Abstract
Pemphigus vulgaris (PV) is an autoimmune blistering disease in which antibodies against the desmosomal cadherin, DSG3 (desmoglein-3), cause acantholysis. It has become increasingly clear that loss of cell-cell adhesion in PV is a complex and active process involving multiple signaling events such as activation of p38MAPK. It has also been demonstrated that incubating keratinocytes with PV IgG causes a redistribution of DSG3 from the cell surface to endosomes, which target these proteins for degradation. This study was undertaken to determine the relationship between p38MAPK and DSG3 endocytosis in pemphigus. In this work, we confirm that PV IgG causes internalization of cell-surface DSG3 into endosomes (as early as 4 h), which are then depleted from both detergent-soluble and detergent-insoluble pools. Cell-surface DSG3 internalization and depletion from both the detergent-soluble and detergent-insoluble fractions were blocked by the p38MAPK inhibitor SB202190. These data suggest that p38MAPK is capable of regulating PV IgG-mediated DSG3 internalization and that previously isolated mechanistic observations may be linked to a common pathway by which pemphigus autoantibodies lead to acantholysis.
Keywords: Adhesion, Endosomes, p38MAPK, Signal Transduction, Skin, Desmoglein, Desmosome, Pemphigus
Introduction
In the autoimmune blistering diseases pemphigus vulgaris (PV)2 and pemphigus foliaceus (PF), pathogenic IgGs that target extracellular epitopes of the desmosome transmembrane cell adhesion proteins DSG3 (desmoglein-3) and DSG1 (desmoglein-1), respectively, induce loss of keratinocyte cell-cell adhesion, resulting in clinical blistering. Two decades ago, ultrastructural studies from mice injected with endemic PF patient sera demonstrated the gradual redistribution of desmosomes from the plasma membrane to the cytoplasm (1). Subsequent studies have shown that following PV or PF IgG binding, DSG3 and DSG1 are internalized into endosomes and targeted for degradation (2–6). It is worth noting that internalization of cell adhesion molecules is not unique to desmosomes; for example, regulation of cadherin internalization has been implicated in embryogenesis, bacterial entry into host cells, and epithelial-to-mesenchymal transitions in cancer (7–9).
In addition to DSG internalization, several laboratories have reported a variety of pemphigus autoantibody-triggered signaling events within the target keratinocytes (10–13). Work from our laboratory has demonstrated that phosphorylation of p38MAPK and HSP25/27 occurs rapidly after exposure of keratinocytes to pemphigus IgG (14). p38MAPK activation may be one of the earliest detectable biochemical changes in PV IgG-treated cells, occurring within minutes of addition of pathogenic IgG to cultures. Furthermore, inhibition of p38MAPK and HSP25/27 phosphorylation blocks pemphigus IgG-induced cytoskeletal changes in vitro and blistering in vivo (14–17), suggesting a mechanistic role for p38MAPK and HSP25/27 in pemphigus acantholysis. Collectively, these observations raise the possibility that signaling and DSG internalization are mechanistically linked. This study was undertaken to investigate the potential relationship between PV IgG-mediated p38MAPK signaling and DSG3 endocytosis.
EXPERIMENTAL PROCEDURES
Materials
Rabbit anti-DSG3 polyclonal antibodies were purchased from Serotec (Oxford, UK). Mouse anti-E-cadherin monoclonal antibodies were purchased from BD Biosciences. Horseradish peroxidase-conjugated sheep anti-human lactate dehydrogenase V and rabbit anti-sheep secondary antibodies were purchased from Cortex Biochemicals (Concord, MA). The pan-cytokeratin antibody AE1/3 was purchased from Invitrogen. The p38MAPK inhibitor SB202190 was from Calbiochem. Normal primary human keratinocytes, Epilife keratinocyte growth medium, human keratinocyte growth supplement, and antibiotics were purchased from Invitrogen.
IgG Preparation
PV IgG was prepared by ammonium sulfate precipitation followed by affinity chromatography on Protein G (HiTrap, GE Healthcare) as described previously (14). The PV IgG used in these experiments was from a single patient with mucocutaneous PV in which antibodies to DSG3 and DSG1 were present. The indirect immunofluorescence titer was 1:640. IgG fractions were dialyzed against phosphate-buffered saline (PBS) and sterile-filtered. Purity was confirmed by SDS-PAGE, and activity was assayed by indirect immunofluorescence and enzyme-linked immunosorbent assay. Normal human (NH) IgG (no activity by indirect immunofluorescence) was prepared in parallel from normal human sera.
Tissue Culture
Normal primary human keratinocytes were passaged and expanded as described (14). Third passage keratinocytes were grown to 80–90% confluence. Keratinocyte medium was supplemented with CaCl2 to a final concentration of 0.5 mm 4 h prior to treating cells. Two hours prior to treating cells, keratinocytes were preincubated with the p38MAPK inhibitor SB202190 (100 μm) or Me2SO vehicle control at 37 °C. Cells were then treated with PBS, NH IgG (2 mg/ml), or PV IgG (2 mg/ml) for the indicated times and harvested.
Confocal Microscopy
Keratinocytes were grown on glass coverslips to 90% confluence, treated, fixed in 3.7% paraformaldehyde at 4 °C for 10 min, and washed three times with 2% bovine serum albumin in PBS for 10 min. Cells were then permeabilized using 0.5% Triton X-100 for 10 min at 4 °C followed by three 5-min washes using 2% bovine serum albumin in PBS. After the cells were blocked in 5% goat serum in PBS for 1 h, they were probed with mouse anti-human DSG3 (1:100; Invitrogen) and chicken anti-human EEA1 (1:100; Invitrogen) overnight. Cy2-conjugated goat anti-mouse (1:75), Cy3-conjugated goat anti-human (1:50), and Cy5-conjugated goat anti-chicken (1:75) secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) were used to probe for DSG3, PV IgG, and EEA1, respectively. Images were analyzed with a Leica SP2 AOBS confocal microscope using excitation wavelengths of 488, 514, and 561 nm. Images were viewed using a 63× objective with a numerical aperture 1.4. Triple-labeled samples were checked for bleed-through by turning off the various lasers and assaying for the absence of image. Independent representative images were assembled using Adobe Photoshop; brightness and contrast were uniformly adjusted across all images.
Cell-surface Biotinylation
Following treatment, keratinocyte cell-surface proteins were labeled using EZ-Link sulfo-NHS-SS-biotin (Pierce) at a concentration of 1 mg/ml at 4 °C on a rocking platform. After 1 h, the biotin was quenched using 500 mm ammonium chloride, and cells were lysed in buffer A (50 mm NaCl, 10 mm PIPES, 3 mm MgCl2, and 1% Triton X-100) using probe sonication. Lysates were clarified by centrifugation at 14,000 rpm for 10 min at 4 °C. Clarified lysates were passed over NeutraAvidin-agarose beads (Pierce) and incubated at room temperature for 1 h in an end-over-end mixer. Following three washes with buffer A, cell-surface proteins were eluted using 1× Laemmli buffer with 50 mm dithiothreitol. Western blot analysis was performed using anti-DSG3 and anti-E-cadherin antibodies.
Preparation of Detergent-soluble and Detergent-insoluble Fractions
Monolayer cells grown to confluence were extracted in cell lysis buffer (1% Nonidet P-40, 150 mm NaCl, 50 mm Tris-HCl, pH 7.4, 1 mm EDTA, 10 μm E-64, 100 μm leupeptin, 10 μm pepstatin, and 1 mm phenylmethylsulfonyl fluoride) at 4 °C for 1 h with rotation and then centrifuged at 13,700 × g for 15 min at 4 °C. The supernatants were collected as detergent-soluble fractions. The pellets were washed twice with PBS, resuspended by incubation in urea lysis buffer (8 m urea, 4% CHAPS, and 10 mm Tris-HCl, pH 7.4) for 1 h at 4 °C, and then centrifuged as described above; the supernatant was used as the detergent-insoluble fraction. Samples were equally loaded on SDS-polyacrylamide gel and separated by SDS-PAGE. Immunoblotting was performed according to established protocols and developed by enhanced chemiluminescence reaction (ECL, Amersham Biosciences).
RESULTS
We hypothesized that p38MAPK acts upstream of DSG3 internalization and that inhibition of p38MAPK blocks DSG3 internalization in PV IgG-treated cells. To investigate this, NH IgG and PV IgG were purified from human sera as described previously (14, 15). Primary human keratinocytes were pretreated for 2 h with the specific p38MAPK inhibitor SB202190 or vehicle and subsequently treated with PBS, NH IgG, or PV IgG for the indicated times. Cells were then fixed and stained with antibodies to human IgG, DSG3, or the early endosome marker EEA1.
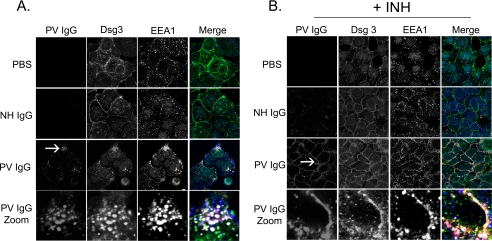
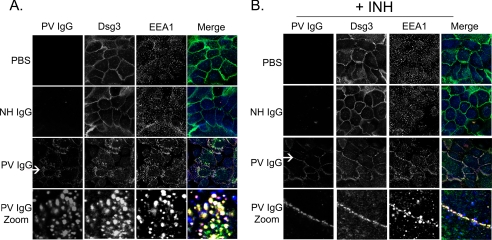
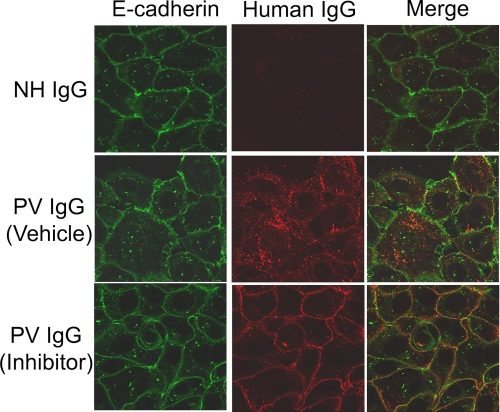
When treated for 4 h with buffer, NH IgG, or PV IgG (Fig. 1A), only the PV IgG-treated cells demonstrated staining with secondary antibodies against human IgG because only PV IgG, but neither buffer nor NH IgG, contains pathogenic anti-DSG3 antibodies capable of binding to the target keratinocytes. After 4 h, DSG3 staining was predominantly and uniformly localized to the plasma membrane in both buffer (PBS) and NH IgG-treated control keratinocytes, whereas EEA1 staining appeared in punctuate structures across the cell; however, both PV IgG and DSG3 were seen in internal structures resembling vesicles in PV IgG-treated cells. Using the endosome marker EEA1, these vesicular structures were demonstrated to be early endosomes; PV IgG, DSG3, and EEA1 all showed co-localization at 4 h in the absence of the p38MAPK inhibitor, analogous to what has previously been shown by Calkins et al. (3). In contrast, inhibition of p38MAPK blocked PV IgG-induced DSG3 internalization (Fig. 1B). Keratinocytes were pretreated with the p38MAPK inhibitor SB202190 for 2 h and then exposed to either buffer or NH IgG controls or to pathogenic PV IgG. As described above, after 4 h, cells were fixed and stained with secondary antibodies to human IgG, DSG3, and EEA1. In SB202190-treated cells, PV IgG and DSG3 co-localized to the cell membrane as opposed to the early endosomes seen in the absence of the p38MAPK inhibitor. PV IgG induced similar changes in DSG3 distribution in cells treated for 18 h (Fig. 2); PV IgG and DSG3 were localized to internal vesicular structures that co-stained with the endosome marker EEA1. In contrast, DSG3 staining was predominantly on the cell surface in control keratinocytes treated with either PBS or NH IgG (Fig. 2A). Pretreating keratinocytes with the p38MAPK inhibitor SB202190 prevented the PV IgG-induced redistribution of DSG3; cell-surface PV IgG and DSG3 staining was preserved in PV IgG-treated keratinocytes pretreated with SB202190 (Fig. 2B). Surprisingly, in SB202190-pretreated cells at both 4 and 18 h, EEA1, which was normally distributed throughout the cell, was visualized near the cell surface as well. In contrast to both DSG3 and PV IgG staining, however, EEA1 appeared as small punctuate structures near the surface. There appeared to be partial co-localization with both DSG3 and PV IgG in these cells (Figs. 1B and 2B). The ability of PV IgG to induce internalization of DSG3 was specific; no change in the adherens junction cell adhesion protein E-cadherin was observed when keratinocytes were treated with PV IgG in the presence or absence of the p38MAPK inhibitor (Fig. 3).
FIGURE 1.
Inhibition of p38MAPK blocks PV IgG-induced DSG3 internalization (4-h time point). Keratinocytes were treated with PBS, NH IgG (2 mg/ml), or PV IgG (2 mg/ml) for 4 h (A) or first pretreated with the p38MAPK inhibitor SB202190 (+INH) followed by PBS, NH IgG, or PV IgG for 4 h (B). Cells were then stained with (i) anti-human secondary antibodies to localize bound control or PV IgG (first column, red in Merge), (ii) murine anti-DSG3 monoclonal antibody followed by anti-mouse secondary antibodies to localize DSG3 (second column, green in Merge), and (iii) chicken anti-EEA1 followed by anti-chicken secondary antibodies to localize the early endosome marker EEA1 (third column, blue in Merge). In the fourth column, merged images are shown. A, in PBS-treated cells, membrane staining for DSG3 is seen with very little EEA1 co-localization. Similarly, in NH IgG-treated cells, DSG3 is seen predominantly at the plasma membrane with very little EEA1 co-staining. However, in PV-treated cells, the immunolocalization of both PV IgG and DSG3 redistributes from the cell surface to punctuate structures within the cytoplasm that co-localize with EEA1 (fourth column, Merge Zoom). B, in contrast, the PV IgG-stimulated redistribution of DSG3 is blocked in cells pretreated with SB202190, as evidenced by the preservation of the membrane staining pattern of PV IgG (first column, red in Merge) and DSG3 (second column, green in Merge). The arrow delineates the region from which the zoomed image was taken.
FIGURE 2.
Inhibition of p38MAPK blocks PV IgG-induced DSG3 internalization (18-h time point). Keratinocytes were treated with PBS, NH IgG (2 mg/ml), or PV IgG (2 mg/ml) for 18 h (A) or first pretreated with the p38MAPK inhibitor SB202190 (+INH) followed by PBS, NH IgG, or PV IgG for 18 h (B). Cells were then stained with (i) anti-human secondary antibodies to localize bound control or PV IgG (first column, red in Merge), (ii) murine anti-DSG3 monoclonal antibody followed by anti-mouse secondary antibodies to localize DSG3 (second column, green in Merge), and (iii) chicken anti-EEA1 followed by anti-chicken secondary antibodies to localize the early endosome marker, EEA1 (third column, blue in Merge). In the fourth column, merged images are shown. A, in PBS-treated cells, membrane staining for DSG3 is seen with very little EEA1 co-localization. Similarly, in NH IgG-treated cells, DSG3 is seen predominantly at the plasma membrane with very little co-staining with EEA1. However, in PV-treated cells, the immunolocalization of both PV IgG and DSG3 redistributes from the cell surface to punctuate structures within the cytoplasm that co-localize with EEA1 (fourth column, Merge Zoom). B, in contrast, the PV IgG-stimulated redistribution of DSG3 is blocked in cells pretreated with SB202190, as evidenced by the preservation of the membrane staining pattern of PV IgG (first column, red in Merge) and DSG3 (second column, green in Merge). The arrow delineates the region from which the zoomed image was taken.
FIGURE 3.
PV IgG specifically triggers endocytosis of DSG3 but not E-cadherin. Primary human keratinocytes were grown to confluency on glass coverslips and pretreated with either vehicle (Me2SO) or inhibitor (SB202190) for 2 h. Cells were subsequently treated with NH IgG (2 mg/ml) or PV IgG (2 mg/ml) for 4 h and then fixed and incubated with Cy2-conjugated antibodies directed against E-cadherin (green) and Cy3-conjugated antibodies against either NH IgG or PV IgG (red). Notice that in the vehicle-treated cells, whereas PV IgG causes internalization of DSG3, E-cadherin remains at the cell surface.
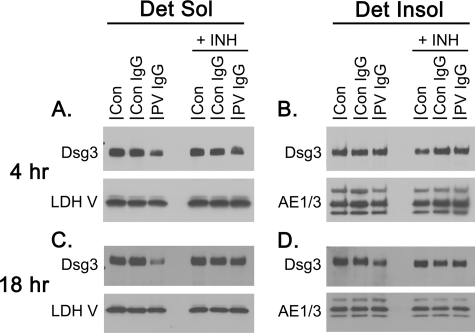
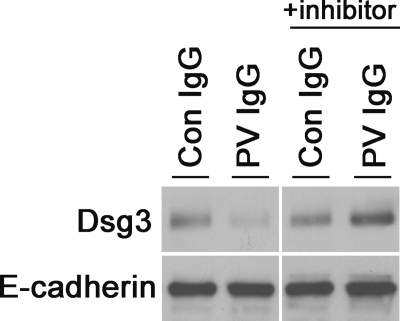
It has previously been demonstrated that prolonged treatment with PV IgG causes the loss of DSG3 from keratinocytes grown in tissue culture. DSG3 distributes into both detergent-soluble and detergent-insoluble fractions, the latter of which serves as a marker for the desmosome and cytoskeleton (3, 5, 18). To further investigate the ability of p38MAPK inhibitors to block DSG3 depletion, detergent-soluble and detergent-insoluble fractions were collected from keratinocytes treated with PBS, NH IgG, and PV IgG in the presence and absence of SB202190 and probed for DSG3 by immunoblotting (Fig. 4). Consistent with previous reports, PV IgG induced DSG3 depletion from both the detergent-soluble and detergent-insoluble cell fractions. In contrast, pretreatment with SB202190 blocked PV IgG-induced depletion of DSG3 from both the detergent-soluble and detergent-insoluble fractions. Furthermore, to differentiate DSG3 distribution into detergent-soluble cell-surface membrane from detergent-soluble intracellular vesicular fractions, biotin labeling studies were performed. Keratinocytes were treated with NH IgG and PV IgG for 4 h in the presence or absence of the p38MAPK inhibitor, and the remaining cell-surface proteins were labeled using a commercially available cell-impermeable biotin. Labeled proteins in the cell extracts were precipitated on NeutraAvidin beads, separated by SDS-PAGE, and probed with antibodies to either DSG3 or E-cadherin as a control (Fig. 5). PV IgG decreased the amount of cell-surface DSG3, but not E-cadherin, available for biotin labeling. Pretreating keratinocytes with SB202190 blocked this effect.
FIGURE 4.
PV IgG-induced depletion of DSG3 is blocked by the p38MAPK inhibitor SB202190. Primary human keratinocytes were pretreated for 1 h with 100 μm SB202190 or vehicle and then treated with PBS (Con), control IgG (Con IgG; 2 mg/ml), or PV IgG (2 mg/ml) for 4 (A, B) or 18 h (C, D). Cells were harvested and separated in detergent -soluble (Det Sol; A, C) and detergent-insoluble (Det Insol; B, D) fractions. Compared with buffer- or NH IgG-treated controls, less DSG3 was detected in the detergent-soluble fraction of keratinocytes treated with PV IgG for 4 and 18 h. This effect was blocked by the p38MAPK inhibitor SB202190 (+INH). Depletion of DSG3 from the detergent-insoluble fraction was observed at 18 h but not at 4 h. As with the detergent-soluble fraction, the depletion of DSG3 from the detergent-insoluble fraction was blocked by the p38MAPK inhibitor (+INH). Immunoblotting was carried out using rabbit anti-DSG3 antibody (1:1000). Immunoblotting for the cytoplasmic enzyme lactate dehydrogenase V (LDH V; sheep anti-human; 1:1000) was used as a loading control for the detergent-soluble fraction, whereas the pan-cytokeratin antibody AE1/3 (1:1000) was used as a loading control in the detergent-insoluble fraction.
FIGURE 5.
SB202190 blocks PV IgG-stimulated DSG3 internalization as measured by cell-surface biotinylation. Keratinocytes were grown to confluency in 12-well plates. Cells were pretreated for 2 h in SB202190 or Me2SO vehicle control and then treated with PBS, NH IgG, or PV IgG. Four hours later, cells were biotinylated using EZ-Link sulfo-NHS-SS-biotin at a concentration of 1 mg/ml at 4 °C on a rocking platform. After 1 h, the biotin was quenched using 500 mm ammonium chloride, and cells were lysed in buffer A using probe sonication. Lysates were passed over NeutraAvidin agarose beads. Following three washes with buffer A, cell-surface proteins were eluted using 1× Laemmli buffer with 50 mm dithiothreitol. Western blot analysis was performed using anti-DSG3 antibodies (1:1000). Anti-E-cadherin antibody (1:1000) was used as a loading control. Con IgG, control IgG.
DISCUSSION
When exposed to pemphigus IgG, keratinocytes lose adhesion to one another, resulting in disruption of epidermal integrity and blistering. Accumulating evidence suggests that this process proceeds through a series of events that will likely provide insight into the mechanism(s) by which desmosome adhesion, assembly, and disassembly are regulated under both normal and pathologic states. We have reported previously that activation of the signaling protein p38MAPK appears to be both an early and key step in the mechanism of both PV and PF IgG-mediated acantholysis (14–17), an observation confirmed by other investigators (12, 13, 19). Loss of DSG from the cell surface, internalization into endosomes, and subsequent degradation result in depletion of cell-surface DSG; this depletion also appears to be an important step in the mechanism by which loss of keratinocyte cell-cell adhesion proceeds in pemphigus, an observation similarly reported by several independent research groups (2–6).
To begin to build a cohesive model of the progressive steps by which acantholysis proceeds, in this work, we explored the relationship between p38MAPK and DSG3 endocytosis. First, we observed that PV IgG, but neither control IgG nor buffer controls, induces loss of DSG3 from the cell surface and internalization into early endosomes. This is consistent with reports from others, including Kowalczyk and coworkers (5), who showed that DSG endocytosis occurs via a clathrin-independent pathway (5). Second, PV IgG did not induce loss of the adherens junction protein E-cadherin from the cell surface, indicating that PV IgG was not inducing a generalized internalization of cell adhesion proteins rather that this effect was specific for the target antigen DSG3. Third, and significantly, inhibition of p38MAPK prevented the loss of DSG3 from the cell surface as measured by three independent assays, including (i) immunoblotting of detergent-soluble and detergent-insoluble fractions from PV IgG-treated keratinocytes, (ii) cell-surface biotin labeling, and (iii) confocal immunofluorescence microscopy.
The ability of p38MAPK inhibitors to block DSG3 internalization demonstrates an essential role for p38MAPK in regulating DSG3 internalization and subsequent degradation, adding to the growing evidence that p38MAPK is a key regulator of pemphigus IgG pathogenicity. The demonstration that p38MAPK may play an important role in receptor endocytosis is not new. Several articles have shown that p38MAPK regulates Rab5 proteins and its effectors (21–23). Rab5 proteins are essential in regulating the endocytosis of numerous receptors. Notably, EEA1 is a protein important in fusion of early endosomes (23) and is a Rab5 effector protein that is recruited to the membrane by Rab5.
Interestingly, we noted that pretreatment of cells with the p38MAPK inhibitor did not prevent the co-localization of PV IgG, DSG3, and EEA1 at the plasma membrane. This suggests (i) that PV IgG binding to DSG3 initiates signaling components acting upstream of p38MAPK and (ii) that these upstream signaling events may function to recruit EEA1 to the membrane, presumably for endocytosis. The surprising finding that EEA1 shows partial membrane localization in the SB202190/PV IgG-treated keratinocytes suggests that this specific localization may be used in future studies to probe for signaling components downstream of PV IgG binding to DSG3 but upstream of p38MAPK.
Significantly, blocking p38MAPK activation prevents internalization of the PV IgG-DSG3 complex. In a different system, it has been demonstrated that p38MAPK-mediated EEA1 phosphorylation regulates endocytosis of the μ opioid receptor (23). Attempts by us to identify p38MAPK-mediated EEA1 phosphorylation in the pemphigus cell culture system were not successful, perhaps due to technical limitations on our part.
The loss of cell-surface DSG3 precedes the loss of cell-cell adhesion, implicating a mechanistic role for DSG3 internalization in the molecular mechanism of acantholysis (5). Furthermore, in pemphigus IgG-treated cells, p38MAPK has been shown to be upstream of Rho kinase, which, in turn, regulates desmosomal adhesion (12). We have demonstrated previously (i) that p38MAPK inhibition prevents PV IgG-induced cytoskeletal changes (14, 17) and (ii) that p38MAPK inhibitors block both histologic and gross blister formation in the PV and PF passive transfer mouse model (15–17). Furthermore, significant p38MAPK and HSP27 phosphorylation has been observed in pemphigus patient skin (24).
We interpret these results to indicate that pemphigus IgG initiates a complicated cascade of inter-related molecular events. Binding of PV IgG to cell-surface DSG3 activates p38MAPK and, subsequently, HSP27 phosphorylation, RhoA inhibition, keratin intermediate filament retraction and actin reorganization, DSG internalization, and loss of adhesion. Although many of these pemphigus IgG-induced cellular changes were initially reported independently of one another, the observation that p38MAPK activation and DSG3 internalization are linked and that DSG3 internalization is dependent upon p38MAPK activity suggests that each of these events may be part of a unified molecular mechanism. We interpret the specificity of the internalization effect for DSG3 to result from the specificity of pemphigus IgG for desmogleins. Binding of PV IgG to DSG3 is hypothesized to bias the equilibrium toward destabilization/disassembly of DSG3 into the desmosome (3, 6). The specificity of the effect suggests molecular autonomy. Over extended time periods, the signaling cascade(s) initiated at DSG3-containing molecular complexes may affect other cell adhesion structures, as suggested by the global loss of adhesive interactions that characterizes acantholysis. Clearly, additional study is warranted to generate a better understanding of how each of these events is related to one another as well as to other described signaling events (11, 13, 20, 25).
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 AI49427 (to D. S. R.) and Grants AR30281 and AR32599 (to L. A. D.). P. B., L. A. D., and D. S. R. are co-inventors on a patent application identifying p38MAPK and HSP27 as targets for the treatment of pemphigus. Neither licensing agreements nor financial benefits have accrued to the inventors.
- PV
- pemphigus vulgaris
- PF
- pemphigus foliaceus
- PBS
- phosphate-buffered saline
- NH
- normal human
- PIPES
- 1,4-piperazinediethanesulfonic acid
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1.Futamura S., Martins C., Rivitti E. A., Labib R. S., Diaz L. A., Anhalt G. J. (1989) J. Invest. Dermatol. 93, 480–485 [DOI] [PubMed] [Google Scholar]
- 2.Aoyama Y., Kitajima Y. (1999) J. Invest. Dermatol. 112, 67–71 [DOI] [PubMed] [Google Scholar]
- 3.Calkins C. C., Setzer S. V., Jennings J. M., Summers S., Tsunoda K., Amagai M., Kowalczyk A. P. (2006) J. Biol. Chem. 281, 7623–7634 [DOI] [PubMed] [Google Scholar]
- 4.Cirillo N., Gombos F., Lanza A. (2007) J. Cell. Physiol. 210, 411–416 [DOI] [PubMed] [Google Scholar]
- 5.Delva E., Jennings J. M., Calkins C. C., Kottke M. D., Faundez V., Kowalczyk A. P. (2008) J. Biol. Chem. 283, 18303–18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao X., Choi E. J., Payne A. S. (2009) J. Invest. Dermatol. 129, 908–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogata S., Morokuma J., Hayata T., Kolle G., Niehrs C., Ueno N., Cho K. W. (2007) Genes Dev. 21, 1817–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sousa S., Cabanes D., Bougnères L., Lecuit M., Sansonetti P., Tran-Van-Nhieu G., Cossart P. (2007) Cell. Microbiol. 9, 2629–2643 [DOI] [PubMed] [Google Scholar]
- 9.Palacios F., Tushir J. S., Fujita Y., D'Souza-Schorey C. (2005) Mol. Cell. Biol. 25, 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seishima M., Iwasaki-Bessho Y., Itoh Y., Nozawa Y., Amagai M., Kitajima Y. (1999) Arch. Dermatol. Res. 291, 606–613 [DOI] [PubMed] [Google Scholar]
- 11.Williamson L., Raess N. A., Caldelari R., Zakher A., de Bruin A., Posthaus H., Bolli R., Hunziker T., Suter M. M., Müller E. J. (2006) EMBO J. 25, 3298–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waschke J., Spindler V., Bruggeman P., Zillikens D., Schmidt G., Drenckhahn D. (2006) J. Cell Biol. 175, 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chernyavsky A. I., Arredondo J., Kitajima Y., Sato-Nagai M., Grando S. A. (2007) J. Biol. Chem. 282, 13804–13812 [DOI] [PubMed] [Google Scholar]
- 14.Berkowitz P., Hu P., Liu Z., Diaz L. A., Enghild J. J., Chua M. P., Rubenstein D. S. (2005) J. Biol. Chem. 280, 23778–23784 [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz P., Hu P., Warren S., Liu Z., Diaz L. A., Rubenstein D. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12855–12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkowitz P., Chua M., Liu Z., Diaz L. A., Rubenstein D. S. (2008) Am. J. Pathol. 173, 1628–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H. E., Berkowitz P., Jolly P. S., Diaz L. A., Chua M. P., Rubenstein D. S. (2009) J. Biol. Chem. 284, 12524–12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato M., Aoyama Y., Kitajima Y. (2000) Lab. Invest. 80, 1583–1592 [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki Y., Aoyama Y., Tsunoda K., Amagai M., Kitajima Y. (2006) Autoimmunity 39, 587–590 [DOI] [PubMed] [Google Scholar]
- 20.Cirillo N., Lanza M., De Rosa A., Femiano F., Gombos F., Lanza A. (2008) Int. J. Immunopathol. Pharmacol. 21, 189–195 [DOI] [PubMed] [Google Scholar]
- 21.Cavalli V., Vilbois F., Corti M., Marcote M. J., Tamura K., Karin M., Arkinstall S., Gruenberg J. (2001) Mol. Cell 7, 421–432 [DOI] [PubMed] [Google Scholar]
- 22.Huang C. C., You J. L., Wu M. Y., Hsu K. S. (2004) J. Biol. Chem. 279, 12286–12292 [DOI] [PubMed] [Google Scholar]
- 23.Macé G., Miaczynska M., Zerial M., Nebreda A. R. (2005) EMBO J. 24, 3235–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkowitz P., Diaz L. A., Hall R. P., Rubenstein D. S. (2008) J. Invest. Dermatol. 128, 738–740 [DOI] [PubMed] [Google Scholar]
- 25.Puviani M., Marconi A., Cozzani E., Pincelli C. (2003) J. Invest. Dermatol. 120, 164–167 [DOI] [PubMed] [Google Scholar]