Abstract
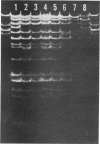
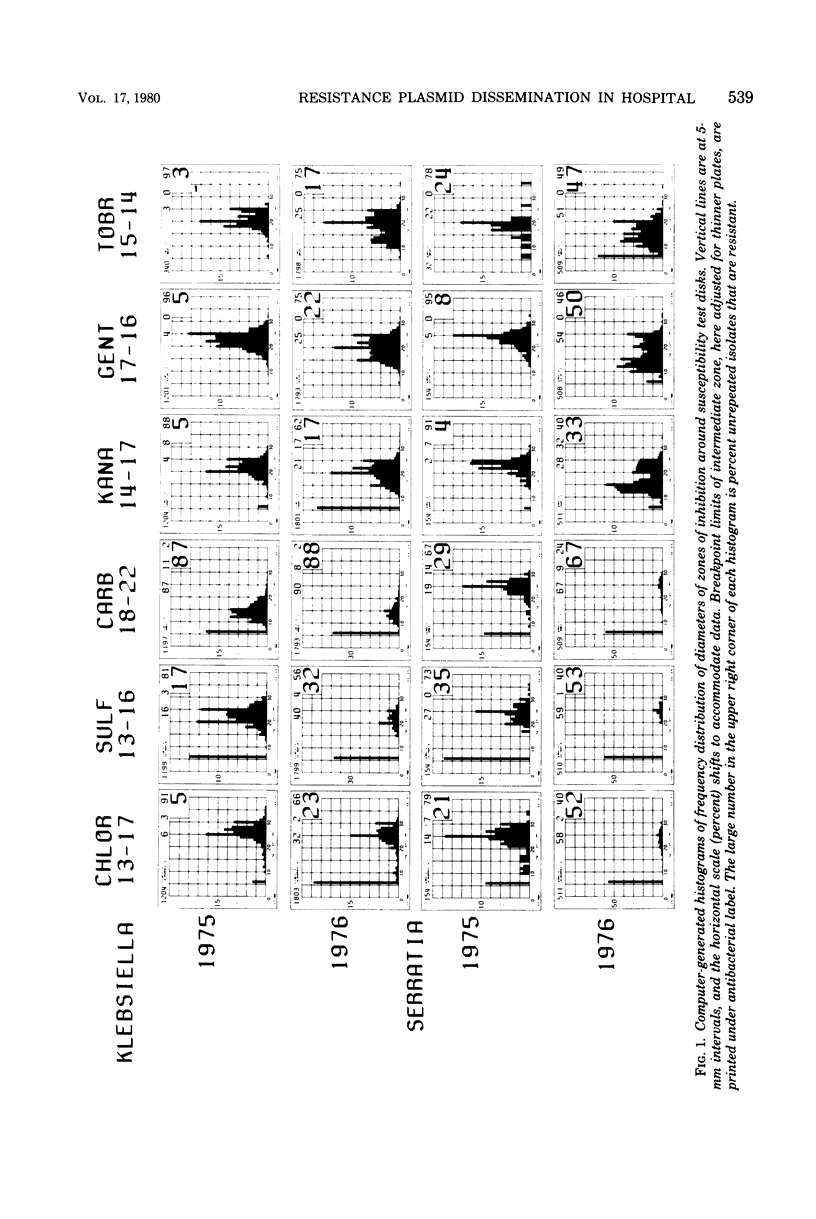
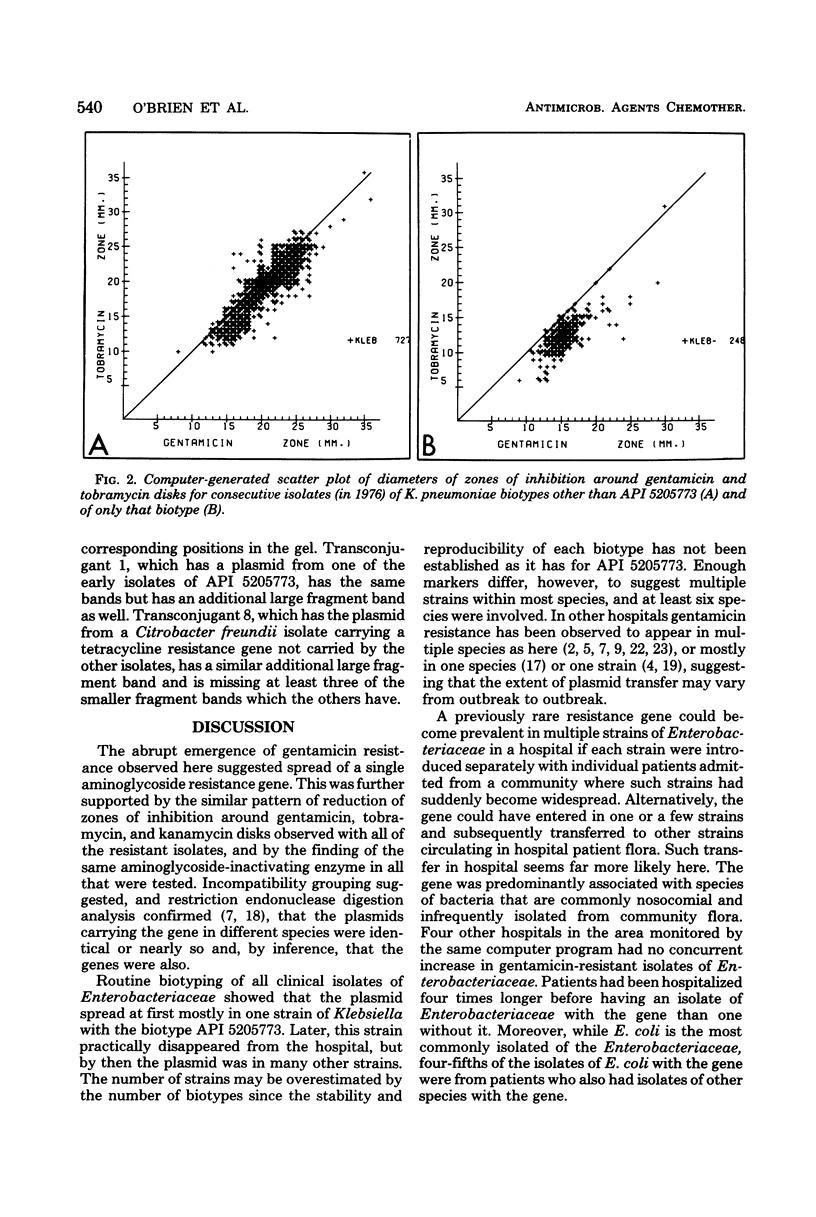
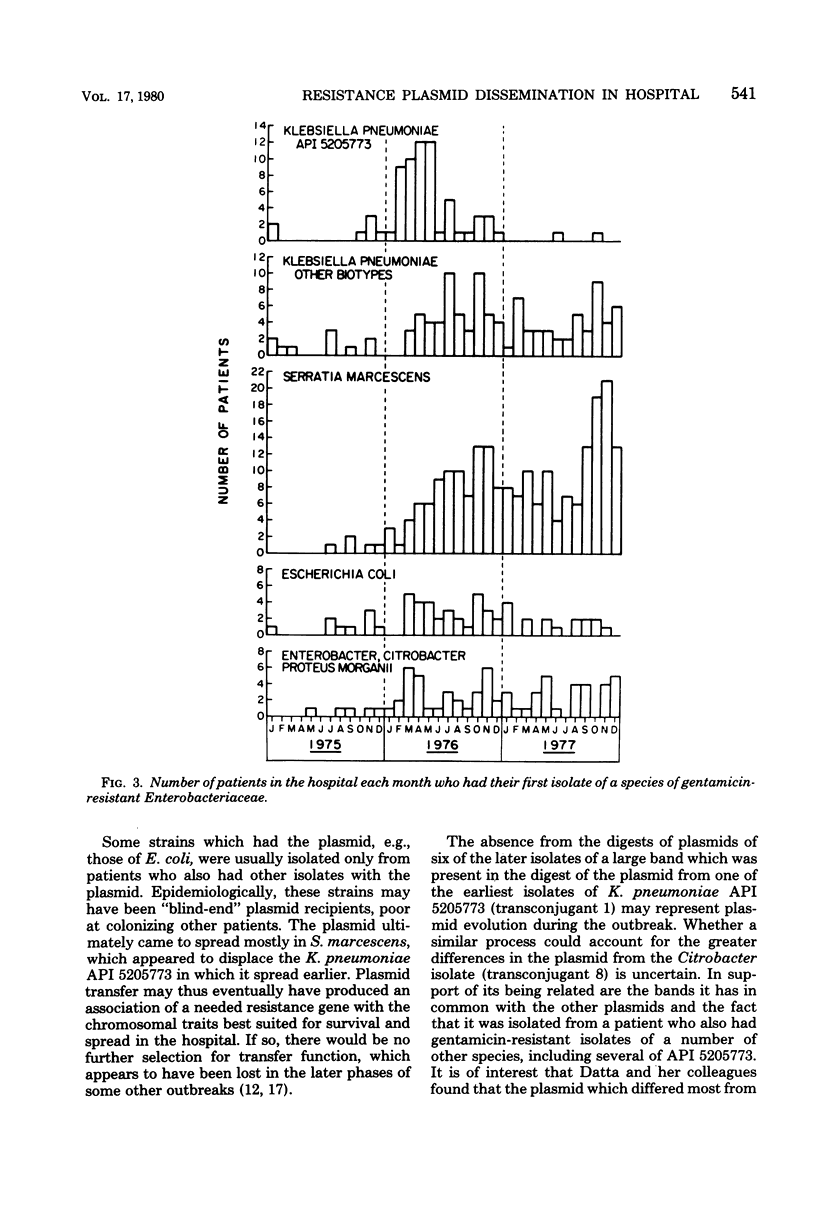
The 2'' aminoglycoside nucleotidyltransferase, AAD (2''), which adenylates gentamicin, tobramycin, and kanamycin, became prevalent over several months in multiple strains and species of Enterobacteriaceae isolated at one hospital. Eight plasmids with the gene for this enzyme purified from different strains and species isolated at different times had similar EcoRI digestion fragments, indicating that the gene had disseminated on one plasmid without transposition. This 56.5-megadalton plasmid of incompatibility group M, which also carried three other resistance genes, spread, at first, largely in one strain of Klebsiella pneumoniae, which later disappeared. It transferred to some strains which tended not to colonize other patients and later circulated predominantly in Serratia marcescens. Computer surveillance of routine hospital laboratory results was able to detect and trace the gene and the plasmid and measure their effect on resistance prevalence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Block C. S. Gentamicin-resistant gram-negative bacilli in hospital patients. Part I. Preliminary epidemiological assessment. S Afr Med J. 1978 Mar 18;53(11):391–395. [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Datta N., Hughes V. M., Nugent M. E., Richards H. Plasmids and transposons and their stability and mutability in bacteria isolated during an outbreak of hospital infection. Plasmid. 1979 Apr;2(2):182–196. doi: 10.1016/0147-619x(79)90037-4. [DOI] [PubMed] [Google Scholar]
- Davey R. B., Pittard J. Plasmids mediating resistance to gentamicin and other antibiotics in Enterobacteriaceae from four hospitals in Melbourne. Aust J Exp Biol Med Sci. 1977 Jun;55(3):299–307. doi: 10.1038/icb.1977.25. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Inamine J. M., Minshew B. H. Common plasmid specifying tobramycin resistance found in two enteric bacteria isolated from burn patients. Antimicrob Agents Chemother. 1978 Feb;13(2):312–317. doi: 10.1128/aac.13.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Buxton A. E., Hughes R. A., Cleary P. P., Arbaczawski J., Stamm W. E. Nosocomial multiply resistant Klebsiella pneumoniae: epidemiology of an outbreak of apparent index case origin. Antimicrob Agents Chemother. 1979 Apr;15(4):608–615. doi: 10.1128/aac.15.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Barker D. F., Ross D. G., Botstein D. Properties of the translocatable tetracycline-resistance element Tn10 in Escherichia coli and bacteriophage lambda. Genetics. 1978 Nov;90(3):427–461. doi: 10.1093/genetics/90.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfhagen T. R., Ferrel J. A., Menefee C. L., Loper J. C. Resistance plasmids of Pseudomonas aeruginosa: change from conjugative to nonconjugative in a hospital population. Antimicrob Agents Chemother. 1976 May;9(5):810–816. doi: 10.1128/aac.9.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. F., Acar J. F., Medeiros A. A., Norton R. A., Goldstein F., Kent R. L. International comparison of prevalence of resistance to antibiotics. JAMA. 1978 Apr 14;239(15):1518–1523. [PubMed] [Google Scholar]
- O'Brien T. F., Kent R. L., Medeiros A. A. Computer surveillance of shifts in the gross patient flora during hospitalization. J Infect Dis. 1975 Feb;131(2):88–96. doi: 10.1093/infdis/131.2.88. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Norton R. A., Kent R. L., Medeiros A. A. International surveillance of prevalence of antibiotic resistance. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):59–66. doi: 10.1093/jac/3.suppl_c.59. [DOI] [PubMed] [Google Scholar]
- Rennie R. P., Duncan I. B. Emergence of gentamicin-resistant Klebsiella in a general hospital. Antimicrob Agents Chemother. 1977 Feb;11(2):179–184. doi: 10.1128/aac.11.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. L., Peterson B. C., Gerding D. N., Cleary P. P. Physical characterization of ten R plasmids obtained from an outbreak of nosocomial Klebsiella pneumoniae infections. Antimicrob Agents Chemother. 1979 Apr;15(4):616–624. doi: 10.1128/aac.15.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Weinstein R. A., Stamm W. E. Epidemics of nosocomial urinary tract infection caused by multiply resistant gram-negative bacilli: epidemiology and control. J Infect Dis. 1976 Mar;133(3):363–366. doi: 10.1093/infdis/133.3.363. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Sugden B., De Troy B., Roberts R. J., Sambrook J. Agarose slab-gel electrophoresis equipment. Anal Biochem. 1975 Sep;68(1):36–46. doi: 10.1016/0003-2697(75)90676-4. [DOI] [PubMed] [Google Scholar]
- Thomas F. E., Jackson R. T., Melly A., Alford R. H. Sequential hospitalwide outbreaks of resistant Serratia and Klebsiella infections. Arch Intern Med. 1977 May;137(5):581–584. [PubMed] [Google Scholar]