Abstract
Previous studies suggest that high glucose-induced RhoA/Rho kinase/CPI-17 activation is involved in diabetes-associated vascular smooth muscle hypercontractility. However, the upstream signaling that links high glucose and RhoA/Rho kinase/CPI-17 activation is unknown. Here we report that calcium-independent phospholipase A2β (iPLA2β) is required for high glucose-induced RhoA/Rho kinase/CPI-17 activation and thereby contributes to diabetes-associated vascular smooth muscle hypercontractility. We demonstrate that high glucose increases iPLA2β mRNA, protein, and iPLA2 activity in a time-dependent manner. Protein kinase C is involved in high glucose-induced iPLA2β protein up-regulation. Inhibiting iPLA2β activity with bromoenol lactone or preventing its expression by genetic deletion abolishes high glucose-induced RhoA/Rho kinase/CPI-17 activation, and restoring expression of iPLA2β in iPLA2β-deficient cells also restores high glucose-induced CPI-17 phosphorylation. Pharmacological and genetic inhibition of 12/15-lipoxygenases has effects on high glucose-induced CPI-17 phosphorylation similar to iPLA2β inhibition. Moreover, increases in iPLA2 activity and iPLA2β protein expression are also observed in both type 1 and type 2 diabetic vasculature. Pharmacological and genetic inhibition of iPLA2β, but not iPLA2γ, diminishes diabetes-associated vascular smooth muscle hypercontractility. In summary, our results reveal a novel mechanism by which high glucose-induced, protein kinase C-mediated iPLA2β up-regulation activates the RhoA/Rho kinase/CPI-17 via 12/15-lipoxygenases and thereby contributes to diabetes-associated vascular smooth muscle hypercontractility.
Keywords: Diseases/Diabetes, Lipid/Phospholipases, Phosphorylation/Phosphatases/Serine-Threonine, Signal Transduction, Signal Transduction/Phospholipase A, Signal Transduction/Phosphoprotein Phosphatases/Serine/Threonine, Tissue/Organ Systems/Muscle/Smooth
Introduction
The prevalence of diabetes has increased dramatically in the United States and worldwide. Diabetes-associated microvascular and macrovascular complications are the major causes of increased mortality and morbidity in diabetic patients (1). It is recognized that abnormal vascular reactivity, including an increase in vasoconstrictive responses and/or a decrease in vasodilatory responses, are present early in diabetes, and this might contribute to the development of diabetes-associated vascular complications (2). Although diabetes-associated endothelial and vasodilatatory dysfunction has been studied extensively, much less is known about vascular smooth muscle dysfunction in diabetes, and the molecular mechanisms that underlie endothelium-independent, diabetes-associated vascular smooth muscle hypercontractility are largely unknown.
G-protein-coupled receptor agonists can induce significant smooth muscle contraction by inhibiting myosin light chain phosphatase at a given Ca2+ concentration by a mechanism designated “Ca2+ sensitization” (3). A small GTP-binding protein RhoA, its downstream effector Rho kinase (ROCK),2 protein kinase C (PKC), and CPI-17 (protein kinase C-potentiated phosphatase inhibitor of 17 kDa) are recognized as four key participants in Ca2+ sensitization of vascular smooth muscle contraction (4). Importantly, abnormal activation of Ca2+ sensitization pathways has been implicated in the pathogenesis of a wide range of cardiovascular diseases, including vasospasm, atherosclerosis, ischemia/reperfusion injury, systemic hypertension, pulmonary hypertension, stroke, and heart failure in both animal models and clinical trials (5). Nonetheless, there is little information about the role of RhoA/ROCK/CPI-17 in diabetes-associated vascular smooth muscle hypercontractility at present.
Recently, studies from our laboratory (6) and others (7–10) suggest that an increase in Ca2+ sensitization signaling in smooth muscle tissues isolated from type 1 and type 2 diabetic animals is involved in diabetes-associated hypercontractility. In particular, we have demonstrated that CPI-17 is activated by high glucose in primary cultured vascular smooth muscle cells (VSMC) and blood vessels from obese and type 2 diabetic db/db mice, and ROCK is the kinase responsible for high glucose-induced CPI-17 phosphorylation and diabetes-associated hypercontractility in db/db mice (6). However, the upstream signaling pathways that link exposure to high concentrations of glucose and activation of RhoA/ROCK/CPI-17 are unknown.
Phospholipases A2 (PLA2) are enzymes that hydrolyze fatty acid substituents from the sn-2 position of phospholipids, and their action results in concomitant production of lysophospholipids and free fatty acids. Based upon their cellular location and the Ca2+ requirement for enzymatic activity, PLA2 can be classified into three groups: secretory PLA2, cytosolic PLA2, and calcium-independent PLA2 (iPLA2) (11). There is a family of iPLA2 intracellular enzymes that do not require Ca2+ for catalytic activity, and its members include iPLA2α, β, γ, δ, ϵ, ξ, and η (12). Of these, iPLA2β was the first recognized and most extensively characterized, and it has a ubiquitous tissue distribution and is implicated in several cellular functions (13).
Bromoenol lactone (BEL) is a suicide substrate of iPLA2 inhibitor and has been widely used for inhibiting iPLA2 activity in many cell types and tissues (14). Using this pharmacological inhibitor, previous studies suggest that iPLA2 is involved in agonist-induced smooth muscle contraction (15–17). Although the mechanism by which iPLA2 mediates smooth muscle contraction remains elusive, Maeda et al. (18) reported that inhibiting iPLA2 with BEL inhibits thrombin-induced ROCK activation in endothelial cells. However, BEL also inhibits many other cellular enzymes, including phosphatidic acid phosphohydrolase (19), serine proteases (20), and serine lipases (21). In addition, BEL inhibits all iPLA2 isoforms (12). Thus, under physiological conditions, the mechanism by which iPLA2β mediates smooth muscle contraction via ROCK has not been established. Moreover, it is unknown whether iPLA2β is involved in high glucose-induced RhoA/ROCK/CPI-17 activation in VSMC and in diabetic vascular smooth muscle hypercontractility.
The current study focuses on the role of iPLA2β in the calcium sensitization of smooth muscle contraction under diabetic conditions. In particular, we specifically test the hypothesis that activation of iPLA2β by hyperglycemia results in RhoA/ROCK/CPI-17 activation and thereby contributes to diabetes-associated vascular smooth muscle hypercontractility.
EXPERIMENTAL PROCEDURES
Materials and Animals
The antibodies against iPLA2β, CPI-17, and phosphorylated CPI-17 (Thr-38) were generated in our laboratory as described previously (6, 22–24). The antibodies against PKCβII, RhoA, MYPT1, and phosphorylated MYPT1 (Thr-853) were purchased from Santa Cruz Biotech (Santa Cruz, CA). The antibody against phosphorylated PKCβII (Thr-641) was purchased from Abcam (Cambridge, MA). The antibody against β-actin was purchased from Cell Signaling (Danvers, MA). BEL, (R)-BEL, (S)-BEL, 17-octadecynoic acid, MK886, baicalein, and luteolin were purchased from Cayman (Ann Arbor, MI). Nordihydroguaiaretic acid and indomethacin were purchased from Biomol (Plymouth Meeting, PA). Other chemicals were purchased from Sigma-Aldrich.
12-Week-old male C57BL/KsJ db/db mice (db/db−/−) and age/gender-matched nondiabetic (db/db+/−) C57BL/KsJ control mice, 8-week-old male and female 12/15-lipoxygenase-deficient mice, and 4-week-old male C57BL/6 mice were purchased from Jackson Lab (Bar Harbor, ME). Male Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). The iPLA2β-null mice were generated in the laboratory of Dr. John Turk, as described elsewhere (25). All of the animals were housed at an animal care facility at the Medical Center of the University of Kentucky that is accredited by the American Association for Accreditation of Laboratory Animal Care. All of the animal protocols were approved by the Institutional Animal Care and Use Committee.
To induce diabetes, 300–500-g Sprague-Dawley rats were induced by intravenous administration of 50 mg/kg of streptozotocin (STZ) or saline as described (26). 7-Week-old male C57BL/6 mice, iPLA2β-null mice, and age- and gender-matched wild-type mice were injected intraperitoneally with 40 mg of STZ or saline/kg of body weight on five consecutive days as described (27). Blood glucose and body weight were measured immediately before injection of STZ and at weekly intervals thereafter as described (28). The presence of diabetes was confirmed by sustained blood glucose values of 300 mg/dl.
Isometric Tension Measurement
Aortic arteries were isolated either from 12-week-old male db/db and gender/age-matched control mice or STZ mice as well as control mice. Isometric contractions in endothelium-denuded aortas were measured by using a “bubble chamber” as described previously (6, 28).
Primary VSMC Culture
The procedure for isolation and culture of primary VSMC from male Sprague-Dawley rats, iPLA2β- or 12/15 lipoxygenase-null mice, and wild-type mice was described previously (6, 22–24).
Real Time PCR
The primers for iPLA2β, 5-LO, platelet-type 12-LO, and leukocyte-type 12 were described in the supplemental materials. The procedure for real time PCR was described previously (22, 28).
Western Blot Analysis
After various treatments as indicated in the text, the cells were frozen with liquid nitrogen and subject to Western blot as described (6, 22–24).
iPLA2 Assay
iPLA2 activity was measured by an iPLA2 assay kit (Cayman, Ann Arbor, MI) under a Ca2+-free condition as described (22, 29).
[3H]AA Release
The method to label VSMC with [3H]AA and measure [3H]AA release and cellular [3H]phospholipid was performed as described (15, 30).
Measurement of Lipoxygenase Product 15(S)-HETE
The 15(S)-HETE in the supernatants of rat VSMC was extracted by C18 reverse phase columns (VWR, West Chester, PA) and measured by a specific 15(S)-HETE enzyme immunoassay kit (Assay Designs, Ann Arbor, MI) following the manufacturer's instructions.
iPLA2β Adenovirus
The procedure of iPLA2β adenovirus generation, purification, and infection has been described previously (6, 15, 22–24).
Statistical Analysis
The data are expressed as the means ± S.E. Statistical analyses were performed by using an unpaired t test or analysis of variance (GraphPad, Prim 4) and appropriate post-hoc analyses.
RESULTS
iPLA2β Is Activated by High Glucose
To explore the potential role of iPLA2β in diabetic vasculopathy, we first determined whether high concentrations of glucose activate and/or up-regulate iPLA2β in primary cultured rat VSMC. We observed that high glucose significantly increased iPLA2β mRNA (Fig. 1, A and B), protein expression (Fig. 1, C and D), and iPLA2 activity (Fig. 1E) in a time-dependent manner. In contrast, high glucose did not change cytosolic PLA2α protein expression (supplemental Fig. S1). Moreover, the same concentration of mannitol did not affect iPLA2β mRNA (Fig. 1, A and B), protein expression (Fig. 1, C and D), and iPLA2 activity (Fig. 1E), indicating that hyperosmolarity is not responsible for high glucose-induced iPLA2β up-regulation/activation.
FIGURE 1.
High glucose increases iPLA2β mRNA, protein expression, and enzymatic activity in a time-dependent manner. 70–80% confluent rat (A–E) or mouse (F) aortic VSMC were incubated in 10% FBS medium containing NG (5.5 mm), HG (25 mm), or mannitol (Mann, 5.5 mm glucose plus 19.5 mm mannitol) for various time periods as indicated. The medium was changed every 12 h. A, a representative DNA acrylamide gel of real time PCR products (NTC, no template control). B, summary of data shown in A; C, a representative Western blot of iPLA2β and β-actin; D, summary of data of shown in C; E, summary of data of iPLA2 assay in rat VSMC; F, summary of data of iPLA2 assay in mouse VSMC. Each experiment was repeated at least three times. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus NG. NS, not significant versus iPLA2β-KO/NG/6 h or iPLA2β-KO/HG/1 h.
The assay used in Fig. 1E measures overall iPLA2 activity (22, 29). The temporal correlation between high glucose-induced iPLA2β protein expression and iPLA2 activity at 1, 6, 12, and 72 h indicates that the increase in iPLA2 activity observed in Fig. 1E may be attributable to iPLA2β. To test this possibility, we conducted similar studies with VSMC from iPLA2β-null and WT mice (22). Fig. 1F illustrates that high glucose-stimulated iPLA2 activity in mouse VSMC, as it did in rat VSMC (Fig. 1E), but high glucose failed to significantly stimulate iPLA2 activity in iPLA2β-deficient mouse VSMC. This suggests that the high glucose-induced increase in VSMC iPLA2 activity primarily results from iPLA2β.
PKC Is Involved in High Glucose-induced iPLA2β Protein Up-regulation
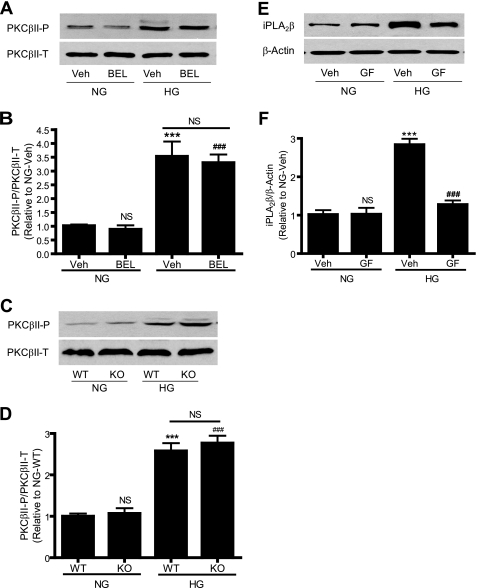
It is well recognized that PKCβII is activated by high glucose and plays an important role in diabetic vascular complications (31). To investigate whether iPLA2β is involved in high glucose-induced PKCβII activation, we determined the effect of genetic deletion of iPLA2β or its inhibition by BEL on high glucose-induced PKCβII activation. BEL is a suicide substrate that selectively inhibits iPLA2 at concentrations that have little effect on secretory or cytosolic PLA2 activities (32). PKCβII activation was determined by Western blotting using a phospho-specific antibody that recognizes PKCβII phosphorylation (33). We found that neither inhibiting iPLA2 with BEL (Fig. 2, A and B) nor genetic deletion of iPLA2β (Fig. 2, C and D) affected high glucose-induced PKCβII activation, suggesting that PKCβII is not downstream of iPLA2β in the response to high glucose stimulation.
FIGURE 2.
BEL inhibition or genetic deletion of iPLA2β does not affect high glucose-induced PKCβII phosphorylation, whereas inhibition of PKC attenuates high glucose-induced iPLA2β protein up-regulation. 70–80% confluent rat (A, B, E, and F) and mouse (C and D) aortic VSMC were incubated with 10% FBS medium containing NG or HG in the presence of BEL (3 μm) for 24 h (A and B), GF109203X (GF, 3 μm) for 12 h (E and F), and vehicle (Veh, Me2SO), respectively. A, C, and E, representative Western blots of iPLA2β, total PKCβII (PKCβII-T), and phosphorylated PKCβII (PKCβII-P); B, D, and F, summary of data shown in A, C, and E, respectively. Each experiment was repeated at least three times. ***, p < 0.001 versus NG/vehicle in B, NG/WT in D, and NG/vehicle in F; ###, p < 0.001 versus NG/vehicle in B, NG/WT in D, and HG/vehicle in F; NS, not significant, HG/vehicle versus HG/BEL in B; HG/WT versus HG/KO in D.
We then tested whether PKCβII is involved in high glucose-induced iPLA2β activation. The cells were incubated with high glucose in the presence of GF109203X, which inhibits both conventional and novel PKCs including PKCβII. Surprisingly, GF109203X abolished high glucose-induced iPLA2β protein up-regulation (Fig. 2, E and F), indicating that PKC is involved in such up-regulation.
iPLA2β Is Required for High Glucose-induced CPI-17 Phosphorylation
To investigate whether iPLA2β plays a role in high glucose-induced CPI-17 phosphorylation, we determined whether inhibiting iPLA2 with BEL blocks high glucose-induced CPI-17 phosphorylation. Fig. 3(A and B) illustrates that, in rat VSMC, BEL abolished the high glucose-induced increase in CPI-17 phosphorylation without affecting the total CPI-17 protein expression or basal CPI-17 phosphorylation. Similar results were also obtained from human VSMC (supplemental Fig. S2).
FIGURE 3.
BEL inhibition or genetic deletion of iPLA2β abolishes high glucose-induced CPI-17 phosphorylation, whereas reconstitution of iPLA2β in iPLA2β-deficient VSMC restores it. 70–80% confluent VSMC were incubated with FBS-free medium containing NG for 24–48 h. Quiescent rat (A and B), mouse WT or iPLA2β-deficient (iPLA2β KO) aortic VSMC (C and D), and iPLA2β adenovirus-infected mouse iPLA2β-KO VSMC (E and F) were incubated with 10% FBS medium containing NG or HG in the presence of BEL (3 μm) or vehicle (Veh, Me2SO) for 48 h. iPLA2β adenoviral expression was induced by doxycycline (1 μg/ml). A, C, and E, representative Western blots Western blots of total CPI-17 (CPI-17-T) and phosphorylated CPI-17 (CPI-17-P). B, D, and F, summary of data shown in A, C, and E, respectively. Each experiment was repeated at least four times. ***, p < 0.001 versus NG/vehicle in B, WT/NG in D, and WT/NG in F; ##, p < 0.01 versus iPLA2β-KO/NG in F; ###, p < 0.001 versus HG/vehicle in B and WT/HG in D; NS, not significant, versus NG/vehicle and HG/BEL in B, WT/NG and iPLA2β-KO/HG in D, and iPLA2β-KO/NG in F.
In addition to inhibiting iPLA2β, BEL also inhibits other iPLA2 family members and a variety of other enzymes (11). To determine whether the inhibition of high glucose-induced CPI-17 phosphorylation by BEL is attributable to inhibition of iPLA2β, we determined whether genetic deletion of iPLA2β in mouse aortic VSMC affects high glucose-induced CPI-17 phosphorylation. Fig. 3 (C and D) illustrates that genetic deletion of iPLA2β diminished high glucose-induced CPI-17 phosphorylation without affecting total CPI-17 protein expression and basal CPI-17 phosphorylation, and this closely mimics the effects of BEL.
To examine further the requirement for iPLA2β in high glucose-induced CPI-17 phosphorylation, we determined whether restoration of iPLA2β expression in iPLA2β-deficient VSMC by tetracycline-inducible, adenovirus-mediated gene transfer would also restore high glucose-induced CPI-17 phosphorylation. Fig. 3 (E and F) illustrates that expressing iPLA2β in iPLA2β-deficient VSMC almost completely restored high glucose-induced CPI-17 phosphorylation. Notably, a double band in phosphor-CPI-17 blot was observed in Fig. 3E, although its identity is currently not known. Importantly, neither adenovirus (in the absence of DOX; Fig. 3, E and F) nor doxycycline alone (data not shown) restored high glucose-induced CPI-17 phosphorylation.
12/15-Lipoxygenases Are Involved in High Glucose-induced CPI-17 Phosphorylation
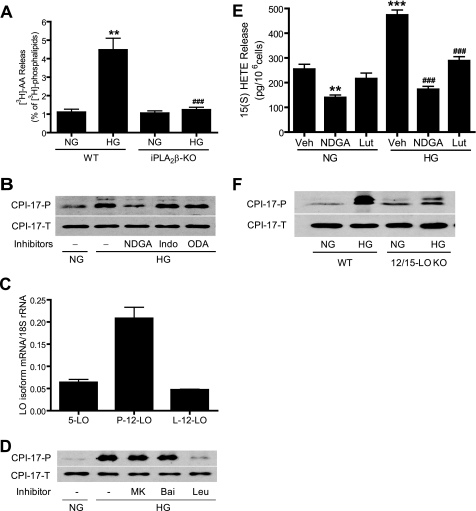
The products of iPLA2 action on phospholipid substrates are a free fatty acid, e.g. AA, and a 2-lysophospholipid, e.g. LPC. AA can be further metabolized to a variety of biologically active eicosanoids via LO, cyclooxygenases (COX), and cytochrome P450-dependent monooxygenases (CytP450) (34). To determine whether AA and/or its metabolites are involved in high glucose-induced CPI-17 phosphorylation, we first determined whether high concentrations of glucose induce AA release and whether this is affected by inhibition of iPLA2β. Fig. 4A illustrates that high glucose markedly stimulated AA release from wild-type mouse VSMC and that such release was abolished in iPLA2β-null cells.
FIGURE 4.
12/15-Lipoxygenases(s) are selectively involved in high glucose-induced CPI-17 phosphorylation. A, 70–80% confluent mouse WT and iPLA2β-deficient VSMC were labeled with [3H]AA and then incubated with NG or HG for 6 h. The medium [3H]AA and cellular [3H]phospholipids were analyzed by thin layer chromatography and quantified by liquid scintillation spectrometry. B, D, E, and F, quiescent rat (B, D, and E) and mouse (F) aortic VSMC were incubated with NG or HG for 24 h (E) or 48 h (B, D, and F) in the presence of vehicle (Veh, Me2SO), NDGA (30 μm), indomethacin (Indo, 50 μm),17-octadecynoic acid (ODA, 10 μm), MK886 (MK, 1 μm), baicalein (Bai, 10 μm), and luteolin (Lut, 50 μm), respectively. Phosphorylated CPI-17 (CPI-17-P) and total CPI-17 (CPI-17-T) were determined by Western blot (B, D, and F). The medium 15(S)-HETE was measured by a specific 15(S)-HETE EIA Kit (E). C, LO isoform expressions were determined by real time PCR in rat VSMC. The results are expressed as the means ± S.E. from three experiments. **, p < 0.01 versus WT/NG in A versus NG/vehicle in E. ***, p < 0.001 versus NG/vehicle in E. ###, p < 0.001 versus WT/HG in A and HG/vehicle in E.
To determine which AA metabolites are involved in high glucose-induced CPI-17 phosphorylation, rat VSMC were incubated with high glucose in the presence of the LO inhibitor nordihydroguaiaretic acid (NDGA) (22), the COX inhibitor indomethacin (28), or the CytP450 inhibitor 17-octadecynoic acid (35), respectively. Fig. 4B and supplemental Fig. S3 illustrate that high glucose-induced CPI-17 phosphorylation was prevented by NDGA but unaffected by indomethacin or 17-octadecynoic acid. This suggests that LO enzyme(s) are involved in high glucose-induced CPI-17 phosphorylation but that COX and CytP450 are not.
Various LO(s) exhibit regiospecificity for oxygenation of AA at the 5-, 8-, 12-, or 15- positions, and these enzymes have been implicated in the pathogenesis of cardiovascular diseases (34). To determine which LO(s) are expressed in VSMC, we performed quantitative reverse transcription-PCR using primers that specifically recognize rat 5-LO, rat leukocyte-type 12-LO, and rat platelet-type 12-LO. Fig. 4C shows that all three LO mRNAs are expressed in rat VSMC.
To determine which LO(s) might be involved in high glucose-induced CPI-17 phosphorylation, we then incubated rat VSMC with high glucose in the presence of MK886 (a 5-LO inhibitor), baicalein (a 12-LO inhibitor), and luteolin (a 15-LO inhibitor), respectively, as described (22). We observed that luteolin diminished high glucose-induced CPI-17 phosphorylation (Fig. 4D and supplemental Fig. S4) but that MK886 and baicalein did not.
To determine whether luteolin and NDGA inhibit 15-LO and thereby inhibit high glucose-induced CPI-17 phosphorylation, we measured 15(S)-HETE, which is a product of rat leukocyte-type 12-LO. That enzyme is highly related to human and rabbit 15-LO, is distinct from rat platelet-type 12-LO, and is known to form both 12(S)-HETE and 15(S)-HETE (36). Fig. 4E illustrates that high concentrations of glucose stimulated 15(S)-HETE release and that such release was abolished by luteolin and NDGA in rat VSMC.
The potential role of 12/15-LO in high glucose-induced CPI-17 phosphorylation was further examined in VSMC from 12/15-LO-null mice. Fig. 4F and supplemental Fig. S5 illustrate that genetic deletion of 12/15-LO abolished high glucose-induced CPI-17 phosphorylation without affecting total CPI-17 protein expression or basal CPI-17 phosphorylation. This indicates that 12/15-LOs are involved in high glucose-induced CPI-17 phosphorylation.
iPLA2β Is Required for High Glucose-induced ROCK and RhoA Activation
We recently reported that activation of ROCK by high glucose is responsible for high glucose-induced CPI-17 phosphorylation in VSMC (6). This raises the possibility that ROCK might link iPLA2β action and CPI-17 phosphorylation in the presence of high concentrations of glucose. To test this possibility, we determined whether inhibiting iPLA2β product generation by BEL or genetic deletion affects high glucose-induced ROCK activation. ROCK activation was determined by Western blotting using a phospho-specific antibody that selectively recognizes myosin phosphatase target subunit phosphorylation at Thr-853 (MYPT1-P), a site phosphorylated exclusively by ROCK (37). We found that inhibiting iPLA2β with BEL (Fig. 5, A and B) or genetic deletion of iPLA2β (Fig. 5, C and D) markedly diminished high glucose-induced ROCK activation without significantly affecting basal ROCK activity or total ROCK protein expression.
FIGURE 5.
BEL inhibition or genetic deletion of iPLA2β diminishes high glucose-induced MYPT1 Thr-853 phosphorylation. Quiescent rat (A and B) or mouse WT or iPLA2β-KO aortic VSMC (C and D) were incubated with 10% FBS medium containing NG or HG in the presence or absence of BEL (3 μm) or vehicle (Veh, Me2SO) for 48 h. A and C, representative Western blots of total MYPT1 (MYPT1-T) and phosphorylated MYPT1 (MYPT1-P). B and D, summary of data shown in A and C, respectively. Each experiment was repeated at least three times. **, p < 0.01 versus NG/vehicle in B and WT/NG in D; ###, p < 0.001 versus HG/vehicle in B and WT/HG in D; NS, not significant, versus NG/vehicle and HG/BEL in B and WT/NG or iPLA2β-KO/HG in D.
It has been demonstrated previously that RhoA activation by high glucose leads to ROCK activation in VSMC (6). Moreover, the free fatty acid AA, which can be liberated by iPLA2β action, stimulates ROCK in a RhoA-independent manner (38). Thus, both RhoA-dependent and RhoA-independent mechanisms could participate in high glucose-induced, iPLA2β-mediated ROCK activation. To distinguish which mechanism is responsible for iPLA2β-mediated ROCK activation, we determined the effects on RhoA activation of inhibiting iPLA2β with BEL or genetic deletion of iPLA2β. RhoA activation was determined by a standard pulldown assay in which the GTP-bound, active form of RhoA is selectively captured (6). Fig. 6 illustrates that inhibiting iPLA2β with BEL (Fig. 6, A and B) or genetic deletion of iPLA2β (Fig. 6, C and D) abolished high glucose-induced RhoA activation. Interestingly, inhibiting iPLA2β affected neither basal RhoA activation nor the total RhoA protein expression level.
FIGURE 6.
BEL inhibition or genetic deletion of iPLA2β alleviates high glucose-induced RhoA activation. Quiescent rat (A and B), mouse WT, or iPLA2β-KO (KO) aortic VSMC (C and D) were incubated with 10% FBS medium containing NG or HG in the presence of BEL (3 μm) or vehicle (Veh, Me2SO) for 48 h. A and C, representative Western blots of total RhoA and GTP-bound RhoA (GTP RhoA). B and D, summary of data shown in A and C, respectively. Each experiment was repeated at least three times. ***, p < 0.001 versus NG/vehicle in B or NG/WT in D; ###, p < 0.001 versus HG/vehicle in B or HG/WT in D; NS, not significant, versus NG/vehicle and HG/BEL in B or NG/WT and HG/KO in D.
iPLA2β Is Activated in Aorta Isolated from Diabetic Mice and Involved in Diabetes-associated Vascular Smooth Muscle Hypercontractility
To further explore the in vivo significance of iPLA2β up-regulation/activation in diabetic vascular dysfunction, we isolated aortae and mesenteric arteries by described methods (6, 15, 28) from mice or rats in which diabetes mellitus had been induced by STZ treatment (a model of type 1 diabetes mellitus) or from db/db mice (a model of type 2 diabetes mellitus). Fig. 7 (A and B) illustrates that iPLA2 activities were significantly higher in aortic vascular smooth muscle tissue isolated from STZ mice (Fig. 7A) or db/db mice (Fig. 7B) compared with nondiabetic controls. Moreover, iPLA2β protein expression was also significantly higher in STZ rat aorta (Fig. 7C and supplemental Fig. S6A) and mesenteric artery (Fig. 7D and supplemental Fig. S6B) compared with nondiabetic controls.
FIGURE 7.
iPLA2β is activated in the diabetic vessel wall and is involved in diabetes-associated vascular smooth muscle hypercontractility. Aorta and/or mesentery artery were isolated from db/db and C57BL/KsJ control mice or STZ and C57BL/6J control mice or rats (Sprague-Dawley). A and B, summary of data of iPLA2 assays. C and D, representative Western blots of iPLA2β and β-actin. E–H, summary of data for isometric tension measurement. Intact thoracic aorta (E), α-toxin permeabilized mesenteric artery (F), and intact abdominal aorta (G and H) were incubated with BEL (10 μm, 30 min), Y-27632 (10 μm), (R)-BEL or (S)-BEL (each 10 μm, 60 min), or vehicle (Veh, Me2SO) for 60 min, respectively, prior to 5-HT (1 μm), GTPγS (50 μm), and PE (10 μm) stimulation, respectively. Each experiment was repeated at least three times. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control in A, B, E, F, and G and WT/control in H; ##, p < 0.01; ###, p < 0.001 versus vehicle in E and F; NS, not significant, in G (vehicle versus (R)-BEL) and in H (iPLA2β-KO/control versus iPLA2β-KO/STZ).
The potential role of iPLA2β in diabetes-associated vascular functional abnormalities was then investigated. Consistent with previous reports by us (6, 28) and others (2), contractile responses to serotonin (5-HT; Fig. 7E) in db/db mouse thoracic aorta and to phenylephrine (PE) in STZ mouse abdominal aorta (Fig. 7G) were significantly greater than those of vessels from nondiabetic control mice. Moreover, a significant increase in Ca2+ sensitization of vascular smooth muscle contraction was also observed in db/db mouse mesenteric artery (Fig. 7F), as reported in aorta from STZ mice (10). The reason that we used different tissue preparations (thoracic aorta in db/db mice versus abdominal aorta in STZ mice) and different contractile stimuli (5-HT in thoracic aorta versus PE in abdominal aorta) is that thoracic aorta has much larger contractile response to 5-HT than PE, but abdominal aorta has much larger contractile response to PE than 5-HT, as described previously (28). Importantly, pretreatment of db/db mouse thoracic aortic tissues with BEL (Fig. 7E) or abdominal aortae from STZ mice with (S)-BEL (which selectively inhibits iPLA2β (39); Fig. 7G) abolished diabetes-associated vascular smooth muscle hypercontractility. Interestingly, pretreatment of mouse abdominal aortae with (R)-BEL (which selectively inhibits iPLA2γ) (39) did not affect diabetes-associated vascular smooth muscle hypercontractility (Fig. 7G).
The potential role of iPLA2β in diabetes-associated vascular functional abnormalities was also examined in abdominal aortae isolated from STZ-treated iPLA2β-null mice and their WT littermates. Fig. 7H illustrates that PE-induced diabetes-associated vascular smooth muscle hypercontractility was reduced in iPLA2β-null mice compared with that in WT mice.
DISCUSSION
The current study, to the best of our knowledge, has several novel findings. First, we illustrate that high glucose is sufficient to induce increases in iPLA2β mRNA and protein expressions and iPLA2 enzymatic activity, and PKC is involved in high glucose-induced iPLA2β protein up-regulation. Second, we demonstrate that iPLA2β plays an essential role in high glucose-induced RhoA/ROCK/CPI-17 activation. Third, we identified 12/15-LOs as downstream iPLA2β effectors in high glucose-induced CPI-17 phosphorylation. Fourth, we show that iPLA2 enzymatic activity and iPLA2β protein expression are increased in aorta and mesenteric arteries isolated from type 1 and type 2 diabetic animal models. Fifth, we illustrate that iPLA2β but not iPLA2γ is involved in diabetes-associated vascular smooth muscle hypercontractility.
CPI-17 is an endogenous myosin phosphatase-inhibitory protein that is primarily expressed in VSMC and is recognized to participate in Ca2+ sensitization of smooth muscle contraction (4). Phosphorylation of Thr-38 in CPI-17 increases its phosphatase inhibitory potency by over 1,000-fold (40). By using a Thr-38 phospho-specific antibody, we have recently reported that both G-protein-coupled receptor agonists and high glucose can stimulate CPI-17 phosphorylation via RhoA/ROCK but that the time courses of these responses differ greatly. G-protein-coupled receptor agonists, such as thrombin and U46619, cause rapid CPI-17 phosphorylation via RhoA/ROCK within minutes of stimulation (23–24), but high glucose induces CPI-17 phosphorylation via RhoA/ROCK after a delay of 24–48 h (6). Thus, different molecular mechanisms are likely to mediate G-protein-coupled receptor agonist- and high glucose-induced activation of RhoA/ROCK/CPI-17. The 24–48-h delay in high glucose-induced CPI-17 phosphorylation suggests involvement of a relatively slow cellular process such as protein synthesis, but the molecular identities of proteins that might link high glucose and activation of RhoA/ROCK/CPI-17 phosphorylation have not yet been established. One of the major novel findings from the current study is that we have identified iPLA2β as a molecular linkage between high glucose and CPI-17 phosphorylation via RhoA/ROCK.
Several lines of evidence from the current study suggest the involvement of iPLA2β in high glucose-induced RhoA/ROCK/CPI-17 activation. First, activation of iPLA2β by high glucose precedes CPI-17 phosphorylation. High glucose-induced iPLA2β up-regulation was first observed after 6 h and achieved maximal levels after about 12 h. In contrast, high glucose-induced CPI-17 phosphorylation occurred after 24 h and achieved maximal levels after 48 h (6). Second, inhibiting iPLA2β by BEL or genetic deletion abolishes RhoA/ROCK/CPI-17 activation by high glucose. Third, restoring expression of iPLA2β in iPLA2β-deficient cells also restores high glucose-induced CPI-17 phosphorylation.
Products of iPLA2β action include 2-lysophospholipids and free fatty acids, such as AA, which can be rapidly metabolized to a variety of mediators by LO, COX, and CytP450 to a host of bioactive eicosanoids (34). AA is recognized to participate in Ca2+ sensitization of smooth muscle contraction (38, 41, 42), but it is not yet clear whether AA itself, its metabolites, or both are involved in such effects. Our studies here demonstrate for the first time that high glucose-induced AA release is largely mediated by iPLA2β and that pharmacologic inhibition or genetic deletion of 12/15-LO isozymes blocks high glucose-induced CPI-17 phosphorylation. Moreover, suppression of COX or CytP450 product generation fails to block such phosphorylation. 12/15-LO has been implicated previously in the pathogenesis of diabetic vasculopathies (34). Our results suggest that 12/15-LO may link iPLA2β and RhoA/ROCK/CPI-17 when cells are exposed to high concentrations of glucose. In agreement with this notion, the action of 12/15-LO is recognized to mediate monocyte adhesion to aortic endothelium through activation of RhoA (43). It will be of interest to determine whether high glucose-induced, iPLA2β-mediated RhoA activation also occurs via 12/15-LO in VSMC.
It remains unclear how the catalytic activity of 12/15-lipoxygenase may activate the RhoA/Rho kinase/CPI-17. We attempted to use 12(S)-HETE and/or 15(S)-HETE to restore high glucose-induced CPI-17 phosphorylation in 12/15-LO-deficient mouse VSMC, but the effort was unsuccessful (data not shown). It is possible that the LO metabolites responsible for high glucose-induced CPI-17 phosphorylation could be compounds (e.g. 12(S)- and 15(S)-HPETE) other than 12(S)- or 15(S)-HETE. It is also possible that the action of 12/15-LO on AA released by iPLA2β might be required, but not sufficient, for high glucose-induced CPI-17 phosphorylation. Alternatively, exogenous 12(S)- and 15(S)-HETE may not fully recapitulate endogenous 12(S)- and 15(S)-HETE action in high glucose-induced CPI-17 phosphorylation because endogenously produced and exogenously added 12(S)- and 15(S)-HETE might penetrate different intracellular compartments and/or achieve different local concentrations at the relevant site(s) of action. Further studies are required to address these issues.
Of particular interest is the participation of PKC in high glucose-induced CPI-17 phosphorylation. We previously reported that inhibiting PKC with GF109203X for 30 min did not inhibit high glucose-induced CPI-17 phosphorylation, and this indicates that PKC does not directly phosphorylate CPI-17 (6). Interestingly, when GF109203X was introduced simultaneously with high concentrations of glucose in the medium and incubation was continued for 48 h, RhoA and ROCK activation was reduced, as was CPI-17 phosphorylation. This suggests that PKC is involved in the up-regulation of protein(s) required for high glucose-induced RhoA/ROCK/CPI-17 activation (6). The results described here identify iPLA2β as a protein whose expression is up-regulated in the presence of high concentrations of glucose in a manner that depends on PKC activity. This is consistent with previous reports that PKC mediates iPLA2-mediated AA release in macrophage-like P388D1 cells (44), endothelial cells (45), and cardiomyocytes (46). Nonetheless, it is not yet clear which isoform of PKC is involved in the up-regulation of iPLA2β, because GF109203X inhibits several PKC isoforms.
It also remains unclear how activation of PKC by high glucose up-regulates expression of iPLA2β. It has been reported that sterol regulatory element-binding protein, a transcription factor, binds to iPLA2β promoter for suppressing iPLA2β mRNA expression (47). It has also been reported that PKC can directly phosphorylate histone H3 in low density lipoprotein receptor promoter (48). PKC has also been reported to be involved in phosphorylation of histone H3 associated with the TBX2 promoter in a sequence that also involves action of mitogen- and stress-activated kinase 1 (49). However, whether these mechanisms are involved in high glucose-induced and PKC-mediated iPLA2β transcriptional up-regulation is not yet known and needs to be addressed in future studies.
Using adenovirus-mediated gene transfer, we have previously demonstrated that overexpressing iPLA2β in the mouse portal vein potentiates 5-HT-induced contraction (15). Consistent with that finding, it has been demonstrated that inhibiting iPLA2 with BEL inhibits agonist-induced smooth muscle contraction in various blood vessels that include rabbit portal veins (15), rabbit carotid artery (16), rat basilar artery (17), and mouse cerebral and mesenteric arteries (16). Whether iPLA2β participates in diabetes-associated vascular smooth muscle hypercontractility is not yet known, but we demonstrate here that iPLA2 activity and iPLA2β protein expression are increased in aortae and mesentery arteries isolated from animal models of type 1 (STZ mice or rats) and type 2 (db/db mice) diabetes. Moreover, we demonstrate that inhibiting iPLA2β with (S)-BEL, which preferentially inhibits iPLA2β over iPLA2γ, or by genetic deletion attenuates diabetes-associated vascular smooth muscle hypercontractility.
Various vascular cells can potentially contribute to the observed iPLA2β up-regulation in diabetic blood vessels, but we demonstrate here that iPLA2β up-regulation does occur in VSMC. Interestingly, there is a clear difference in the time course of iPLA2β mRNA and protein up-regulation. Transcriptional regulation, mRNA stability, and translational control might account for this difference. These potential mechanisms need to be investigated in future studies.
The inhibition on the vascular smooth muscle hypercontractility by genetic deletion of iPLA2β is less complete than that achieved with BEL. The underlying mechanism that accounts for this difference is not yet known, but it might reflect compensatory overexpression of other iPLA2 members in the iPLA2β knock-out mice. In support of this possibility, we found that iPLA2γ and iPLA2ζ mRNA levels were dramatically increased in VSMC from iPLA2β-null mice by ∼12- and ∼22-fold (data not shown). Interestingly, Moon et al. (30) have reported that iPLA2γ and cytosolic PLA2α are up-regulated in iPLA2β-deficient VSMC and suggested that this is likely to occur to compensate for the loss of iPLA2β activity in the regulation of Ca2+ entry.
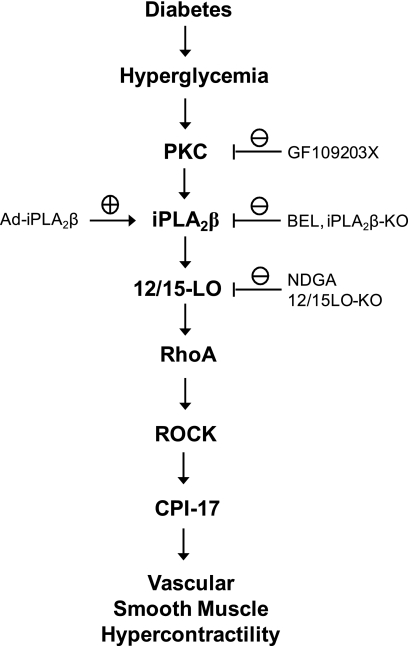
In summary, our results demonstrate that iPLA2β is activated in the vasculature of animal models of type 1 and type 2 diabetes and in primary cultured VSMC incubated with high concentrations of glucose. Fig. 8 illustrates a model that integrates our findings and outlines the sequence of events in a pathway by which high concentrations of glucose result in vascular smooth muscle hypercontractility. These events include activation of PKC, which results in up-regulation of iPLA2β expression that leads to activation of RhoA/Rho kinase/CPI-17 signaling in a manner that requires 12/15-lipoxygenase action. These processes may contribute to diabetes-associated vascular smooth muscle hypercontractility, and our findings suggest that iPLA2β is a potential novel therapeutic target for preventing and/or treating diabetes-associated cardiovascular disease.
FIGURE 8.
Model for diabetes-induced iPLA2β protein up-regulation and the role of iPLA2β in diabetes-induced RhoA/ROCK/CPI-17 activation in VSMC and diabetes-associated vascular smooth muscle hypercontractility. The pharmacological, molecular, and genetic approaches used in this study are also indicated.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL088389 (to Z. G.) and HL082791 (to M. C. G.). This work was also supported by funds from the Commonwealth of Kentucky Diabetes Research Trust Fund (to Z. G.) and American Diabetes Association Career Development Award 1-04-CD-04 (to M. C. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- ROCK
- Rho kinase
- VSMC
- vascular smooth muscle cell(s)
- HG
- high glucose
- NG
- normal glucose
- PLA2
- phospholipase(s) A2
- iPLA2
- calcium-independent phospholipase A2
- PKC
- protein kinase C
- AA
- arachidonic acid
- LO
- lipoxygenase(s)
- COX
- cyclooxygenase(s)
- CytP450
- cytochrome P450-dependent monooxygenase(s)
- BEL
- bromoenol lactone
- NDGA
- nordihydroguaiaretic acid
- STZ
- streptozotocin
- NS
- not significant
- WT
- wild-type
- PE
- phenylephrine
- FBS
- fetal bovine serum
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate.
REFERENCES
- 1.Brownlee M. (2001) Nature 414, 813–820 [DOI] [PubMed] [Google Scholar]
- 2.De Vriese A. S., Verbeuren T. J., Van de Voorde J., Lameire N. H., Vanhoutte P. M. (2000) Br. J. Pharmacol. 130, 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somlyo A. P., Somlyo A. V. (1994) Nature 372, 231–236 [DOI] [PubMed] [Google Scholar]
- 4.Somlyo A. P., Somlyo A. V. (2003) Physiol. Rev. 83, 1325–1358 [DOI] [PubMed] [Google Scholar]
- 5.Shimokawa H., Takeshita A. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1767–1775 [DOI] [PubMed] [Google Scholar]
- 6.Xie Z., Su W., Guo Z., Pang H., Post S. R., Gong M. C. (2006) Cardiovasc. Res. 69, 491–501 [DOI] [PubMed] [Google Scholar]
- 7.Mueed I., Zhang L., MacLeod K. M. (2005) Br. J. Pharmacol. 146, 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S., Hypolite J. A., DiSanto M. E., Changolkar A., Wein A. J., Chacko S. (2006) Am. J. Physiol. Renal Physiol. 290, F650–F656 [DOI] [PubMed] [Google Scholar]
- 9.Lagaud G. J., Masih-Khan E., Kai S., van Breemen C., Dubé G. P. (2001) J. Vasc. Res. 38, 578–589 [DOI] [PubMed] [Google Scholar]
- 10.Nobe K., Sakai Y., Maruyama Y., Momose K. (2002) Br. J. Pharmacol. 136, 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis E. A. (1994) J. Biol. Chem. 269, 13057–13060 [PubMed] [Google Scholar]
- 12.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. (2004) J. Biol. Chem. 279, 48968–48975 [DOI] [PubMed] [Google Scholar]
- 13.Ma Z., Turk J. (2001) Prog. Nucleic Acid Res. Mol. Biol. 67, 1–33 [DOI] [PubMed] [Google Scholar]
- 14.Balsinde J., Balboa M. A., Insel P. A., Dennis E. A. (1999) Annu. Rev. Pharmacol. Toxicol. 39, 175–189 [DOI] [PubMed] [Google Scholar]
- 15.Guo Z., Su W., Ma Z., Smith G. M., Gong M. C. (2003) J. Biol. Chem. 278, 1856–1863 [DOI] [PubMed] [Google Scholar]
- 16.Park K. M., Trucillo M., Serban N., Cohen R. A., Bolotina V. M. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H1183–H1187 [DOI] [PubMed] [Google Scholar]
- 17.Ji X., Nishihashi T., Trandafir C. C., Wang A., Shimizu Y., Kurahashi K. (2007) Eur. J. Pharmacol. 577, 109–114 [DOI] [PubMed] [Google Scholar]
- 18.Maeda A., Ozaki Y., Sivakumaran S., Akiyama T., Urakubo H., Usami A., Sato M., Kaibuchi K., Kuroda S. (2006) Genes Cells 11, 1071–1083 [DOI] [PubMed] [Google Scholar]
- 19.Fuentes L., Pérez R., Nieto M. L., Balsinde J., Balboa M. A. (2003) J. Biol. Chem. 278, 44683–44690 [DOI] [PubMed] [Google Scholar]
- 20.Daniels S. B., Cooney E., Sofia M. J., Chakravarty P. K., Katzenellenbogen J. A. (1983) J. Biol. Chem. 258, 15046–15053 [PubMed] [Google Scholar]
- 21.van Tienhoven M., Atkins J., Li Y., Glynn P. (2002) J. Biol. Chem. 277, 20942–20948 [DOI] [PubMed] [Google Scholar]
- 22.Xie Z., Gong M. C., Su W., Turk J., Guo Z. (2007) J. Biol. Chem. 282, 25278–25289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang H., Guo Z., Su W., Xie Z., Eto M., Gong M. C. (2005) Am. J. Physiol. Cell Physiol. 289, C352–C360 [DOI] [PubMed] [Google Scholar]
- 24.Pang H., Guo Z., Xie Z., Su W., Gong M. C. (2006) Am. J. Physiol. Cell Physiol. 290, C892–C899 [DOI] [PubMed] [Google Scholar]
- 25.Bao S., Miller D. J., Ma Z., Wohltmann M., Eng G., Ramanadham S., Moley K., Turk J. (2004) J. Biol. Chem. 279, 38194–38200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHowat J., Creer M. H., Hicks K. K., Jones J. H., McCrory R., Kennedy R. H. (2000) Am. J. Physiol. Endocrinol. Metab. 279, E25–E32 [DOI] [PubMed] [Google Scholar]
- 27.Müller A., Schott-Ohly P., Dohle C., Gleichmann H. (2002) Immunobiology 205, 35–50 [DOI] [PubMed] [Google Scholar]
- 28.Guo Z., Su W., Allen S., Pang H., Daugherty A., Smart E., Gong M. C. (2005) Cardiovasc. Res. 67, 723–735 [DOI] [PubMed] [Google Scholar]
- 29.Smani T., Zakharov S. I., Csutora P., Leno E., Trepakova E. S., Bolotina V. M. (2004) Nat. Cell Biol. 6, 113–120 [DOI] [PubMed] [Google Scholar]
- 30.Moon S. H., Jenkins C. M., Mancuso D. J., Turk J., Gross R. W. (2008) J. Biol. Chem. 283, 33975–33987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Way K. J., Katai N., King G. L. (2001) Diabet. Med. 18, 945–959 [DOI] [PubMed] [Google Scholar]
- 32.Hazen S. L., Zupan L. A., Weiss R. H., Getman D. P., Gross R. W. (1991) J. Biol. Chem. 266, 7227–7232 [PubMed] [Google Scholar]
- 33.Ramana K. V., Friedrich B., Tammali R., West M. B., Bhatnagar A., Srivastava S. K. (2005) Diabetes 54, 818–829 [DOI] [PubMed] [Google Scholar]
- 34.Natarajan R., Nadler J. L. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 1542–1548 [DOI] [PubMed] [Google Scholar]
- 35.Fatima S., Khandekar Z., Parmentier J. H., Malik K. U. (2001) J. Pharmacol. Exp. Ther. 298, 331–338 [PubMed] [Google Scholar]
- 36.Funk C. D. (1996) Biochim. Biophys. Acta 1304, 65–84 [DOI] [PubMed] [Google Scholar]
- 37.Velasco G., Armstrong C., Morrice N., Frame S., Cohen P. (2002) FEBS Lett. 527, 101–104 [DOI] [PubMed] [Google Scholar]
- 38.Feng J., Ito M., Kureishi Y., Ichikawa K., Amano M., Isaka N., Okawa K., Iwamatsu A., Kaibuchi K., Hartshorne D. J., Nakano T. (1999) J. Biol. Chem. 274, 3744–3752 [DOI] [PubMed] [Google Scholar]
- 39.Jenkins C. M., Han X., Mancuso D. J., Gross R. W. (2002) J. Biol. Chem. 277, 32807–32814 [DOI] [PubMed] [Google Scholar]
- 40.Ito M., Nakano T., Erdodi F., Hartshorne D. J. (2004) Mol. Cell Biochem. 259, 197–209 [DOI] [PubMed] [Google Scholar]
- 41.Gong M. C., Fuglsang A., Alessi D., Kobayashi S., Cohen P., Somlyo A. V., Somlyo A. P. (1992) J. Biol. Chem. 267, 21492–21498 [PubMed] [Google Scholar]
- 42.Gong M. C., Kinter M. T., Somlyo A. V., Somlyo A. P. (1995) J. Physiol. 486, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolick D. T., Srinivasan S., Whetzel A., Fuller L. C., Hedrick C. C. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1260–1266 [DOI] [PubMed] [Google Scholar]
- 44.Akiba S., Ohno S., Chiba M., Kume K., Hayama M., Sato T. (2002) Biochem. Pharmacol. 63, 1969–1977 [DOI] [PubMed] [Google Scholar]
- 45.Meyer M. C., Kell P. J., Creer M. H., McHowat J. (2005) Am. J. Physiol. Cell Physiol. 288, C475–C482 [DOI] [PubMed] [Google Scholar]
- 46.Steer S. A., Wirsig K. C., Creer M. H., Ford D. A., McHowat J. (2002) Am. J. Physiol. Cell Physiol. 283, C1621–C1626 [DOI] [PubMed] [Google Scholar]
- 47.Seashols S. J., del Castillo Olivares A., Gil G., Barbour S. E. (2004) Biochim. Biophys. Acta 1684, 29–37 [DOI] [PubMed] [Google Scholar]
- 48.Huang W., Mishra V., Batra S., Dillon I., Mehta K. D. (2004) J. Lipid Res. 45, 1519–1527 [DOI] [PubMed] [Google Scholar]
- 49.Teng H., Ballim R. D., Mowla S., Prince S. (2009) J. Biol. Chem. 284, 26368–26376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.