Abstract
The dopamine D2 receptor (D2R) plays a critical role in diverse neurophysiological functions. D2R knock-out mice (D2R−/−) show reduced food intake and body weight while displaying an increased basal energy expenditure level, compared with their wild type littermates. Thus, these mice show a lean phenotype. D2R−/− mice displayed increased leptin sensitivity, and leptin injection induced increased phosphorylation of the hypothalamic signal transducer and activator of transcription 3 (STAT3) in D2R−/− mice relative to wild type littermates. Using double immunofluorescence histochemistry, we have demonstrated that D2Rs are present in leptin-sensitive STAT3-positive cells in the arcuate nucleus of the hypothalamus and that leptin injection induces STAT3 phosphorylation in hypothalamic neurons expressing D2Rs. Stimulation of D2R by the D2R agonist quinpirole suppressed the leptin-induced STAT3 phosphorylation and nuclear trans-localization of phospho-STAT3 in the hypothalamus of wild type mice. However, this regulation was not detected in the D2R−/− mice. Treatment of D2R agonist and antagonist could modulate the leptin-induced food intake and body weight changes in wild type mice but not in D2R−/− mice. Together, our findings suggest that the interaction between the dopaminergic system and leptin signaling in hypothalamus is important in control of energy homeostasis.
Keywords: Diseases/Obesity, Metabolism, Receptors/Neurotransmitters, Signal Transduction, Signal Transduction/JAK-STAT, Tissue/Organ Systems/Brain, Energy Homeostasis, Hypothalamus
Introduction
Dopamine is the main catecholamine in the brain. It serves important regulatory roles in many neural functions, including the control of locomotion, neuroendocrine hormone release, cognition, emotive behavior, reward, and memory (1). Dopamine is also a critical neurotransmitter in the control of feeding behavior. It is generally accepted that the dopaminergic pathway is primarily involved in the regulation of reward-related behaviors, including feeding behaviors. Dopamine regulates these behaviors through the mesolimbic dopaminergic pathways, which project from the ventral tegmental area (VTA)4 to the limbic region, including the nucleus accumbens. Dopamine signaling in these reward-related circuits seems to control for different reward values, including food rewards (2, 3).
The cellular and molecular mechanism by which dopamine controls food intake through these dopaminergic mesolimbic pathways remains largely unknown. Recent findings propose that hormones regulating energy homeostasis, such as leptin and insulin, can modulate the midbrain dopaminergic system to regulate feeding behavior (4, 5). However, it is not known if the action of these hormones in VTA regions of the midbrain would be physiologically relevant, because there are very few functional receptors of leptin or insulin in this region (3–5).
Dopamine is presumed to regulate feeding via hypothalamic circuits in the brain (6–10). Dopaminergic neurons project to the arcuate nucleus and to the median eminence of the hypothalamus. These projections may influence the function of hypothalamic neurons involved in ingestive behaviors. Pharmacological studies using D1- and D2-like receptor agonists and antagonists demonstrated that modulation of dopamine receptor activity in the hypothalamus could regulate food intake (11–16). However, the mechanism by which dopaminergic neurotransmission in the hypothalamus regulates food intake has yet to be defined. Different reports on the role of dopamine on food intake have yielded contradictory results. Contradictions may be due to the differential actions of dopamine on different hypothalamic areas or the involvement of different receptor subtypes.
Binding of dopamine to the dopamine D2 receptor is crucial for the regulation of diverse physiological functions, such as the control of locomotor activity, reward-related behavior, and the synthesis of pituitary hormones (17). Generation and analysis of dopamine D2 receptor knock-out mice indeed demonstrated that the D2 receptor plays a key role in dopaminergic neurotransmission (18–22).
Dopamine D2 receptors are localized in several hypothalamic regions, including the lateral, dorsomedial, ventromedial, and arcuate nuclei (9, 23). These hypothalamic regions contain receptors for several key factors that regulate energy homeostasis, such as leptin and insulin, as well as other obesity-related peptides and hormones, such as melanin-concentrating hormone (MCH) and orexin (24–27).
In humans, genetic studies with obese individuals showed possible association of the polymorphisms of the D2R gene among different dopamine receptor subtypes with obesity (28–34). A recent report also suggested that activation of D2R by bromocriptine lowers circulating leptin levels in an obese woman, indicating a role of dopaminergic neurotransmission via D2R in control of leptin levels in humans (35). Although the precise mechanism has not been uncovered, these observations indicate that normal D2R function might be important in the maintenance of normal eating behavior and also in the regulation of energy balance.
We have observed decreased body weight and food intake in D2R−/− mice as compared with wild type littermates (18), indicating a role of the D2 receptor in control of food intake and body weight. Thus, we undertook an investigation of the underlying mechanisms of dopaminergic regulation of body weight and food intake to better understand how dopamine signaling, via D2Rs in the hypothalamus, can regulate energy homeostasis.
EXPERIMENTAL PROCEDURES
Mice
Experiments were mostly conducted with D2R knock-out mice, official strain designation B6;129S2-Drd2tmllow, which were obtained from the Induced Mutant Resource at The Jackson Laboratory (Bar Harbor, ME). Mutant and WT mice were obtained by heterozygote crossings, and siblings were used as controls. WT, heterozygous, and knock-out mice were identified by PCR of genomic DNA, via protocols provided by The Jackson Laboratory. D2R−/− mice and WT littermates used in the initial phase of this study originated from the breeding of heterozygous D2R−/− mice described by Baik et al. (18) and were identified by Southern hybridization analyses as described previously (21). Most of experiments have been performed with male mice to exclude possible female hormonal influence. Mice were kept within an SPF barrier area, and were housed in groups of four or five with mixed genotypes in an air-conditioned room on a 12:12 h light/dark schedule, under constant conditions of temperature and humidity. Food (Purina Certified Rodent Diet) and tap water (membrane filter-purified and autoclaved water) were provided ad libitum. Animal care and handling was carried out according to the standards approved by the Institutional Animal Care and Use Committee.
Measurement of Basal Food Intake and Body Composition
For basal food intake measurement, age-matched WT and D2R−/− mice were housed individually and allowed to acclimate in cage for 1 week before food-intake measurements. Fresh food pellets of standard chow diet (Purina Certified Rodent Diet) were weighed and placed in the food pellet holder of the cage each day, and then the cumulative food intake was monitored daily at 10:00 a.m. over a 1-week-period. Body weight and food intake were recorded and averaged for each day. To minimize error attributable to loss of food particles, cages were carefully monitored before and after the experiment to capture any spilled food. Each food intake measurement was corrected for spillage. Mice at 10–12 weeks of age were anesthetized using Zoletil (1.6 μg/g, Verbac Laboratories, 06516 Carros, France) and 0.05 μl/g Rompun (Bayer) for dual-energy x-ray absorptiometry (Norland pDEXA, Orthometrix Inc., New York) analysis. Fat mass was normalized to total body mass.
Effect of Leptin on Food Intake and Body Weight
Intracerebroventricular administration of leptin was performed with using 10–12-week-old mice. At least 1 week before experimentation, each mouse received an i.c.v. cannulation. Animals were anesthetized with 1.6 μl/g Zoletil and 0.05 μl/g Rompun (Bayer) intraperitoneally and placed in a stereotaxic apparatus (Kopf Instruments). A 26-gauge stainless steel guide cannula was implanted in the lateral ventricle of the brain with the following coordinates: 0.3 mm posterior and 1 mm lateral relative to bregma and 3 mm depth. Injection position was verified at the end of the experiment by dye administration and histological analysis. Prior to leptin administration, mice were fasted for 14 h. Either recombinant mouse leptin (1, 2, and 5 μg, R & D Systems, National Hormone and Peptide Program) in 5 μl of 0.9% saline or a 5-μl saline control was administered (injection rate 1.0 μl/min). Food intake was measured at 2, 6, 12, and 24 h after injection.
Effect of D2R Agonist and Antagonist on Leptin Action
Cannulation surgery was performed as mentioned above in leptin i.c.v. administration, and 10–12-week-old mice were used. After surgery, mice recovered for at least 1 week. Prior to administration, mice were fasted for 14 h. Either recombinant mouse leptin (2 μg, National Hormone and Peptide Program reagent) or quinpirole (0.7 μg/g, Tocris) or haloperidol (50 nmol, Sigma) in 4–8 μl of saline or a 4–8-μl saline control was administered in 1.0 μl/min rate. Haloperidol was dissolved in a drop of glacial acetic acid, and the pH was adjusted with NaOH and made up to volume with PBS/saline solution. Quinpirole or haloperidol was pre-injected before leptin treatment. Body weight, food intake, and also locomotor activity were measured at 2, 6, 12, and 24 h after injection. Locomotor activity was measured using an activity monitoring system (Iwoo Science, Korea) with a photo-beam sensor in the home cage of the animal.
Pair-feeding Experiment
Male mice at 6 weeks of age were housed individually and maintained on a 12-h light/dark cycle, under constant conditions of temperature (22 ± 1 °C) and humidity (50%). Pair-fed study was performed from 7 weeks of age for 6 weeks. WT mice were divided into a pair-fed group and an ad libitum group, and separated groups of D2R−/− mice were allowed ad libitum food access. For the pair-fed WT group, animals were pair-fed to the amount of daily food intake consumed by the ad libitum groups of D2R−/− mice the previous day. The amount of daily food was divided into two portions, and portions were given twice a day at 10:00 a.m. and 06:00 p.m. Body weight and food intake were measured daily for the period of the pair-fed experiment.
Measurement of Plasma Leptin Concentration
Plasma was obtained from the collected blood samples by immediate centrifugation and stored at −70 °C until analysis. Plasma leptin concentrations were measured using a rat leptin ELISA kit (Linco Research Inc., St. Charles, MO) according to the manufacturer's instructions. The sensitivity of this assay was 0.05 ng/ml, and the intra- and interassay coefficients of variation were 2 and 4%, respectively.
Hypothalamic Protein Extraction after Leptin Administration
Before drug administration, mice were made to fast for 14 h. 15 min after 1 μg of leptin or saline i.c.v. administration, brain were removed, and hypothalami were extracted within a minute. Then hypothalami were homogenized in lysis buffer (50 mm Tris, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mm Na3VO4, 1 mm NaF) using a Teflon potter in 1.5-ml tubes. Two hypothalamic homogenates were centrifuged for 15 min at 4 °C at 23,000 × g, and supernatants were placed in a fresh tube. Protein concentrations were measured using the protein assay solution (Bio-Rad).
Nuclear Fraction Extraction
At least 1 week before experimentation, each mouse received an i.c.v. cannulation as described above. Before drug administration, mice were made to fast for 14 h. Mice received i.c.v. injections of 5 μl of saline or 1 μg/5 μl leptin or quinpirole (0.7 μg/g) + leptin (1 μg). One h after i.c.v. administration, the hypothalami were dissected and homogenized in 100:1 (v/v) buffer A (10 mm HEPES, pH 7.9, 10 mm KCl, 1 mm EDTA, 1 mm EGTA, 1 mg/ml aprotinin, 100 mm leupeptin, 1 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, and 0.5% Nonidet P-40). The samples were centrifuged at 2000 × g for 10 min at 4 °C. The pellets were resuspended in a 4:1 ratio of buffer A and centrifuged at 2000 × g for 10 min at 4 °C. The pellets were resuspended in 2 volumes of buffer B (10 mm HEPES, pH 7.9, 420 mm NaCl, 25% glycerol, 5 mm MgCl2, 0.1 mm EDTA, 0.1 mm EGTA, 10 mg/ml aprotinin, 100 mm leupeptin, 1 mm phenylmethylsulfonyl fluoride, and 1 mm dithiothreitol) and centrifuged at 13,000 × g for 10 min to remove debris. The supernatant was collected and labeled as the nuclear fraction. Both cytoplasmic and nuclear fractions were assayed for protein concentration using the protein assay solution (Bio-Rad).
Western Blotting
After hypothalamic protein extraction as described above, 200 μg of protein lysates were subjected to 10% SDS-PAGE followed by transfer onto pre-wetted polyvinylidene difluoride nitrocellulose membranes (Millipore, MA), using transfer buffer (50 mm Tris, 20 mm glycine, 20% methanol). For detection of nuclear p-STAT3, 80 μg of nuclear fractionated lysates were loaded for each sample. Membranes were blocked in 5% skim milk and incubated with anti-P-STAT3 (catalog no. 9138, Cell Signaling), STAT3 (catalog no. 9132, Cell Signaling), pJAK2, (catalog no. 3771S, Cell Signaling), and mouse anti-lamin B1 (33-2000, Zymed Laboratories Inc.) antibody (1:1000) overnight at 4 °C. Membranes were then washed and incubated with secondary antibodies (anti-rabbit horseradish peroxidase-coupled, 1:5000; Amersham Biosciences). Specific bands were detected via enhanced chemiluminescence (Amersham Biosciences) and analyzed using an LAS3000 image analysis system (Fuji, Tokyo, Japan). Hypothalami from two mice were pooled for each point used in an experiment, and four independent experiments were performed (n = 8 mice per group).
Measurement of Energy Expenditure
Mice of 10–12 weeks of age were allowed to acclimate in the chamber before initiating the measurement. To reduce the effect of the exposure to the novel environment, their locomotor activity in the initial period of measurement was monitored. Oxygen consumption (VO2) was determined simultaneously for multiple animals by indirect calorimetry using the Oxymax System (Columbus Instruments, Columbus, OH). Measurements were taken for a 24-h period, encompassing a full 12:12 h light/dark cycle. Data were normalized to body weight. Food and water were provided ad libitum throughout.
Luciferase Reporter Gene Assay
Expression constructs for the ObR and the D2R were cotransfected with the luciferase reporter gene pSTAT3 response element-LUC (P950M4/Luc, kindly provided by Dr. Pravin B. Sehgal, New York Medical College, Valhalla, NY) and pCH110 into HEK293T cells in 6-well dishes using Polyfect (Qiagen) for 36 h. Following transfection, the culture medium was changed to serum-free Dulbecco's modified Eagle's medium for 6 h and then murine leptin (R & D Systems, Abingdon, Oxon, UK) with or without quinpirole (Tocris, UK) was added every 12 h. After treatment, the cells were lysed and assayed for luciferase activity using the Promega luciferase assay system (Promega). Luciferase and β-galactosidase activities were measured in cell lysates, and luciferase activity was normalized to β-galactosidase activity to control for transfection efficiency.
Double-labeled Immunofluorescence and Confocal Laser Microscopy
Double-labeled immunofluorescence was applied to specifically identify the cell type(s) expressing the D2R and pSTAT3. Double immunostaining was performed on 40-μm-thick sections of D2R knock-out and WT mice. Mice received i.c.v. injections of 5 μl of saline or 1 μg/5 μl leptin or quinpirole (0.7 μg/g) + leptin (1 μg). One h after injection, mice were anesthetized with Zoletil (Virbac, 1.6 μl/g, intraperitoneally) and 0.05 μl/g Rompun (Bayer) and perfused with filter-sterilized 0.1 m PBS and then fixed by 4% paraformaldehyde/PBS solution (Sigma). The brains were then removed and post-fixed for 6 h with ice-cold fixative as above. The brains were then dehydrated in 30% sucrose/PBS for 2 days. Brains were then frozen and 40-μm-thick consecutive coronal sections were prepared on a cryostat (Leica CM 1900, Germany). Sections were pre-treated with 1% NaOH and 1% H2O2 for 20 min, then incubated in 0.3% glycine in PBS for 10 min, and then blocked with a blocking solution (3% bovine serum albumin, and 0.3% Triton X-100 in PBS, pH 7.4) for 1 h at room temperature. Tissue sections were incubated overnight at 4 °C with a rabbit anti-D2R antibody (AB5084P, Chemicon International, Temecula, CA) and a mouse monoclonal antibody against phospho-STAT3 (catalog no. 9138S, Cell Signaling Technology, Beverly, MA). After rinsing in PBS, the double-stained sections were incubated at room temperature for 1 h with goat anti-rabbit IgG (fluorescence emission at 568 nm; Vector Laboratories, Burlingame, CA) for the D2R and goat anti-mouse IgG (fluorescence emission at 488 nm; Vector Laboratories, Burlingame, CA) for pSTAT3. After rinsing in PBS, the sections were mounted in Vectashield (Vector Laboratories) to prevent fading of the immunofluorescence stain. Sections were examined on a C1 Plan Apo ×40/1.4 water confocal laser scanning system, LSM 510 META (Zeiss, Berlin, Germany).
In Situ Hybridization
12-Week-old male WT and D2R−/− mice were sacrificed, and whole brains were immediately extracted and stored at −70 °C until cryosectioning. The prepared brains were mounted in a cryostat maintaining −20 °C, serially sectioned into 10-μm slices, and placed on glass slides. Antisense NPY was prepared by linearizing the plasmid pBLNPY-1, which contains 511 bp of the rat NPY gene, with FspI (provided by Dr. Steven L. Sabol). Antisense MCH was prepared by linearizing the plasmid pGEM4-MCH, which contains 700 bp of the rat MCH gene (provided by Dr. R. Thompson at University of Michigan), with XbaI. The AGRP template was prepared from mouse hypothalamic RNA by RT-PCR with the N-terminal primer, 5′-TGA CTG CAA TGT TGC TGA GTT GTG-3′, and the C-terminal primer, 5′-TAG GTG CGA CTA CAG AGG TTC GTG-3′, as described previously (36). The amplified fragments were gel-purified and then subcloned into pBluescript (Stratagene). Antisense AGRP was then prepared by linearizing the plasmid pBluescript-AGRP, which contains 300 bp of the mouse AGRP gene, with EcoRI. The POMC gene was provided by Dr. Jacques Drouin, and 900 bp of fragment was subcloned into the HindIII and EcoRI sites of the pBluescript. [35S]cRNA probes were prepared by transcribing 1 μg of each linearized DNA with T3 polymerase (NPY and POMC), T7 polymerase (galanin and AGRP), or SP6 polymerase (MCH) for 90 min at 37 °C, in a reaction mixture containing 35S-CTP (Amersham Biosciences) using a riboprobe in vitro transcription kit (Promega, Madison, WI). Hybridization was performed as described earlier (21). The dark field images were processed using an image digitalizer (Imaging Technology) to quantify the in situ hybridization. The digitalized images were then analyzed on a Metamorph image analysis system (Universal Imaging Corp.).
Real Time RT PCR
12-Week-old male WT and D2R−/− mice were decapitated and hypothalami removed in a minute and frozen by liquid nitrogen. Then RNA was extracted by LiCl precipitation and phenol/chloroform purification. 1 μg of RNA was reverse-transcribed using SuperScript III reverse transcriptase (10 units) (Invitrogen). Primers encoding NPY, AGRP, POMC, MCH, and β-actin were used as described previously (37). PCR was performed on a LightCycler 480 (Roche Diagnostics) using a 100-ng sample of hypothalamic cDNA added to the commercially available LightCycler PCR master mix (LightCycler 480 SYBR Green I Master, Roche Diagnostics). Neuropeptide mRNA expression levels were normalized to β-actin mRNA content and expressed as a percent of the mean value of WT control mice by using 2−ΔΔCT method (38).
Statistical Analysis
All results are expressed as mean ± S.E. For two group comparisons, unpaired Student's t test was used, although a paired Student's t test was used for within group comparisons. Two-way or one-way analysis of variance was used when appropriate with a matched set of groups to analyze the interaction between genotype and treatment. Probability values were less than 0.05.
RESULTS
Decreased Basal Body Weight and Food Intake and Altered Body Composition in D2R−/− Mice
Basal body weights and food intake of WT and D2R−/− mice were measured between 1 and 6 months of age. The body weight of D2R−/− mice was 20% lower than their wild type littermates (Fig. 1A). In addition, the basal food intake of D2R−/− mice was also reduced, compared with their wild type littermates (Fig. 1B).
FIGURE 1.
Basal body weight, food intake, energy expenditure, and body composition of WT and D2R−/− mice. A, body weight of WT and D2R−/− mice from 1 to 7 months of age. Body weight curve for WT and D2R−/− mice (n ≥7 per age group) is shown. B, food intake was measured for 7 days in 1–6-month-old WT and D2R−/− mice (A and B, n ≥7 per age group). C, metabolic rate was indicated by O2 consumption. Basal O2 consumption and CO2 production were measured with an indirect calorimeter. Oxygen consumption (VO2) and heat production were measured over 24 h and corrected for body mass (g) in age-matched male WT versus D2R−/− mice housed at room temperature (n = 15 per group). D, food intake normalized by oxygen consumption in WT and D2R−/− mice. Food intake amount (g) of 2-month-old WT and D2R mice was normalized by oxygen consumption (VO2) and displayed as relative value to WT mice (n = 9 for WT and n = 7 for D2R−/− mice). E, analysis of body composition by dual-energy x-ray absorptiometry (DEXA) revealed body fat in WT and D2R−/− mice (n = 8 for WT and n = 6 for D2R−/− mice). F, effect of pair-feeding on body weight in WT mice from 7 to 13 weeks of age. WT male mice were pair-fed to the amount of food intake consumed by ad libitum-fed D2R−/− mice over the preceding 24 h, beginning at 7 week of age and continuing for 6 weeks. Separated groups of WT and D2R−/− mice had ad libitum access to food. Body weight and food intake were measured daily for the period of the pair-fed experiment (n = 4 for each group). All values are expressed as means ± S.E. (*, p; †, p < 0.05; **, p; ††, p < 0.01; and ***, p; †††, p < 0.001; *, p, WT pair-fed versus WT ad libitum; †, p, WT pair-fed versus D2R−/− ad libitum.)
We examined the level of energy expenditure in WT and D2R−/− mice. Energy expenditure was measured using an indirect calorimeter. The level of oxygen consumption was significantly higher in D2R−/− mice as compared with WT littermates (Fig. 1C), and the ratio of energy intake versus energy expenditure was obtained by normalizing the food intake by VO2 in WT and D2R−/− mice (Fig. 1D), showing a significant difference between WT and D2R−/− mice. These results indicate that not only was the food intake of D2R−/− mice decreased, but the basal energy expenditure in D2R−/− mice was also significantly increased. Whole body fat content was measured with the dual-energy x-ray absorptiometry method. Whole body fat content in D2R−/− mice was significantly decreased to approximately 55% of WT mice, suggesting a decreased adiposity in D2R−/− mice (Fig. 1E).
To clarify the involvement of food intake regulation and metabolism in the lean phenotype of D2R−/− mice, age-matched WT male mice were pair-fed to the amount of food intake consumed by ad libitum-fed D2R−/− mice, beginning at 7 weeks of age and continuing for 7–13 weeks of age. Separated groups of WT and D2R−/− mice had ad libitum access to food. By 13 weeks of age, pair-fed WT mice attained average body weights intermediate between WT ad libitum and the D2R−/− mice-ad libitum fed group (Fig. 1F). These results indicate that the absence of D2R interferes not only in food intake but also in energy metabolism. Thus, the absence of D2R seems to substantially alter energy homeostasis in D2R−/− mice.
Leptin Effects in WT and D2R−/− Mice
The regulation of body weight involves a balance between energy intake and energy expenditure. Leptin is a key element of this regulation. Leptin decreases energy intake, increases energy expenditure, and promotes body weight loss. We investigated whether the altered energy balance status of D2R−/− mice involved changes in leptin-mediated signaling in the hypothalamus. We compared the ability of leptin to reduce food intake and weight gain in WT and D2R−/− mice. Different dose of leptin (1, 2, and 5 μg) was administered into brain by i.c.v. injection after fasting, and cumulative food intake of WT and D2R−/− mice was measured after leptin injection. Central leptin injections produced greater body weight loss and food intake suppression in D2R−/− mice than in WT mice throughout the leptin doses we analyzed (Fig. 2, A and B). In WT mice, i.c.v. injection of 1, 2, and 5 μg of leptin induced the decrease of food intake by 15, 60, and 75%, respectively, as compared with saline-injected WT mice. In D2R−/− mice, the same dose of leptin injection induced the decrease of food intake by 49, 84, and 85% respectively, as compared with saline-injected D2R−/− mice (Fig. 2A), showing a more profound effect of leptin in D2R−/− mice. Similarly, i.c.v. injection of leptin produced a significantly lower regain of body weight after fasting in D2R−/− mice (Fig. 2B). This suggests that D2R−/− mice are more sensitive to leptin treatment than WT mice.
FIGURE 2.
Effect of leptin and regulation of leptin signaling in WT and D2R−/− mice. A and B, before leptin i.c.v. administration, mice were fasted for 14 h. A, after injection, cumulative food intake was measured for 6 h after leptin injection (left panel) and normalized by saline control of each WT and D2R−/− group (right panel). B, body weight regain of WT and D2R−/− mice was measured for 6 h after leptin injection (left panel) and then normalized by weight regain of each saline control group of WT and D2R−/− mice (right panel) (n = 7–13 per group; n = 4–8 per 5 μg of leptin-treated group). C, plasma leptin levels were measured from blood of 10-week-old WT and D2R−/− mice (n = 6 for WT mice and n = 5 for D2R−/− mice). D, leptin-induced phosphorylation of STAT3 in hypothalamus of WT and D2R−/− mice was measured by Western blotting. Quantification of leptin-induced P-STAT3 in each sample from WT and D2R−/− mice was normalized to the level of β-actin in the same sample. Hypothalamus from two mice was pooled for each point used in the experiment, and four independent experiments were performed (n = 8 mice per group for WT and D2R−/− mice). All values are expressed as means ± S.E. For matched two-group comparisons, two-way analysis of variance was used; otherwise, analysis of variance and unpaired Student's t test between genotype was used. (*, p; †, p < 0.05; **, p; ††, p < 0.01; ***, p; †††, p < 0.001; †, p versus saline-injected control; *, p, versus WT mice.)
We measured the basal plasma level of leptin in WT and D2R−/− mice. The plasma leptin concentration in D2R−/− mice was reduced to 75% of WT littermates (Fig. 2C).
It has been shown that leptin activates signal transduction and transcription 3 (STAT3) in the hypothalamus of mice via leptin receptor b-mediated signaling (39–42). Leptin activates leptin receptor b, which induces phosphorylation of STAT3 (43–45). Phosphorylated STAT3 proteins, in turn, stimulate the transcription of target genes that mediate some cellular effects of leptin (46).
Because D2R−/− mice displayed a more sensitized leptin response at a reduced leptin level, we examined whether the downstream signaling of leptin was altered. We analyzed the activation of hypothalamic STAT3 in WT and D2R−/− mice. Fasted WT and D2R−/− mice were administrated leptin by i.c.v., and hypothalamic extracts were prepared; Western blotting analysis was performed using an anti-phospho-STAT3 (Tyr(P)-705) antibody. The phosphorylation levels of hypothalamic STAT3 in D2R−/− mice were significantly increased to a level ∼160% higher than their WT littermates (Fig. 2D).
Altered Hypothalamic Neuropeptide Expression in D2R−/− Mice
Many orexigenic and anorexigenic genes in the central nervous system are reported to be regulated by leptin. NPY, AGRP, and POMC neurons express leptin receptors and are regulated by leptin (47–49). MCH, an orexigenic peptide, is located in the lateral hypothalamic area/perifornical area. MCH neurons are considered to be second-order neurons involved in leptin signaling (49).
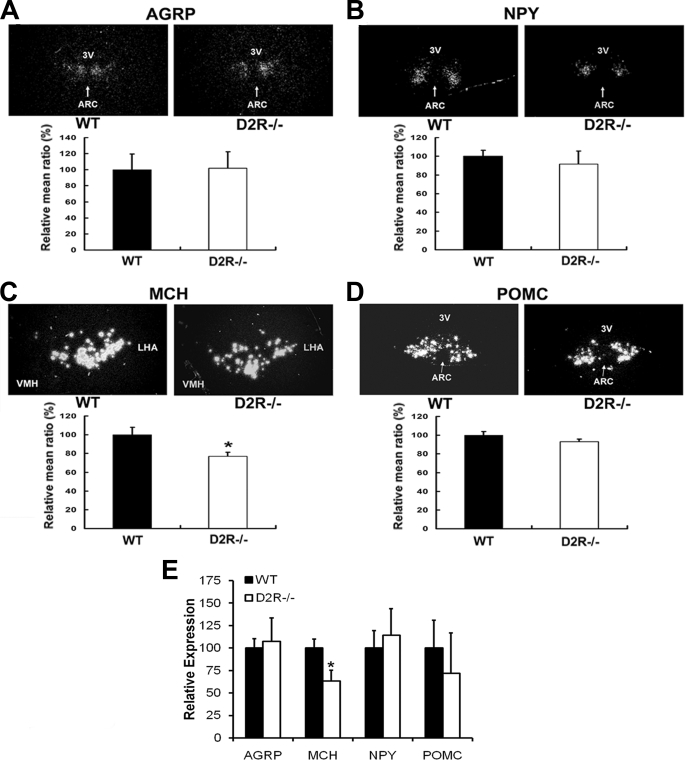
To investigate whether these neuropeptides are involved in altered energy homeostasis status of D2R−/− mice, in association with enhanced leptin signaling in these mice, the expression of AGRP, NPY, MCH, and POMC was measured by in situ hybridization on brain sections from WT and D2R−/− mice. Hypothalamic MCH mRNA expression level was decreased in D2R−/− mice to 70% of the level in WT littermates (Fig. 3C). However, the expression levels of orexigenic peptides, NPY and AGRP, were not significantly altered (Fig. 3, A and B). The expression level of an anorexigenic peptide, POMC, was not significantly altered in D2R−/− mice (Fig. 3D). We performed an additional analysis of the expression level of these peptides by real time RT-PCR on hypothalamic RNA from WT and D2R−/− mice (Fig. 3E). We obtained similar results as in situ hybridization analysis, showing a significant decrease in hypothalamic MCH expression in D2R−/− mice. These data suggest that MCH may be involved in D2R-mediated hypothalamic control of leptin signaling. Decreased MCH expression could be one of the factors responsible for the lean phenotype of the D2R−/− mice.
FIGURE 3.
In situ hybridization and real time RT-PCR analysis of hypothalamic neuropeptide gene expression in WT and D2R−/− mice. The expression levels of hypothalamic AGRP (A), NPY (B), MCH (C), and POMC (D) mRNA in WT and D2R−/− were assessed by in situ hybridization. The mRNA levels were expressed as percentage of WT controls. ARC and 3V indicate arcuate nucleus and 3rd ventricle. LHA, lateral hypothalamic area; VMH, ventromedial hypothalamus. All values are expressed as means ± S.E., p ≤ 0.05, n = 4 each. E, hypothalamic levels of mRNA encoding feeding-related neuropeptides (expressed relative to β-actin) as measured by real time PCR in WT and D2R−/− mice. The mRNA levels were expressed as percentage of WT controls. All values are expressed as means ± S.E., n = 4 each. *, p < 0.05.
Dopamine via D2R Negatively Regulates Leptin Action
Because we observed enhanced leptin signaling and leptin sensitivity in D2R−/− mice, we hypothesized that dopamine, via the D2R, may negatively regulate leptin action. To address this question, we first determined whether D2R stimulation might induce an inhibition of leptin signaling. We conducted a STAT3-response element-dependent reporter gene activation assay on leptin receptor-b (OB-Rb) activation by cotransfecting leptin receptor (OB-Rb) with D2R into HEK293T cells, coupled with a luciferase reporter gene construct (p950M4) containing four copies of the STAT3-binding DNA element from the human angiotensinogen promoter (50).
Leptin treatment (10 nm to 1 μm) elicited dose-dependent luciferase activation, resulting in a 10–14-fold increase, compared with control levels (Fig. 4A). Treatment with quinpirole, a D2R agonist, significantly blocked this leptin-induced STAT3 response element-dependent transcriptional increase (Fig. 4A). The D2R-mediated inhibition of STAT3 response element activity, which was stimulated by leptin, was reversed by treatment with haloperidol, a D2R antagonist. This shows that transactivation of leptin is specifically suppressed by D2R-mediated signaling (Fig. 4A).
FIGURE 4.
Effect of a dopamine D1R/D2R agonist on the action of leptin in vitro and in vivo. A, transfected HEK cells were stimulated by treatment with 10 nm, 0.1 μm, or 1 μm leptin, with or without 100 μm quinpirole and 10 μm of haloperidol. Relative luciferase activities are expressed as fold increases, as indicated, compared with the corresponding leptin-only controls (n = 8). B, leptin, quinpirole, or SKF81297 or quinpirole/leptin or SKF81297/leptin were administered intracerebroventricularly into WT and D2R−/− mice that had been fasted for 14 h. Cumulative food intake of WT and D2R−/− mice was measured for 6 h after drug administration (n = 5–8 for each WT group, n = 5–10 for D2R−/− saline, leptin groups and n = 3 for D2R−/− SKF81297 + leptin groups). All values are expressed as means ± S.E. *, p; †, p < 0.05; **, ##, p < 0.01 and ###, p < 0.001. C, either leptin or haloperidol or both were administered intracerebroventricularly into 10–12-week-old WT and D2R−/− mice that had been fasted for 14 h. Cumulative food intake (upper panel), body weight (middle panel), and activity (lower panel) measured at 10 h after injection (n = 4–8 mice/group for each WT and D2R−/− groups) are shown. All values are expressed as means ± S.E. (*, p; †, p < 0.05; **, p; ††, p < 0.01; ***, p; †††, p < 0.001; †, p versus saline-injected control; *, p versus WT mice.)
To determine whether the inhibition of D2R-mediated leptin signaling reflects the effect of leptin on food intake, we examined the effect of a D2 receptor agonist on leptin action in WT and D2R−/− mice. When the D2R agonist quinpirole was pre-injected (i.c.v.) before leptin treatment, quinpirole-injected WT mice displayed a significant reduction in the effect of leptin, compared with the leptin-only injected WT mice. In contrast, inhibition of the effect of leptin by quinpirole was not detected in D2R−/− mice (Fig. 4B). In addition, the injection of quinpirole alone, or another dopaminergic drug, the D1R agonist SKF81297, did not alter the food intake (Fig. 4B).
We also tested the effect of haloperidol, a D2R-specific antagonist, on the effect of leptin in WT and D2R−/− mice. Haloperidol (50 nmol) was pre-injected (i.c.v.) before leptin treatment in WT and D2R−/− mice, and food intake and body weight changes were measured from 2 to 24 h (Fig. 4C and supplemental Fig. S1). Because haloperidol can alter locomotor activity, we monitored the locomotor activity by home-cage activity monitoring system. The locomotor activity in WT mice has been decreased by 50% after 2 h of injection of haloperidol but gradually recovered by 70% of WT saline-injected mice at 10 h post-injection. When compared, the food intake and body weight changes after saline- and leptin-injected WT and D2R−/− mice and D2R−/− mice showed a more significant effect of leptin (Fig. 4C). Injection of haloperidol alone slightly decreased food intake and body weight, but generally it did not significantly affect the food intake and body weight in both groups of mice as compared with the leptin-only treated group. Interestingly, when haloperidol was pre-injected prior to leptin treatment (haloperidol/leptin), WT mice showed a strong decrease in food intake and in body weight as compared with leptin-only injected mice, whereas there was no difference between leptin-only and haloperidol/leptin-injected group in D2R−/− mice (Fig. 4C). Similar results were observed at 6 and 24 h after injection (supplemental Fig. S1). Leptin treatment also induced a decrease in locomotor activity, and it is possible that this is due to the overnight fasting before leptin injection in our experimental condition. Although leptin treatment showed a decrease in locomotor activity, there was no significant difference in locomotor activity between the leptin-treated and haloperidol/leptin-treated group. Thus, it appears that the greater reduction in food intake and body weight induced by haloperidol/leptin treatment is not merely attributable to the decrease of locomotor activity. These data demonstrate that the leptin effect was enhanced by antagonizing D2R by haloperidol in WT mice but not in D2R−/− mice, supporting our hypothesis that dopamine via D2R negatively regulates leptin action.
We next examined more closely the role of D2R in the regulation of leptin signaling on the cellular level by determining the localization of leptin-responsive cells in WT and D2R−/− mice. We performed dual immunofluorescence immunohistochemistry on hypothalamic regions in these mice. As shown in Fig. 5A and supplemental Fig. S2, the i.c.v. injection of leptin elicited the STAT3 activation in cells located in the arcuate nucleus in WT mice with an increase in the nuclearly localized phospho-STAT3-positive cells. Interestingly, when quinpirole, a D2R agonist, was pre-injected, the phospho-STAT3 (pSTAT3) signal was significantly decreased in WT mice (Fig. 5, A and D, and supplemental Fig. S2A). This inhibition was not detected in D2R−/− mice, and no obvious changes in the number of pSTAT3-positive cells were observed with quinpirole treatment in these mice, as evidenced by double immunofluorocytochemical analysis coupled with confocal microscopy (Fig. 5, B and D, supplemental Fig. S2B).
FIGURE 5.
Effects of D2R activation on leptin-mediated pSTAT3 induction in the arcuate nucleus (ARC) of hypothalamus from WT and D2R−/− mice; colocalization of the D2 receptor with pSTAT3. Mice received i.c.v. injection of 5 μl of saline or 1 μg/5 μl of leptin or 1 μg of leptin with 0.7 μg/g of quinpirole. 1 h after injection, mice were sacrificed, and samples were prepared. A and B, representative immunofluorescence images of leptin-induced phosphorylation levels of STAT3 with or without quinpirole treatment in the hypothalamic ARC region of WT and D2R−/− mice. C, colocalization of pSTAT3 with D2R-positive neurons of leptin and quinpirole + leptin-treated WT mice. Filled arrows indicate D2R-nuclear translocated-pSTAT3 colocalized cells, and open arrows indicate D2R-cytosolic pSTAT3 colocalized cells. Asterisks indicate cells those have signals either D2R or pSTAT3 only. DAPI, 4′,6-diamidino-2-phenylindole. D, quantification of pSTAT3-positive cells and D2R-pSTAT3-colocalized cells in each sample. Relative numbers of pSTAT3 were normalized with respect to the average number of WT saline samples. Relative numbers of D2R-pSTAT3-colocalized cells were normalized with number of total D2R-positive cells (n = 5–7 per group). Quin, quinpirole. E, quantification of ratio of nuclearly localized pSTAT3-positive cells and of nuclearly localized pSTAT3-D2R-colocalized cells in each sample. Relative numbers of nuclearly localized pSTAT3-positive cells, and D2R-expressing cells were normalized with respect to the average number of WT saline samples. Relative numbers of nuclearly localized pSTAT3-D2R-colocalized were normalized with respect to the number of D2R-positive cells in arcuate nucleus region in hypothalamus. All values are expressed as means ± S.E. (n = 3 per group: †, p < 0.05; ††, p < 0.01; †††, p < 0.001; **, p < 0.01; and ***, p < 0.001.) F, nuclearly localized p-STAT3 after administration of saline (Sal), leptin (Lep), or quinpirole + leptin (Q + L) in hypothalamus of WT and D2R−/− mice were measured by Western blotting. Hypothalamus from two mice was pooled for each point used in the experiment, and four independent experiments were performed (n = 8 mice per group). Band intensity of each sample from WT and D2R−/− mice was normalized to the level of nuclear protein lamin B1 in each sample. All values are expressed as means ± S.E.
Moreover, we observed a colocalization of pSTAT3 in D2R-positive cells in the arcuate nucleus in WT mice (Fig. 5, A and C, filled arrows, D2R nuclearly translocated pSTAT3 colocalized cells; open arrows, D2R cytosolic pSTAT3 colocalized cells). The number of pSTAT3-D2R colocalized cells was increased by leptin treatment, up to about 50% of total D2R-positive cells as compared with that of saline-injected mice (Fig. 5D). We could observe a more significant increase in proportion of nuclearly localized pSTAT3 in D2R−/− mice upon leptin treatment as compared with the WT counterpart (Fig. 5, A, B, and E). However, quinpirole/leptin treatment elicited a decrease in the number of nuclearly localized pSTAT3 cells, nearly to the basal levels in WT mice (Fig. 5, A, C, and E), whereas these changes were not detected in the hypothalamus of D2R−/− mice (Fig. 5, B and E).
We also examined the nuclear translocation of pSTAT3 by leptin injection from the hypothalamus of WT and D2R−/− mice by Western analysis. As shown in Fig. 5F, we confirm that injection of leptin induced nuclear p-STAT3 localization, as evidenced by the presence of nuclear protein lamin B1 and that the leptin-induced pSTAT3 signal has been suppressed by cotreatment with quinpirole in nuclear protein extracts of WT hypothalamus. In D2R−/− mice, we could observe a more pronounced pSTAT3 signal by leptin injection in the nuclear extract when normalized by a signal ratio of nuclear protein lamin B1 and that the effect of cotreatment with quinpirole was absent (Fig. 5F). Taken together, these data strongly suggest that D2R colocalizes with the leptin receptor and that dopamine regulates leptin signaling in the hypothalamus via the D2R.
DISCUSSION
There is much evidence to show the importance of dopaminergic neurotransmission in the regulation of feeding behavior. Regulation of feeding behaviors includes sensory input, reflexes, and the reward system. Often, the dopaminergic regulation of feeding behavior is considered a role of the dopaminergic pathway in the reward system, which is composed of the mesolimbic pathway projecting from VTA to the nucleus accumbens. Nevertheless, it is important to emphasize that the regulation of food intake is critically associated with the metabolic circuit, which modulates motivational aspects of feeding behaviors in parallel with homeostatic systems. In this regard, the role of dopaminergic neurotransmission on energy homeostasis remains to be unraveled. Here, we demonstrate that dopaminergic signaling, via the dopamine D2R, in the hypothalamus plays an important role in the regulation of food intake and energy homeostasis. We suggest a new connection, in which D2R inhibits leptin signaling to regulate energy homeostasis in the hypothalamus.
It has been reported that dopamine deficiency, either generated by genetic manipulation or pharmacologically, markedly alters the food intake of an animal. Dopamine-deficient mice become hypophagic and die of starvation by 3–4 weeks of age, unless dopamine is restored by daily treatment with l-3–4-dihydroxyphenylalanine (51). Dopamine-deficient mice can execute behaviors required for seeking and ingesting food, but they do not eat enough to survive (51, 52). Pharmacological depletion of dopamine also results in serious feeding deficits in animals (7, 53). Thus, dopaminergic neurotransmission seems to be important for the regulation of feeding behavior by the central nervous system.
Dopamine executes its physiological role via dopamine receptors. D2R is one of the dominant dopamine receptor subtypes, present not only in postsynaptic regions but also in presynaptic regions such as substantia nigra or VTA in the midbrain (23). Interestingly, D2R is expressed in the hypothalamus (23); hypothalamic D2R expression is relatively abundant compared with other dopamine receptors. D2R−/− mice showed a reduction in body weight and food intake (18, 54). Previously, it was reported that D2R null mice present a decrease in spontaneous locomotor activity (18). However, these mice retain exploratory behaviors despite the decrease in spontaneous locomotor activity (55). Thus, reduction in food intake and body weight cannot be attributed to decreased locomotor activity.
It is possible that decreased food intake is related to a lack of motivation in D2R−/− mice, as the dopaminergic system is not only closely involved in the rewarding or motivational characteristics of drugs of abuse but also of natural stimuli such as food. Total suppression of morphine-rewarding properties was observed in D2R−/− mice, but these mice behaved the same as WT mice when food was used as reward (55). This indicates that the D2R is involved in the motivational component of drug abuse but not in natural rewards such as foods (55). Therefore, even though we cannot rule out the possible involvement of behavioral alterations mentioned above in the reduction in food intake, the metabolic alterations observed in D2R−/− mice strongly indicate that these mice have altered energy homeostasis associated with the hypothalamic homeostatic system. This hypothesis was further supported by our pair-feeding study data, where we found that the absence of D2R interferes not only in food intake but also in energy metabolism.
Indeed, in this study, we demonstrate that D2R−/− mice consumed more energy with less food intake and that these mice have increased leptin sensitivity, thus displaying lean phenotype. We have also demonstrated that the treatment of D2R agonist and antagonist can affect the food intake and body weight changes induced by leptin, indicating that dopamine via D2R negatively regulates leptin action. Interestingly, haloperidol, a D2R antagonist treatment, enhanced the leptin response in WT mice but not in D2R−/− mice, supporting our observations in D2R−/− mice. Although it is sometimes compromising to interpret the response of haloperidol with its effect on the locomotor activity, our experiment of blocking D2R with haloperidol by imitating the conditions of D2R−/− mice, upon leptin treatment, showed an increased leptin response as compared with the leptin-only treated group in WT mice.
Furthermore, we have demonstrated the colocalization of D2R and pSTAT3-positive cells in arcuate nucleus regions in the hypothalamus and that stimulation of D2R by the D2R agonist quinpirole suppressed the leptin-induced STAT3 phosphorylation and nuclear localization of phospho-STAT3 in the hypothalamus of WT mice. However, this regulation was not detected in the D2R−/− mice. These data strongly suggest a tight association of the hypothalamic leptin signaling with the dopaminergic system via the D2R.
Recently, it has been reported that in leptin-deficient mice (Lep ob/ob mice), dopamine release in the nucleus accumbens is decreased, having only ∼60% the normal amount of dopamine (5). Similarly, it has been suggested that leptin-mediated signaling occurs in midbrain dopaminergic neurons and suppresses dopamine neuron activity (4). However, if leptin suppresses dopaminergic neuronal activity in the VTA- nucleus accumbens, then Lep ob/ob mice should consequently have increased dopamine signaling. However, this is not the case (4, 5). Rather, these obese Lep ob/ob mice have decreased dopamine signaling that does not prevent hyperphagia by these obese animals. In addition, injection of leptin increased pSTAT3 in the VTA region, but there was actually very little pSTAT3 in the VTA of normal mice. This raises questions about the physiological relevance of leptin regulation within the VTA region of the brain (3–5). Thus, as already described elsewhere (3), there seems to be a disconnection between dopamine signaling and eating behavior that remains to be understood with respect to leptin signaling in the midbrain.
Interestingly, in D2R−/− mice, MCH expression level in the hypothalamus was decreased compared with WT mice. It has been reported that leptin-deficient ob/ob mice have increased levels of MCH expression (56) and that MCH−/− mice are hypophagic and lean (57). Dopaminergic signaling is modified in MCH−/− mice (58), suggesting an interaction between these two systems. Intriguingly, we could not detect change in the expression level of NPY, AGRP, or POMC in D2R−/− mice as compared with WT mice. It has been known that hypothalamic neuronal populations are heterogeneous and that not all POMC neurons express leptin receptors in the hypothalamus for example (42). It has also been suggested that despite the importance of STAT3 signaling in the regulation of feeding and energy balance, the regulation of neuropeptide expression and activity in orexigenic arcuate nucleus neurons is independent of leptin receptor-mediated STAT3 signaling (59).
Recent findings demonstrated that in addition to the STAT-dependent pathway, leptin receptor signaling can also induce activation of phosphoinositide 3-kinase (PI3K) signaling (60–63). PI3K promotes Akt activity, and it has been reported that Akt can induce phosphorylation and inactivation of FOXO1, a forkhead family transcription factor, which is an important regulator of hypothalamic neuropeptides such as POMC, NPY, and AGRP (64–66). In addition, it appears that PI3K signaling can modulate leptin and insulin-mediated electrical responses of POMC and AGRP neurons (67, 68), indicating a distinct role for PI3K signaling in hypothalamic control of energy homeostasis. It would be worthwhile to examine whether dopaminergic signaling via D2R in hypothalamus can affect the PI3K-Akt pathway in relation to leptin signaling. It has been demonstrated that dopamine D2R activates phosphorylation of Akt, a major target of PI3K (69–71), but also some contradictory data showed that D2R induces inhibition of Akt (72). Taken together, it will be necessary to explore how downstream of leptin signaling is translated in the hypothalamus in D2R-positive cells under dopaminergic tone, how their neuronal excitabilities are regulated mutually, and also to understand how dopamine signaling interacts with the hypothalamic leptin system in control of energy balance.
Diaz-Torga et al. (54) reported that D2R−/− mice have altered growth hormone control. However, we and Diaz-Torga (54) did not observe any significant growth retardation, as the body length of D2R−/− mice was similar to WT mice (9.9 ± 008 in WT versus 9.4 ± 0.05 cm in D2R−/− mice) (54). This indicates that growth hormone alteration is not a major factor in reduced body weight and food intake. Here, we analyzed the D2R−/− mice from Baik et al. (18) and from Kelly et al. (19). We did not find significant differences in either line of mice. The fat mass was also decreased in their report (46) (inguinal fat (g) 1.88 ± 0.11 in WT versus 1.25 ± 0.07 in D2R−/− mice) to about 66% of WT, comparable with our data (55%), analyzed using a dual-energy x-ray absorptiometry instrument.
Food intake regulation in humans is undoubtedly highly complex. It will be interesting to find a relationship between brain dopamine and obesity. Increasing reports are emerging that describe polymorphisms of the D2 receptor genes among different dopamine receptor subtypes in obese patients (28–34). For example, the presence of the TaqIA1 allele has been associated with a decrease in D2R density, and a recent study with human obese subjects suggested that decreased dopaminergic activity in the striatum in subjects with the TaqIA1 allele polymorphism can be linked to the development of obesity (33, 34). Wang et al. (73) and Stice et al. (34) observed lower dopamine D2R availability in obese subjects, providing direct evidence of a deficit in D2Rs in obese individuals. Nevertheless, this study cannot determine whether D2R changes in obese individuals are a consequence or a cause of the obesity.
During the course of our manuscript preparation, Leinninger et al. (74) have reported that leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Lateral hypothalamic area is another hypothalamic area where leptin receptors are abundantly expressed, and it has been suggested to represent a component of reward circuit in the hypothalamus (74, 75). As we mentioned above, it is important to emphasize that the regulation of food intake is critically associated with the metabolic circuit, which modulates motivational aspects of feeding behaviors in parallel with homeostatic systems. Our present study is focusing more on the role of dopaminergic neurotransmission via dopamine D2R on energy homeostasis in arcuate nucleus hypothalamic regions that represent the center of energy homeostasis. Therefore, even though we cannot rule out the possible involvement of reward-related behaviors mentioned above in the reduction in food intake, the metabolic alterations observed in D2R−/− mice strongly indicate that these mice have altered energy homeostasis associated with the hypothalamic homeostatic system.
Taken together, our data unveil an important potential of the dopaminergic system, linking dopamine, a classical reward-related neurotransmitter, with the homeostatic system of energy balance, suggesting a previously unknown role for dopamine D2R in the homeostatic aspects of feeding. Because feeding behavior is dependent upon both nutritional status and emotional status, it is possible that our proposed interaction between leptin and dopamine can also change and adapt to different physiological situations, depending on the balance between these homeostatic and hedonic systems. Further studies should explore and clarify the dopamine-associated regulation of leptin signaling and the brain circuitry involved in dopamine-mediated regulation of energy homeostasis and feeding behaviors. An animal model with genetically manipulated conditional restriction of D2R in leptin receptor-expressing cells using Cre-LoxP approach would probably elucidate the role of the dopaminergic system via D2R in association with leptin in hypothalamic circuitry in control of energy balance.
Supplementary Material
Acknowledgments
We thank Dr. D. H. Lee (Department of Medicine, College of Medicine, Cheju National University, Cheju, South Korea) and Dr. Bradford Lowell (Harvard Medical School, Boston) for their help and advice. We also thank Dr. R. Thompson (University of Michigan), Dr. S. Sabol (National Institutes of Health), Dr. Jacques Drouin (Institut de Recherches Cliniques de Montréal, Canada), and Dr. Sehgal (New York Medical College) for providing us with the MCH, NPY, POMC cDNAs, and the STAT3 response element-luciferase construct, respectively. We thank all members of our laboratory for help and discussions.
This work was supported by Korea Research Foundation Research Grant KRF-2006-311-C00478, funded by the Korean Government (MOEHRD), Grants M103KV010015-07K2201-01510 and M103KV010014-08K2201-01410 from the Brain Research Center of the 21st Century Frontier Research Program, Grant M1-0311-00-0069 from the Korea Science and Engineering Foundation, funded by the Korean Ministry of Science and Technology, and a grant award from the AstraZeneca VRI Program.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- VTA
- ventral tegmental area
- D2R
- dopamine D2 receptor
- WT
- wild type
- POMC
- pro-opiomelanocortin
- AGRP
- agouti-related protein
- NPY
- neuropeptide Y
- MCH
- melanin-concentrating hormone
- PBS
- phosphate-buffered saline
- RT
- reverse transcription
- i.c.v.
- intracerebroventricular
- PI3K
- phosphoinositide 3-kinase.
REFERENCES
- 1.Hornykiewicz O. (1966) Pharmacol. Rev. 18, 925–964 [PubMed] [Google Scholar]
- 2.Berridge K. C. (2007) Psychopharmacology 191, 391–431 [DOI] [PubMed] [Google Scholar]
- 3.Palmiter R. D. (2007) Trends Neurosci. 30, 375–381 [DOI] [PubMed] [Google Scholar]
- 4.Hommel J. D., Trinko R., Sears R. M., Georgescu D., Liu Z. W., Gao X. B., Thurmon J. J., Marinelli M., DiLeone R. J. (2006) Neuron 51, 801–810 [DOI] [PubMed] [Google Scholar]
- 5.Fulton S., Pissios P., Manchon R. P., Stiles L., Frank L., Pothos E. N., Maratos-Flier E., Flier J. S. (2006) Neuron 51, 811–822 [DOI] [PubMed] [Google Scholar]
- 6.Zigmond M. J., Stricker E. M. (1972) Science 177, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 7.Zigmond M. J., Stricker E. M. (1973) Science 182, 717–720 [DOI] [PubMed] [Google Scholar]
- 8.Orosco M., Nicolaidis S. (1992) Physiol. Behav. 52, 1015–1019 [DOI] [PubMed] [Google Scholar]
- 9.Meguid M. M., Fetissov S. O., Varma M., Sato T., Zhang L., Laviano A., Rossi-Fanelli F. (2000) Nutrition 16, 843–857 [DOI] [PubMed] [Google Scholar]
- 10.Fetissov S. O., Meguid M. M., Chen C., Miyata G. (2000) Neuroscience 101, 657–663 [DOI] [PubMed] [Google Scholar]
- 11.Zigmond M. J., Heffner T. G., Stricker E. M. (1980) Prog. Neuropsychopharmacol. 4, 351–362 [DOI] [PubMed] [Google Scholar]
- 12.Sato T., Meguid M. M., Fetissov S. O., Chen C., Zhang L. (2001) Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1907–R1916 [DOI] [PubMed] [Google Scholar]
- 13.Yang Z. J., Meguid M. M. (1995) Neuroreport 6, 1191–1194 [DOI] [PubMed] [Google Scholar]
- 14.Bina K. G., Cincotta A. H. (2000) Neuroendocrinology 71, 68–78 [DOI] [PubMed] [Google Scholar]
- 15.Fetissov S. O., Meguid M. M., Sato T., Zhang L. H. (2002) Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R905–R910 [DOI] [PubMed] [Google Scholar]
- 16.Ramos E. J., Meguid M. M., Campos A. C., Coelho J. C. (2005) Nutrition 21, 269–279 [DOI] [PubMed] [Google Scholar]
- 17.Picetti R., Saiardi A., Abdel Samad T., Bozzi Y., Baik J. H., Borrelli E. (1997) Crit. Rev. Neurobiol. 11, 121–142 [DOI] [PubMed] [Google Scholar]
- 18.Baik J. H., Picetti R., Saiardi A., Thiriet G., Dierich A., Depaulis A., Le Meur M., Borrelli E. (1995) Nature 377, 424–428 [DOI] [PubMed] [Google Scholar]
- 19.Kelly M. A., Rubinstein M., Asa S. L., Zhang G., Saez C., Bunzow J. R., Allen R. G., Hnasko R., Ben-Jonathan N., Grandy D. K., Low M. J. (1997) Neuron 19, 103–113 [DOI] [PubMed] [Google Scholar]
- 20.Saiardi A., Bozzi Y., Baik J. H., Borrelli E. (1997) Neuron 19, 115–126 [DOI] [PubMed] [Google Scholar]
- 21.An J. J., Bae M. H., Cho S. R., Lee S. H., Choi S. H., Lee B. H., Shin H. S., Kim Y. N., Park K. W., Borrelli E., Baik J. H. (2004) Mol. Cell. Neurosci. 25, 732–741 [DOI] [PubMed] [Google Scholar]
- 22.Kim S. Y., Choi K. C., Chang M. S., Kim M. H., Kim S. Y., Na Y. S., Lee J. E., Jin B. K., Lee B. H., Baik J. H. (2006) J. Neurosci. 26, 4567–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansour A., Meador-Woodruff J. H., Bunzow J. R., Civelli O., Akil H., Watson S. J. (1990) J. Neurosci. 10, 2587–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahu A. (2003) Front. Neuroendocrinol. 24, 225–253 [DOI] [PubMed] [Google Scholar]
- 25.Mercer J. G., Hoggard N., Williams L. M., Lawrence C. B., Hannah L. T., Trayhurn P. (1996) FEBS Lett. 387, 113–116 [DOI] [PubMed] [Google Scholar]
- 26.Trivedi P., Yu H., MacNeil D. J., Van der Ploeg L. H., Guan X. M. (1998) FEBS Lett. 438, 71–75 [DOI] [PubMed] [Google Scholar]
- 27.Kokkotou E. G., Tritos N. A., Mastaitis J. W., Slieker L., Maratos-Flier E. (2001) Endocrinology 142, 680–686 [DOI] [PubMed] [Google Scholar]
- 28.Thomas G. N., Tomlinson B., Critchley J. A. (2000) Hypertension 36, 177–182 [DOI] [PubMed] [Google Scholar]
- 29.Comings D. E., Gade R., MacMurray J. P., Muhleman D., Peters W. R. (1996) Mol. Psychiatry 1, 325–335 [PubMed] [Google Scholar]
- 30.Fang Y. J., Thomas G. N., Xu Z. L., Fang J. Q., Critchley J. A., Tomlinson B. (2005) Int. J. Cardiol. 102, 111–116 [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson C. P., Hanson R., Cray K., Wiedrich C., Knowler W. C., Bogardus C., Baier L. (2000) Int. J. Obes. Relat. Metab. Disord. 24, 1233–1238 [DOI] [PubMed] [Google Scholar]
- 32.Tataranni P. A., Baier L., Jenkinson C., Harper I., Del Parigi A., Bogardus C. (2001) Diabetes 50, 901–904 [DOI] [PubMed] [Google Scholar]
- 33.Epstein L. H., Temple J. L., Neaderhiser B. J., Salis R. J., Erbe R. W., Leddy J. J. (2007) Behav. Neurosci. 121, 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stice E., Spoor S., Bohon C., Small D. M. (2008) Science 322, 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim R. Y., Shin S. W., Kim B. J., Lee W., Baik J. H. (2005) Biochem. Biophys. Res. Commun. 329, 1178–1185 [DOI] [PubMed] [Google Scholar]
- 36.Kok P., Roelfsema F., Frölich M., van Pelt J., Meinders A. E., Pijl H. (2006) J. Clin. Endocrinol. Metab. 91, 3236–3240 [DOI] [PubMed] [Google Scholar]
- 37.Sindelar D. K., Ste Marie L., Miura G. I., Palmiter R. D., McMinn J. E., Morton G. J., Schwartz M. W. (2004) Endocrinology 145, 3363–3368 [DOI] [PubMed] [Google Scholar]
- 38.Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 39.Vaisse C., Halaas J. L., Horvath C. M., Darnell J. E., Jr., Stoffel M., Friedman J. M. (1996) Nat. Genet. 14, 95–97 [DOI] [PubMed] [Google Scholar]
- 40.Banks A. S., Davis S. M., Bates S. H., Myers M. G., Jr. (2000) J. Biol. Chem. 275, 14563–14572 [DOI] [PubMed] [Google Scholar]
- 41.Tartaglia L. A. (1997) J. Biol. Chem. 272, 6093–6096 [DOI] [PubMed] [Google Scholar]
- 42.Münzberg H., Huo L., Nillni E. A., Hollenberg A. N., Bjørbaek C. (2003) Endocrinology 144, 2121–2131 [DOI] [PubMed] [Google Scholar]
- 43.Håkansson M. L., Meister B. (1998) Neuroendocrinology 68, 420–427 [DOI] [PubMed] [Google Scholar]
- 44.Håkansson M., de Lecea L., Sutcliffe J. G., Yanagisawa M., Meister B. (1999) J. Neuroendocrinol. 11, 653–663 [DOI] [PubMed] [Google Scholar]
- 45.Hâkansson M. L., Brown H., Ghilardi N., Skoda R. C., Meister B. (1998) J. Neurosci. 18, 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bates S. H., Dundon T. A., Seifert M., Carlson M., Maratos-Flier E., Myers M. G., Jr. (2004) Diabetes 53, 3067–3073 [DOI] [PubMed] [Google Scholar]
- 47.Flier J. S., Maratos-Flier E. (1998) Cell 92, 437–440 [DOI] [PubMed] [Google Scholar]
- 48.Elmquist J. K., Elias C. F., Saper C. B. (1999) Neuron 22, 221–232 [DOI] [PubMed] [Google Scholar]
- 49.Schwartz M. W., Woods S. C., Porte D., Jr., Seeley R. J., Baskin D. G. (2000) Nature 404, 661–671 [DOI] [PubMed] [Google Scholar]
- 50.Shah M., Patel K., Fried V. A., Sehgal P. B. (2002) J. Biol. Chem. 277, 45662–45669 [DOI] [PubMed] [Google Scholar]
- 51.Zhou Q. Y., Palmiter R. D. (1995) Cell 83, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 52.Szczypka M. S., Rainey M. A., Kim D. S., Alaynick W. A., Marck B. T., Matsumoto A. M., Palmiter R. D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12138–12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stricker E. M., Zigmond M. J. (1984) Int. J. Obes. 8, Suppl. 1,39– 50 [PubMed] [Google Scholar]
- 54.Díaz-Torga G., Feierstein C., Libertun C., Gelman D., Kelly M. A., Low M. J., Rubinstein M., Becú-Villalobos D. (2002) Endocrinology 143, 1270–1279 [DOI] [PubMed] [Google Scholar]
- 55.Maldonado R., Saiardi A., Valverde O., Samad T. A., Roques B. P., Borrelli E. (1997) Nature 388, 586–589 [DOI] [PubMed] [Google Scholar]
- 56.Qu D., Ludwig D. S., Gammeltoft S., Piper M., Pelleymounter M. A., Cullen M. J., Mathes W. F., Przypek R., Kanarek R., Maratos-Flier E. (1996) Nature 380, 243–247 [DOI] [PubMed] [Google Scholar]
- 57.Shimada M., Tritos N. A., Lowell B. B., Flier J. S., Maratos-Flier E. (1998) Nature 396, 670–674 [DOI] [PubMed] [Google Scholar]
- 58.Smith D. G., Tzavara E. T., Shaw J., Luecke S., Wade M., Davis R., Salhoff C., Nomikos G. G., Gehlert D. R. (2005) J. Neurosci. 25, 914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Münzberg H., Jobst E. E., Bates S. H., Jones J., Villanueva E., Leshan R., Björnholm M., Elmquist J., Sleeman M., Cowley M. A., Myers M. G., Jr. (2007) J. Neurosci. 27, 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niswender K. D., Morton G. J., Stearns W. H., Rhodes C. J., Myers M. G., Jr., Schwartz M. W. (2001) Nature 413, 794–795 [DOI] [PubMed] [Google Scholar]
- 61.Duan C., Li M., Rui L. (2004) J. Biol. Chem. 279, 43684–43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu A. W., Kaelin C. B., Takeda K., Akira S., Schwartz M. W., Barsh G. S. (2005) J. Clin. Invest. 115, 951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao A. Z., Huan J. N., Gupta S., Pal R., Sahu A. (2002) Nat. Neurosci. 5, 727–728 [DOI] [PubMed] [Google Scholar]
- 64.Kim M. S., Pak Y. K., Jang P. G., Namkoong C., Choi Y. S., Won J. C., Kim K. S., Kim S. W., Kim H. S., Park J. Y., Kim Y. B., Lee K. U. (2006) Nat. Neurosci. 9, 901–906 [DOI] [PubMed] [Google Scholar]
- 65.Kitamura T., Feng Y., Kitamura Y. I., Chua S. C., Jr., Xu A. W., Barsh G. S., Rossetti L., Accili D. (2006) Nat. Med. 12, 534–540 [DOI] [PubMed] [Google Scholar]
- 66.Tang E. D., Nuñez G., Barr F. G., Guan K. L. (1999) J. Biol. Chem. 274, 16741–16746 [DOI] [PubMed] [Google Scholar]
- 67.Hill J. W., Williams K. W., Ye C., Luo J., Balthasar N., Coppari R., Cowley M. A., Cantley L. C., Lowell B. B., Elmquist J. K. (2008) J. Clin. Invest. 118, 1796–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Qassab H., Smith M. A., Irvine E. E., Guillermet-Guibert J., Claret M., Choudhury A. I., Selman C., Piipari K., Clements M., Lingard S., Chandarana K., Bell J. D., Barsh G. S., Smith A. J., Batterham R. L., Ashford M. L., Vanhaesebroeck B., Withers D. J. (2009) Cell Metab. 10, 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brami-Cherrier K., Valjent E., Garcia M., Pagès C., Hipskind R. A., Caboche J. (2002) J. Neurosci. 22, 8911–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair V. D., Sealfon S. C. (2003) J. Biol. Chem. 278, 47053–47061 [DOI] [PubMed] [Google Scholar]
- 71.Bolan E. A., Kivell B., Jaligam V., Oz M., Jayanthi L. D., Han Y., Sen N., Urizar E., Gomes I., Devi L. A., Ramamoorthy S., Javitch J. A., Zapata A., Shippenberg T. S. (2007) Mol. Pharmacol. 71, 1222–1232 [DOI] [PubMed] [Google Scholar]
- 72.Beaulieu J. M., Sotnikova T. D., Yao W. D., Kockeritz L., Woodgett J. R., Gainetdinov R. R., Caron M. G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5099–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang G. J., Volkow N. D., Logan J., Pappas N. R., Wong C. T., Zhu W., Netusil N., Fowler J. S. (2001) Lancet 357, 354–357 [DOI] [PubMed] [Google Scholar]
- 74.Leinninger G. M., Jo Y. H., Leshan R. L., Louis G. W., Yang H., Barrera J. G., Wilson H., Opland D. M., Faouzi M. A., Gong Y., Jones J. C., Rhodes C. J., Chua S., Jr., Diano S., Horvath T. L., Seeley R. J., Becker J. B., Münzberg H., Myers M. G., Jr. (2009) Cell Metab. 10, 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leshan R. L., Björnholm M., Münzberg H., Myers M. G., Jr. (2006) Obesity 14, Suppl. 5,208S–212S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.