Abstract
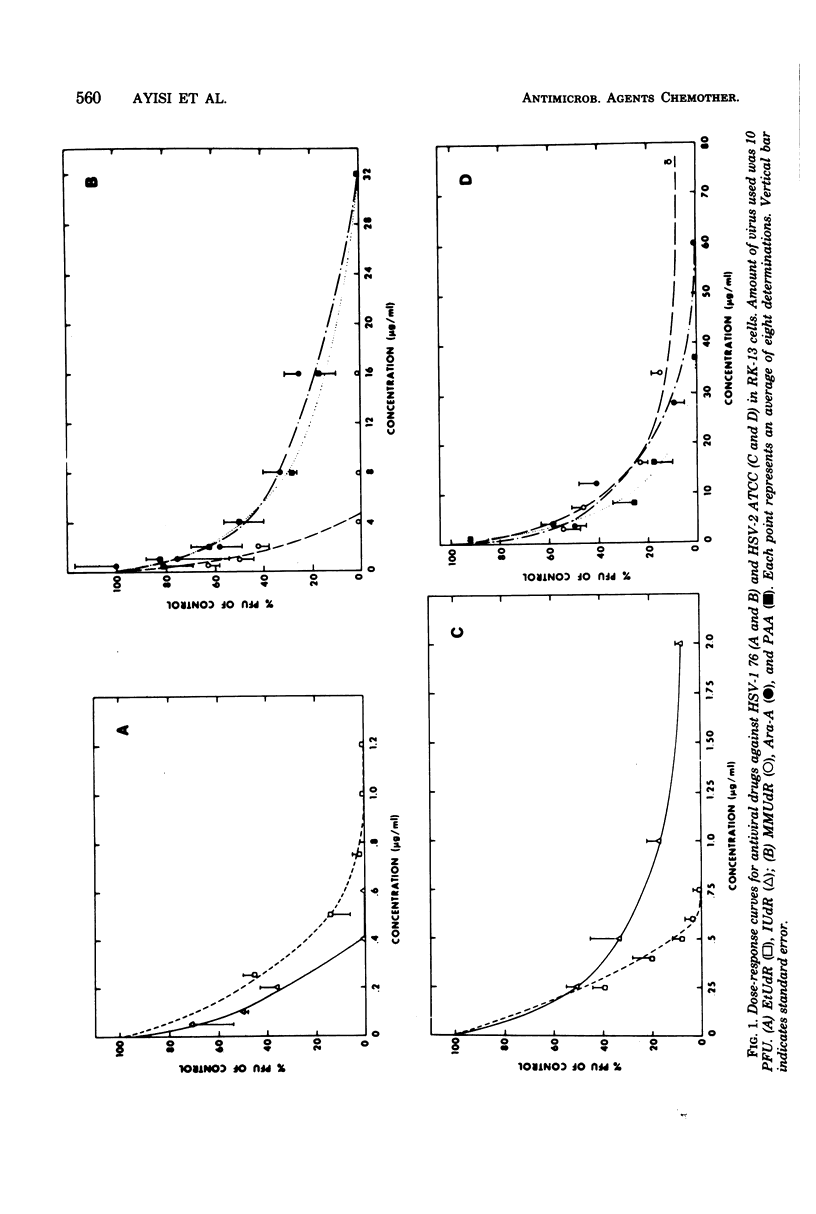
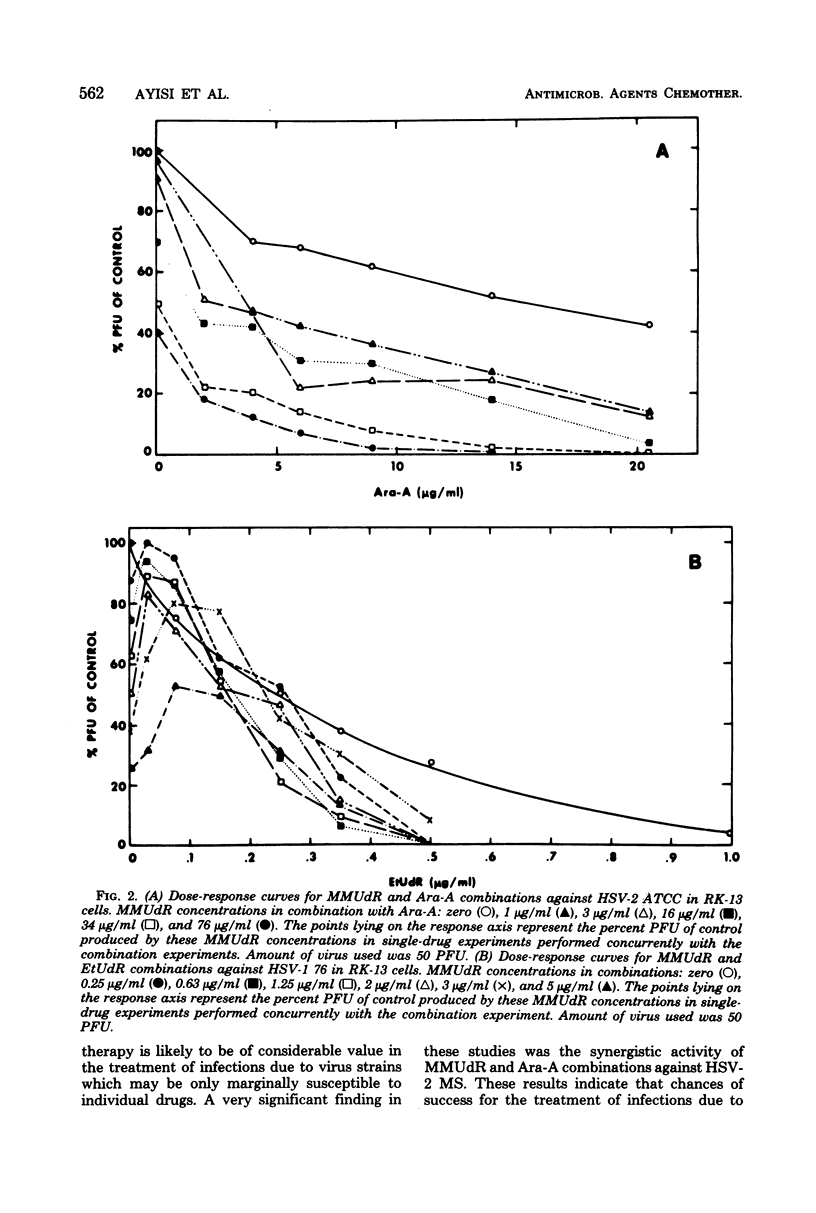
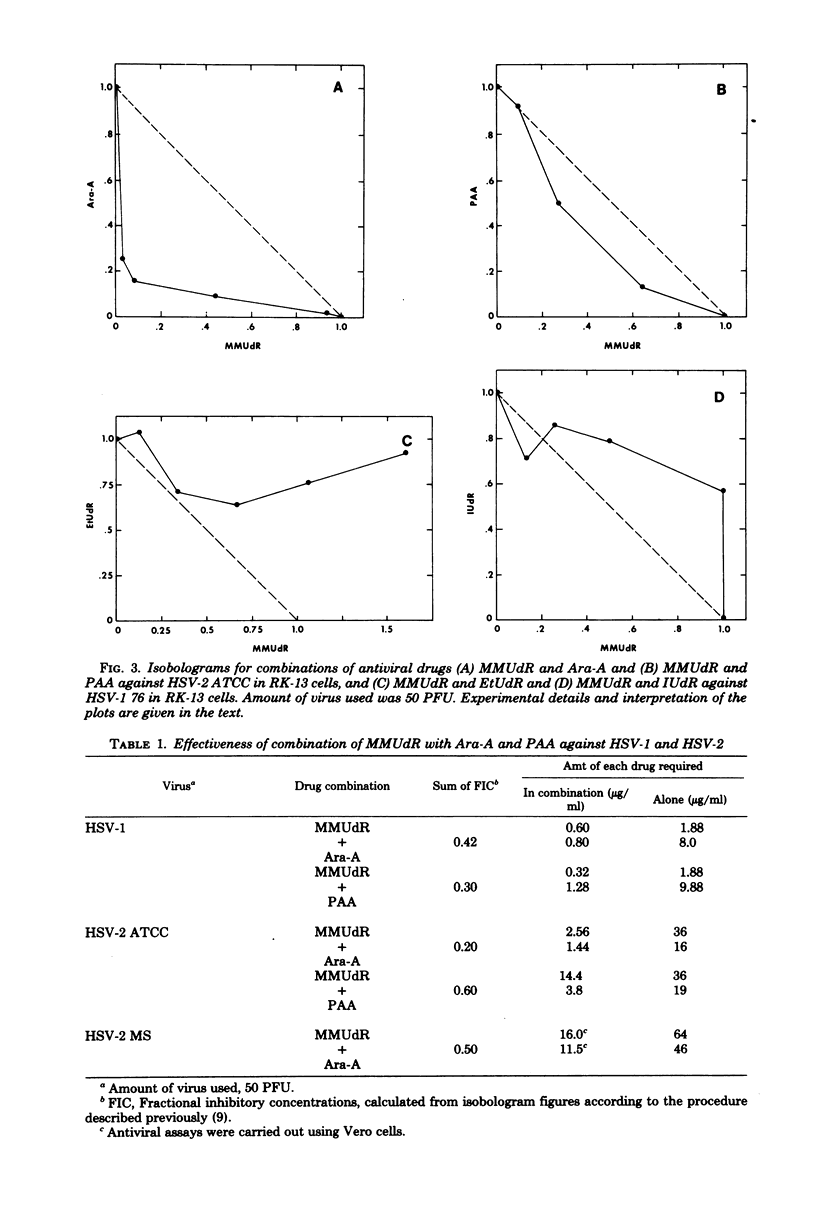
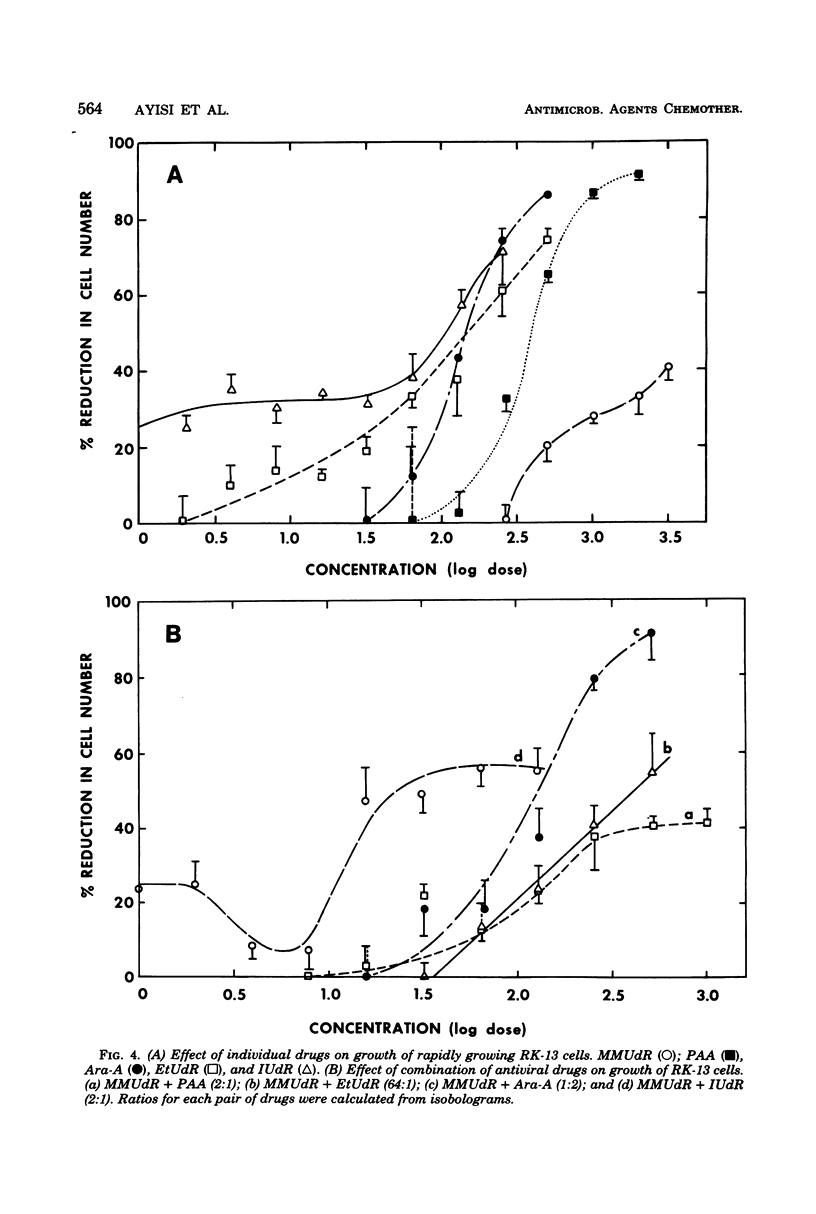
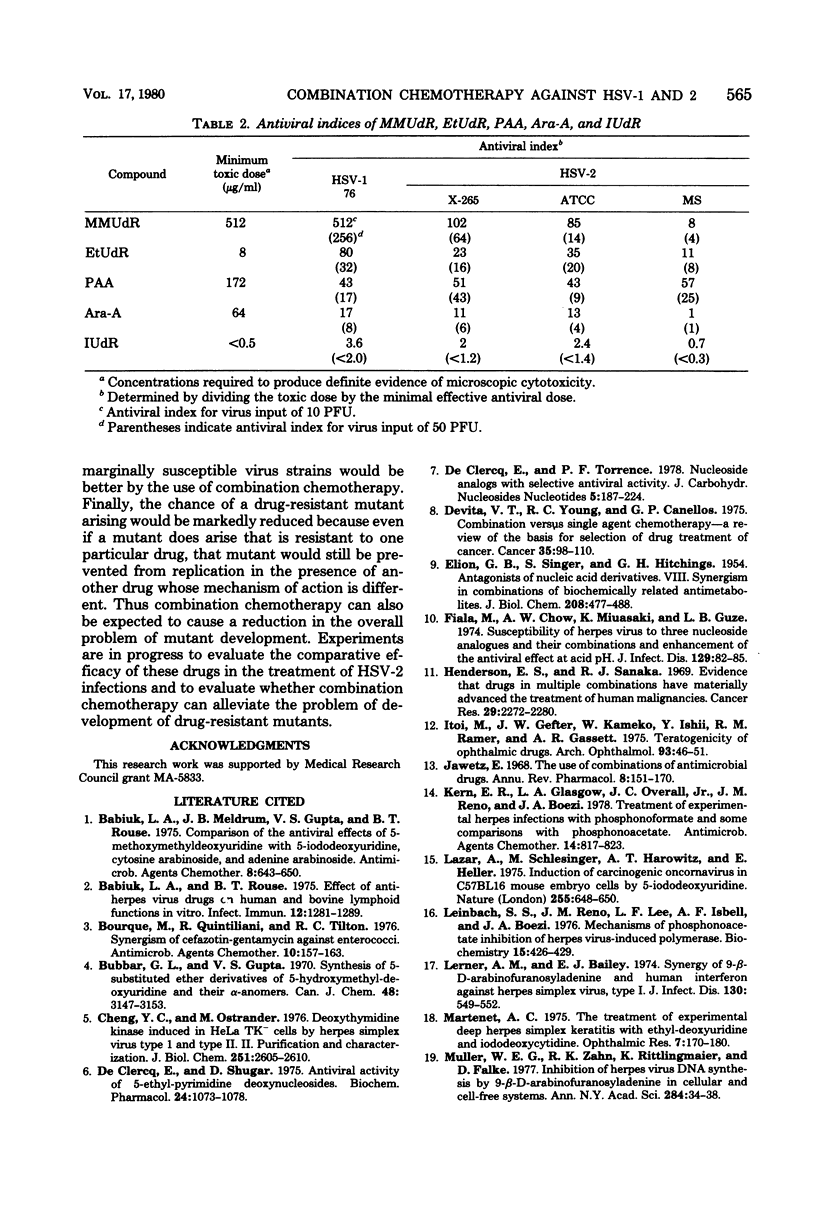
The antiviral activity of 5-methoxymethyl-2'-deoxyuridine (MMUdR) was compared with that of 5-iodo-2'-deoxyuridine (IUdR), 5-ethyl-2'-deoxyuridine (EtUdR), adenine arabinoside (Ara-A), and phosphonoacetic acid (PAA) against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). MMUdR was more potent than Ara-A and PAA but less active than EtUdR and IUdR against HSV-1 in rabbit kidney (RK-13) cells. In Vero cells, the antiviral activities of MMUdR, Ara-A, and PAA against HSV-1 were of the same order of magnitude. The antiviral potency against HSV-2 varied with the strain of virus used. All strains of HSV-2 were markedly inhibited by EtUdR and IUdR and to a lesser degree by PAA. However, considerable variation was noticed in the susceptibility of HSV-2 strains to Ara-A and MMUdR. Interaction of MMUdR with Ara-A, EtUdR, IUdR, and PAA was investigated by the method of response isobolograms. MMUdR showed synergistic activity in combination with Ara-A and PAA but antagonistic activity in combination with EtUdR and IUdR against herpesviruses. Minimum toxic dose (concentration required to produce definite evidence of microscopic cytotoxicity in rapidly growing RK-13 cells) was determined for each compound and was found to be 512, 172, 64, 8, and less than 0.5 microgram/ml for MMUdR, PAA, Ara-A, EtUdR, and IUdR, respectively. MMUdR was found to have the maximum antiviral index against HSV-1 (512) and HSV-2 strains X-265 (102) and ATCC (85). Antiviral index was defined as the minimum toxic dose divided by the dose that reduced plaque numbers by 50%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Meldrum B., Gupta V. S., Rouse B. T. Comparison of the antiviral effects of 5-methoxymethyl-deoxyuridine with 5-iododeoxyuridine, cytosine arabinoside, and adenine arabinoside. Antimicrob Agents Chemother. 1975 Dec;8(6):643–650. doi: 10.1128/aac.8.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., Rouse B. T. Effect of anti-herpesvirus drugs on human and bovine lymphoid function in vitro. Infect Immun. 1975 Dec;12(6):1281–1289. doi: 10.1128/iai.12.6.1281-1289.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque M., Quintiliani R., Tilton R. C. Synergism of cefazolin-gentamicin against enterococci. Antimicrob Agents Chemother. 1976 Jul;10(1):157–163. doi: 10.1128/aac.10.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- De Clercq E., Shugar D. Antiviral activity of 5-ethyl pyrimidine deoxynucleosides. Biochem Pharmacol. 1975 May 15;24(10):1073–1078. doi: 10.1016/0006-2952(75)90192-6. [DOI] [PubMed] [Google Scholar]
- DeVita V. T., Jr, Young R. C., Canellos G. P. Combination versus single agent chemotherapy: a review of the basis for selection of drug treatment of cancer. Cancer. 1975 Jan;35(1):98–110. doi: 10.1002/1097-0142(197501)35:1<98::aid-cncr2820350115>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Fiala M., Chow A. W., Miyasaki K., Guze L. B. Susceptibility of herpesviruses to three nucleoside analogues and their combinations and enhancement of the antiviral effect of acid pH. J Infect Dis. 1974 Jan;129(1):82–85. doi: 10.1093/infdis/129.1.82. [DOI] [PubMed] [Google Scholar]
- Itoi M., Gefter J. W., Kaneko N., Ishii Y., Ramer R. M., Gasset A. R. Teratogenicities of ophthalmic drugs. I. Antiviral ophthalmic drugs. Arch Ophthalmol. 1975 Jan;93(1):46–51. doi: 10.1001/archopht.1975.01010020050009. [DOI] [PubMed] [Google Scholar]
- Jawetz E. The use of combinations of antimicrobial drugs. Annu Rev Pharmacol. 1968;8:151–170. doi: 10.1146/annurev.pa.08.040168.001055. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Glasgow L. A., Overall J. C., Jr, Reno J. M., Boezi J. A. Treatment of experimental herpesvirus infections with phosphonoformate and some comparisons with phosphonoacetate. Antimicrob Agents Chemother. 1978 Dec;14(6):817–823. doi: 10.1128/aac.14.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar A., Schlesinger M., Horowitz A. T., Heller E. Induction of a carcinogenic oncornavirus in C57BL/6 mouse embryo cells by 5-iododeoxyuridine. Nature. 1975 Jun 19;255(5510):648–650. doi: 10.1038/255648a0. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Reno J. M., Lee L. F., Isbell A. F., Boezi J. A. Mechanism of phosphonoacetate inhibition of herpesvirus-induced DNA polymerase. Biochemistry. 1976 Jan 27;15(2):426–430. doi: 10.1021/bi00647a029. [DOI] [PubMed] [Google Scholar]
- Lerner A. M., Bailey E. J. Synergy of 9-beta-D-arabinofuranosyladenine and human interferon against Herpes simplex virus, type 1. J Infect Dis. 1974 Nov;130(5):549–552. doi: 10.1093/infdis/130.5.549. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Bittlingmaier K., Falke D. Inhibition of herpesvirus DNA synthesis by 9-beta-D-arabinofuranosyladenine in cellular and cell-free systems. Ann N Y Acad Sci. 1977 Mar 4;284:34–48. doi: 10.1111/j.1749-6632.1977.tb21935.x. [DOI] [PubMed] [Google Scholar]
- NICHOLS W. W. IN VITRO CHROMOSOME BREAKAGE INDUCED BY ARABINOSYLADENINE IN HUMAN LEUKOCYTES. Cancer Res. 1964 Sep;24:1502–1505. [PubMed] [Google Scholar]
- Pavan-Langston D., Dohlman C. H. A double blind clinical study of adenine arabinoside therapy of viral keratoconjunctivitis. Am J Ophthalmol. 1972 Jul;74(1):81–88. doi: 10.1016/0002-9394(72)91130-0. [DOI] [PubMed] [Google Scholar]
- Pavan-Langston D., Langston R. H., Geary P. A. Prophylaxis and therapy of experimental ocular herpes simplex. Comparison of idoxuridine, adenine arabinoside, and hypoxanthine arabinoside. Arch Ophthalmol. 1974 Nov;92(5):417–421. doi: 10.1001/archopht.1974.01010010429012. [DOI] [PubMed] [Google Scholar]
- Person D. A., Sheridan P. J., Herrmann E. C. Sensitivity of Types 1 and 2 Herpes Simplex Virus to 5-Iodo-2'-Deoxyuridine and 9-beta-d-Arabinofuranosyladenine. Infect Immun. 1970 Dec;2(6):815–820. doi: 10.1128/iai.2.6.815-820.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusoff W. H., Ward D. C. Nucleoside analogs with antiviral activity. Biochem Pharmacol. 1976 Jun 1;25(11):1233–1239. doi: 10.1016/0006-2952(76)90083-6. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A., Gupta V. S. The effect of the antiherpesvirus drug 5-methoxymethyl-2'-deoxyuridine on the humoral immune response in vivo. Can J Microbiol. 1977 Aug;23(8):1059–1061. doi: 10.1139/m77-158. [DOI] [PubMed] [Google Scholar]
- Sartorelli A. C. Some approaches to the therapeutic exploitation of metabolic sites of vulnerability of neoplastic cells. Cancer Res. 1969 Dec;29(12):2292–2299. [PubMed] [Google Scholar]
- Schardein J. L., Hentz D. L., Petrere J. A., Fitzgerald J. E., Kurtz S. M. The effect of vidarabine on the development of the offspring of rats, rabbits, and monkeys. Teratology. 1977 Jun;15(3):231–241. doi: 10.1002/tera.1420150304. [DOI] [PubMed] [Google Scholar]
- Tattersall M. H., Harrap K. R. Combination chemotherapy: the antagonism of methotrexate and cytosine arabinoside. Eur J Cancer. 1973 Mar;9(3):229–232. doi: 10.1016/s0014-2964(73)80023-4. [DOI] [PubMed] [Google Scholar]


