.1007/s004420100739) [DOI] [PubMed] [Google Scholar]
- Johnson S. D.1994Evidence for Batesian mimicry in a butterfly-pollinated orchid. Biol. J. Linn. Soc. 53, 91–104 [Google Scholar]
- Johnson S. D.1996Pollination, adaptation and speciation models in the Cape flora of South Africa. Taxon 45, 59–66 (doi:10.2307/1222585) [Google Scholar]
- Johnson S. D.1997Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Bot. J. Linn. Soc. 123, 225–235 [Google Scholar]
- Johnson S. D.2000Batesian mimicry in the non-rewarding orchid Disa pulchra, and its consequences for pollinator behaviour. Biol. J. Linn. Soc. 71, 119–132 (doi:10.1006/bijl.1999.0430) [Google Scholar]
- Johnson S. D.2006Pollinator-driven speciation in plants. In The ecology and evolution of flowers (eds Harder L. D., Barrett S. C. H.), pp. 295–310 Oxford, UK: Oxford University Press [Google Scholar]
- Johnson S. D., Bond W. J.1994Red flowers and butterfly pollination in the fynbos of South Africa. In Plant–animal interactions in Mediterranean-type ecosystems (eds Arianoutsou M., Groves R. H.), pp. 137–148 Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- Johnson S. D., Bond W. J.1997Evidence for widespread pollen limitation of fruiting success in Cape wildflowers. Oecologia 109, 530–534 (doi:10.1007/s004420050113) [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Midgley J. J.1997Fly pollination of Gorteria diffusa (Asteraceae), and a possible mimetic function for dark spots on the capitulum. Am. J. Bot. 84, 429–436 (doi:10.2307/2446018) [PubMed] [Google Scholar]
- Johnson S. D., Midgley J. J.2001Pollination by monkey beetles (Scarabaeidae: Hopliini): do color and dark centers of flowers influence alighting behavior? Environ. Entomol. 30, 861–868 [Google Scholar]
- Johnson S. D., Morita S.2006Lying to Pinocchio: floral deception in an orchid pollinated by long-proboscid flies. Bot. J. Linn. Soc. 152, 271–278 (doi:10.1111/j.1095-8339.2006.00571.x) [Google Scholar]
- Johnson S. D., Steiner K. E.1997Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution 51, 45–53 (doi:10.2307/2410959) [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Steiner K. E.2000Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 15, 190–193 (doi:10.1016/S0169-5347(99)01811-X) [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Steiner K. E.2003Specialized pollination systems in southern Africa. S. Afr. J. Sci. 99, 345–348 [Google Scholar]
- Johnson S. D., Linder H. P., Steiner K. E.1998Phylogeny and radiation of pollination systems in Disa (Orchidaceae). Am. J. Bot. 85, 402–411 (doi:10.2307/2446333) [PubMed] [Google Scholar]
- Johnson S. D., Pauw A., Midgley J.2001Rodent pollination in the African lily Massonia depressa (Hyacinthaceae). Am. J. Bot. 88, 1768–1773 (doi:10.2307/3558351) [PubMed] [Google Scholar]
- Johnson S. D., Edwards T. J., Carbutt C., Potgieter C.2002Specialization for hawkmoth and long-proboscid fly pollination in Zaluzianskya section Nycterinia (Scrophulariaceae). Bot. J. Linn. Soc. 138, 17–27 (doi:10.1046/j.1095-8339.2002.00005.x) [Google Scholar]
- Johnson S. D., Peter C. I., Nilsson L. A., Agren J.2003Pollination success in a deceptive orchid is enhanced by co-occurring rewarding magnet plants. Ecology 84, 2919–2927 (doi:10.1890/02-0471) [Google Scholar]
- Johnson S. D., Neal P. R., Peter C. I., Edwards T. J.2004Fruiting failure and limited recruitment in remnant populations of the hawkmoth-pollinated tree Oxyanthus pyriformis subsp pyriformis (Rubiaceae). Biol. Conserv. 120, 31–39 (doi:10.1016/j.biocon.2004.01.028) [Google Scholar]
- Johnson S. D., Hargreaves A. L., Brown M.2006Dark bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology 87, 2709–2716 (doi:10.1890/0012-9658(2006)87[2709:DBNFAA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Torninger E., Agren J.2009Relationships between population size and pollen fates in a moth-pollinated orchid. Biol. Lett. 5, 282–285 (doi:10.1098/rsbl.2008.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay K., Schemske D.2004Geographic patterns in plant–pollinator mutualistic networks: comment. Ecology 85, 875–878 (doi:10.1890/03-3016) [Google Scholar]
- Kleizen C., Midgley J., Johnson S. D.2008Pollination systems of Colchicum (Colchicaceae) in southern Africa: evidence for rodent-pollination. Ann. Bot. 102, 747–755 (doi:10.1093/aob/mcn157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. M., et al. 2005Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 36, 467–497 (doi:10.1146/annurev.ecolsys.36.102403.115320) [Google Scholar]
- Kramer E. M., Hodges S. A.2010Aquilegia as a model system for the evolution and ecology of petals. Phil. Trans. R. Soc. B 365, 477–490 (doi:10.1098/rstb.2009.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder H. P.2003The radiation of the Cape flora, southern Africa. Biol. Rev. 78, 597–638 (doi:10.1017/S1464793103006171) [DOI] [PubMed] [Google Scholar]
- Lloyd D. G.1965Evolution of self-compatibility and racial differentiation in Leavenworthia (Cruciferae). Contr. Gray Herb. Harv. Univ. 195, 3–134 [Google Scholar]
- Macior L. W.1982Plant community and pollinator dynamics in the evolution of pollination mechanisms in Pedicularis (Scrophulariaceae). In Pollination and evolution (eds Armstrong J. A., Powell J. M., Richards A. J.), pp. 29–45 Sydney, NSW: Royal Botanic Gardens [Google Scholar]
- Manning J. C., Brothers D. J.1986Floral relations of four species of Rediviva in Natal. J. Entomol. Soc. S. Afr. 49, 107–114 [Google Scholar]
- Manning J. C., Goldblatt P.1996The Prosoeca peringueyi (Diptera: Nemestrinidae) pollination guild in southern Africa: long-tongued flies and their tubular flowers. Ann. Mo. Bot. Gard. 83, 67–86 (doi:10.2307/2399969) [Google Scholar]
- Manning J. C., Goldblatt P.1997The Moegistorhynchus longirostris (Diptera: Nemestrinidae) pollination guild: long-tubed flowers and a specialized long-proboscid fly pollination system in southern Africa. Plant Syst. Evol. 206, 51–69 (doi:10.1007/BF00987941) [Google Scholar]
- Manning J., Goldblatt P.2002The pollination of Tritoniopsis parviflora (Iridaceae) by the oil-collecting bee Rediviva gigas (Hymenoptera: Melittidae): the first-record of oil-secretion in African Iridaceae. S. Afr. J. Bot. 68, 171–176 [Google Scholar]
- Manning J. C., Goldblatt P.2005Radiation of pollination systems in the Cape genus Tritoniopsis (Iridaceae: Crocoideae) and the development of bimodal pollination strategies. Int. J. Plant Sci. 166, 459–474 (doi:10.1086/428703) [Google Scholar]
- McMullen C. K.1990Reproductive biology of Galapagos Islands angiosperms. Monogr. Syst. Bot. Mo. Bot. Gard. 32, 35–45 [Google Scholar]
- Morgan M. T., Wilson W. G.2005Self-fertilization and the escape from pollen limitation in variable pollination environments. Evolution 59, 1143–1148 [PubMed] [Google Scholar]
- Nilsson L. A.1988The evolution of flowers with deep corolla tubes. Nature 334, 147–149 (doi:10.1038/334147a0) [Google Scholar]
- Olesen J. M., Jordano P.2002Geographic patterns in plant–pollinator mutualistic networks. Ecology 83, 2416–2424 [Google Scholar]
- Olesen J. M., Bascompte J., Dupont Y. L., Jordano P.2007The modularity of pollination networks. Proc. Natl Acad. Sci. USA 104, 19 891–19 896 (doi:10.1073/pnas.0706375104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J.1996Reconciling ecological processes with phylogenetic patterns: the apparent paradox of plant–pollinator systems. J. Ecol. 84, 767–769 (doi:10.2307/2261338) [Google Scholar]
- Ollerton J., Johnson S. D., Cranmer L., Kellie S.2003The pollination ecology of an assemblage of grassland asclepiads in South Africa. Ann. Bot. 92, 807–834 (doi:10.1093/aob/mcg206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J., Johnson S. D., Hingston A. B.2006Geographical variation in diversity and specificity of pollination systems. In Plant–pollinator interactions: from specialization to generalization (eds Waser N. M., Ollerton J.), pp. 283–308 Chicago, IL: Chicago University Press [Google Scholar]
- Ollerton J., Alarcon R., Waser N., Price M., Watts S., Cranmer L., Hingston A., Peter C., Rottenberry J.2009A global test of the pollination syndrome hypothesis. Ann. Bot. 103, 1471–1480 (doi:10.1093/aob/mcp031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson T. B., Givnish T. J.2004Geographic cohesion, chromosomal evolution, parallel adaptive radiations, and consequent floral adaptations in Calochortus (Calochortaceae): evidence from a cpDNA phylogeny. New Phytol. 161, 253–264 (doi:10.1046/j.1469-8137.2003.00951.x) [Google Scholar]
- Pauw A.2004Variation in pollination across a fragmented landscape at the Cape of Africa. PhD thesis, University of Cape Town, Cape Town, South Africa [Google Scholar]
- Pauw A.2005Inversostyly: a new stylar polymorphism in an oil-secreting plant, Hemimeris racemosa (Scrophulariaceae). Am. J. Bot. 92, 1878–1886 (doi:10.3732/ajb.92.11.1878) [DOI] [PubMed] [Google Scholar]
- Pauw A.2006Floral syndromes accurately predict pollination by a specialized oil-collecting bee (Rediviva peringueyi, Melittidae) in a guild of South African orchids (Coryciinae). Am. J. Bot. 93, 917–926 (doi:10.3732/ajb.93.6.917) [DOI] [PubMed] [Google Scholar]
- Pauw A.2007Collapse of a pollination web in small conservation areas. Ecology 88, 1759–1769 (doi:10.1890/06-1383.1) [DOI] [PubMed] [Google Scholar]
- Pauw A., Stofberg J., Waterman R. J.2009Flies and flowers in Darwin's race. Evolution 63, 268–279 (doi:10.1111/j.1558-5646.2008.00547.x) [DOI] [PubMed] [Google Scholar]
- Potgieter C. J., Edwards T. J.2005The Stenobasipteron wiedemanni (Diptera, Nemestrinidae) pollination guild in Eastern Southern Africa. Ann. Mo. Bot. Gard. 92, 254–267 [Google Scholar]
- Price J. P., Wagner W. L.2004Speciation in Hawaiian angiosperm lineages: cause, consequence, and mode. Evolution 58, 2185–2200 [DOI] [PubMed] [Google Scholar]
- Ramsey J., Bradshaw H. D., Schemske D. W.2003Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57, 1520–1534 [DOI] [PubMed] [Google Scholar]
- Rebelo A. G., Siegfried W. R., Crowe A. A.1984Avian pollinators and the pollination syndromes of selected mountain fynbos plants. S. Afr. J. Bot. 2, 285–396 [Google Scholar]
- Rieseberg L. H., Widmer A., Arntz A. M., Burke J. M.2002Directional selection is the primary cause of phenotypic diversification. Proc. Natl Acad. Sci. USA 99, 12 242–12 245 (doi:10.1073/pnas.192360899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandring S., Agren J.2009Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution 63, 1292–1300 (doi:10.1111/j.1558-5646.2009.00624.x) [DOI] [PubMed] [Google Scholar]
- Schemske D. W.1983Limits to specialization and coevolution in plant–animal mutualisms. In Coevolution (ed. Nitecki M. H.), pp. 67–109 Chicago, IL: University of Chicago Press [Google Scholar]
- Schemske D. W., Bradshaw H. D., Jr1999Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proc. Natl Acad. Sci. USA 96, 11 910–11 915 (doi:10.1073/pnas.96.21.11910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation Oxford, UK: Oxford University Press [Google Scholar]
- Shuttleworth A., Johnson S. D.2006Specialized pollination by large spider-hunting wasps and self-incompatibility in the African milkweed Pachycarpus asperifolius. Int. J. Plant Sci. 167, 1177–1186 (doi:10.1086/507685) [Google Scholar]
- Shuttleworth A., Johnson S. D.2008Bimodal pollination by wasps and beetles in the African milkweed Xysmalobium undulatum. Biotropica 40, 568–574 (doi:10.1111/j.1744-7429.2008.00418.x) [Google Scholar]
- Shuttleworth A., Johnson S. D.2009A key role for floral scent in a wasp-pollination system in Eucomis (Hyacinthaceae). Ann. Bot. 103, 715–725 (doi:10.1093/aob/mcn261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth A., Johnson S. D.2009a. The importance of scent and nectar filters in a specialized wasp-pollination system. Funct. Ecol. 23, 931–940 (doi:10.1111/j.1365-2435.2009.01573.x) [Google Scholar]
- Shuttleworth A., Johnson S. D.2009b. Palp-faction: an African milkweed dismembers its wasp pollinators. Environ. Entomol. 38, 741–747 (doi:10.1603/022.038.0326) [DOI] [PubMed] [Google Scholar]
- Stebbins G. L.1970Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annu. Rev. Ecol. Syst. 1, 307–326 (doi:10.1146/annurev.es.01.110170.001515) [Google Scholar]
- Steiner K. E.1989The pollination of Disperis (Orchidaceae) by oil-collecting bees in southern Africa. Lindleyana 4, 164–183 [Google Scholar]
- Steiner K. E.1993Has Ixianthes (Schrophulariaceae) lost its special bee? Plant Syst. Evol. 185, 7–16 (doi:10.1007/BF00937717) [Google Scholar]
- Steiner K., Cruz B.2009Hybridization between two oil-secreting orchids in South Africa. Plant Syst. Evol. 277, 233–243 (doi:10.1007/s00606-008-0119-7) [Google Scholar]
- Steiner K. E., Whitehead V. B.1988The association between oil-producing flowers and oil-collecting bees in the Drakenberg of southern Africa. Monogr. Syst. Bot. Mo. Bot. Gard. 25, 259–277 [Google Scholar]
- Steiner K. E., Whitehead V. B.1990Pollinator adaptation to oil-secreting flowers—Redivivia and Diascia. Evolution 44, 1701–1707 (doi:10.2307/2409348) [DOI] [PubMed] [Google Scholar]
- Steiner K. E., Whitehead V. B.1991Oil flowers and oil bees: further evidence for pollinator adaptation. Evolution 45, 1493–1501 (doi:10.2307/2409895) [DOI] [PubMed] [Google Scholar]
- Takebayashi N., Morrell P. L.2001Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. Am. J. Bot. 88, 1143–1150 (doi:10.2307/3558325) [PubMed] [Google Scholar]
- Terry I., Walter G. H., Moore C., Roemer R., Hull C.2007Odor-mediated push–pull pollination in cycads. Science 318, 70 (doi:10.1126/science.1145147) [DOI] [PubMed] [Google Scholar]
- Thompson J. N.1994The coevolutionary process Chicago, IL: Chicago University Press [Google Scholar]
- Toju H., Sota T.2006Imbalance of predator and prey armament: geographic clines in phenotypic interface and natural selection. Am. Nat. 167, 105–117 (doi:10.1086/498277) [DOI] [PubMed] [Google Scholar]
- Tripp E. A., Manos P. S.2008Is floral specialization and evolutionary dead end? Pollination system transitions in Ruellia (Acanthaceae). Evolution 62, 1712–1737 (doi:10.1111/j.1558-5646.2008.00398.x) [DOI] [PubMed] [Google Scholar]
- Van der Niet T., Johnson S. D.2009Patterns of plant speciation in the Cape floristic region. Mol. Phylogenet. Evol. 51, 85–93 (doi:10.1016/j.ympev.2008.11.027) [DOI] [PubMed] [Google Scholar]
- Van der Niet T., Johnson S. D., Linder H. P.2006Macroevolutionary data suggest a role for reinforcement in pollination system shifts. Evolution 60, 1596–1601 (doi:10.1554/05-705.1) [DOI] [PubMed] [Google Scholar]
- Van Kleunen M., Nanni I., Donaldson J. S., Manning J. C.2007The role of beetle marks and flower colour on visitation by monkey beetles (Hopliini) in the greater cape floral region, South Africa. Ann. Bot. 100, 1483–1489 (doi:10.1093/aob/mcm256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venail J., Dell'Olivo A., Kuhlemeier C.2010Speciation genes in the genus Petunia. Phil. Trans. R. Soc. B 365, 461–467 (doi:10.1098/rstb.2009.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S.1954Blütenbiologische Typen als Elemente der Sippengliederug, dargestellt anhand der Flora Südafrikas Jena: Fischer [Google Scholar]
- Ward M., Johnson S. D.2005Pollen limitation and demographic structure in small fragmented populations of Brunsvigia radulosa (Amaryllidaceae). Oikos 108, 253–262 (doi:10.1111/j.0030-1299.2005.13468.x) [Google Scholar]
- Waser N. M., Real L. A.1979Effective mutualism between sequentially flowering plants species. Nature 281, 670–672 (doi:10.1038/281670a0) [Google Scholar]
- Waser N. M., Chittka L., Price M. V., Williams N. M., Ollerton J.1996Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060 (doi:10.2307/2265575) [Google Scholar]
- Weller S. G., Sakai A. K.1999Using phylogenetic approaches for analysis of plant breeding system evolution. Annu. Rev. Ecol. Syst. 30, 167–199 (doi:10.1146/annurev.ecolsys.30.1.167) [Google Scholar]
- Whitehead V. B., Steiner K. E.2001Oil-collecting bees of the winter rainfall area of South Africa (Melittidae, Rediviva). Ann. S. Afr. Mus. 108, 143–277 [Google Scholar]
- Whitehead V. B., Steiner K. E., Eardley C. D.2008Oil collecting bees mostly of the summer rainfall area of southern Africa (Hymenoptera: Melittidae: Rediviva). J. Kans. Entomol. Soc. 81, 122–141 (doi:10.2317/JKES-703.12.1) [Google Scholar]
- Whittall J. B., Hodges S. A.2007Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–712 (doi:10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- Whittall J., Hodges S.2007Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–709 (doi:10.1038/nature05857) [DOI] [PubMed] [Google Scholar]
- Wiens D., Rourke J. P., Casper B. B., Rickart E. A., Lapine T. R., Peterson C. J.1983Non-flying mammal pollination of southern African Proteas: a non-coevolved system. Ann. Mo. Bot. Gard. 70, 1–31 (doi:10.2307/2399006) [Google Scholar]
- Wilson P., Thomson J. D.1991Heterogeneity among floral visitors leads to discordance between removal and deposition of pollen. Ecology 72, 1503–1507 (doi:10.2307/1941124) [Google Scholar]