Abstract
This is a combination of review and original data on floral structure and diversity in the two earliest diverging lineages of the Ericales, i.e. the balsaminoids, comprising Balsaminaceae, Marcgraviaceae and Tetrameristaceae, and the polemonioids, comprising Fouquieriaceae and Polemoniaceae. Each clade is strongly supported in molecular studies, while structural synapomorphies have largely been lacking. For the balsaminoid families, we compare floral morphology, anatomy and histology among selected taxa and find that the entire clade is strongly supported by the shared presence of nectariferous tissue in the floral periphery, thread-like structures on anthers, truncate stigmas, secretion in the ovary, as well as mucilage cells, raphides and tannins in floral tissues. A possible sister group relationship between Balsaminaceae and Tetrameristaceae is supported by the shared presence of post-genital fusion of filaments and ovary and a star-shaped stylar canal. For polemonioids, we document unexpected diversity of floral features in Polemoniaceae, partly providing structural links to Fouquieriaceae. Features include cochlear and quincuncial corolla aestivation, connective protrusions, ventrifixed anthers and nectariferous tissue in the base of the ovary. In addition, we outline future directions for research on floral structure in the Ericales and briefly discuss the general importance of structural studies for our understanding of plant phylogeny and evolution.
Keywords: asterids, Balsaminaceae, floral diversity, Marcgraviaceae, Polemoniaceae, Tetrameristaceae
1. Introduction
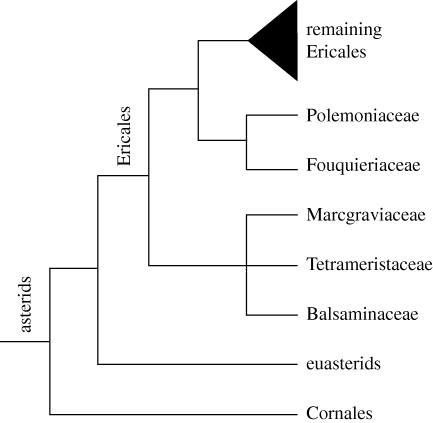
The stem node of the Ericales has been estimated to have its origin in the Mid-Early Cretaceous (Bremer et al. 2004), and the first major diversification, which gave rise to all major lineages of the order, probably took place 109–103 Myr ago (Sytsma et al. 2006). By the Early Eocene, i.e. ca 50 Myr ago, almost all extant families had already diverged (Bremer et al. 2004). Today, Ericales comprise ca 6 per cent of the eudicot species diversity (Magallón et al. 1999) and include many well-known tropical as well as temperate groups. In many tropical rainforests, they are an important component of the understorey vegetation (ca 10% of the total species diversity; Davis et al. 2005). They include ca 11 000 species in 347 genera and 25 families (Stevens 2001). In pre-molecular classification systems, members of Ericales were usually assigned to between 10 and 12 different angiosperm orders (e.g. Cronquist 1981; Dahlgren 1983), many of which were thought to be distantly related (table 1). Only the more recent rise of molecular phylogenetics made possible our current understanding of the evolutionary history of the Ericales. Molecular studies have not only demonstrated the monophyly of the order (Soltis et al. 2000; Albach et al. 2001b), but also resolved the Ericales as sister to the euasterids (figure 1; Bremer et al. 2002). Within the order, families are generally easily recognized based on morphological features and are mostly well supported in molecular studies. It has, however, proved difficult to disentangle interfamilial relationships as most of the deeper nodes in the ericalean phylogeny remained unresolved or only weakly supported (Anderberg et al. 2002; Geuten et al. 2004). However, with the addition of more sequence data and a denser taxon sampling, subsequent studies have managed to improve resolution also at deeper levels in the ericalean tree (Schönenberger et al. 2005; Sytsma et al. 2006).
Table 1.
Currently recognized families of Ericales (Stevens 2001; APG 2003) and pre-molecular, ordinal classifications according to Cronquist (1981) and Dahlgren (1983). Subclass abbreviations: A, Asteridae; C, Corniflorae; D, Dilleniidae; M, Magnoliflorae; P, Primuliflorae; R, Rosidae; Ru, Rutiflorae; S, Solaniflorae; T, Theiflorae.
| family names (no. of genera/species according to Stevens 2001) | ordinal classification of earlier authors |
|
|---|---|---|
| Cronquist (1981) | Dahlgren (1983) | |
| Actinidiaceae (3/355) | Theales (D) | Ericales (C) |
| Balsaminaceae (2/1001) | Geraniales (R) | Balsaminales (Ru) |
| Clethraceae (2/75) | Ericales (D) | Ericales (C) |
| Cyrillaceae (2/2) | Ericales (D) | Ericales (C) |
| Diapensiaceae (6/18) | Diapensiales (D) | Ericales (C) |
| Ebenaceae (4/548) | Ebenales (D) | Ebenales (P) |
| Ericaceae (126/3395) | Ericales (D) | Ericales (C) |
| Fouquieriaceae (1/11) | Violales (D) | Fouquieriales (C) |
| Lecythidaceae (25/310) | Lecythidales (D) | Theales (T) |
| Maesaceae (1/150) | Primulales (D) | Primulales (P) |
| Marcgraviaceae (7/130) | Theales (D) | Theales (T) |
| Mitrastemonaceae (1/2) | Rafflesiales (R) | Rafflesiales (M) |
| Myrsinaceae (41/1435) | Primulales (D) | Primulales (P) |
| Pentaphylacaceae (14/340) | Theales (D) | Theales (T) |
| Polemoniaceae (18/385) | Solanales (A) | Solanales (S) |
| Primulaceae (9/900) | Primulales (D) | Primulales (P) |
| Roridulaceae (1/2) | Rosales (R) | Ericales (C) |
| Sapotaceae (53/1100) | Ebenales (D) | Ebenales (P) |
| Sarraceniaceae (3/15) | Nepenthales (D) | Sarraceniales (C) |
| Styracaceae (11/160) | Ebenales (D) | Ebenales (P) |
| Symplocaceae (2/320) | Ebenales (D) | Cornales (C) |
| Tetrameristaceae (3/5) | Theales (D) | Theales (T) |
| Theaceae (7/195) | Theales (D) | Theales (T) |
| Theophrastaceae (5/105) | Primulales (D) | Primulales (P) |
Figure 1.
Phylogenetic relationships of Ericales and its two first diverging subclades; tree topology is based on Bremer et al. (2002), Schönenberger et al. (2005) and Sytsma et al. (2006).
Why were the Ericales not recognized as a natural group in the pre-molecular era? Part of the answer may lie in the considerable age of many of the families and the corresponding long time spans of anagenic stem lineage evolution, which may have obscured many structural synapomorphies. Today, members of the Ericales exhibit a vast diversity at all levels of their biology, including, for instance, mycorrhizal associations (e.g. Ericaceae p.p.), mycorrhizal parasitism (saprophytism, Ericaceae p.p.), holoparasitism (Mitrastemonaceae) and carnivory (Roridulaceae, Sarraceniaceae). However, undoubtedly most important in this context is the broad range of diversity that has evolved at the level of floral structure and function. Remarkable floral features that show extensive homoplasy when analysed across the order include, for instance, sympetaly, stamen number, integument number and type of endosperm formation (Schönenberger et al. 2005). Already Darwin was intrigued by the diversity and biology of ericalean flowers. For instance, in his groundbreaking book The different forms of flowers on plants and the same species (Darwin 1877), he collected a mass of data from morphological observations and pollination experiments about phenomena such as heterostyly and cleistogamy. An important part of his data is based on the study of ericalean taxa including various representatives from Primulaceae, Polemoniaceae and Balsaminaceae.
At present, there are no clear-cut non-molecular synapomorphies neither for the order as a whole, nor for most of the major ericalean clades spanning more than one family. The only feature shared by all taxa is tenuinucellate ovules, but this is a characteristic of most asterids (Albach et al. 2001a). One important reason for our inability to describe synapomorphies for larger clades within the Ericales, and, for that matter, for many other large angiosperm clades, is that our current knowledge of the floral structure and diversity of most families is highly fragmentary. In recent years, comparative structural analyses have not kept pace with the ever faster evolving methods for acquiring and analysing molecular sequence data, and it is therefore currently difficult to test sequence-based phylogenetic hypothesis and to conduct combined morphological/molecular analyses. This situation has been recognized as a fundamental problem in angiosperm phylogenetics (Crane et al. 2004), and the great need for new comparative structural studies in the light of the modern phylogenetic framework of the angiosperms has been stressed repeatedly (Endress 2002; Weber 2003; Judd & Olmstead 2004; Schönenberger 2005; Matthews & Endress 2006). Rare, recent examples of broad-scale, comparative studies have provided invaluable structural data that have led to a considerably better understanding of floral evolution in various angiosperm clades (Endress & Igersheim 2000; Endress 2001; Matthews & Endress 2002, 2004, 2005, 2008).
Here, we highlight salient aspects of the diversity and evolution of floral structure and diversity among early diverging ericalean lineages. First, as an example for an interfamilial comparative study, we provide new comparative structural data and identify potential synapomorphies for the first diverging lineage of the Ericales, i.e. the balsaminoid clade comprising Balsaminaceae, Marcgraviaceae and Tetrameristaceae. Second, in order to provide a striking example about the fragmentary status of our current knowledge of floral structure and diversity in many ericalean families, we highlight selected floral features of Polemoniaceae, which, in a recent study, have been shown to be considerably more diverse than previously thought (Schönenberger 2009). Finally, we highlight potential future directions of research on the diversity and evolution of ericalean flowers.
2. Material and methods
See the electronic supplementary material.
3. Results and discussion
(a). Comparative floral structure of the balsaminoid clade
The deepest split in the ericalean phylogeny is between the balsaminoid clade comprising Balsaminaceae, Marcgraviaceae and Tetrameristaceae (including Pellicieraceae) and the clade uniting the remaining families of the order (figure 1). The balsaminoid clade was first identified in a molecular study by Morton et al. (1996) and is one of the few larger ericalean clades that repeatedly has found strong support in phylogenetic studies dealing with the order (Källersjö et al. 1998; Anderberg et al. 2002; Bremer et al. 2002; Geuten et al. 2004; Schönenberger et al. 2005). Interfamilial relationships in the clade, however, are problematic, apparently because of conflicting phylogenetic signals from chloroplast and mitochondrial data (Geuten et al. 2004; Schönenberger et al. 2005).
The clade comprises ca 1136 species in 12 genera. Marcgraviaceae and Tetrameristaceae are almost exclusively Neotropical while Balsaminaceae are widespread, but mainly in the Old World. Even based on non-molecular data, Marcgraviaceae and Tetrameristaceae have usually been classified close to each other (Cronquist 1981; Takhtajan 1997), and Hallier (1916) even treated them as a single family. The systematic position of Balsaminaceae, however, was always much debated, and the family has never been considered to be closely related to the other families based on non-molecular characters (see also table 1). With their herbaceous habit and their monosymmetric flowers, Balsaminaceae differ considerably from the two other families (figure 2a–c). Previously, the only known potential synapomorphy for the balsaminoid clade as a whole was the presence of calcium oxalate raphides in parenchymatic tissues (Morton et al. 1996; Anderberg et al. 2002). Marcgraviaceae and Tetrameristaceae share a number of features including wood anatomical characters (Lens et al. 2005, 2007), palynological similarities (Janssens et al. 2005) and general floral organization, i.e. flowers are bisexual, polysymmetric, hypogynous and basically pentamerous. Balsaminaceae and Marcgraviaceae share micropylar endosperm haustoria (Anderberg et al. 2002); embryology of Tetrameristaceae is not known.
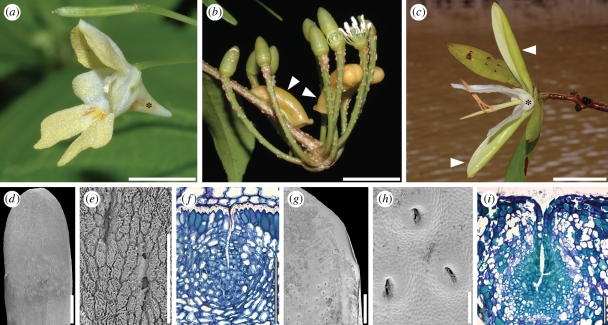
Figure 2.
Balsaminaceae/Marcgraviaceae/Tetrameristaceae. (a–c) Anthetic flowers. (a) Impatiens parviflora (Balsaminaceae); asterisk indicates spurred sepal. (b) Marcgravia caudata (Marcgraviaceae); arrowheads indicate nectariferous bracts. (c) Pelliciera rhizophorae (Pellicieraceae); asterisk indicates petaloid calyx, arrowheads indicate petaloid prophylls. (d–i) Sepal structure of Tetrameristaceae. (d–f) Pentamerista neotropica. (d) Sepal with glandular pits, adaxial view. (e) Close-up of glandular pits. (f) Transverse section of sepal with glandular pit, adaxial side of sepal up. (g–i) Pelliciera rhizophorae. (g) Part of sepal with glandular pits, adaxial view. (h) Close-up of glandular pits. (i) Transverse section of sepal with glandular pit, adaxial side of sepal up. Scale bars: (a–c) = 10 mm; (d) = 1 mm; (e,f,i) = 100 µm; (g) = 2 mm; (h) = 200 µm.
Geuten et al. (2006) describe expression patterns of SEPALLATA3-like genes in representatives of Balsaminaceae and Marcgraviaceae. SEPALLATA3-like genes are usually expressed in the three innermost organs categories of eudicot flowers. However, in the two latter families, they are expressed outside these inner organ whorls: in Balsaminaceae IhSEP3 is expressed in a spurred, petaloid, nectariferous sepal, and in Marcgraviaceae MuSEP3 is expressed in modified, nectariferous bracts, which often are brightly coloured, apparently playing an important role in pollinator attraction (Dressler 2004). Geuten et al. (2006) interpret these expression patterns as a feature supporting the close relationship of the two families. Nectariferous elaborations are also present in the perianth of Pentamerista and Pelliciera (Tetrameristaceae), where the sepals have distinct patches of glandular pits mainly on their adaxial side (figure 2d–f; Kubitzki 2004a,b). The nectaries are of the epithelial (epidermis) type (sensu Vogel 1977; Endress 1994), with several layers of protoplasma-rich tissue underlying the pits and nectar secretion via the epidermis (figure 2f,i). In addition, both in Pentamerista and in Pelliciera, sepals are petaloid in their appearance: in the former taxon they are white or reddish like the petals (figure 2c; Tomlinson 1986), and in the latter genus sepals are yellowish-petaloid and apparently attractive. Pentamerista flowers are further characterized by two large, showy and petaloid prophylls, which may be white or red and probably play a role in pollinator attraction (figure 2c; Howe 1911; Tomlinson 1986). It can be speculated that also in the flowers of Tetrameristaceae, the expression of SEPALLATA3-like genes extends outside the three inner organ categories and perhaps even to extrafloral organs, and thus represents a potential synapomorphy for the balsaminoid clade.
Floral organs and in particular reproductive organs of all three families are in close association with each other in bud as well as during anthesis (figure 3), and organs are partly shaped by their tight packing. For instance in Marcgraviaceae, stamen filaments leave distinct imprints on the ovary surface when removed from the flower (figure 3a–d). In Tetrameristaceae, androecium and gynoecium are even more intimately synorganized: the basal parts of the filaments are post-genitally fused with the ovary by interlocking epidermal cells (Pentamerista; figure 3e,f) or by interlocking epidermal cells and cuticular excrescences (Pelliciera; figure 3g,h) forming a gynostegium. In Balsaminaceae, the distal-most parts of adjacent filaments and proximal parts of anthers are post-genitally united forming an androecial tube around the gynoecium (figure 3i,m; see also Caris et al. 2006). In addition and similar to Pentamerista (Tetrameristaceae), filaments are also partly post-genitally fused to the gynoecium surface by interlocking epidermal cells (figure 3j). It is likely that the close synorganization of stamens and pistil in the flowers of the balsaminoid clade is a means of guaranteeing precise mechanical application of pollen during a pollinator's visit. Pollination biology and floral mechanisms have been studied in a few species of Impatiens (Wilson 1995; for a short summary, see Fisher 2004) and various representatives of Marcgraviaceae (for a summary, see Dressler 2004), but are apparently unknown for Tetrameristaceae.
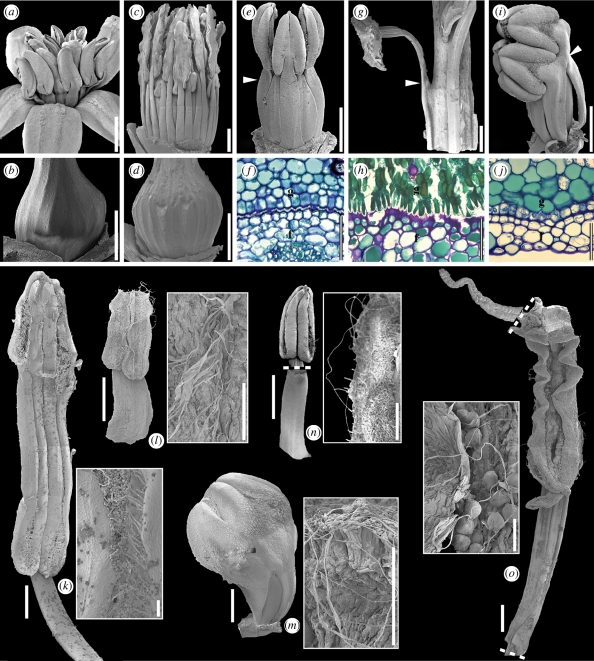
Figure 3.
Marcgraviaceae/Tetrameristaceae/Balsaminaceae. (a–i) Synorganization of androecium and gynoecium. (a,b) Schwartzia brasiliensis (Marcgraviaceae). (a) Anthetic flower, lateral view. (b) Ovary, lateral view, showing imprint marks of stamens. (c,d) Marcgravia rectiflora (Marcgraviaceae). (c) Pre-anthetic flower with perianth removed, lateral view. (d) Ovary, lateral view, showing imprint marks of stamens. (e,f) Pentamerista neotropica (Tetrameristaceae). (e) Pre-anthetic flower with perianth removed, lateral view; arrowhead indicates the distal limit of post-genital fusion between filament and gynoecium. (f) Transverse section of filament [f] and gynoecium [g] showing post-genital fusion. (g,h) Pelliciera rhizophorae (Tetrameristaceae). (g) Partial lateral view of androecium and gynoecium; arrowhead indicates the distal limit of post-genital fusion between filament and gynoecium. (h) Transverse section of filament [f] and gynoecium [g] showing post-genital fusion. (i,j) Impatiens auricoma (Balsaminaceae). (i) Pre-anthetic flower with perianth removed, lateral view; arrowhead indicates the region of post-genital fusion of filaments and gynoecium. (j) Transverse section of filament [f] and gynoecium [g] showing post-genital fusion. (k–o) Stamen structure. (k) Marcgravia rectiflora (Marcgraviaceae); stamen, adaxial view; close-up of thecal threads. (l) Schwartzia brasiliensis (Marcgraviaceae); stamen, adaxial view; close-up of thecal threads. (m) Impatiens auricoma (Balsaminaceae); entire androecium, lateral view; close-up of thecal threads. (n) Pentamerista neotropica (Tetrameristaceae); anther in adaxial view, filament in abaxial view; close-up of thecal threads. (o) Pelliciera rhizophorae (Tetrameristaceae); adaxial views of connective protrusion, partial anther and the free part of the filament; close-up of thecal threads. Scale bars: (a,c,g) = 2 mm; (b,d,e,i,k,m,o) = 1 mm; (f,h,j) = 50 µm; (l,n) = 100 µm.
The androecium of Balsaminaceae and Tetrameristaceae is haplostemonous, and stamens are arranged in a single whorl (Fisher 2004; Kubitzki 2004a,b). The androecium is also haplostemonous in many Marcgraviaceae (e.g. Souroubea, Ruyschia), but higher stamen numbers are found in several genera (e.g. in Schwartzia, Marcgraviastrum and Marcgravia (figures 2b and 3a,c); Dressler 2004). In all three families, the stamen filaments are broad and dorsiventrally flattened (figure 3k–o). At least in Balsaminaceae and Tetrameristaceae, this has to be seen in connection with the synorganization of the filaments and the ovary described above. Anthers are basifixed, slightly to distinctly sagittate, and introrse; dehiscence is by longitudinal slits that extend over the entire length of the thecae (figure 3k–o); sterile connective protrusions are only present in Tetrameristaceae (figure 3n,o). In all taxa studied, the dehiscence process of the anthers involves the formation of distinct thread-like structures along the stomium (figure 3k–o). These threads are formed by the disintegration of the thecal septum as well as the outer epidermal cells lining the stomium. Among members of the balsaminoid clade, such threads were earlier only known for Impatiens (Balsaminaceae; Vogel & Coccuci 1988) and Schwartzia brasiliensis (Marcgraviaceae; Pinheiro et al. 1995). The threads on the anthers of Impatiens are apparently involved in pollen presentation, forming a ‘pollen basket’ entangling the pollen grains, whereas in S. brasiliensis they may function as pollen-connecting vectors forming pollen dispersal units. This feature has been hypothesized to be an adaptation to relatively large pollinators (Endress 1996; Hesse et al. 2000), which fits well with the bat and hummingbird pollination syndromes of many Marcgraviaceae (Tschapka et al. 2006).
The gynoecium is superior, syncarpous and generally pentamerous (figure 4), although deviations from pentamery apparently occur in all families studied (Balsaminaceae four to five (Shimizu & Takao 1982); Marcgraviaceae two to eight (in rare cases up to 20) (Dressler 2004); Tetrameristaceae four or five (Kubitzki 2004b)). Pelliciera is generally reported to have a two-carpellate gynoecium and a bifid stigma (Kobuski 1951; Stevens 2001; Kubitzki 2004a). However, our studies show a pentamerous stigma (figure 4n), an extended pentamerous stylar region (figure 4o) as well as a pentamerously organized gynoecium vascularization (not illustrated in detail). Thus, it seems likely that the ovary of Pelliciera is basically pentamerous and only appears dimerous during older developmental stages with three locules reduced/suppressed. This hypothesis needs to be confirmed by an ontogenetic study of the gynoecium. The gynoecia of all three families are similar in the distal region where they end in an indistinct, truncate stigmatic region (figure 4a,e,i,m,q); stigmas are minutely lobed in Tetrameristaceae and Balsaminaceae (figure 4i,j,m,n,q,r), while the gynoecia of the here-studied Marcgraviaceae have a punctiform stigma, in which the minute lobes are post-genitally united with each other (figure 4a,b,e,f). However, some representatives of the family are described to have distally radiating stigmatic lobes (e.g. Souroubea; Dressler 2004). In Marcgraviaceae, a meandering network of post-genitally united pollen tube transmitting tracts forming a compitum is extending over the entire length of the style (figure 4c,g). In, Balsaminaceae and Pelliciera (Tetrameristaceae), however, carpels are open in the symplicate region, and a star-shaped central stylar canal lined with pollen tube transmitting tissue and filled with secretion is present (figure 4o,s). In the symplicate region of Pentamerista (Tetrameristaceae), a star-shaped, secretion-filled central stylar canal is restricted to the distal part of the style while carpels are post-genitally united at the base of the style (figure 4k). A larger (Marcgraviaceae) or smaller (Tetrameristaceae, Balsaminaceae) amount of secretion is present in the ovary of all taxa (figure 4d,h,l,p,t), most likely providing the substrate for pollen tube growth in the ovary.
Figure 4.
Marcgraviaceae/Tetrameristaceae/Balsaminaceae. Gynoecium structure. (a–d) Schwartzia brasiliensis (Marcgraviaceae). (a) Uppermost part of gynoecium, lateral view. (b) Transverse section at the level of the gynoecium tip. (c) Transverse section at the level of the style. (e–h) Marcgravia coriacea (Marcgraviaceae). (d) Transverse section at the level of the ovary, secretion shown. (e) Uppermost part of gynoecium, lateral view. (f) Transverse section at the level of the gynoecium tip. (g) Transverse section at the level of the style. (h) Transverse section at the level of the ovary, secretion shown. (i–l) Pentamerista neotropica (Tetrameristaceae). (i) Uppermost part of gynoecium, lateral view. (j) Transverse section at the level of the gynoecium tip. (k) Transverse section at the level of the style. (l) Transverse section at the level of the ovary, secretion shown. (m–p) Pelliciera rhizophorae (Tetrameristaceae). (m) Uppermost part of gynoecium, lateral view. (n) Transverse section at the level of the gynoecium tip. (o) Transverse section at the level of the style. (p) Transverse section at the level of the ovary, secretion shown. (q–t) Impatiens parviflora (Balsaminaceae). (q) Uppermost part of gynoecium, lateral view. (r) Transverse section at the level of the gynoecium tip. (s) Transverse section at the level of the style. (t) Transverse section at the level of the ovary, secretion shown. Scale bars: (a,k) = 500 µm; (b–d,f,g,j,m–o,q–s) = 100 µm; (e,i) = 1 mm; (h,l,p,t) = 50 µm.
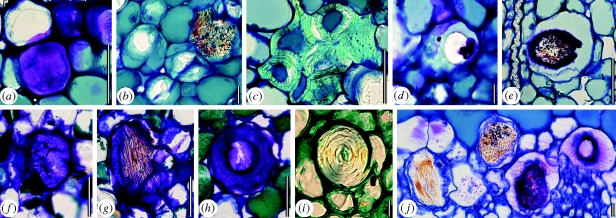
At the histological level, calcium oxalate raphides, tannins, stone cells (brachysclereids) and mucilage cells are common in the balsaminoid clade. The former three of these features have been identified as deterrents to herbivore feeding in various groups of angiosperms, (Mauseth 1988; Bennett & Wallsgrove 1994; Salminen & Lempa 2002). It is therefore likely that they are also part of a biochemical/mechanical defence system protecting the flowers of the balsaminoid species studied here. Mucilage cells are present in many different systematic groups of angiosperms and also in many different types of plant organs and tissues (Matthews & Endress 2006). It is therefore not surprising that mucilage secretion fulfils various functions in plants. For instance, mucilage in the seed coat may serve in seed dispersal and mucilage in the parenchyma of succulent plants may allow for water storage (Fahn 1979). Their exact function in floral organs such as sepals, petals and stamens, however, remains elusive. They may perhaps also play a role in protection against herbivores.
In the balsaminoid clade, tannins and raphides are found in all floral organs of all three families. Stone cells are abundant in all floral organs of Marcgraviaceae (figure 5c) and Pelliciera (Tetrameristaceae; figure 5i), but absent in Balsaminaceae and Pentamerista (Tetrameristaceae). Almost all floral organs of Balsaminaceae and Tetrameristaceae are rich in mucilage cells, whereas they are less abundant in Marcgraviaceae (figure 5a,b,d–h,j). The mucilage cells are unlike the specialized cells described by Matthews & Endress (2006), which have thickened mucilaginous inner cell walls. Instead, mucilage cells either have regular, non-thickened cell walls (figure 5a,f) or uniformly thickened cell walls surrounding a mucilaginous cell centre (figure 5b,d,e,g,h,j). Mucilage cells may, in addition, contain raphides in representatives of all three families (figure 5b,e,g,j).
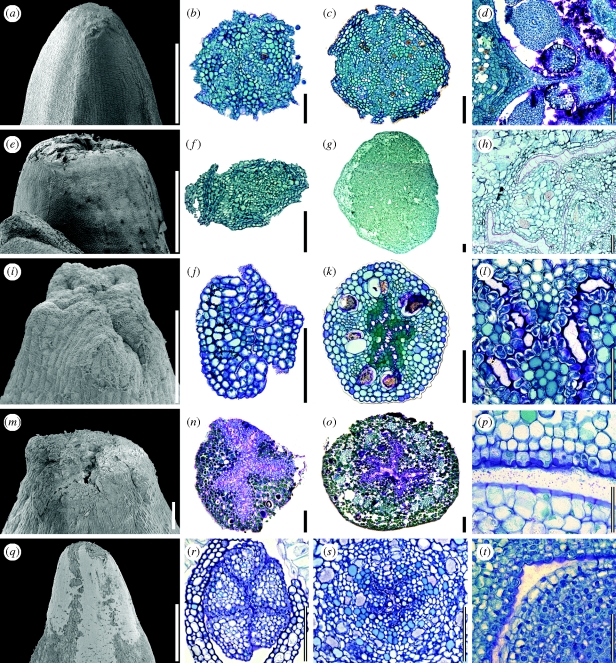
Figure 5.
Marcgraviaceae/Tetrameristaceae/Balsaminaceae. Histology; mucilage cells, raphides and stone cells in floral organs. (a–d) Schwartzia brasiliensis (Marcgraviaceae). (a) Petal, mucilage cell with non-thickened cell wall. (b) Gynoecium, cells with uniformly thickened cell walls containing raphides. (c) Petal, group of stone cells with thickened, layered cell walls. (d) Sepal, cell with uniformly thickened cell wall and mucilaginous cell centre. (e) Pentamerista neotropica (Tetrameristaceae); petal, cell with uniformly thickened cell wall containing both mucilage and raphides. (f–i) Pelliciera rhizophorae (Tetrameristaceae). (f) Sepal, mucilage cell with non-thickened cell wall. (g) Sepal, mucilage cell with non-thickened cell wall containing raphides. (h) Sepal, cell with uniformly thickened cell wall and mucilaginous cell centre. (i) Gynoecium, stone cell with thickened, layered cell wall. (j) Impatiens parviflora (Balsaminaceae); gynoecium, mucilaginous cells with both non-thickened (on the left) and uniformly thickened cells walls containing raphides. Scale bars: (a,c,e,f–i) = 50 µm; (b,d,j) = 10 µm.
In summary, although the present comparative analysis of the floral structure of balsaminoid families is far from comprehensive, we found a surprising number of new floral features supporting the clade as being monophyletic. Prominent shared features include the presence of nectariferous tissue in the floral periphery (on bracts in Marcgraviaceae; on sepals in the two other families), broad and dorsiventrally flattened filaments, thread-like structures along thecal stomia, truncate or only shortly lobed stigmas, secretion in the style and ovary as well as mucilage cells, raphides and tannins in the parenchymatic tissue of most floral organs. None of these features is present in the next diverging lineage of the Ericales (the clade with Polemoniaceae and Fouquieriaceae; Schönenberger 2009), and they may well turn out to be synapomorphic for the balsaminoid clade. In addition, we also found features that are shared between Balsaminaceae and Tetrameristaceae, indicating a possible closer relationship of these two families. Such features include the partial fusion of filaments and ovary surface by interlocking epidermal cells and cuticular excrescences as well as a star-shaped central stylar canal.
(b). Floral diversity in Polemoniaceae
The second split in the ericalean phylogeny is between the strongly supported clade consisting of Polemoniaceae and Fouquieriaceae (the polemonoid clade) and the clade with all remaining families (figure 1; Sytsma et al. 2006). The two families differ considerably in their vegetative morphology: while most Polemoniaceae are annuals or herbaceous perennials, Fouquieriaceae are woody shrubs or small succulent trees. In addition, the two families differ also in a couple of floral characters that often have been considered highly indicative for phylogenetic relationships (e.g. stamen number, integument number and type of endosperm formation). These differences are most likely the main reasons why earlier authors usually classified them far apart from each other (table 1) even if a possible closer relationship of the two families had been suggested repeatedly in the pre-molecular era (Nash 1903; Henrickson 1967; Thorne 1968). A recent comparative investigation of the floral structure of the two families found a series of shared characters, many of which may turn out to be synapomorphic for the clade (Schönenberger 2009). The study also revealed that our knowledge of the floral structure and diversity of Polemoniaceae was far from comprehensive and in some cases even misleading. Here, we highlight just four prominent floral features of the latter family in order to provide an example of how modern comparative structural studies may improve our understanding of the floral structure and diversity of a given family.
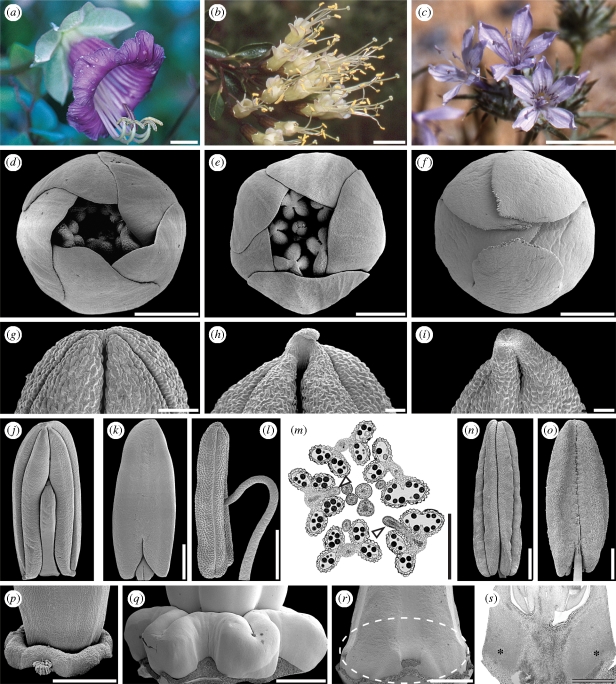
With probably less than 400 species in 18 genera, Polemoniaceae (figure 6a–c) is a relatively small family distributed primarily in North America and extending into Central and South America. A few species are also present in Eurasia. A floral character that had been thought ubiquitous in the family is a contort corolla aestivation (illustrated here with Linanthus californicus, figure 6d; Cronquist 1981; Wilken 2004). The only known exceptions to this pattern were Cantua pyrifolia (Johnson et al. 1999) and Phlox paniculata (Schoute 1935), which were reported to deviate from the contort pattern. However, of the seven species of Polemoniaceae studied by Schönenberger (2009), only three seem contort throughout (Polemonium reptans, Cantua coerulea, Phlox divaricata). In each of the four other species, two or more distinct aestivation patterns were found: corolla aestivation of Gilia achilleifolia is mostly cochlear (figure 6e), but contort aestivation occurs as well; Cantua flexuosa has flowers with quincuncial (figure 6f) or cochlear petal aestivation; Acanthogilia gloriosa seems most often contort, but occasionally cochlear aestivation is present as well; and finally, Ipomopsis tenuifolia may have contort, quincuncial or cochlear petal aestivation (all on the same individual). Corolla aestivation is obviously much more diverse in Polemoniaceae than previously suspected, even down to the level of individual plants. Of particular interest is the occurrence of quincuncial corolla aestivation as this pattern is present in all representatives of the sister family Fouquieriaceae (Henrickson 1972; Schönenberger 2009), and therefore provides a structural link to the latter family. The question about the adaptive significance of this variation in corolla aestivation patterns in Polemoniaceae is currently difficult to answer. In general, aestivation patterns may have an impact on the potential synorganization of the organs and on mechanical properties (e.g. structural stability, opening and reclosing of flowers) of the flower as a whole (Endress 1994). Clearly, further studies on corolla aestivation, based on a broader taxon sampling of Polemoniaceae, are advisable in order to explore the potential systematic value of this character as well as its adaptive significance.
Figure 6.
Polemoniaceae. (a–c) Anthetic flowers. (a) Cobaea scandens. (b) Cantua flexuosa. (c) Eriastrum eremicum. (d–s) Floral structure. (d–f) Corolla aestivation. (d) Linanthus californicus, contort (e) Gilia achilleifolia; cochlear (f) Cantua flexuosa; quincuncial. (g–i) Distal part of anthers/connective protrusion, ventral view. (g) Eriastrum eremicum; no protrusion. (h) Acanthogilia gloriosa; distinct protrusion. (i) Cantua coerulea; distinct protrusion. (j–o) Anther attachment. (j–k) Cantua flexuosa; ventrifixed. (j) Anther, ventral view. (k) Anther, dorsal view. (l,m) Loeselia cordifolia; ventrifixed. (l) Anther, lateral view, ventral side to the right. (k) Transverse section in the distal part of a floral bud showing ventrifixed anther attachment (arrowheads); perianth removed. (n–o) Linanthus californicus; basifixed. (n) Anther, ventral view. (o) Anther, dorsal view. (p–s) Nectary structure. (m) Phlox divaricata. Truncate nectary disc around the base of the ovary, lateral view. (q) Cobaea scandens. Lobed nectary disc around the base of the ovary, lateral view. (r–s) Cantua flexuosa. (r) Ovary, lateral view; dashed line indicates region of nectary. (s) Ovary, longitudinal section; asterisks indicate nectariferous tissue. Scale bars: (a–c) = 10 mm; (d–f,j–p) = 500 µm; (g–i) = 100 µm; (q) = 2 mm; (r,s) = 1 mm.
A feature of the anthers of Polemoniaceae, which previously has been overlooked or at least has not been reported for the family, is the presence of sterile connective protrusions. Although not present in all species (e.g. Eriastrum eremicum, figure 6g; P. divaricata; Schönenberger 2009), connective protrusions seem particularly well developed in taxa belonging to early diverging lineages of the family (for phylogenetic relationship within Polemoniaceae, see Johnson et al. 2008) such as A. gloriosa (figure 6h) and C. coerulea (figure 6j) (Schönenberger 2009). Again, this is particularly interesting, because conspicuous connective protrusions are characteristics of the sister family Fouquieriaceae (Henrickson 1972; Schönenberger 2009).
Yet another interesting feature of the androecium of Polemoniaceae is the way in which the filament attaches to the anther. The general descriptive literature agrees on that anther attachment is either basifixed or dorsifixed (Brand 1907; Cronquist 1981; Wilken 2004). However, among the species studied by Schönenberger (2009), most have ventrifixed anthers (C. coerulea, A. gloriosa, G. achilleifolia, I. tenuifolia), while anthers are basifixed in P. reptans and P. divaricata. Here, we add data for C. flexuosa (figure 6j,k) and Loeselia cordifolia (figure 6l,m), both of which have distinctly ventrifixed anthers, as well as for L. californicus (figure 6n,o), in which anthers are basifixed. Ventrifixed anthers are also mentioned for Cobaea scandens by Leins & Boecker (1982). Apparently, ventral attachment is combined with versatility of the anthers in Polemoniaceae (Schönenberger 2009), which may help directing the anther opening towards the pollinator's body at the slightest touch and thereby facilitating pollen transfer, as is the case in many other angiosperm taxa (Endress 1994). It is noteworthy that none of the species studied here or in Schönenberger (2009) has dorsifixed anthers. These results indicate a general misinterpretation (the ventrifixed condition earlier misinterpreted as dorsifixed) of anther attachment in Polemoniaceae. Among ericalean families, ventrifixed anthers are otherwise only reported for Clethraceae (but see Lechner 1915; Schneider & Bayer 2004). The sister family Fouquieriaceae is characterized by dorsifixed anthers (Schönenberger 2009). As all representatives of early diverging polemoniaceous lineages studied so far (species of Cantua, Cobaea, Acanthogilia) have ventrifixed anthers, it is likely that the ventrifixed condition has evolved along the stem lineage of Polemoniaceae.
Finally, also floral nectaries of Polemoniaceae have been shown to be far from uniform across the family (Schönenberger 2009). Most species are characterized by prominent nectary discs surrounding the base of the ovary. The disc may be truncate as in P. divaricata (figure 6p) or distinctly lobed as in C. scandens (figure 6q) and A. gloriosa (Schönenberger 2009). In C. flexuosa, a disc-shaped nectary is apparently lacking (figure 6r). Instead, a ring of nectariferous tissue is incorporated into the base of the ovary (figure 6s). This latter condition is matched by all representatives of the sister family Fouquieriaceae (Henrickson 1972; Schönenberger 2009) and therefore provides another structural link between the two families.
This comparative study in Polemoniaceae shows that our knowledge of floral diversity in this family was, and probably still is, far from comprehensive. It is surprising that even basic features of floral organization and construction such as corolla aestivation patterns or the type of anther attachment have been known only fragmentarily or even have been misinterpreted. This is even more surprising considering the fact that Polemoniaceae is a mainly north-temperate family and has been studied by numerous botanists and ecologists during the past decades. One may expect that similar studies in taxa with mainly subtropical or tropical distributions will reveal even more surprising results.
4. General discussion and conclusions
Additional structural studies will be needed to provide a solid basis for our understanding of floral evolution as well as of phylogenetic relationships within the Ericales. These studies are likely to allow for the identification of synapomorphies for suprafamilial clades, which is an important and at the same time challenging task. Currently, the structural descriptions of larger ericalean clades comprising more than one family usually lack clear-cut structural synapomorphies (Anderberg et al. 2002; Schönenberger et al. 2005). As shown here, new comparative structural studies conducted in a modern and well-supported phylogenetic framework are most useful not only because they have the potential to reveal synapomorphies for newly circumscribed taxa, but also because they fill many of the gaps that still exist in our knowledge of floral structure and diversity of a given group. Another advantage is that such comparative structural studies investigate simultaneously many taxa in a consistent and standardized fashion, which facilitates later use of characters for phylogenetic analyses. Ideally, the taxon sampling of such studies should be congruent with past and ongoing molecular studies (Schönenberger et al. 2005; Sytsma et al. 2009), which will make it possible to conduct subsequent combined phylogenetic analyses.
Which ericalean taxa are in most urgent need of new comparative structural studies? Next to the balsaminoid and the polemonoid lineages, recent molecular studies have revealed a series of other large and well-supported clades with a previously unexpected or even surprising taxonomic composition (Anderberg 1992; Schönenberger et al. 2005; Sytsma et al. 2006). Salient examples include ((Actinidiaceae, Roridulaceae) Sarraceniaceae) and ((Diapensiaceae, Styracaceae) Symplocaceae). Like the balsaminoids and the polemonoids, these two clades had earlier not been recognized as natural groups (table 1). Unequivocal structural synaporphies for these two clades are currently unavailable, and new comparative studies are necessary and potentially highly rewarding. Another interesting candidate for a comparative study of floral structure is the enigmatic, holoparasitic family Mitrastemonaceae, which recently has been placed within the Ericales (Barkman et al. 2004). However, in spite of considerable efforts, its exact placement among families of Ericales remains elusive (Sytsma et al. 2009). The floral structure of Mitrastemonaceae has never been studied in detail, let alone compared with any ericalean taxon. A new comparative study may help us to identify the closest relatives of Mitrastemonaceae among other ericalean lineages.
A better understanding of floral structure and diversity in the Ericales will also allow for a more accurate phylogenetic placement of fossil reproductive structures with affinities to the order. In many cases—not only in Ericales—a detailed comparison of fossil specimens with extant taxa is not so much hampered by what we know about the fossils, but more by our incomplete understanding of the structure of extant taxa (Friis et al. 2005; Schönenberger 2005). Like in most other groups of organisms, much of the total diversity of Ericales is likely to be extinct, and the incorporation of fossils into phylogenetic and structural analyses is pivotal for any comprehensive understanding of the evolutionary history of the order. The fossil record of ericalean reproductive structures is relatively extensive and dates back far into the Cretaceous (Keller et al. 1996; Schönenberger & Friis 2001; Crepet et al. 2004; Martínez-Millán et al. 2009). A more accurate systematic placement of fossil taxa will not only help us to reach a better understanding of floral diversity and evolution, but will also allow for a more precise estimation of the divergence times of different lineages within the order. This is especially interesting in connection with the previously formulated hypothesis of a possible rapid radiation of the major ericalean lineages during the Cretaceous (Anderberg et al. 2002; Bremer et al. 2004; Schönenberger et al. 2005).
Clearly, a multifaceted approach including phylogenetics, developmental genetics, functional ecological studies, palaeobotany, as well as comparative morphology, is the most promising way to go if we are to establish a comprehensive understanding of the floral evolution and phylogeny of the Ericales. And it is also clear that morphology plays a central part in this scientific process as it is morphology as a discipline that interconnects the other disciplines with each other. As Darwin (1859), in The origin of species, put it so pointedly one and a half centuries ago: ‘This [morphology] is one of the most interesting departments of natural history, and may almost be said to be its very soul’. This is true even today.
Acknowledgements
For providing most valuable floral material, we thank Peter Endress, Tasha LaDoux, Mark Porter as well as the Botanical Garden of the University of Zürich, Switzerland; The Fairchild Tropical Botanic Garden, Miami, FL, USA; and the Rancho Santa Ana Botanic Garden, Claremont, CA, USA. We are grateful to Julie Cantrill, Linda Lundmark and Anna-Karin Lindh for technical assistance. Finally, we would like to thank Else Marie Friis and an anonymous colleague for their constructive reviews and the organizers of the discussion meeting for their invitation. Funding for this work was provided by the Swedish Research Council (research grant to J.S.) and by the Swiss National Science foundation (fellowship to M.B.).
Footnotes
One contribution of 16 to a Discussion Meeting Issue ‘Darwin and the evolution of flowers’.
References
- Albach D. C., Soltis P. S., Soltis D. E.2001aPatterns of embryological and biochemical evolution in the Asterids. Syst. Biol. 26, 242–262 [Google Scholar]
- Albach D. C., Soltis P. S., Soltis D. E., Olmstead R. G.2001bPhylogenetic analysis of asterids based on sequences of four genes. Ann. Mo. Bot. Gard. 88, 163–212 (doi:10.2307/2666224) [Google Scholar]
- Anderberg A. A.1992The circumscription of the Ericales, and their cladistic relationships to other families of higher dicotyledons. Syst. Bot. 17, 660–675 (doi:10.2307/2419734) [Google Scholar]
- Anderberg A. A., Rydin C., Källersjö M.2002Phylogenetic relationships in the order Ericales s. l.: Analyses of molecular data from five genes from the plastid and mitochondrial genomes. Am. J. Bot. 89, 677–687 (doi:10.3732/ajb.89.4.677) [DOI] [PubMed] [Google Scholar]
- APG II (Angiosperm Phylogeny Group II) 2003An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 141, 399–436 (doi:10.1046/j.1095-8339.2003.t01-1-00158.x) [Google Scholar]
- Barkman T. J., Lim S. H., Salleh K. M., Nais J.2004Mitochondrial DNA sequences reveal the photosynthetic relatives of Rafflesia, the world's largest flower. Proc. Natl Acad. Sci. USA 101, 787–792 (doi:10.1073/pnas.0305562101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. N., Wallsgrove R. M.1994Secondary metabolites in plant defence mechanisms. New Phytol. 127, 617–633 (doi:10.1111/j.1469-8137.1994.tb02968.x) [DOI] [PubMed] [Google Scholar]
- Brand v. A.1907Polemoniaceae. In Das Pflanzenreich. Regnis vegetabilis conspectus (ed. Engler A.), pp. 1–19 Leipzig, Germany: Engelman [Google Scholar]
- Bremer B., Bremer K., Heidari N., Erixon P., Olmstead R. G., Anderberg A. A., Källersjö M., Barkhordarian E.2002Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Mol. Phylogenet. Evol. 24, 274–301 (doi:10.1016/S1055-7903(02)00240-3) [DOI] [PubMed] [Google Scholar]
- Bremer K., Friis E. M., Bremer B.2004Molecular phylogenetic dating of asterid flowering plants shows early Cretaceous diversification. Syst. Biol. 53, 496–505 (doi:10.1080/10635150490445913) [DOI] [PubMed] [Google Scholar]
- Caris P. L., Geuten K. P., Janssens S. B., Smets E. F.2006Floral development in three species of Impatiens (Balsaminaceae). Am. J. Bot. 93, 1–14 (doi:10.3732/ajb.93.1.1) [Google Scholar]
- Crane P. R., Herendeen P. S., Friis E. M.2004Fossils and plant phylogeny. Am. J. Bot. 91, 1683–1699 (doi:10.3732/ajb.91.10.1683) [DOI] [PubMed] [Google Scholar]
- Crepet W. L., Nixon K. C., Gandolfo M. A.2004Fossil evidence and phylogeny: the age of major angiosperm clades based on mesofossil and macrofossil evidence from cretaceous deposits. Am. J. Bot. 91, 1666–1682 (doi:10.3732/ajb.91.10.1666) [DOI] [PubMed] [Google Scholar]
- Cronquist A.1981An integrated system of classification of flowering plants New York, NY: Columbia University Press [Google Scholar]
- Dahlgren R.1983General aspects of angiosperm evolution and macrosystematics. Nord. J. Bot. 3, 119–149 (doi:10.1111/j.1756-1051.1983.tb01448.x) [Google Scholar]
- Darwin C.1859The origin of species London, UK: Murray [Google Scholar]
- Darwin C.1877The different forms of flowers on plants of the same species London, UK: Murray [Google Scholar]
- Davis C. C., Webb C. O., Wurdack K. J., Jaramillo C. A., Donoghue M. J.2005Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. Am. Nat. 165, E36–E65 (doi:10.1086/428296) [DOI] [PubMed] [Google Scholar]
- Dressler S.2004Marcgraviaceae. In The families and genera of vascular plants. Flowering plants—dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, vol. 6 (ed. Kubitzki K.), pp. 258–265 Berlin, Germany: Springer [Google Scholar]
- Endress P. K.1994Diversity and evolutionary biology of tropical flowers. Cambridge Tropical Biology Series. Cambridge, UK: Cambridge University Press [Google Scholar]
- Endress P. K.1996Diversity and evolutionary trends in angiosperm anthers. In The anther: form, function and phylogeny (eds D'Arcy W. G., Keating R. C.), pp. 92–110 Cambridge, UK: Cambridge University Press [Google Scholar]
- Endress P. K.2001The flowers in extant basal angiosperms and inferences on ancestral flowers. Int. J. Plant. Sci. 162, 1111–1140 (doi:10.1086/321919) [Google Scholar]
- Endress P. K.2002Morphology and angiosperm systematics in the molecular era. Bot. Rev. 68, 545–570 (doi:10.1663/0006-8101(2002)068[0545:MAASIT]2.0.CO;2) [Google Scholar]
- Endress P. K., Igersheim A.2000Gynoecium structure and evolution in basal angiosperms. Int. J. Plant Sci. 161(Suppl.), S211–S223 [Google Scholar]
- Fahn A.1979Secretory tissues in plants New York, NY: Academic Press [Google Scholar]
- Fischer E.2004Balsaminaceae. In The families and genera of vascular plants. Flowering plants—dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, vol. 6 (ed. Kubitzki K.), pp. 20–25 Berlin, Germany: Springer [Google Scholar]
- Friis E. M., Pedersen K. R., Crane P. R.2005When Earth started blooming: insights from the fossil record. Curr. Opin. Plant Biol. 8, 5–12 (doi:10.1016/j.pbi.2004.11.006) [DOI] [PubMed] [Google Scholar]
- Geuten K., Smets E., Schols P., Yuan Y.-M., Janssens S., Küpfer P., Pyck N.2004Conflicting phylogenies of balsaminoid families and the polytomy in Ericales: combining data in a Bayesian framework. Mol. Phylogenet. Evol. 31, 711–729 (doi:10.1016/j.ympev.2003.09.014) [DOI] [PubMed] [Google Scholar]
- Geuten K., Becker A., Kaufmann K., Caris P., Janssens S., Viaene T., Theissen G., Smets E.2006Petaloidy and petal identity MADS-box genes in the balsaminoid genera Impatiens and Marcgravia. Plant J. 47, 501–518 (doi:10.1111/j.1365-313X.2006.02800.x) [DOI] [PubMed] [Google Scholar]
- Hallier H.1916Beiträge zur Flora von Borneo. Beih. Bot. Centralbl. 34, 34–50 [Google Scholar]
- Henrickson J.1967Pollen morphology of the Fouquieriaceae. Aliso 6, 137–160 [Google Scholar]
- Henrickson J.1972A taxonomic revision of the Fouquieriaceae. Aliso 7, 439–537 [Google Scholar]
- Hesse M., Vogel S., Halbritter H.2000Thread-forming structures in angiosperm anthers: their diverse role in pollination ecology. Plant. Syst. Evol. 222, 281–292 (doi:10.1007/BF00984107) [Google Scholar]
- Howe M. A.1911A little-known mangrove from Panama. J. NY Bot. Gard. 12, 61–72 [Google Scholar]
- Janssens S., Lens F., Dressler S., Geuten K., Smets E., Vinckier S.2005Palynological variation in balsaminoid Ericales. II. Balsaminaceae, Tetrameristaceae, Pellicieraceae and general conclusions. Ann. Bot. 96, 1061–1073 (doi:10.1093/aob/mci257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. A., Soltis D. E., Soltis P. S.1999Phylogenetic relationships of Polemoniaceae inferred from 18S ribosomal DNA sequences. Plant. Syst. Evol. 214, 65–89 (doi:10.1007/BF00985732) [Google Scholar]
- Johnson L. A., Chan L. M., Weese T. L., Busby L. D., McMurry S.2008Nuclear and cpDNA sequences combined provide strong inference of higher phylogenetic relationships in the phlox family (Polemoniaceae). Mol. Phylogenet. Evol. 48, 997–1012 (doi:10.1016/j.ympev.2008.05.036) [DOI] [PubMed] [Google Scholar]
- Judd W. S., Olmstead R. G.2004A survey of tricolpate (eudicot) phylogenetic relationships. Am. J. Bot. 91 (doi:10.3732/ajb.91.10.1627) [DOI] [PubMed] [Google Scholar]
- Källersjö M., Farris J. S., Chase M. W., Bremer B., Fay M. F., Humphries C. J., Peterson G., Seberg O., Bremer K.1998Simultaneous parsimony jackknife analysis of 2538 rbcL DNA sequences reveals support for major clades of green plants, land plants, seed plants and flowering plants. Plant Syst. Evol. 213, 259–287 (doi:10.1007/BF00985205) [Google Scholar]
- Keller J. A., Herendeen P. S., Crane P. R.1996Fossil flowers and fruits of the Actinidiaceae from the Campanian (Late Cretaceous) of Georgia. Am. J. Bot. 83, 528–539 (doi:10.2307/2446221) [Google Scholar]
- Kobuski C. E.1951Studies in the Theaceae, XXIII—the genus Pelliciera. J. Arnold Arbor. 32, 256–262 [Google Scholar]
- Kubitzki K.2004aPellicieraceae. In The families and genera of vascular plants. Flowering plants—dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, vol. 6 (ed. Kubitzki K.), pp. 297–299 Berlin, Germany: Springer [Google Scholar]
- Kubitzki K.2004bTetrameristaceae. In The families and genera of vascular plants. Flowering plants—dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, vol. 6 (ed. Kubitzki K.), pp. 461–462 Berlin, Germany: Springer [Google Scholar]
- Lechner S.1915Anatomische Untersuchungen über die Gattungen Actinidia, Saurauia, Clethra und Clematoclethra mit besonderer Berücksichtigung ihrer Stellung im System. Beih. Bot. Centralbl. 32, 431–467 [Google Scholar]
- Leins P., Boecker K.1982Entwickeln sich Staubgefässe wie Schildblätter? [Do stamens develop like peltate leaves?]. Beitr. Biol. Pflanzen 56, 317–327 [Google Scholar]
- Lens F., Dressler S., Jansen S., van Evelghem L., Smets E.2005Relationships within balsaminoid Ericales: a wood anatomical approach. Am. J. Bot. 92, 941–953 (doi:10.3732/ajb.92.6.941) [DOI] [PubMed] [Google Scholar]
- Lens F., Schönenberger J., Baas P., Jansen S., Smets E.2007The role of wood anatomy in phylogeny reconstruction of Ericales. Cladistics 23, 229–254 (doi:10.1111/j.1096-0031.2006.00142.x) [DOI] [PubMed] [Google Scholar]
- Magallón S., Crane P. R., Herendeen P. S.1999Phylogenetic pattern, diversity and diversification of eudicots. Ann. Mo. Bot. Gard. 86, 297–372 (doi:10.2307/2666180) [Google Scholar]
- Martínez-Millán M., Crepet W. L., Nixon K. C.2009Pentpetalum trifasciculandricus gen. et sp. nov., a thealean fossil flower from the Raritan Formation, New Jersey, USA (Turonian, Late Cretaceous). Am. J. Bot. 96, 933–949 (doi:10.3732/ajb.0800347) [DOI] [PubMed] [Google Scholar]
- Matthews M. L., Endress P. K.2002Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). Bot. J. Linn. Soc. 140, 321–381 (doi:10.1046/j.1095-8339.2002.00105.x) [Google Scholar]
- Matthews M. L., Endress P. K.2004Comparative floral structure and systematics in Cucurbitales (Corynocarpaceae, Coriariaceae, Tetramelaceae, Datiscaceae, Begoniaceae, Cucurbitaceae, Anisophylleaceae). Bot. J. Linn. Soc. 145, 129–185 (doi:10.1111/j.1095-8339.2003.00281.x) [Google Scholar]
- Matthews M. L., Endress P. K.2005Comparative floral structure and systematics in Crossosomatales (Crossosomataceae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae). Bot. J. Linn. Soc. 147, 1–46 (doi:10.1111/j.1095-8339.2005.00347.x) [Google Scholar]
- Matthews M. L., Endress P. K.2006Floral structure and systematics in four orders of rosids, including a broad survey of floral mucilage cells. Plant Syst. Evol. 260, 199–221 [Google Scholar]
- Matthews M. L., Endress P. K.2008Comparative floral structure and systematics in Chrysobalanaceae s. l. (Chrysobalanaceae, Dichapetalaceae, Euphroniaceae, Trigoniaceae; Malpighiales). Bot. J. Linn. Soc. 157, 249–309 (doi:10.1111/j.1095-8339.2008.00803.x) [Google Scholar]
- Mauseth J. D.1988Plant anatomy Menlo Park, CA: Benjamin/Cummings [Google Scholar]
- Morton C. M., Chase M. W., Kron K. A., Swensen S. M.1996A molecular evaluation of the monophyly of the Order Ebenales based upon rbcL sequence data. Syst. Bot. 21, 567–586 (doi:10.2307/2419616) [Google Scholar]
- Nash G. V.1903A revision of the family Fouquieriaceae. Bull. Torrey Bot. Club 30, 449–459 (doi:10.2307/2478732) [Google Scholar]
- Pinheiro M. C., Teixeira O. W., Alves de Lima H., Rodrigues Correia M. C.1995Biologia da reproducao de Norantea brasiliensis Choisy (Marcgraviaceae). Revista Brasil. Biol. 55(Suppl. 1), 79–88 [Google Scholar]
- Salminen J.-P., Lempa K.2002Effects of hydrolysable tannins on a herbivorous insect: fate of individual tannins in insect digestive tract. Chemoecology 12, 203–211 (doi:10.1007/PL00012670) [Google Scholar]
- Schneider J. V., Bayer C.2004Clethraceae. The families and genera of vascular plants. Flowering plants—dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, vol. 6 (ed. Kubitzki K.), pp. 69–73 Berlin, Germany: Springer [Google Scholar]
- Schönenberger J.2005Rise from the ashes—the reconstruction of charcoal fossil flowers. Trends Plant Sci. 10, 436–443 (doi:10.1016/j.tplants.2005.07.006) [DOI] [PubMed] [Google Scholar]
- Schönenberger J.2009Comparative floral structure and systematics of Fouquieriaceae and Polemoniaceae (Ericales). Int. J. Plant Sci. 170, 1132–1167 (doi:10.1086/605875) [Google Scholar]
- Schönenberger J., Friis E. M.2001Fossil flowers of ericalean affinity from the Late Cretaceous of Southern Sweden. Am. J. Bot. 88, 467–480 (doi:10.2307/2657112) [PubMed] [Google Scholar]
- Schönenberger J., Anderberg A. A., Sytsma K. J.2005Molecular phylogenetics and patterns of floral evolution in the Ericales. Int. J. Plant Sci. 166, 265–288 (doi:10.1086/427198) [Google Scholar]
- Schoute J. C.1935On corolla aestivation and phyllotaxis of floral phyllomes. Verh. K. Akad. Wet. Amsterdam Afd Natuurkunde Sec 2 34, 1–77 [Google Scholar]
- Shimizu T., Takao S.1982Taxonomic significance of the inner structure of the ovary in the genus Impatiens (Balsaminaceae). Bot. Mag. Tokyo 95, 89–99 (doi:10.1007/BF02488576) [Google Scholar]
- Soltis D. E., et al. 2000Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot. J. Linn. Soc. 133, 381–461 [Google Scholar]
- Stevens P. F.2001Angiosperm Phylogeny Website. Version 9 June 2008. See http://www.mobot.org/MOBOT/research/APweb/ [Google Scholar]
- Sytsma K. J., Walker J. B., Schönenberger J., Anderberg A. A.2006. Phylogenetics, biogeography, and radiation of Ericales. Annual BSA Meeting, Botany 2006, Chicago, CA, Abstract volume: 71 [Google Scholar]
- Sytsma K. J., Kleist T. J., Nickrent D., Barkman T. J., Schönenberger J.2009. Phylogenetics in Ericales—the utility of mtDNA gene sequences and the placement of the holoparasite Mitrastema. Annual BSA Meeting, Botany & Mycology 2009, Snowbird, UT, Abstract [Google Scholar]
- Takhtajan A.1997Diversity and classification of flowering plants New York, NY: Columbia University Press [Google Scholar]
- Thorne R. F.1968Synopsis of a putatively phylogenetic classification of flowering plants. Aliso 6, 57–66 [Google Scholar]
- Tomlinson P. B.1986The botany of mangroves. Cambridge Tropical Biology Series New York, NY: Cambridge University Press [Google Scholar]
- Tschapka M., Dressler S., von Helversen O.2006Bat visits to Marcgravia pittieri and notes on the inflorescence diversity within the genus Marcgravia (Marcgraviaceae). Flora 201, 383–388 [Google Scholar]
- Vogel S.1977Nektarien und ihre ökologische Bedeutung. Apidologie 8, 321–335 [Google Scholar]
- Vogel S., Coccuci A.1988Pollen threads in Impatiens: their nature and function. Beitr. Biol. Pflanzen 63, 271–287 [Google Scholar]
- Weber A.2003What is morphology and why is it time for its renaissance in plant systematics. In Deep morphology: towards a renaissance of morphology in plant systematics (eds Stuessy T. F., Hörandl E., Mayer V.), pp. 3–34 Ruggell, Liechtenstein: Gantner [Google Scholar]
- Wilken D. H.2004Polemoniaceae. In The families and genera of vascular plants. Flowering plants—dicotyledons. Celastrales, Oxalidales, Rosales, Cornales, Ericales, vol. 6 (ed. Kubitzki K.), pp. 300–312 Berlin, Germany: Springer [Google Scholar]
- Wilson P.1995Selection for pollination success and the mechanical fit of Impatiens flowers around bumblebee bodies. Biol. J. Linn. Soc. 55, 355–383 [Google Scholar]