Abstract
Previous studies have shown that the combined presence of two cytochrome P450 enzymes (P450s) can affect the function of both enzymes, results that are consistent with the formation of heteromeric P450·P450 complexes. The goal of this study was to provide direct evidence for a physical interaction between P450 1A2 (CYP1A2) and P450 2B4 (CYP2B4), by determining if the interactions required both enzymes to reside in the same lipid vesicles. When NADPH-cytochrome P450 reductase (CPR) and a single P450 were incorporated into separate vesicles, extremely slow reduction rates were observed, demonstrating that the enzymes were anchored in the vesicles. Next, several reconstituted systems were prepared: 1) CPR·CYP1A2, 2) CPR·CYP2B4, 3) a mixture of CPR·CYP1A2 vesicles with CPR·CYP2B4 vesicles, and 4) CPR·CYP1A2·CYP2B4 in the same vesicles (ternary system). When in the ternary system, CYP2B4-mediated metabolism was significantly inhibited, and CYP1A2 activities were stimulated by the presence of the alternate P450. In contrast, P450s in separate vesicles were unable to interact. These data demonstrate that P450s must be in the same vesicles to alter metabolism. Additional evidence for a physical interaction among CPR, CYP1A2, and CYP2B4 was provided by cross-linking with bis(sulfosuccinimidyl) suberate. The results showed that after cross-linking, antibody to CYP1A2 was able to co-immunoprecipitate CYP2B4 but only when both proteins were in the same phospholipid vesicles. These results clearly demonstrate that the alterations in P450 function require both P450s to be present in the same vesicles and support a mechanism whereby P450s form a physical complex in the membrane.
Keywords: Cytochromes/Cytochrome P450, Electron Transfer, Enzymes/Kinetics, Membrane/Enzymes, Membrane/Reconstitution, Metabolism/Drug, Protein/Protein-Protein Interactions
Introduction
The cytochrome P450 gene superfamily is composed of a diverse array of monooxygenases that are expressed in virtually all species and involved in both xenobiotic and endogenous compound metabolism (1, 2). Mammalian liver expresses at least 10 different P4502 enzymes that are primarily involved in the metabolism of xenobiotics (3), with activation of the orphan nuclear receptors by xenobiotics influencing the relative levels of these enzymes in a tissue-specific manner (4). The mixed function monooxygenation reactions catalyzed by the P450 enzymes require electrons that are primarily provided by a separate redox partner, NADPH cytochrome P450 reductase (CPR) (5).
One feature of the P450 system in the liver is that the P450 enzymes are 10–20-fold more abundant than the CPR (6). The physiologic rationale for the apparent limit in the abundance of the CPR is not understood. Furthermore, it is not known how the low CPR/P450 ratio influences metabolism by the different P450s.
It has been suggested that aggregation of the P450 enzymes may serve to minimize the effects of limiting CPR on P450-mediated metabolism. There is considerable evidence that the enzymes do aggregate in solution (7, 8), in reconstituted systems with phospholipid (9, 10), and in cellular systems (11–13). Physical methods to estimate the size of P450 complexes in solution (e.g. size exclusion chromatography and sucrose density centrifugation) have generated measurements consistent with aggregates of 6–20 enzyme units, depending on experimental conditions (9, 10, 14). Other evidence for the intermolecular association of P450 enzymes has come from studies using detergents (7, 8), fluorescent labels (15–17), rotational diffusion (18), and exposure to high pressure (19, 20). Despite these studies, it is unclear whether the enzymes of the P450 system exist in the endoplasmic reticulum as functional monomers that interact with CPR or as components of higher order complexes (21).
One intriguing aspect of P450 enzyme aggregation is the possibility that the association of different P450 enzymes in a multienzyme complex may affect the catalytic function of the constituent enzymes. This effect was first reported by examining the effect of the interaction of CYP1A2 and CYP2B4 on the metabolism of 7-pentoxyresorufin and 7-ethoxyresorufin (7ER) (21, 22). Interestingly, when the CPR concentration was limiting and both P450 enzymes were present together in a mixed reconstituted system, the metabolism of the CYP1A2-selective substrate 7-ER was stimulated, whereas that of the CYP2B4-selective substrate 7-pentoxyresorufin was strongly inhibited. Detailed kinetic analysis showed that the changes in 7-alkoxyresorufin metabolism in the presence of both P450 enzymes were not caused by a simple competition for the CPR but were consistent with the formation of a heteromeric CYP1A2·CYP2B4 complex (23). Similar effects were also observed with CYP1A2 and CYP2E1, providing further evidence for the formation of a heteromeric P450 complex (24). These complexes could be disrupted by an increase in the ionic strength of the buffer, suggesting that the interactions between the P450s were electrostatic in nature (23, 24). Recently, a similar type of interaction also has been reported for CYP2C9 and CYP2C19 in which the activity of CYP2C9 is activated, whereas that of CYP2C19 is inhibited by the presence of the alternate enzyme (25). Thus, it seems that some P450-P450 interactions may have an important role in the regulation of enzyme activity.
In order to assess the function and interactions of the P450 system, both CPR and the P450s need to be reconstituted into phospholipid. Early studies showed that of the various phospholipids examined, dilauroylphosphatidylcholine (DLPC) was particularly effective in supporting P450-dependent monooxygenase activities (26). As a result of these studies, DLPC has commonly been used for reconstitution of P450-dependent activities; however, the conditions used by different laboratories are quite divergent with regard to 1) how the lipid is suspended prior to preincubation with proteins, 2) the lipid to protein ratio, 3) the duration of preincubation of proteins prior to measurement of enzyme activities, and 4) the concentration of the proteins in the preincubation mixture. Each of these parameters has been shown to affect the activity of the P450s in these systems (10, 27–29). Additionally, with many of the standard methods for preparation of reconstituted systems, the proteins were not stably anchored in the membranes (9, 10, 30).
We recently evaluated several methods used to prepare reconstituted systems and the effectiveness with which the enzymes were incorporated into the phospholipid bilayer (10). Preparations where the proteins are preincubated with preformed vesicles permitted only partial anchoring of CPR and P450 into the membrane. However, when the phospholipids and proteins are solubilized in an anionic detergent and the detergent is subsequently removed by dialysis or gel filtration, the proteins are integrally incorporated into the lipid membrane (31, 32). An easier and more rapid method for incorporation of CPR and P450 into phospholipid membranes has recently been reported, which uses adsorption of the detergent onto Biobeads as the method for detergent removal (29). These procedures result in reconstituted systems where the proteins are integrally incorporated in the phospholipid bilayer.
The goal of the present study was to utilize this method for CPR and P450 incorporation into membranes to determine if the functional changes observed in reconstituted systems containing both CYP2B4 and CYP1A2 required both proteins to be present in the same vesicles. These studies showed that 1) the proteins were anchored in the membrane and that electron transfer to the P450 enzymes and their ability to metabolize substrates required the incorporation of both CPR and P450 in the same vesicle and 2) CYP1A2 and CYP2B4 interactions were obtained only when both P450s were present in the same membrane, providing additional evidence that the alterations in substrate metabolism caused by the presence of multiple P450 enzymes required a physical interaction between these proteins. Finally, chemical cross-linking with bis(sulfosuccinimidyl) suberate (BS3) provided evidence for the formation of physical complexes between CYP1A2 and CYP2B4 only when the enzymes were present in the same lipid vesicles.
EXPERIMENTAL PROCEDURES
Materials
Bovine liver phosphatidylcholine (BPC) was purchased from Avanti Polar Lipids (Alabaster, AL). 7ER, 7-ethoxycoumarin (7EC), and DLPC were purchased from Sigma. 7-Ethoxy-4-trifluoromethylcoumarin (7EFC) was purchased from Molecular Probes, Inc. (Eugene, OR). Benzphetamine was a gift from The Upjohn Co. p-Nitroanisole was purchased from Sigma-Aldrich. The P450GLO CYP1A2 assay kit was purchased from Promega (Madison, WI). The antibody for CYP2B4 was purchased from Discovery Labware-BD Biosciences, the antibody for CYP1A2 was provided by Dr. Kristopher Krausz (National Institutes of Health), and the anti-CYP1A2 antibody used for immunoprecipitation was purchased from Abcam (Cambridge MA). BS3 was purchased from Pierce.
Enzyme Sources
Rabbit NADPH cytochrome P450 reductase was expressed from a recombinant plasmid, containing the wild type cDNA insert in a vector using the T7 promoter, which was provided by Dr. Lucy Waskell (University of Michigan) and has been described previously (23). Recombinant CYP2B4 was expressed and purified from Escherichia coli as described previously (23), and CYP1A2 was isolated and purified from β-naphthoflavone-treated rabbit liver microsomes (33). P450 levels were determined by measuring the carbon monoxy· ferrous complex (34). After preparation, the enzymes were stored at −80 °C in a solution of 50 mm potassium phosphate (pH 7.4) and 20% (v/v) glycerol.
Preparation of Lipid Vesicles Containing P450 Enzymes and CPR in BPC
Three different types of vesicle reconstituted systems were prepared. Unless otherwise stated, all of the systems were prepared at a 500:1 BPC/enzyme ratio. Simple reconstituted systems contained only one enzyme (CYP1A2, CYP2B4, or CPR) with the BPC. Binary reconstituted systems contained CPR and either CYP2B4 or CYP1A2 at a 0.6:1 CPR/P450 ratio (preparation of the vesicles typically resulted in less efficient incorporation of CPR than P450 enzyme; the final CPR/P450 ratio was ∼0.5:1) (10, 29). Ternary reconstituted systems contained CPR and equimolar concentrations of both P450 enzymes at a 0.6:1 CPR/total P450 ratio (again the final ratio after detergent removal was about 0.5:1).
CPR and P450 were incorporated into BPC vesicles using the DBB method that was described previously (29). Briefly, bovine phosphatidylcholine was dissolved in chloroform, lyophilized overnight, and bath-sonicated until clarified in 250 mm HEPES (pH 7.5) containing 5% (w/v) sodium glycocholate. The solubilized lipid solution (0.025 ml) was then mixed with the enzymes and MgCl2 (to final concentrations of 5 μm and 15 mm, respectively) (29). Nitrogen was layered over the solution to prevent oxidation of the phospholipids, and the samples were gently inverted to mix the solutions after the addition of the lipid/detergent mixture in 4 25-μl aliquots. After the entire detergent-lipid solution was added to the CPR and P450 enzymes, the mixture was incubated at 4 °C for 1 h before adding 0.3 g of Biobeads SM-2, which was prepared as described (35). The sample was rocked for 2 h at 4 °C and was washed twice with 0.05 m HEPES buffer (pH 7.5) containing 15 mm MgCl2, drawn into a syringe with a 32-gauge needle, and then filtered through a 5-μm syringe filter (Osmonics, Minnetonka, MN).
Reconstitution of P450 and CPR into Preformed DLPC Vesicles
Reconstituted systems of CPR and P450 in DLPC were prepared at a 0.5:1 CPR/P450 ratio and a 160:1 DLPC/P450 ratio. This ratio of DLPC/P450 has been previously shown to be optimal for catalytic activity (28). For these preparations, the DLPC was suspended at a concentration of 8 mm in a solution of 50 mm potassium phosphate (pH 7.25), 20% (v/v) glycerol, 0.1 m sodium chloride, and 5 mm EDTA (36). The DLPC suspension was bath-sonicated until clarified (about 20 min) before combining with CPR and P450. The P450, DPLC, and CPR were incubated at an elevated concentration (P450 concentration >10 μm) for 2 h at room temperature. After the 2-h preincubation, the reconstituted system was diluted to the desired concentration with buffer and other assay components.
Enzymatic Assays
The rate of N-demethylation of benzphetamine was determined by measuring the production of formaldehyde by fluorescence after the reaction with a modified Nash reagent (37). The spectrofluorometer was set at excitation and emission wavelengths of 410 and 510 nm, respectively. The reactions (1 ml) contained the reconstituted system (0.075 μm CPR and 0.15 μm P450 for binary reconstituted systems or 0.15 μm CPR, 0.15 μm CYP2B4, and 0.15 μm CYP1A2 for ternary reconstituted systems) and 500 μm benzphetamine in 25 mm potassium phosphate (pH 7.4). Reactions were initiated by the addition of NADPH to a final concentration of 0.4 mm, incubated at 37 °C for 5 min, and terminated with 110 μl of a 0.1:1 mixture of 50 mm semicarbazide (the pH of the semicarbazide solution was adjusted to ∼7.0 with an aqueous solution of sodium hydroxide before use) plus 25% ZnSO4 (w/v), followed immediately by 100 μl of a saturated solution of barium hydroxide as described previously (38).
The metabolism of 7ER was measured by monitoring the fluorescence change associated with the formation of the product 7-hydroxyresorufin (39). The reactions contained the reconstituted system (0.05 μm CPR and 0.1 μm P450 for binary reconstituted systems or 0.1 μm CPR, 0.1 μm CYP2B4, and 0.1 μm CYP1A2 for ternary systems) and the alkoxyresorufin substrate in 50 mm HEPES (pH 7.5), 15 mm MgCl2, and 0.1 mm EDTA. The reactions were initiated by the addition of NADPH to a final concentration of 0.4 mm and incubated at 37 °C. The reactions were measured in real time by excitation at 535 nm and emission at 585 nm, and initial rates were calculated from the linear portion of the fluorescence versus time curve. Substrate was dissolved at a concentration of 5 μm in the buffer by drying off the solvent from a 2 mm acetonitrile stock solution under a stream of N2 and then stirring in the buffer at a temperature of 70 °C for 1 h.
7EC and 7EFC dealkylation were also determined by fluorescence change: O-dealkylation of 7EC (excitation, 390 nm; emission, 440 nm) and 7EFC (excitation, 410 nm; emission, 510 nm). The reactions contained the reconstituted system (0.1 μm CPR and 0.2 μm P450 for binary reconstituted systems or 0.2 μm CPR, 0.2 μm CYP2B4, and 0.2 μm CYP1A2 for ternary systems), 10 mm glucose 6-phosphate, 0.5 unit of glucose-6-phosphate dehydrogenase, and substrate (200 μm 7EC or 400 μm 7EFC) in 50 mm HEPES (pH 7.5), 15 mm MgCl2, and 0.1 mm EDTA. The substrates added to the assays were diluted 100-fold from stock solutions in acetonitrile. The reactions were initiated by the addition of NADPH to a final concentration of 0.4 mm and incubated at 37 °C. The rate of product formation was determined from standard curves generated from 7-hydroxycoumarin and 7-hydroxy-4-trifluoromethylcoumarin (38).
The O-dealkylation of 4-nitroanisole was measured by the change in the absorbance (405–490 nm, ϵ = 0.01287 μm−1 cm−1). The 0.1-ml reactions contained the reconstituted system (0.4 μm CPR and 0.8 μm P450 for binary reconstituted systems or 0.8 μm CPR, 0.8 μm CYP2B4, and 0.8 μm CYP1A2 for ternary systems), 10 mm MgCl2, 10 mm glucose-6-phosphate, 0.5 unit of glucose-6-phosphate dehydrogenase, and 0.4 mm 4-nitroanisole in 100 mm potassium phosphate (pH 7.25). The reactions were initiated by the addition of NADPH to a final concentration of 0.4 mm and incubated at 37 °C.
The P450GLO CYP1A2 assay was performed according to the manufacturer's instructions, and the luciferin product was quantified by luminescence of a luciferin standard curve. The final concentrations of assay components in the 0.05-ml reactions were the reconstituted system (0.05 μm CPR and 0.1 μm P450 for the binary system and 0.1 μm CPR, 0.1 μm CYP1A2, and 0.1 μm CYP2B4 for the ternary reconstituted system). The luciferin-ME substrate was added to the reactions at a concentration of 400 μm. The reaction at 37 °C was initiated by the addition of 0.4 mm NADPH. The reaction was terminated after 10 min by the addition of 0.05 ml of the stopping solution provided with the kit. After mixing thoroughly, the samples were kept in the dark for 20 min before reading the luminescence with an integration time of 500 ms. All other conditions used were those specified in the manufacturer's instructions.
FPLC Characterization of Reconstituted Systems of CPR and P450
An aliquot of the reconstituted systems was applied to a Tricorn Superose 6 column equilibrated in 0.05 m potassium phosphate (pH 7.4), 0.2 mm EDTA, and 100 mm NaCl and run on an Akta FPLC system (GE Healthcare). Using this column, the sample components with a molecular mass greater than 5,000,000 Da were eluted in the void volume. The elution of cytochrome P450 from the FPLC was monitored by absorbance at 405 nm. Molecular weight standards were used to estimate sizes of the complexes.
Determination of the Rate of Reduction of P450 in BPC and DLPC Reconstituted Systems
NADPH-dependent P450 reduction was measured by monitoring the rate of formation of the ferrous P450·CO complex with an OLIS RSM-1000 (Bogart, GA) stopped flow spectrophotometer. There were two different types of reconstituted systems with which the rates of reduction were measured, and each type involved a slightly different preparation for stopped flow spectrophotometry.
For the reduction of binary reconstituted systems, the first syringe contained the reconstituted system containing CPR and P450 in phospholipid that was prepared as described above and a deoxygenating system (composed of 200 μm protocatecheuic acid and 0.4 unit/ml protocatecheuic acid dioxygenase) in 100 mm potassium phosphate buffer, pH 7.4. The P450 concentration for these reconstituted systems was 7 μm. The second syringe contained 0.4 mm NADPH and the deoxygenating system (200 μm protocatecheuic acid and 0.4 unit/ml protocatecheuic acid dioxygenase) in 100 mm potassium phosphate buffer, pH 7.4. Prior to the addition of the protocatecheuic acid dioxygenase, the sample for each syringe was bubbled for 2–3 min with CO. After adding the protocatecheuic acid dioxygenase, the solutions were drawn into syringes and loaded into different ports of the stopped flow spectrophotometer. The solutions were mixed in the stopped flow spectrophotometer, and the absorbance from 275 to 565 nm was measured (62.5 scans/s for 75 s).
In order to determine if the enzymes were able to transfer electrons between vesicles, the reduction of P450 was measured by mixing simple lipid reconstituted systems of CPR and P450 in the stopped flow spectrophotometer. In this case, the first syringe of the stopped flow spectrophotometer contained 7 μm P450 reconstituted into lipid (the BPC/P450 ratio was 500:1, and the DLPC/P450 ratio was 160:1), 0.33 mm NADPH, and the deoxygenating system in phosphate buffer. The second syringe contained 3.5 μm CPR reconstituted into phospholipid (at the lipid/protein ratios indicated above) and the deoxygenating system in phosphate buffer. The syringes were rapidly mixed, and 32 scans from 275 to 565 nm were taken every second for 75 s.
The rate constants and the amplitudes of reduction were determined by fitting the data for the change in absorbance at 450 nm using the successive integration option in the OLIS software and a multiphasic, exponential process with offset involving sequential, irreversible reduction of oxidized species of enzyme and one product (the ferrous·CO complex). All fits had an S.D. value of the residual fit of <5 × 10−4 and a Durbin-Watson ratio between 0.77 and 1.3.
Cross-linking and Immunoprecipitation of BPC Reconstituted Systems Containing CYP1A2 and/or CYP2B4
Reconstituted systems were prepared by the detergent-Biobeads method as described above at a 500:1 phospholipid to P450 ratio. The following vesicle systems were prepared: CYP1A2 in BPC (A), CYP2B4 in BPC (B), a mixture of CYP1A2-containing vesicles with CYP2B4-containing vesicles in BPC (C), and CYP1A2 and CYP2B4 reconstituted into the same BPC vesicles (D). Each of these reconstituted systems was diluted to a concentration of 0.4 μm P450 for the simple systems (A and B) and a 0.4 μm concentration of each P450 in the binary systems (C and D) in 50 mm HEPES buffer pH 7.5 containing 15 mm MgCl2, 0.1 mm EDTA. Each of the reconstituted systems was cross-linked with the water-soluble, homobifunctional cross-linking agent BS3, which reacts with primary amino groups. Each 100-μl reaction mixture was treated with 1 mm BS3 for 2 min at 4 °C and then quenched with 96 μl of ice-cold 2× radioimmune precipitation buffer (50 mm Tris, pH 7.6, containing 300 mm NaCl, 2% (v/v) Nonidet P-40, 2% (w/v) sodium deoxycholate, 0.2% SDS (w/v)) and 500 mm glycine and placed in an ice bath. Samples remained on ice for 15 min before the addition of 4 μl of goat polyclonal antibody to rabbit CYP1A2 (Abcam, Cambridge, MA). Samples were then allowed to incubate for 2 h on ice. At the end of the 2-h incubation, 100 μl of 25% (w/v) Protein G-agarose bead (Pierce) slurry was added, and each sample was rocked/incubated for 1 h at 4 °C. Samples were spun for 10 s at 3500 × g, the supernatants were transferred to clean microcentrifuge (1.5-ml) tubes, and the bead pellets were washed four times with 100 μl of radioimmune precipitation buffer. After aspirating the final wash, 100 μl of 2× loading buffer (6% (w/v) SDS, 0.01% (w/v) bromphenol blue, 24% (w/v) sucrose, 500 mm glycine, and 50 mm Tris (pH 6.8)) was added to each sample. The samples were treated with 5 μl of β-mercaptoethanol before boiling for 10 min. Thirty μl of each sample was loaded onto 4–12% (w/v) SDS-polyacrylamide gels and run for 45 min at 30 mA before running an additional 50 min at 200 V. The gels were transferred to nitrocellulose membranes and subjected to immune blot analysis. Briefly, the membrane used for CYP1A2 identification was incubated with a goat polyclonal antibody to rat CYP1A2 (Abcam) for primary antibody binding and purified recombinant Protein G-peroxidase conjugate (Pierce) for detection of primary antibody binding. The membrane used for P450 2B4 detection was subjected to a mouse monoclonal antibody to rat P450 2B1 (Oxford Biomedical, Rochester Hills, MI) for primary antibody binding and anti-mouse IgG-peroxidase conjugate for detection. Treated blots were visualized using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Waltham, MA) on a Versa Doc imager (Bio-Rad).
RESULTS
Our laboratory has demonstrated that the presence of one P450 enzyme will affect the catalytic behavior of another P450 when together in mixed reconstituted systems (21–23, 40). This was shown by experiments where the co-reconstitution of both CYP2B4 and CYP1A2 with CPR dramatically inhibited CYP2B4-selective metabolism and synergistically stimulated CYP1A2-dependent activities, an effect that was shown to be consistent with the formation of a CYP1A2·CYP2B4 complex. The goal of this study was to clearly demonstrate that CYP1A2 and CYP2B4 interact only when both P450s are localized into the same lipid vesicle. The strategy used to accomplish this goal was to compare the catalytic behavior of CYP1A2 and CYP2B4 when present in the same vesicles with their behavior under conditions where both proteins were present in the same solution but in different vesicles.
FPLC Comparison of Reconstituted Systems Using BPC Vesicles and DLPC Liposomes
As a first step, it was important to establish that proteins reconstituted into lipid were indeed anchored into the phospholipid membrane and did not readily translocate between vesicles. Therefore, we compared the characteristics of reconstituted systems prepared with DLPC, where the proteins were combined with preformed vesicles, with those prepared with BPC, where the proteins were present at the time that the lipid vesicles were formed. These reconstituted systems were applied to a Superose 6 gel filtration column to measure the size of resulting components. The results in Fig. 1 show that these reconstituted systems had very different characteristics. Both DLPC (generated by sonication) and BPC (generated by the DBB method) reconstituted systems produced liposomal vesicles with a molecular size greater than 5000 kDa (10, 29). However, the distribution of proteins was dramatically affected by the method of membrane preparation. As shown in Fig. 1 (curve A), CYP1A2 (based on absorbance at 405 nm) readily associated with the large BPC vesicles that eluted in the void volume of the Superose 6 column. Similarly, as reported previously, CPR (as measured by cytochrome c reduction) also eluted with the void volume in the BPC vesicles (data not shown) (10, 29). In contrast, when the proteins were reconstituted with preformed DLPC vesicles, a different elution profile was observed. Although about 60% of the CYP1A2 eluted with the void volume in the DLPC system, the remainder eluted as lower molecular weight complexes (Fig. 1, curve B). Similarly, a significant proportion of the CPR (∼60%) eluted as lower molecular weight complexes (∼1250 kDa in size) that did not appear to incorporate into the phospholipid (data not shown).
FIGURE 1.
Comparison of the effectiveness of P450 incorporation into BPC vesicles prepared by DBB method to the incorporation into preformed DLPC vesicles. Superose 6 gel filtration chromatograms of binary reconstituted systems, containing CPR and CYP1A2 in BPC (curve A) and DLPC (curve B) and CPR and CYP2B4 in BPC (curve C) and DLPC (curve D). The BPC and DLPC reconstituted systems were prepared as described under “Experimental Procedures.”
Generally, a similar elution pattern was observed for the CYP2B4-containing reconstituted systems. When CYP2B4 was incorporated into BPC vesicles prepared by the DBB method, both CYP2B4 and CPR eluted with the void volume (Fig. 1, curve C), consistent with their physical incorporation into the lipid. In contrast, incubation of CPR and CYP2B4 with preformed DLPC vesicles led to the elution of only a small amount of the proteins (10% of the P450 and 5% of the CPR) with the void volume. The majority of the proteins were associated as smaller molecular weight complexes. Nearly 40% of the CYP2B4 was associated with a 325-kDa sized assemblage that also contained ∼5% of the CPR. Interestingly, the CPR was found to be mainly associated (∼90%) with a 1250-kDa sized aggregate that was also associated with the remaining 50% of the CYP2B4. These results demonstrate that the reconstituted systems in BPC prepared by the DBB method lead to the anchoring of the proteins in the lipid membrane. In contrast, although the incubation of proteins with preformed DLPC vesicles readily supports P450-dependent monooxygenase activities (28), the proteins are distributed as complexes that are heterogeneous in nature and are not integrally attached to the membrane.
Using P450 Reduction to Measure the Ability of the Enzymes to Translocate between Lipid Vesicles
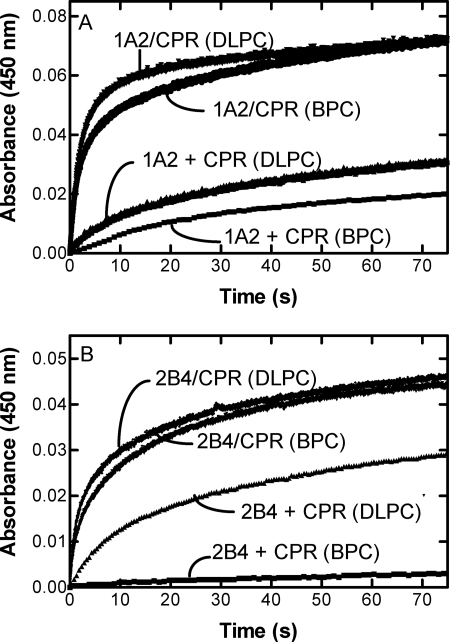
The next experiments were designed to measure the rate of translocation of proteins between vesicles using both the BPC and DLPC reconstituted systems. This was accomplished by reconstituting CPR and P450 into separate vesicles, rapidly mixing them in a stopped flow spectrophotometer in the presence of NADPH, and measuring the rate of first electron transfer to the P450 (Fig. 2). Because CPR·P450 complex formation is required for efficient electron transfer to P450, this method permits the determination of the rate of complex formation between the separately reconstituted components. P450 reduction was also measured with CPR and P450 in a preformed complex, which provides a measure of the inherent rate of electron transfer between these proteins.
FIGURE 2.
Effect of mixing of CPR-containing and P450-containing vesicles; comparison of BPC and DLPC systems. The scans were generated by measuring the anaerobic reduction of CYP1A2-containing (A) or CYP2B4-containing vesicles (B) by CPR when the enzymes were reconstituted together (CPR/P450) or separately (CPR + P450) in phospholipid (either BPC or DLPC, as indicated). When CPR and P450 were reconstituted separately in phospholipid, each reconstituted system was placed in separate syringes of the stopped flow spectrophotometer, the NADPH was added to the syringe containing the P450, and the time course of reduction was monitored as described under “Experimental Procedures.” When the NADPH-dependent reduction of the binary system containing CPR, P450, and lipid was measured, the reconstituted system from one arm of the stopped flow spectrophotometer was mixed with a solution of NADPH from the other arm of the instrument as described under “Experimental Procedures.”
Fig. 2A shows that the mixing of CPR-containing BPC vesicles with CYP1A2-containing BPC vesicles resulted in a slow rate of electron transfer (Fig. 2A). This is in contrast to the much more rapid reduction observed when both CPR and CYP1A2 were incorporated into the same BPC vesicles. These results demonstrate that CPR and CYP1A2 interact much more efficiently when anchored into the same vesicles.
Qualitatively similar results were obtained when DLPC vesicles were used. When CPR and CYP1A2 were reconstituted into separate preformed DLPC vesicles, the rate of electron transfer was slower than that observed when both proteins were incorporated into the same phospholipid vesicles. The rate of complex formation using separate vesicles (as measured by electron transfer) was more rapid in DLPC than observed with the BPC system, consistent with the incomplete anchoring of CYP1A2 and CPR during the reconstitution process.
Similar results were observed with the CYP2B4 system when reconstituted with BPC using the DBB method. Mixing of CPR-containing BPC vesicles with CYP2B4-containing vesicles showed an extremely slow rate of electron transfer (Fig. 2B), in contrast to the much more rapid reduction observed when CPR and CYP2B4 were reconstituted into the same vesicles. Again, these results demonstrate that both proteins need to be anchored into the same vesicles to permit physical complex formation and efficient electron transfer.
Reconstitution of the CYP2B4 into DLPC showed different results from those obtained with BPC (Fig. 2B) and with CYP1A2 in DLPC (Fig. 2A). Although reconstitution of CPR and CYP2B4 into separate vesicles led to a slower rate of reduction, when compared with their co-reconstitution, the difference was not nearly as dramatic as observed with the BPC system (Fig. 2B). This could be the result of the less efficient physical incorporation of CPR and CYP2B4 into the DLPC membranes, as shown in Fig. 1, which would increase the ability of CPR and CYP2B4 to interact, even when reconstituted separately. These results also differ from those obtained with CYP1A2 in DLPC. The rates are much faster when CPR and CYP2B4 are associated with separate vesicles of DLPC as compared with CYP1A2. These results may be due to the more efficient incorporation of CYP1A2 into the DLPC vesicles as shown in Fig. 1. Estimated kinetic parameters for the experimental data are shown in the supplemental material.
Effect of CYP1A2 and CYP2B4 Interactions on Substrate Metabolism in Mixed Reconstituted Systems
In the ER, P450s and other protein partners form complexes, at least in part, through electrostatic interactions (23, 24, 41). With all of these proteins present in the ER network, it is likely that the interactions occur by translational motion through the membrane. We prepared reconstituted systems with different combinations of CYP1A2, CYP2B4, and CPR using both DLPC and BPC systems. The purpose of these experiments was to demonstrate that CYP2B4 and CYP1A2 must be incorporated in the same phospholipid vesicle to interact and form a complex. Thus, we compared metabolism of several P450 substrates using binary systems (containing CPR and a single P450), mixed binary (where the vesicles from two binary reconstituted systems were mixed), and ternary reconstituted systems (containing CPR and both P450s reconstituted into the same membrane).
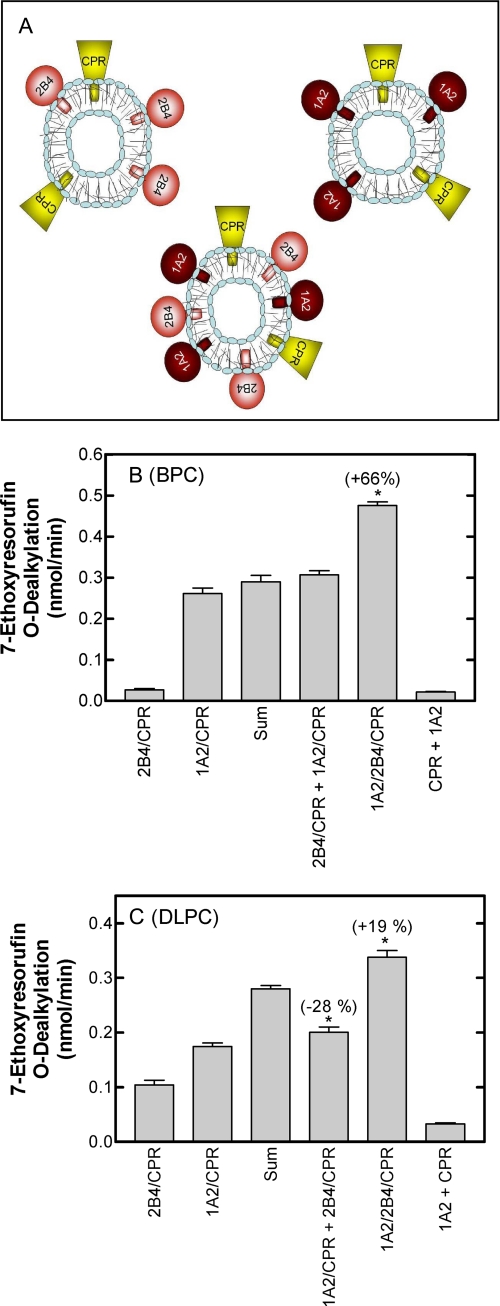
The rationale is diagrammed in Fig. 3A. Reconstituted systems containing (a) CPR and CYP1A2, (b) CPR and CYP2B4, and (c) CPR, CYP1A2, and CYP2B4 were incorporated into BPC membranes prepared using the DBB method (29). If the P450 enzymes were organized in the same manner in the ternary reconstituted system and in the binary systems, the metabolic rate in the ternary system would simply be the sum of the rates of the two binary systems. However, if one P450 enzyme affected the function of another P450, a non-additive effect in the ternary system would be expected. This approach has previously been used to identify functional interactions between different P450 enzymes in reconstituted systems using DLPC as the phospholipid source (21). The advantage of incorporation of the proteins into BPC using the DBB method is that the proteins are integrally incorporated into the lipid vesicle. Thus, in this study using BPC-Biobeads, we also examined substrate metabolism in a system where CPR·CYP1A2 vesicles and CPR·CYP2B4 vesicles were mixed to determine if metabolism was affected when both P450 enzymes were present in the assay but were unable to physically interact.
FIGURE 3.
Interactions among CYP1A2, CYP2B4, and CPR require the proteins to reside in the same vesicles. A, this schematic describes the experimental strategy used to demonstrate physical interactions between CYP1A2 and CYP2B4. The figure shows three different types of reconstituted systems represented by the P450s (either CYP2B4 or CYP1A2) and CPR. Substrate metabolism was compared using a mixture of the two binary systems containing CPR and one of the two P450 enzymes (the two upper systems in the panel) and a ternary system containing the CPR and both P450 enzymes (lower system in the panel). B, the rates of 7ER metabolism by binary, mixed binary, and ternary reconstituted systems of CYP1A2, CYP2B4, and CPR in BPC. The rate of 7ER metabolism by binary systems of CYP2B4 and CPR (2B4/CPR) and CYP1A2 and CPR (1A2/CPR), mixed binary systems (2B4/CPR + 1A2/CPR), and the ternary system (1A2/2B4/CPR) was measured in BPC using the glycocholate-detergent method for preparation of the reconstituted system. In addition, the sum of the means of the rates by the binary systems is shown to represent the rate that would be expected by the mixed systems if there was no interaction between the P450 enzymes that influenced metabolism. A negative control of a mixture of the simple systems in which the CPR and the P450 enzyme were incorporated in separate lipid vesicles is also shown (1A2 + CPR). *, a significant difference from the “sum” group (p < 0.05). C, rates of 7ER metabolism by binary, mixed binary, and ternary reconstituted systems of CYP1A2, CYP2B4, and CPR in DLPC. Reconstituted systems were the same as described in B except that the proteins were reconstituted into preformed DLPC vesicles. A negative control of a mixture of the simple systems in which the CPR and the P450 enzyme were incorporated in separate lipid vesicles is also shown (1A2 + CPR).
First, and as confirmation of the data on first electron transfer, the data show that incorporation of CPR and CYP1A2 into separate membranes was unable to adequately support 7-ethoxyresorufin dealkylation (Fig. 3B). The rate of 7ER metabolism (CPR·BPC + 1A2·BPC in Fig. 3B) was less than 6% of the rate of the CPR·CYP1A2 binary reconstituted system. The low rate of turnover when the CPR and P450 were reconstituted into separate vesicles indicates that the enzymes were effectively “anchored” in their respective vesicles over the time course of the reaction. These results demonstrate that CPR and CYP1A2 cannot effectively interact when anchored into different vesicles; a physical interaction between CPR and CYP1A2 is required for 7-ethoxyresorufin metabolism to occur.
Fig. 3B also shows the potential for interactions between CYP1A2 and CYP2B4 using the strategy described above. 7ER is predominantly a CYP1A2-selective substrate, exhibiting a 7-fold higher rate of metabolism with CPR·CYP1A2 than with CPR·CYP2B4 in binary reconstituted systems (Fig. 3B). As reported previously with the DLPC system (22), the combination of CYP2B4 and CYP1A2 in the same reconstituted system (CPR·1A2·2B4) resulted in a dramatic activation of 7-ER metabolism, producing a 66% increase over the sum of the rates of the two binary reconstituted systems. Furthermore, mixing of the two binary reconstituted systems produced a rate that was equal to the sum of the individual binary systems, indicating that the presence of both P450 enzymes in the same incubation was not, in itself, sufficient to affect the rate of substrate dealkylation; both P450s needed to be in the same vesicles in order to interact.
Fig. 3C shows the rates of 7ER metabolism associated with reconstituted systems prepared in DLPC. Again, 7ER was shown to be predominantly a CYP1A2-selective substrate, although the reaction was better supported by CYP2B4 in the DLPC reconstituted system than was observed in BPC. When CPR, CYP2B4, and CYP1A2 were reconstituted into the same DLPC vesicles, a synergistic stimulation of 7ER metabolism was observed, although the response was only elevated by about 20%. This was a much smaller response than that observed with the BPC vesicles. This stimulation was not observed when the two binary systems were mixed; in fact, the rate was slightly less than the sum of the rates by the binary systems.
The potential for CPR in one vesicle to support CYP1A2 metabolism (in another vesicle) was also shown in Fig. 3C using the DLPC system. Consistent with the data observed with the BPC system, the rate of 7-ER metabolism when CPR and CYP1A2 were reconstituted into separate vesicles was much lower than when both proteins were co-reconstituted. The slightly higher rate of metabolism in DLPC was probably due to the less efficient incorporation of the proteins into DLPC (Fig. 1) and their greater potential to interact.
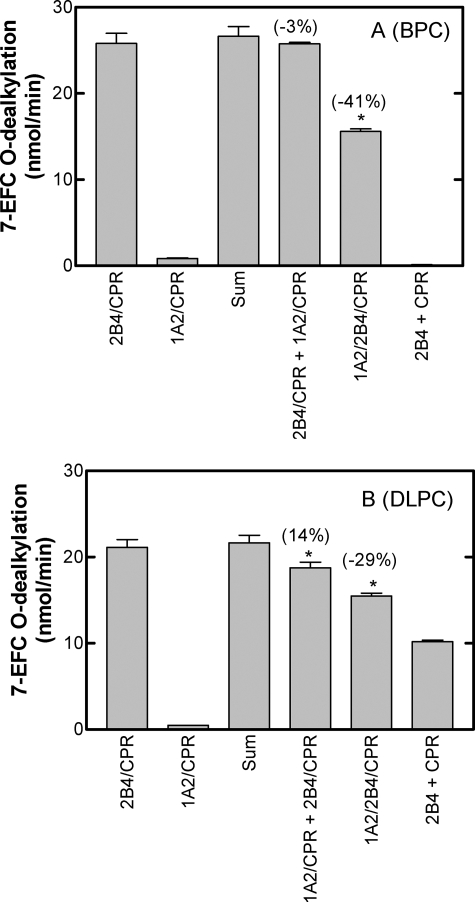
Fig. 4 shows the results obtained when metabolism by CYP1A2 and CYP2B4 was measured using 7EFC, a substrate that is selective for CYP2B4. In contrast to the stimulation observed with the CYP1A2-selective substrate, 7-EFC metabolism was inhibited by 40% when both proteins were in the same BPC vesicles (Fig. 4A). This inhibition was not observed when the P450 proteins were anchored into separate vesicles, supporting the idea that the proteins need to be in the same vesicles to interact. The complex between CYP1A2 and CYP2B4 takes on more of a CYP1A2 character when the enzymes are present in mixed reconstituted systems than is expected by the catalytic characteristics of the individual P450s as described previously (22, 23).
FIGURE 4.
Rates of 7EFC metabolism by binary, mixed binary, and ternary reconstituted systems. The rates of metabolism for the CYP2B4-selective substrate 7EFC were measured in reconstituted systems as described in the legend to Fig. 3A and under “Experimental Procedures.” *, a significant difference from the “sum” group (p < 0.05). A, reconstituted systems prepared using BPC by the DBB method. B, proteins were incorporated into preformed DLPC vesicles.
Analogous experiments using the DLPC system produced qualitatively similar but less clear cut results. Mixed reconstituted systems (containing both P450s and CPR in the same vesicles) exhibited about a 30% inhibition of 7-EFC metabolism. However, a small but significant amount of inhibition (15%) was also observed when CYP1A2 and CYP2B4 were reconstituted into separate DLPC vesicles. This inhibition was probably due to the less efficient anchoring of the proteins into their respective DLPC membranes, permitting cross-vesicle interactions to occur.
When CPR-containing vesicles were mixed with CYP2B4-containing vesicles, different results were obtained in the BPC and DLPC systems. Whereas 7EFC was not metabolized in the BPC system when mixing of CPR vesicles with CYP2B4 vesicles (Fig. 4A), the same experiment in DLPC yielded a significant amount of product formation (Fig. 4B). Again, these results can be explained due to the inefficient anchoring of proteins in their respective DLPC vesicles, allowing exchange of proteins between the different DLPC pools.
Tables 1 and 2 show the rates of metabolism of several additional substrates by binary, mixed binary, and ternary systems of CYP1A2, CYP2B4, and CPR reconstituted in liposomes of BPC (Table 1) and DLPC (Table 2). In each case, CYP1A2-selective substrates showed a synergism of their activities (7-ER and luciferin methyl ester) when both P450s were present in the same vesicles (ternary reconstituted systems), ranging from 66 to 105% stimulation. In contrast, CYP2B4-selective substrates (7EFC, benzphetamine, 7EC, and p-nitroanisole) were inhibited in the ternary reconstituted systems. Using the BPC vesicle systems, the degree of inhibition varied from 28% (benzphetamine) to 50% (p-nitroanisole). Similar results were observed with the DLPC systems.
TABLE 1.
Effect of reconstitution of multiple P450 enzymes on monooxygenase function: BPC vesicles
Reconstituted systems containing CPR plus CYP2B4 (CPR·2B4) or CPR plus CYP1A2 (CPR·1A2); a mixture of these two binary systems (CPR·1A2 + CPR·2B4); and a ternary system containing CPR, CYP1A2, and CYP2B4 (CPR·1A2·2B4) were incorporated into BPC membranes prepared using the DBB method and were used to measure the rate of metabolism of the listed substrates as described under “Experimental Procedures.” The abbreviations in the top row represent the components contained in the different reconstituted systems tested. When the components are separated by a chemical point, the enzymes were incorporated in the same lipid vesicles as a binary or ternary system. The plus sign is used to indicate the mixing of separate reconstituted systems immediately before performing the enzyme assays. The values represent the mean (expressed as nmol/min) ± S.D. of triplicate determinations. ND, not detected.
| Substrate | CPR·2B4 | CPR·1A2 | SUMa | CPR·1A2 + CPR·2B4 (change)b | CPR·1A2·2B4 (change) | 1A2 or 2B4 + CPR |
|---|---|---|---|---|---|---|
| nmol/min | nmol/min | nmol/min | nmol/min | nmol/min | nmol/min | |
| Luciferin-ME | ND | 0.2 ± 0.0 | 0.2 | 0.2 ± 0.0 (+11%) | 0.4 ± 0.1 (+105%) | NDc |
| 7ER | <0.1 | 0.3 ± 0.0 | 0.3 | 0.3 ± 0.0 (+0%) | 0.5 ± 0.0 (+66%) | <0.1c |
| 7EFC | 25.8 ± 2 | 0.8 ± 0.1 | 26.6 | 25.8 ± 0.3 (−3%) | 15.6 ± 0.6 (−41%) | <0.1d |
| Benzphetamine | 8.8 ± 0.4 | 0.7 ± 0.0 | 9.5 | 8.9 ± 0.4 (−6.3%) | 6.9 ± 0.3 (−27.5%) | 0.7 ± 0.0d |
| 7EC | 10.0 ± 0.1 | 0.5 ± 0.1 | 10.5 | 8.9 ± 0.6 (−15%) | 6.1 ± 0.3 (−42.1%) | 0.4 ± 0.3d |
| p-Nitroanisole | 1.2 ± 0.1 | 0.3 ± 0.0 | 1.3 | 1.1 ± 0.0 (−15.3%) | 0.7 ± 0.1 (−49.0%) | 0.2 ± 0.0d |
a The sum of the rates of the two binary systems containing CPR and one of the P450 enzymes are shown to indicate the rates expected if the two P450s did not interact with one another in the mixed systems.
b The numbers in parentheses represent the percentage change when compared with the sum of the binary systems. With all of the substrates, the rates of metabolism by the mixed binary and the ternary systems were statistically significant (p < 0.01) when analyzed by an unpaired t test.
c Metabolism was measured after mixing a simple reconstituted system of CYP1A2 in BPC with a simple system of CPR in BPC. This condition served as a negative control for CYP1A2-specific substrates to show that the CPR and P450 did not interact when in separate BPC vesicles.
d Metabolism was measured after mixing a simple reconstituted system of CYP2B4 in BPC with a simple system of CPR in BPC.
TABLE 2.
Effect of reconstitution of multiple P450 enzymes on monooxygenase function: Preformed DLPC vesicles
Reconstituted systems and mixtures of systems described in Table 1 were prepared in DLPC and used to measure the metabolism of the listed substrates as described under “Experimental Procedures.” The abbreviations used are the same as those indicated in Table 1. The values represent the mean (nmol/min) ± S.D. of triplicate determinations. ND, not detected.
| Substrate | 2B4/CPR | 1A2/CPR | SUM | 1A2/CPR/2B4 (change) |
|---|---|---|---|---|
| Luciferin-ME | ND | 0.3 ± 0.1 | 0.3 | 0.6 ± 0.1 (+152%) |
| 7ER | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.3 | 0.3 ± 0.0 (+17%) |
| 7EFC | 21.2 ± 1.5 | 0.5 ± 0.1 | 21.7 | 15.5 ± 0.6 (−30%) |
| Benzphetamine | 10.2 ± 1.6 | 0.7 ± 0.2 | 10.9 | 8.4 ± 0.0 (−22%) |
| 7EC | 20.3 ± 0.7 | 0.3 ± 0.10 | 20.6 | 10.4 ± 1.4 (−49.5%) |
| p-Nitroanisole | 1.4 ± 0.2 | 0.3 ± 0.1 | 1.7 | 0.9 ± 0.1 (−46%) |
Evidence for Physical Complex Formation between CYP1A2 and CYP2B4 by Chemical Cross-linking
In order to provide additional evidence for the formation of physical complexes between CYP1A2 and CYP2B4, the bifunctional amine-reactive compound BS3 was used to chemically cross-link the following BPC reconstituted systems: 1) vesicles containing only CYP1A2, 2) vesicles containing only CYP2B4, 3) a mixture of the CYP1A2 vesicles with the CYP2B4 vesicles, and 4) a reconstituted system containing both CYP1A2 and CYP2B4 in the same vesicles. Each of these cross-linked samples was immunoprecipitated with anti-CYP1A2, subjected to SDS-PAGE, and blotted with anti-CYP1A2 and anti-CYP2B4 antibody (Fig. 5).
FIGURE 5.
Cross-linking of P450s in simple and binary BPC reconstituted systems with BS3 followed by immunoprecipitation with anti-CYP1A2 and immune blotting. The following reconstituted systems were prepared using BPC: 1) vesicles containing only CYP1A2, 2) vesicles containing only CYP2B4, 3) a mixture of CYP1A2 vesicles with CYP2B4 vesicles, and 4) a reconstituted system containing both CYP1A2 and CYP2B4 in the same vesicles. As described under “Experimental Procedures,” the systems were cross-linked (X-L) with BS3, immunoprecipitated (IP) with anti-CYP1A2, subjected to SDS-PAGE, and blotted with anti-CYP1A2 (A) or anti-CYP2B4 antibody (B). The reconstituted systems in lanes 1–4 were cross-linked with BS3, whereas those in lanes 7–10 were immunoprecipitated but not cross-linked. Lanes 1 and 7, CYP1A2-containing vesicles; lanes 2 and 8, CYP2B4-containing vesicles; lanes 3 and 9, a mixture of the CYP1A2 vesicles with the CYP2B4 vesicles; lanes 4 and 10, CYP1A2 and CYP2B4 in the same vesicles. Lane 5, a control using the immunoprecipitating CYP1A2 antibody without any reconstituted system; lane 6, molecular weight standards with the mass of the band designated (kDa). The figure also shows the approximate band locations for monomeric, dimeric, trimeric, and tetrameric complexes of P450 enzymes.
Cross-linking led to multiple high molecular weight bands on both the CYP1A2 and CYP2B4 immune blots. Cross-linking and immunoprecipitation of vesicles containing only CYP1A2 showed high molecular weight complexes when immune blotted with anti-CYP1A2 (Fig. 5A, lane 1). These results show that CYP1A2 is capable of forming homomeric complexes. Similar complexes were observed with reconstituted systems containing separate and combined vesicles (Fig. 5A, lanes 3 and 4). High molecular weight complexes were not immunoprecipitated from vesicles containing only CYP2B4, attesting to the specificity of the antibody. Some higher molecular weight complexes were observed without cross-linking of the proteins (Fig. 5A, lanes 7–10).
In Fig. 5B, samples that were immune blotted with anti-CYP2B4 antibody provided evidence for the formation of CYP1A2·CYP2B4 complexes. Cross-linked CYP1A2-containing vesicles showed no immunoreactivity with anti-CYP2B4, showing the specificity of the antibody (Fig. 5B, lane 1). Vesicles containing only CYP2B4 were unable to be immunoprecipitated with anti-CYP1A2 and consequently showed no evidence of higher molecular weight complexes (Fig. 5B, lane 2). A similar result was observed when CYP1A2-containing vesicles were mixed with CYP2B4-containing vesicles (Fig. 5B, lane 3). Although both P450s were present during the immunoprecipitation with anti-CYP1A2, the antibody was unable to immunoprecipitate CYP2B4. However, when both proteins were present in the same vesicles, significant evidence for high molecular weight CYP2B4-containing complexes was found (Fig. 5B, lane 4). Such complexes were not observed without cross-linking of the samples (lanes 7–10). These results conclusively demonstrate that CYP1A2 and CYP2B4 form heteromeric complexes when present in a phospholipid membrane and that complex formation requires both proteins to reside in the same membranes.
DISCUSSION
Several studies have shown that the metabolism of numerous substrates by a given P450 enzyme is altered in the presence of other P450s (21, 25, 42–44). The specific interaction with mixed reconstituted systems containing both CYP1A2 and CYP2B4 was observed kinetically as a dramatic inhibition of CYP2B4-selective activities and a synergism of CYP1A2-selective activities, a characteristic that was more pronounced at subsaturating CPR levels. This process could not be explained by a simple competition between these P450s for the CPR (22) but could best be explained through the formation of a CYP2B4·CYP1A2 complex, having functional characteristics that differed from the individual P450s. A similar effect was shown with the CYP1A2·CYP2E1 system (23).
Yamazaki et al. (45) reported that CYP3A4-mediated testosterone 6β-hydroxylation was stimulated by the presence of CYP1A2 but not other P450s. Evidence for homomeric P450-P450 interactions was also shown with CYP3A4 (46). In this study, first electron transfer to CYP3A4 was examined in reconstituted systems, which allowed for CYP3A4-CYP3A4 interactions, and with monomeric P450 (in nanodiscs or detergent systems). Although the reconstituted systems exhibited a biphasic reduction, the reduction of the monomeric CYP3A4 systems was monophasic, consistent with the P450-P450 interactions affecting function via conformational effects on oligomerization (46). Evidence for homomeric complexes with CYP2E1 has also been obtained (47).
Not all P450s appear to form complexes that affect P450 function. Tan et al. (43) demonstrated that CYP2A6-dependent coumarin hydroxylation was inhibited by the presence of CYP2E1, a response that was relieved at elevated CPR. Li et al. (44) reported similar results by co-expressing CYP2D6, CYP3A4 and subsaturating CPR in E. coli. Our laboratory obtained similar findings with CYP2B4 and CYP2E1, exhibiting kinetic behavior similar to competition between monomeric P450s and CPR (24). Results from all three studies are consistent with two P450 enzymes simply competing for the limiting CPR. These data can be readily explained without the requirement for the formation of heteromeric P450·P450 complexes.
Recent studies have shown that allowing the vesicles to form around the proteins (as with the detergent-dialysis, -gel filtration, and -Biobeads methods) leads to more efficient incorporation and anchoring into the phospholipid membrane (10, 29). When the proteins were integrally incorporated into separate vesicles, as shown with the BPC reconstituted system, their mixing did not lead to significant interactions between the P450s. The differences between the BPC and DLPC reconstituted systems can also be explained based on the efficiency of incorporation. When the binary systems were combined, the reaction rates in the DLPC system were higher than observed with BPC, which is consistent with the proteins in DLPC having an increased ability to translocate and form functional complexes. Gel filtration analysis confirmed the less efficient anchoring of these proteins into DLPC vesicles. This difference in the ability of proteins to be anchored into phospholipid vesicles may be partly due to differences in methodology for vesicle production and protein incorporation (i.e. proteins added to preformed vesicles as opposed to the formation of vesicles around the proteins) but also is a function of the phospholipid employed. In the latter case, more efficient CYP2B4 incorporation was observed when longer chain phospholipids were used, even when the proteins were simply added to preformed BPC vesicles (10).
There are several conclusions to be drawn from these experiments. First, CPR and P450 need to be present in the same vesicle in order to effectively interact, which is supported by the extremely slow rates of electron transfer observed when CPR and P450 were integrally incorporated into different vesicles (Fig. 2).
Second, there does not appear to be a diffusible species (e.g. superoxide or peroxide) that can support these monooxygenation reactions, at least for the P450 enzymes and substrates examined in this study. This conclusion is supported by the inefficient substrate metabolism when CPR and P450 are reconstituted into separate vesicles. These preparations exhibit slow rates of both electron transfer and substrate turnover.
Third, the changes in monooxygenase function resulting from the interaction between different P450 enzymes require that both P450s exist in the same vesicles. When different P450 enzymes are present in separate vesicles, the inhibition of CYP2B4-selective activities or the synergism of CYP1A2-selective activities was not observed. Similarly, when the P450 enzymes were incorporated in separate vesicles, there was little tendency for the formation of heteromeric complexes when the separate, simple systems were cross-linked with BS3. However, when the enzymes were incorporated in the same lipid vesicles, the formation of mixed CYP1A2·CYP2B4 complexes was evident.
An unexpected result from the cross-linking experiments (Fig. 5) was that instead of only a few discrete high molecular weight bands representing dimers, trimers, tetramers, and higher order complexes, additional bands were obtained that became a smear on the gel, particularly above 250 kDa. There are two possible reasons for this effect. First, cross-linking may differentially influence the mobility of the protein aggregates due to reactions with different amounts of chemical cross-linker. Complexes may contain different amounts of cross-linker attached. This could lead to variability in SDS association with the proteins and result in altered electrophoretic mobility, producing indistinct “smears” on the gels at molecular masses greater than 250 kDa. A second reason for the band smearing is probably a result of the difference in size between CYP2B4 and CYP1A2. CYP2B4 has a molecular mass of 48 kDa, whereas CYP1A2 has a molecular mass of 53 kDa. Thus, homomeric and heteromeric complexes of the enzymes would be expected to have different electrophoretic mobilities. For example, there are two trimers that could be immunoprecipitated having different mobilities: 2B4· 2B4·1A2 and 2B4·1A2·1A2. The multiplicity of these complexes would become more pronounced as complex size is increased.
We have put forth a mechanism to explain how the presence of multiple P450 enzymes changes the metabolism by the individual isoforms. It is presumed that CYP1A2 and CYP2B4 form a heteromeric complex that results in the CYP1A2 moiety of the complex having a higher CPR-binding affinity than it does when not associated with the CYP2B4. Interestingly, a study utilizing computer modeling of the existing crystal structures of P450 enzymes has provided support for this idea by showing that specific heterodimers of P450 enzymes (which are formed in the presence of some substrates) induce changes in the CPR-binding affinity of the constituent P450 enzymes in the complex (48). Our findings suggest that the binding of most, if not all, of the CYP1A2 and CYP2B4 substrates induce this putative heteromeric complex formation. However, judging by the relative magnitude of the effects, the substrates probably have varying tendencies to induce the formation of these complexes.
The drug metabolic capacity of the liver phase 1 enzymes has been considered to be the sum of the metabolic activities of the individual components. Because of the presence of multiple P450 enzymes, higher levels of a specific isoform would be expected to proportionally influence flux through a particular pathway. However, several factors may affect this simplified description. First, the limiting levels of NADPH-cytochrome P450 CPR may affect overall drug disposition by restricting electron flow through particular P450 enzymes. Second, the presence of cytochrome b5 may differentially affect drug disposition in a substrate- and isoform-dependent manner. Finally, the results of the current study clearly demonstrate that organizational factors can have a significant influence on P450 function as a result of the potential of different P450 enzymes to form heteromeric complexes. Consequently, the predicted behavior of a particular P450 enzyme in a simple reconstituted system may not adequately predict its behavior in microsomal systems where other P450 enzymes may be present.
Supplementary Material
Acknowledgments
We thank Dr. Andrew Catling for guidance with the immunoprecipitation experiments. We also thank Dr. Kristopher Krausz (National Institutes of Health, Bethesda, MD) for providing a CYP1A2 antibody.
This work was supported, in whole or in part, by National Institutes of Health, NIEHS, United States Public Health Service Grant R01-ES004344.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables A and B.
- P450
- cytochrome P450
- CPR
- NADPH-cytochrome P450 reductase
- luciferin-ME
- 7-methyl ester of luciferin
- 7EC
- 7-ethoxycoumarin
- 7EFC
- 7-ethoxy-4-trifluoromethylcoumarin
- 7ER
- 7-ethoxyresorufin
- DBB
- detergent Bio-bead method for preparing BPC vesicles
- DLPC
- dilaurylphosphatidylcholine
- BPC
- bovine phosphatidylcholine
- BS3
- bis(sulfosuccinimidyl) suberate
- FPLC
- fast protein liquid chromatography.
REFERENCES
- 1.Nelson D. R., Koymans L., Kamataki T., Stegeman J. J., Feyereisen R., Waxman D. J., Waterman M. R., Gotoh O., Coon M. J., Estabrook R. W., Gunsalus I. C., Nebert D. W. (1996) Pharmacogenetics 6, 1–42 [DOI] [PubMed] [Google Scholar]
- 2.Guengerich F. P. (2001) Chem. Res. Toxicol. 14, 611–650 [DOI] [PubMed] [Google Scholar]
- 3.Anzenbacher P., Anzenbacherová E. (2001) Cell. Mol. Life Sci. 58, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waxman D. J. (1999) Arch. Biochem. Biophys. 369, 11–23 [DOI] [PubMed] [Google Scholar]
- 5.Wang M., Roberts D. L., Paschke R., Shea T. M., Masters B. S., Kim J. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson J. A., Ebel R. E., O'Keeffe D. H., Matsubara T., Estabrook R. W. (1976) J. Biol. Chem. 251, 4010–4016 [PubMed] [Google Scholar]
- 7.Wagner S. L., Dean W. L., Gray R. D. (1984) J. Biol. Chem. 259, 2390–2395 [PubMed] [Google Scholar]
- 8.Dean W. L., Gray R. D. (1982) J. Biol. Chem. 257, 14679–14685 [PubMed] [Google Scholar]
- 9.French J. S., Guengerich F. P., Coon M. J. (1980) J. Biol. Chem. 255, 4112–4119 [PubMed] [Google Scholar]
- 10.Reed J. R., Kelley R. W., Backes W. L. (2006) Drug Metab. Dispos. 34, 660–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alston K., Robinson R. C., Park S. S., Gelboin H. V., Friedman F. K. (1991) J. Biol. Chem. 266, 735–739 [PubMed] [Google Scholar]
- 12.Baskin L. S., Yang C. S. (1980) Biochemistry 19, 2260–2264 [DOI] [PubMed] [Google Scholar]
- 13.Baskin L. S., Yang C. S. (1982) Biochim. Biophys. Acta 684, 263–271 [DOI] [PubMed] [Google Scholar]
- 14.Kanaeva I. P., Dedinskii I. R., Skotselyas E. D., Krainev A. G., Guleva I. V., Sevryukova I. F., Koen Y. M., Kuznetsova G. P., Bachmanova G. I., Archakov A. I. (1992) Arch. Biochem. Biophys. 298, 395–402 [DOI] [PubMed] [Google Scholar]
- 15.Szczesna-Skorupa E., Mallah B., Kemper B. (2003) J. Biol. Chem. 278, 31269–31276 [DOI] [PubMed] [Google Scholar]
- 16.Ozalp C., Szczesna-Skorupa E., Kemper B. (2005) Drug Metab. Dispos. 33, 1382–1390 [DOI] [PubMed] [Google Scholar]
- 17.Davydov D. R., Knyushko T. V., Kanaeva I. P., Koen Y. M., Samenkova N. F., Archakov A. I., Hui Bon Hoa G. (1996) Biochimie 78, 734–743 [DOI] [PubMed] [Google Scholar]
- 18.Kawato S., Gut J., Cherry R. J., Winterhalter K. H., Richter C. (1982) J. Biol. Chem. 257, 7023–7029 [PubMed] [Google Scholar]
- 19.Davydov D. R., Petushkova N. A., Archakov A. I., Hoa G. H. (2000) Biochem. Biophys. Res. Commun. 276, 1005–1012 [DOI] [PubMed] [Google Scholar]
- 20.Davydov D. R., Deprez E., Hoa G. H., Knyushko T. V., Kuznetsova G. P., Koen Y. M., Archakov A. I. (1995) Arch. Biochem. Biophys. 320, 330–344 [DOI] [PubMed] [Google Scholar]
- 21.Backes W. L., Kelley R. W. (2003) Pharmacol. Ther. 98, 221–233 [DOI] [PubMed] [Google Scholar]
- 22.Backes W. L., Batie C. J., Cawley G. F. (1998) Biochemistry 37, 12852–12859 [DOI] [PubMed] [Google Scholar]
- 23.Kelley R. W., Reed J. R., Backes W. L. (2005) Biochemistry 44, 2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley R. W., Cheng D., Backes W. L. (2006) Biochemistry 45, 15807–15816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hazai E., Kupfer D. (2005) Drug Metab. Dispos. 33, 157–164 [DOI] [PubMed] [Google Scholar]
- 26.Strobel H. W., Lu A. Y., Heidema J., Coon M. J. (1970) J. Biol. Chem. 245, 4851–4854 [PubMed] [Google Scholar]
- 27.Ingelman-Sundberg M., Johansson I. (1980) Biochemistry 19, 4004–4011 [DOI] [PubMed] [Google Scholar]
- 28.Causey K. M., Eyer C. S., Backes W. L. (1990) Mol. Pharmacol. 38, 134–142 [PubMed] [Google Scholar]
- 29.Reed J. R., Brignac-Huber L. M., Backes W. L. (2008) Drug Metab. Dispos. 36, 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingelman-Sundberg M., Glaumann H. (1980) Biochim. Biophys. Acta 599, 417–435 [DOI] [PubMed] [Google Scholar]
- 31.Gut J., Richter C., Cherry R. J., Winterhalter K. H., Kawato S. (1982) J. Biol. Chem. 257, 7030–7036 [PubMed] [Google Scholar]
- 32.Ingelman-Sundberg M., Blanck J., Smettan G., Ruckpaul K. (1983) Eur. J. Biochem. 134, 157–162 [DOI] [PubMed] [Google Scholar]
- 33.Coon M. J., van der Hoeven T. A., Dahl S. B., Haugen D. A. (1978) Methods Enzymol. 52, 109–117 [DOI] [PubMed] [Google Scholar]
- 34.Omura T., Sato R. (1964) J. Biol. Chem. 239, 2370–2378 [PubMed] [Google Scholar]
- 35.Holloway P. W. (1973) Anal. Biochem. 53, 304–308 [DOI] [PubMed] [Google Scholar]
- 36.Huang C. H. (1969) Biochemistry 8, 344–352 [DOI] [PubMed] [Google Scholar]
- 37.de Andrade J. B., Bispo M. S., Reboucas M. V., Carvalho M. L., Pinheiro H. L. (1996) Am. Lab. 28, 56–58 [Google Scholar]
- 38.Hanna I. H., Reed J. R., Guengerich F. P., Hollenberg P. F. (2000) Arch. Biochem. Biophys. 376, 206–216 [DOI] [PubMed] [Google Scholar]
- 39.Lubet R. A., Mayer R. T., Cameron J. W., Nims R. W., Burke M. D., Wolff T., Guengerich F. P. (1985) Arch. Biochem. Biophys. 238, 43–48 [DOI] [PubMed] [Google Scholar]
- 40.Cawley G. F., Batie C. J., Backes W. L. (1995) Biochem. 34, 1244–1247 [DOI] [PubMed] [Google Scholar]
- 41.Tamburini P. P., Schenkman J. B. (1986) Mol. Pharmacol. 30, 178–185 [PubMed] [Google Scholar]
- 42.Kaminsky L. S., Guengerich F. P. (1985) Eur. J. Biochem. 149, 479–489 [DOI] [PubMed] [Google Scholar]
- 43.Tan Y., Patten C. J., Smith T., Yang C. S. (1997) Arch. Biochem. Biophys. 342, 82–91 [DOI] [PubMed] [Google Scholar]
- 44.Li D. N., Pritchard M. P., Hanlon S. P., Burchell B., Wolf C. R., Friedberg T. (1999) J. Pharmacol. Exp. Ther. 289, 661–667 [PubMed] [Google Scholar]
- 45.Yamazaki H., Gillam E. M., Dong M. S., Johnson W. W., Guengerich F. P., Shimada T. (1997) Arch. Biochem. Biophys. 342, 329–337 [DOI] [PubMed] [Google Scholar]
- 46.Davydov D. R., Fernando H., Baas B. J., Sligar S. G., Halpert J. R. (2005) Biochemistry 44, 13902–13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jamakhandi A. P., Kuzmic P., Sanders D. E., Miller G. P. (2007) Biochemistry 46, 10192–10201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hazai E., Bikádi Z., Simonyi M., Kupfer D. (2005) J. Comput. Aided Mol. Des. 19, 271–285 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.