Abstract
Alzheimer disease is an age-related neurodegenerative disorder characterized by amyloid-β (Aβ) peptide deposition into cerebral amyloid plaques. The natural polyphenol resveratrol promotes anti-aging pathways via the activation of several metabolic sensors, including the AMP-activated protein kinase (AMPK). Resveratrol also lowers Aβ levels in cell lines; however, the underlying mechanism responsible for this effect is largely unknown. Moreover, the bioavailability of resveratrol in the brain remains uncertain. Here we show that AMPK signaling controls Aβ metabolism and mediates the anti-amyloidogenic effect of resveratrol in non-neuronal and neuronal cells, including in mouse primary neurons. Resveratrol increased cytosolic calcium levels and promoted AMPK activation by the calcium/calmodulin-dependent protein kinase kinase-β. Direct pharmacological and genetic activation of AMPK lowered extracellular Aβ accumulation, whereas AMPK inhibition reduced the effect of resveratrol on Aβ levels. Furthermore, resveratrol inhibited the AMPK target mTOR (mammalian target of rapamycin) to trigger autophagy and lysosomal degradation of Aβ. Finally, orally administered resveratrol in mice was detected in the brain where it activated AMPK and reduced cerebral Aβ levels and deposition in the cortex. These data suggest that resveratrol and pharmacological activation of AMPK have therapeutic potential against Alzheimer disease.
Keywords: Diseases/Alzheimer Disease, Diseases/Amyloid, Proteases/Secretases, Signal Transduction/Calcium, Signal Transduction/Protein Kinases, Autophagy, Resveratrol
Introduction
Alzheimer disease (AD)2 is a progressive neurodegenerative disorder and the first cause of dementia. Amyloid-β (Aβ) peptides have a central role in the pathogenesis of the disease and represent the core components of the senile plaques, the lesions invariably found in the neocortex and hippocampus of the AD brains (1, 2). In the amyloidogenic pathway, the amyloid-β precursor protein (APP) is sequentially cleaved by the aspartic protease β-secretase/BACE1 and by the γ-secretase proteolytic complex to produce various Aβ peptides, including the most abundant isoforms Aβ1–40 and Aβ1–42 (3, 4).
Epidemiological data suggest that moderate consumption of red wine is associated with a lower incidence of dementia and AD (5). The naturally occurring polyphenol resveratrol (trans-3,4′,5-trihydroxystilbene), which is found in abundance in red wine, has antioxidant and neuroprotective properties in vitro and could explain, in part, the beneficial effects of wine consumption in AD (6, 7). Importantly, resveratrol controls Aβ levels by facilitating its proteolytic clearance in cultured cell lines (8). However, the exact molecular mechanism by which resveratrol controls Aβ metabolism is currently unknown. Furthermore, evidence is missing to support the notion that orally administered resveratrol is bioavailable and bioactive in the brain.
A growing body of literature has demonstrated the beneficial effect of resveratrol on age-related metabolic deterioration and its protective role in metabolic diseases, such as type 2 diabetes and obesity. Resveratrol mimics caloric restriction by extending the lifespan of different small organisms, including Saccharomyces cerevisiae and Caenorhabditis elegans (9, 10), and by delaying several aging phenotypes in mice (11). Resveratrol also appears to be protective against the deregulation of energy homeostasis observed in mouse models for metabolic syndromes via the activation of key metabolic sensor proteins, such as the AMP-activated protein kinase (AMPK) and the deacetylase from the sirtuin family SIRT1 (12, 13).
AMPK is a Ser/Thr protein kinase formed by a heterotrimeric complex comprising a catalytic α subunit and regulatory β and γ subunits. AMPK is activated by different upstream kinases via phosphorylation within its activation loop at Thr-172 (14, 15). The main AMPK-activating kinase is LKB1, a protein expressed ubiquitously and recruited for AMPK phosphorylation after an elevation of the AMP/ATP ratio. The calcium/calmodulin-dependent protein kinase kinase-β (CaMKKβ), a kinase with a more restricted expression in neural tissue, also activates AMPK. AMPK phosphorylation at Thr-172 by CaMKKβ is triggered by an increase in cytosolic calcium levels. AMPK targets several proteins involved in cellular energy balance, including a regulator of fatty acid biosynthesis, acetyl-CoA carboxylase (ACC). The calcium/CaMKKβ/AMPK signaling pathway also controls mechanisms relevant to protein degradation by controlling mTOR (mammalian target of rapamycin) signaling and autophagy (16). Indeed, mTOR is a potent repressor of autophagy and is negatively controlled by AMPK (14, 15).
In recent years, several studies have focused on the potential relationship between AD and metabolic diseases. Obesity and diabetes significantly increase cognitive decline and AD risk (17), supporting the notion that molecular mechanisms of cellular energy homeostasis are linked to AD pathogenesis. Here we identify the mechanism involved in the anti-amyloidogenic effect of resveratrol by showing that this polyphenol lowered Aβ accumulation via activation of the metabolic sensor AMPK in different cell lines and in mouse primary neurons. Resveratrol activated AMPK by increasing intracellular calcium levels and by promoting AMPK phosphorylation at Thr-172 by CaMKKβ. Activation of AMPK by resveratrol resulted in mTOR inhibition and initiation of autophagy and lysosomal clearance of Aβ. Importantly, we also demonstrate that resveratrol, orally administered in mice, reached the brain where it activated AMPK and significantly reduced Aβ levels and deposition in the cerebral cortex, showing that resveratrol is both bioavailable and bioactive in the brain after oral dosing.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
AICAR (5-aminoimidazole-4-carboxamide-1-β-riboside), bafilomycin A1, deoxy-d-glucose, antimycin A, STO-609, and compound C were purchased from Calbiochem. Synthetic resveratrol, catechin, and thapsigargin were from Sigma. Natural resveratrol was purchased from Chromadex. Constitutively active T172D-AMPK (CA-AMPK) and dominant negative T172A-AMPK (DN-AMPK) cDNAs were kindly provided by Dr. David Carling (MRC Clinical Sciences Centre, Imperial College, London, UK). Anti-Aβ-(1–17) (6E10) and anti-Aβ-(17–24) (4G8) antibodies were from Signet. Anti-APP-(1–200) (LN27) antibody was from Zymed Laboratories Inc., and anti-APP C-terminal domain (R1) antibody was provided by Dr. P. D. Mehta (Institute for Basic Research in Developmental Disabilities, Staten Island, NY). Antibodies directed against AMPK, pAMPK, ACC, pACC, p70S6K, p-p70S6K, pS6, peIF4B, LC3, CREB, pCREB, c-Fos, and glial fibrillary acidic protein were from Cell Signaling Technology. Anti-actin antibody was from BD Transduction Laboratories. Anti-Myc (9E10) and anti-NeuN antibodies were from Chemicon.
Cell Lines and Drug Treatments
HEK293 (APP-HEK293) and N2a (APP-N2a) cells stably transfected with human APP695 were treated at confluence with the different drugs and for the indicated concentrations and incubation times. Culture medium was changed, and treatments were continued for another 3 h to allow Aβ secretion. Cells were transiently transfected with CA-AMPK and DN-AMPK cDNAs using Lipofectamine 2000 reagent (Invitrogen), as per the manufacturer's instructions. All cell lines were tested negative for mycoplasma contaminants (18).
Transgenic Mice and Brain Extraction
All animal experiments were performed according to procedures approved by the Feinstein Institute for Medical Research Institutional Animal Care and Use Committee. Fifteen-week-old male APP/PS1 transgenic mice (B6C3-Tg(APPswe, PSEN1dE9)85Dbo/J, The Jackson Laboratory) were randomly assigned to resveratrol or control groups. The control groups received a standard AIN-93G diet (Testdiet), and the resveratrol groups received a standard AIN-93G diet supplemented with 0.35% resveratrol. Resveratrol (Sigma-Aldrich) was mixed to homogeneity during manufacturing of the diet (Testdiet) and stored at −20 °C. Diet was replaced every 3 days. Food intake and body weight were monitored weekly throughout the study. At 30 weeks of age, mice were sacrificed. Brains were excised and hemi-dissected, and immediately one hemi-brain was fixed with 4% paraformaldehyde in 0.1 m phosphate-buffered saline, pH 7.6, whereas the other hemi-brain was sequentially extracted, as described (19). Briefly, brains were homogenized and sonicated in Tris-buffered saline containing 2% SDS and 1× Complete protease inhibitor mixture (Roche Applied Science) and centrifuged at 100,000 × g for 1 h at 4 °C. The supernatant was then removed, and the resulting pellet was extracted with 70% formic acid in water. For brain Aβ ELISA, 2% SDS extracts were diluted 1:40. Formic acid extracts were neutralized initially by a 1:20 dilution into 1 m Tris phosphate buffer, pH 11, and then diluted as necessary. Brain Aβ1–40 and Aβ1–42 were quantified by ELISA.
Primary Neuronal Cultures
Primary neurons were prepared as described before (20) from J20 APP transgenic mice (B6.Cg-Tg(PDGFB-APPSwInd)20Lms/2J, The Jackson Laboratory). Briefly, females were sacrificed at 17.5 days of gestation. Fetuses were processed separately to obtain pure transgenic cultures. Genotyping was carried out by isolating tail DNA and as described online in The Jackson Laboratory data base. Forebrains were dissected in ice-cold Hanks' balanced salt solution (Invitrogen) plus 0.5% w/v d-glucose (Sigma) and 25 mm Hepes (Invitrogen) under a dissection microscope. Dissociation was carried out mechanically in ice-cold dissection medium containing 0.01% w/v papain (Worthington), 0.1% w/v dispase (Roche Applied Science), and 0.01% w/v DNase (Worthington) and by incubation at 37 °C twice for 15 min. Cells were then spun down at 220 × g for 5 min at 4 °C, resuspended in Neurobasal medium with 2% B27, 1 mm sodium pyruvate, 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mm Glutamax (Invitrogen), filtered through a 40-μm cell strainer (Fisher), counted, and plated on poly-l-ornithine- and laminin-coated plates at a density of about 106 cells/well. Culture medium was completely replaced after 16–20 h, and new medium (30% of starting volume) was added every 3 days until the end of the culture period.
Western Blot (WB) Analyses and Aβ ELISA
5–20 μg of cell extracts were analyzed by SDS-PAGE using antibodies listed above. Secreted and intracellular total Aβ or Aβ1–40 and Aβ1–42 were analyzed by WB or ELISA as described before (8) and in the supplemental Materials and Methods.
Human Phosphoprotein Array and Calcium Measurements
APP-HEK293 cells were treated with 40 μm resveratrol (Sigma-Aldrich) or vehicle (DMSO) for 24 h. Cell lysates (250 μg of total proteins per array) were applied to the phosphoprotein array following the manufacturer's instructions (Proteome Profiler Human Phosphokinase Array kit, R&D Systems). Free cytosolic calcium was measured using the fluorescent calcium indicator Fluo-4 in cells plated on poly-l-lysine-coated 35-mm-diameter culture dishes, as per the manufacturer's recommendations (Fluo-4 NW Calcium Assay kit, Molecular Probes). Calcium add-back assays were performed as described previously (21) and in the supplemental Materials and Methods.
Immunohistochemistry
For Aβ staining, paraformaldehyde-fixed brain hemispheres were paraffin-embedded and processed according to standard protocols. In brief, sections were pretreated with 70% formic acid for 15 min and immersed in 1% H2O2 for 30 min. Sections were then incubated with 5% milk in Tris-buffered saline for 1 h and with 6E10 primary antibody (1:1000 dilution) overnight at 4 °C. Incubation with biotin-coupled anti-mouse IgG1 secondary antibodies (1:1000 dilution) was performed before incubation with streptavidin-horseradish peroxidase (Southern Biotech) and diaminobenzidine tetrahydrochloride. For immunofluorescence, brains were immersion-fixed in 4% paraformaldehyde overnight at 4 °C. Staining was performed on mouse brain sagittal Vibratome sections (50 μm). Sections were blocked in 5% milk in 0.25% Triton X-100 phosphate-buffered saline for 1 h at room temperature. Sections were then incubated in the presence of primary antibodies directed against NeuN, glial fibrillary acidic protein, and pAMPK for 16 h at 4 °C. After washing, sections were incubated with appropriate secondary antibody conjugated to Alexa fluorophores (Invitrogen). Finally, sections were incubated with Sudan Black B (0.3%, 10 min), mounted on glass slides using Vectashield (Vector laboratories), and observed using a confocal microscope.
Analysis of Resveratrol Stability and Content in the Supplemented Diet
Resveratrol-supplemented diet samples were exposed to air under room temperature conditions without direct sun light for different time points. The samples were then analyzed using a developed LC/UV/MS method, as described in the supplemental Materials and Methods.
Resveratrol Pharmacokinetics and Brain Accumulation in Rodents
All animal procedures were approved by the Purdue Animal Care and Use Committee. Sprague-Dawley rats were dosed orally by gavage with resveratrol in a dose escalation schedule and after an oral 400-mg/kg dose. Blood sampling was obtained in the CulexTM automated system (Bioanalytical Systems, West Lafayette, IN). Rats were then fasted for 8 h, dosed orally with 400 mg/kg resveratrol, and sacrificed 1 h post-dose. The vascular system was flushed with cold saline. The brains were excised and snap-frozen in liquid nitrogen for subsequent MS analysis. In addition to pharmacokinetic assessment in rats, wild type C57BL/6J mice (The Jackson Laboratory) were fed with AIN-93G diet supplemented or not with resveratrol for 2 weeks. Mice were then sacrificed, and brains were excised and hemi-dissected. One hemi-brain was snap-frozen in liquid nitrogen and stored at −80 °C until extraction for WB analysis. The other hemi-brain was placed in a saline solution containing 0.1% ascorbic acid and frozen at −80 °C for subsequent MS analysis. Blood was collected by intracardiac puncture. Resveratrol was extracted from plasma and homogenized brain tissue (22). Resveratrol metabolites were extracted from plasma using solid phase extraction, as described before (23) and in the supplemental Materials and Methods. LC-MS and MS/MS analyses were completed using an Agilent 1100 HPLC system equipped with an MSD-TOF and a model 6400 QQQ. Separations were conducted using a Varian C18-amide column (2.1 × 150 mm) (Varian Inc., Palo Alto, CA) maintained at a temperature of 30 °C. Resveratrol and resveratrol-glucuronide were detected at 227 and 403 m/z, respectively, in rat plasma and brain extracts. Multiple reaction monitoring mode using the transition from m/z 227.1 → 143 was used for detection of low levels of resveratrol in mouse brain and plasma extracts. Quantification was performed from a standard response curve prepared from analysis of authentic standards.
RESULTS
Our previous studies demonstrated that a 24-h incubation with resveratrol significantly reduced extracellular Aβ levels in APP-transfected cells (8). Both secreted Aβ1–40 and Aβ1–42 were similarly diminished by resveratrol treatments, with a comparable apparent IC50 of about 30 μm (see Ref. 8 and supplemental Fig. S1). At the same concentrations, resveratrol did not noticeably affect the steady state levels of full-length APP or APP C-terminal fragments (Ref. 8 and supplemental Fig. S1), showing that resveratrol reduced Aβ levels without affecting APP processing.
In this study we sought to identify the signaling pathway implicated in the control of Aβ levels by resveratrol. Using a kinase screen analyzing 28 major phosphoproteins, we determined that phosphorylation at Thr-172 on AMPK α2 subunit was the most robust effect of resveratrol in HEK293 cells (Fig. 1, A–C). Consistent with an activation of AMPK, resveratrol treatment also resulted in a significant elevation of AMPK α1 subunit phosphorylation (Fig. 1, A–C). Additional positive hits were identified, including the AMPK target CREB (24, 25) and the previously reported target of resveratrol, the checkpoint kinase Chk2 (26) (Fig. 1, A–C). The effect of resveratrol on AMPK and CREB phosphorylation was confirmed by WB analyses. Resveratrol increased the phosphorylation of AMPK and its target ACC in a dose-dependent manner and at concentrations consistent with the effect of this polyphenol on Aβ levels (Fig. 1, D and E). In the same concentration range, resveratrol also strongly increased dose-dependently the phosphorylation of CREB and ATF1, another CREB/ATF family member (Fig. 1, D and E). Notably, resveratrol treatment resulted in a robust increase in c-Fos protein levels (Fig. 1D). c-Fos gene promoter contains cyclic AMP response elements, and CREB is a strong regulator of the transcription of this gene (27, 28), suggesting that resveratrol not only increased CREB phosphorylation but also activated its transcriptional activity. Together, these results indicate that one of the primary effects of resveratrol is to target AMPK to increase its phosphorylation at Thr-172 and to promote its activation, as demonstrated by the increased phosphorylation of ACC and CREB upon resveratrol treatment.
FIGURE 1.
Resveratrol activates AMPK. A–C, APP-HEK293 cells were treated for 24 h with 40 μm resveratrol or DMSO (Control). Cell extracts were then probed on human phosphoprotein arrays. Results were expressed as a % of the control levels (A). Representative phosphoprotein array analyses are shown in B and C. Boxes 1–3 indicate phospho-Thr-174 AMPKα1, phospho-Thr-172 AMPKα2, and phospho-Ser-133 CREB, respectively. D, APP-HEK293 cells were treated for 24 h with the indicated concentrations of resveratrol (RSV). Two independent sources of polyphenol were tested: synthetic resveratrol (Sigma, >99%, GC) and purified natural resveratrol isolated from Polygonum cuspidatum (Chromadex, 99%, HPLC). Cell extracts were then analyzed by WB for secreted total Aβ, and cellular phospho-AMPK (pAMPK), AMPK, phospho-ACC (pACC), ACC, actin, phospho-CREB (pCREB), CREB, and c-Fos levels. E, shown are densitometric analysis and quantification of the ratios pAMPK/AMPK, pACC/ACC, and pCREB/CREB in three independent experiments as in D. a.u., arbitrary units. F and G, APP-HEK293 cells were treated for 24 h with the indicated concentrations of catechin or with 40 μm RSV. Secreted Aβ1–40 and Aβ1–42 levels were analyzed by ELISA (F). The levels of the indicated proteins were analyzed by WB (G). Histograms in E and F show the means ± S.D. of three independent experiments. CTRL, control.
Like resveratrol, (+)-catechin is a powerful plant-derived antioxidant (29). Although catechin has some structural similarities with resveratrol, it is ineffective at reducing Aβ levels in cultured cells at concentrations as high as 40 μm (see Fig. 1, F and G, and Ref. 8). We found that catechin had no effect on AMPK phosphorylation (Fig. 1G), suggesting that the effect on Aβ levels and AMPK activation is relatively specific to resveratrol and independent of its antioxidant properties.
The activating phosphorylation of AMPK at Thr-172 is primarily controlled by two kinases, LKB1 and CaMKKβ. LKB1 activation is tightly controlled by ATP levels via changes in the AMP/ATP ratio (14, 15). Because resveratrol was proposed to influence cellular ATP levels (30), we examined whether resveratrol affects ATP levels upon conditions inhibiting Aβ accumulation in HEK293 cells. We found no effect of 20 or 40 μm resveratrol on ATP levels (Fig. 2A). Furthermore, resveratrol treatment was still able to promote AMPK phosphorylation in the LKB1-deficient HeLa cells (Fig. 2B), indicating that LKB1 activation is not required for the effect of resveratrol on AMPK phosphorylation. We then asked whether resveratrol modulates cytosolic calcium levels in treated cells. Measurements of intracellular calcium levels were conducted under resting conditions in the presence of physiological levels of extracellular calcium. To reveal possible changes in the rate of calcium entry in treated cells, measurements were also performed under extracellular calcium add-back conditions, as previously described (21). These conditions are obtained after a transient external calcium depletion to generate a driving force for calcium entry into the cells. Using the calcium fluorescent dye Fluo-4, we found that resveratrol significantly increased cytosolic calcium levels both at resting conditions and after calcium add-back (Fig. 2, C and D). Because cytosolic calcium levels are also controlled by release of the ion from intracellular stores, such as the endoplasmic reticulum (ER), we also evaluated the effect of resveratrol treatment on ER calcium levels. Measurements of ER calcium levels were performed by blocking the sarco/endoplasmic reticulum calcium-ATPase (SERCA), a manipulation that generates a passive leak of calcium from the ER to the cytosol. In Fluo-4-loaded cells, in the absence of extracellular calcium, and in the presence of the SERCA inhibitor thapsigargin, resveratrol dose-dependently reduced the levels of ER calcium (Fig. 2E). Together these results show that resveratrol impaired intracellular calcium homeostasis by significantly increasing cytosolic calcium levels and by facilitating both calcium entry and ER calcium depletion. Because CaMKKβ is activated by an increase in cytosolic calcium, we then asked whether CaMKKβ is involved in the activation of AMPK by resveratrol. We found that the CaMKKβ inhibitor STO-609 effectively reduced the effect of resveratrol on the phosphorylation of both AMPK and ACC (Fig. 2F), indicating that resveratrol increased cytosolic calcium to promote CaMKKβ-dependent phosphorylation and activation of AMPK.
FIGURE 2.
Resveratrol increases intracellular calcium levels and promotes CaMKKβ-dependent phosphorylation of AMPK. A, shown are intracellular ATP levels in APP-HEK293 cells treated for 1 or 24 h with the indicated concentrations of resveratrol (RSV) or with 2-deoxy-d-glucose + antimycin A (2DG/AM; used as control for ATP production inhibition). B, shown are WB analyses of pAMPK, AMPK, actin, and LKB1 levels in HEK293 (lane 1) and HeLa (lanes 2–6) cells treated with the indicated concentrations of resveratrol. C, shown are cytosolic calcium measurements with Fluo-4 loading and calcium add-back conditions in APP-HEK293 cells treated for 24 h with the indicated concentrations of RSV. Cells were incubated in calcium-free buffer (0 CaCl2) and then challenged with physiological extracellular calcium concentrations (1.4 mm CaCl2) to monitor the progressive restoration of basal cytoplasmic calcium levels. Traces illustrate the mean relative fluorescence units (RFU) ± S.D. (shaded area) of three independent experiments. CTRL, control. D, peak and steady state of cytosolic calcium measurements are as in C, expressed in ΔF/F0. Histograms show the mean ± S.D. of three independent experiments. *, p < 0.001 (Student's t test). E, shown are cytosolic calcium measurements in cells treated with RSV as in C and incubated with 2 μm thapsigargin in calcium-free buffer. F, shown are WB analyses of pAMPK, pACC, and actin levels in APP-HEK293 cells incubated for 24 h with the indicated concentrations of STO-609 and in the absence (−) or presence (+) of 40 μm RSV.
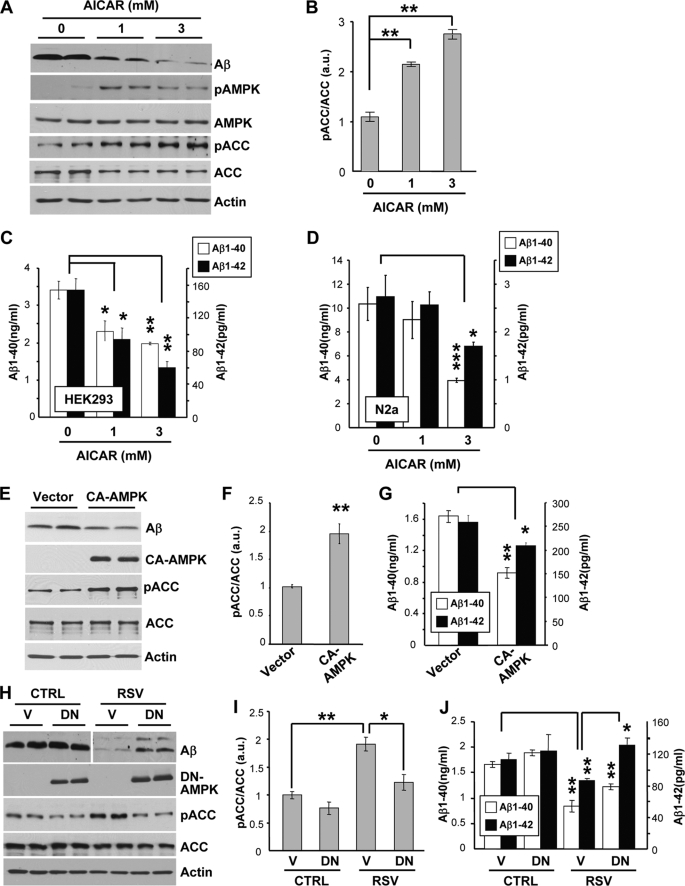
We then sought to determine whether direct activation of AMPK controls Aβ metabolism. Pharmacological activation of AMPK by the use of the AMP analog AICAR strongly and significantly lowered extracellular Aβ levels in HEK293 and N2a cells (Fig. 3, A–D). AICAR treatments at concentrations reducing Aβ resulted in a robust increase in the phosphorylation of AMPK and ACC (Fig. 3, A and B), confirming the activation of AMPK in these conditions. In addition, genetic activation of AMPK by transfection of a constitutively active form of AMPK α1 subunit (31) led to a robust increase of ACC phosphorylation and to a significant reduction of Aβ levels (Fig. 3, E–G). Thus, AMPK activation in cell lines lowered extracellular Aβ accumulation, including Aβ1–40 and Aβ1–42. Furthermore, expression of a dominant-negative form of AMPK (31) significantly inhibited the effect of resveratrol on ACC phosphorylation (Fig. 3, H and I) and on the levels of secreted Aβ (Fig. 3H), including Aβ1–40 and Aβ1–42 (Fig. 3J). Together these data show that resveratrol lowered Aβ levels by activating AMPK.
FIGURE 3.
Resveratrol lowers Aβ levels by activating AMPK. A, APP-HEK293 cells were treated for 24 h with the indicated concentrations of AICAR. Secreted Aβ and cellular pAMPK, AMPK, pACC, ACC, and actin levels were analyzed by WB. B, shown are densitometric analysis and quantification of the pACC/ACC ratio in cells treated as in A. a.u., arbitrary units. C and D, shown are ELISA measurements of secreted Aβ1–40 and Aβ1–42 levels from APP-HEK293 (C) or APP-N2a (D) cells treated with AICAR as in A. E, APP-HEK293 cells were transfected for 24 h with control vector (Vector) or with a Myc-tagged constitutively active form of AMPK (CA-AMPK). Myc-AMPK, pACC, ACC, actin, and secreted Aβ levels were analyzed by WB. F, shown are densitometric analysis and quantification of the pACC/ACC ratio in cells treated as in E. G, shown are ELISA measurements of secreted Aβ1–40 and Aβ1–42 levels from cells treated as in E. H, APP-HEK293 cells were transfected with control vector (V) or with a Myc-tagged dominant negative form of AMPK (DN). 24 h post-transfection, cells were treated for 24 h with DMSO (CTRL) or 40 μm resveratrol (RSV). Myc-AMPK, pACC, ACC, actin, and secreted Aβ levels were analyzed by WB. I, shown are densitometric analysis and quantification of the pACC/ACC ratio in cells treated as in H. J, shown are ELISA measurements of secreted Aβ1–40 and Aβ1–42 levels from cells treated as in H. Histograms in B–D, F, G, I, and J show the mean ± S.D. of 3–4 independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Student's t test).
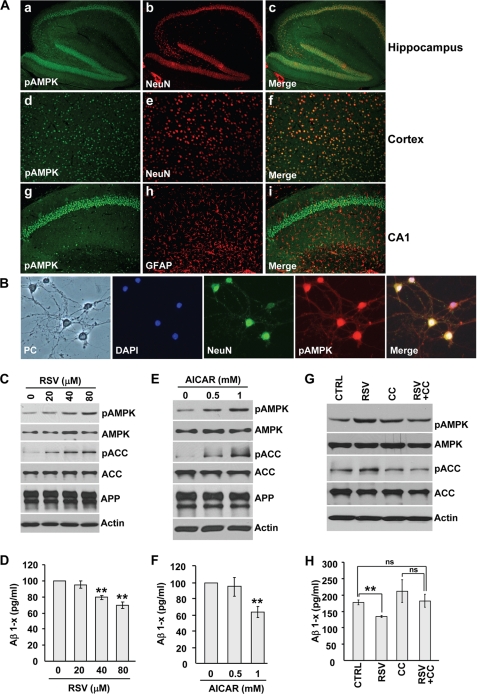
AMPK is expressed in many cell types and tissues. In the adult brain, all AMPK isoforms predominantly localize in neurons (32, 33). A more restricted expression of some AMPK subunits was also found in hippocampal astrocytes (32). Although AMPK activation has been extensively studied in hypothalamic neurons for its role in food intake (34, 35), little is known about the constitutive levels of activation of AMPK in neurons or in glial cells in other brain regions, such as the hippocampus and cortex. By immunofluorescence in adult mouse brain, we found that activated AMPK is present in most cortical and hippocampal neurons, as shown by the strong colocalization between phosphorylated AMPK and the neuronal marker NeuN (Fig. 4A, panels a–f). No significant staining for phosphorylated AMPK was found in hippocampal astrocytes (Fig. 4A, panels g–i), indicating that under constitutive conditions in the brain, AMPK is mostly active in neurons. Using immunocytochemistry, we confirmed that activated AMPK is present in primary neuronal cultures (Fig. 4B).
FIGURE 4.
Resveratrol lowers Aβ levels by activating AMPK in primary neurons. A, shown is pAMPK (panels a, d, and g), NeuN (panels b and e), GFAP (panel h), and merged (panels c, f, and i) staining of sagittal brain sections of an adult mouse. Hippocampus (panels a–c and g–i) and cerebral cortex (panels d–f) are shown. B, shown is phase contrast (PC), 4′,6-diamidino-2-phenylindole (DAPI), NeuN, pAMPK, and merged staining of 15 days in vitro primary neuronal cultures from J20 APP transgenic mice. C–H, WB analyses of pAMPK, AMPK, pACC, ACC, APP, and actin (C, E, and G) and ELISA measurements of secreted Aβ1-x levels (D, F, and H) in 15 days in vitro J20 mouse primary neurons treated for 24 h with the indicated concentrations of resveratrol (RSV, C and D) or AICAR (E and F). Primary neurons in G and H were treated for 24 h with 80 μm resveratrol or its vehicle (DMSO, control (CTRL)) in the absence or presence of compound C (CC, 20 μm). **, p < 0.01; ns, not significant (Student's t test).
In this context and to confirm our findings in a more physiologically relevant system, we assessed the effect of resveratrol on AMPK activation and Aβ levels in primary neurons. We found that resveratrol significantly and in a dose-dependent manner reduced secreted Aβ levels in primary neurons isolated from APP transgenic mouse forebrain (Fig. 4D), whereas full-length APP levels remained unchanged (Fig. 4C). At concentrations reducing Aβ, resveratrol led to an increase in phosphorylated AMPK and ACC (Fig. 4C), showing that resveratrol can activate AMPK in forebrain neurons. Direct activation of AMPK with AICAR also resulted in a significant decrease in neuronal Aβ levels (Fig. 4, E and F). Importantly, pretreatment with the AMPK inhibitor, compound C, significantly prevented the effect of resveratrol on neuronal Aβ levels (Fig. 4, G and H). Together, these results show that in neurons resveratrol reduced secreted Aβ accumulation by activating AMPK.
To gain insight into the mechanism by which resveratrol promotes a reduction of Aβ levels, we asked whether the polyphenol facilitates intracellular degradation of Aβ. Our previous work has shown that resveratrol does not significantly affect APP processing and Aβ production but instead facilitates intracellular Aβ clearance (8). AMPK is a key regulator of autophagy (or macroautophagy), an evolutionary conserved lysosomal pathway involved in protein and organelle turnover via the formation of vacuoles known as autophagosomes (36). Autophagy is deregulated in AD brain and participates in intracellular Aβ degradation in vitro and in vivo (37, 38). AMPK activation promotes autophagy by repressing the Ser/Thr protein kinase mTOR, which represents a key blocker of autophagosome formation (39). Interestingly, resveratrol was found to induce autophagy in cancer cell lines (40). In this context we asked whether resveratrol (i) inhibits mTOR activity, (ii) promotes autophagy, and (iii) leads to intracellular degradation of Aβ by the lysosomal system.
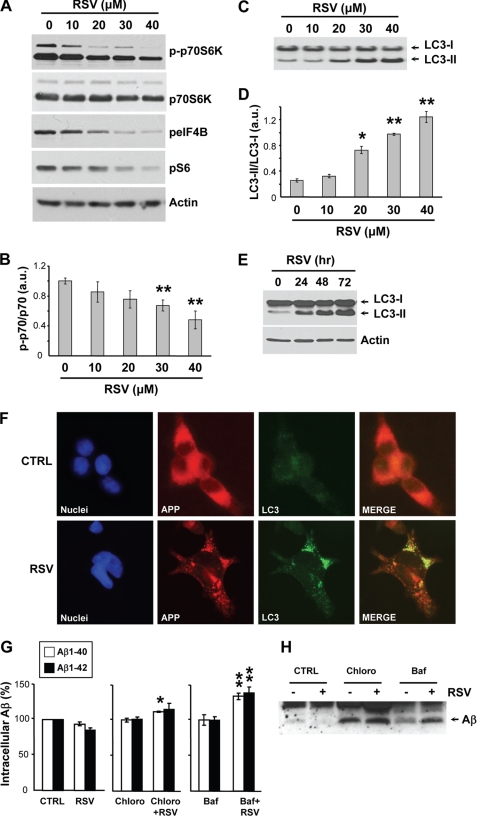
We observed that treatments of HEK293 cells with resveratrol resulted in a dose-dependent inhibition of the phosphorylation of p70-S6 kinase, eIF4B (eukaryotic initiation factor 4B), and S6 ribosomal protein, three proteins downstream from mTOR activation (Fig. 5, A and B). Importantly, resveratrol treatments also led to a strong and dose-dependent conversion of the light chain 3 (LC3) protein from LC3-I to LC3-II, which represents a marker for autophagy induction (Fig. 5, C–E). Furthermore, formation of autophagosomes immunoreactive for LC3 was observed in the presence of resveratrol (Fig. 5F). Finally, treatment with the lysosomotropic drugs bafilomycin A1 and chloroquine, which neutralize lysosomal degradation, resulted in a significant increase of intracellular Aβ accumulation in the presence of resveratrol (Fig. 5, G and H). In summary, these data show that resveratrol inhibited mTOR to induce autophagy and intracellular degradation of Aβ by the lysosomal system.
FIGURE 5.
Resveratrol induces autophagy and lysosomal degradation of Aβ. A–D, APP-HEK293 cells were treated for 24 h with the indicated concentrations of resveratrol (RSV). Cell extracts were then analyzed by WB for phospho-p70S6K (p-p70S6K), p70S6K, phospho-eIF4B (peIF4B), phospho-S6 (pS6), and actin (A) and for LC3 (C). Densitometric analysis and quantification of the p-p70S6K/p70S6K (B) and LC3-II/LC3-I (D) ratios are shown. a.u., arbitrary units. E, WB analyses of LC3 and actin in APP-HEK293 cells treated for the indicated times with 40 μm RSV. F, immunocytochemistry with anti-APP (red) and anti-LC3 (green) antibodies in APP-HEK293 cells incubated with for 72 h in the absence (CTRL) or presence of 40 μm RSV. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). G and H, shown are ELISA measurements of intracellular Aβ1–40 and Aβ1–42 levels (G) and WB analysis of immunoprecipitated intracellular Aβ (H) from APP-HEK293 cells treated for 24 h with 40 μm RSV or its vehicle (DMSO, CTRL) in the absence or presence of chloroquine (Chloro, 100 μm) or bafilomycin A1 (Baf, 100 nm). Histograms in B, D, and G show the mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01 (Student's t test).
Based on these in vitro results in cell lines and in primary neurons, we tested the ability of resveratrol to control AMPK activation and Aβ accumulation in the brain of APP/PS1 transgenic mice. This mouse model co-expresses the familial AD-linked mutants of APP (Swedish) and presenilin-1 (PSEN1ΔE9) (41) and exhibits high levels of soluble brain Aβ and develops a robust amyloid pathology in different brain regions of the hippocampus and cerebral cortex by 30 weeks of age (41).
An efficient metabolic effect of resveratrol has recently been achieved in vivo in mice by oral administration of a supplemented diet (42, 43). This effect was found to be mediated by SIRT1 and AMPK activation. The authors indicated that a dose of 0.4% resveratrol in the diet was well tolerated by the mice for 15 weeks of treatment (43). Because our studies show that AMPK is involved in the effect of resveratrol on Aβ levels in cultured cells, we followed the same protocol to evaluate the in vivo efficacy of resveratrol on Aβ accumulation and amyloid deposition in APP/PS1 mice. Two independent groups of 10 male mice (15 weeks old) were fed an AIN-93G diet supplemented or not with 0.4% resveratrol for a period of 15 weeks.
Before administration in animals, the content of resveratrol in the diet was verified using LC/UV/MS methods. The results show that the variation of resveratrol content in the samples was 0.347 ± 0.053% (n = 10). We also analyzed the stability of this polyphenol in the supplemented diet under room conditions during different incubation periods. Resveratrol-supplemented diet samples were exposed to air at room temperature and under conditions without direct sunlight and then analyzed using LC/UV/MS. We found that resveratrol in solid status (mixed in the diet) is very stable under room conditions. No decomposed peak was detected in both UV and MS detections in the samples up to 4 days under room conditions, whereas only trace levels of trans-resveratrol conversion into cis-resveratrol was observed after 7 days (Fig. 6A). These results show that resveratrol is detected on average at 0.35% in the supplemented diet and, in line with previous studies (44), appears to be very stable under ambient typical room conditions for durations comparable with the one required for the animal treatments.
FIGURE 6.
Resveratrol stability, bioavailability, and bioactivity in the brain. A, shown are overlaid UV chromatograms (enlarged) of resveratrol-supplemented diet samples at 7 days (red) and 19 days (blue) under room conditions. cis-RSV, cis-resveratrol; trans-RSV, trans-resveratrol. The inset shows the entire UV (a) and MS (b) chromatograms of resveratrol-supplemented diet samples at the time point of 19 days. B, shown is the plasma pharmacokinetic response of RSV and its major metabolite, resveratrol-glucuronide (RSV-gluc), after oral gavage of 400 mg/kg body weight of resveratrol after pretreatment with dose escalation of resveratrol, as described under “Experimental Procedures.” Data represent the mean ± S.E., n = 4 rats. C, shown is LC-MS separation of RSV and resveratrol-glucuronide (RSV-gluc) from an extract of plasma and brain from rats administered resveratrol at 400 mg/kg body weight by oral gavage. Extracted ion chromatograms at 227 and 403 m/z are shown for RSV and RSV-gluc, respectively. D, detection of RSV in extracts of plasma and brain from mice fed the diet supplemented with 0.35% resveratrol for 2 weeks is shown. Extracted ion chromatograms collected in multiple reaction monitoring mode (transitions 227 → 143; m/z) are shown in mouse plasma (Treated plasma) or brain extracts (Treated brain) from resveratrol-treated mice or in brain extracts from non-treated control mice (Control brain). E and F, brain extracts from mice fed for 2 weeks a diet supplemented (RSV) or not (CTRL) with 0.35% resveratrol were analyzed by WB for pAMPK, AMPK, pACC, and ACC. a.u., arbitrary units. F, shown are densitometric analysis and quantification of the pAMPK/AMPK and pACC/ACC ratios in brain extracts from mice treated as in E. Histograms show the mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01 (Student's t test).
We determined that the mice ingested ∼350 mg/kg of body weight daily of resveratrol from the supplemented diet during the 15-week period (Fig. 7B). Based on this information, absorption of resveratrol and accumulation by the brain was investigated in rodents treated either by intragastric gavage or by oral administration through the diet. Plasma and brain levels of resveratrol and its primary metabolite, resveratrol-glucuronide, were evaluated by LC-MS in Sprague-Dawley rats dosed by gavage in escalation at 100, 250, and 400 mg/kg for 3 days at each dose before an acute 400 mg/kg dose of resveratrol (see “Experimental Procedures”). Plasma resveratrol profiles indicated a rapid rise of resveratrol and resveratrol-glucuronide content in the plasma at 4 h after administration followed by a slow decline, indicating a lack of complete clearance of the polyphenol during the test period with repeated dosing (Fig. 6B). Importantly, in the same animals resveratrol was detected in perfused brain at a concentration of ∼1.7 nmol/g wet weight (Fig. 6C). The levels of resveratrol and resveratrol-glucuronide were also analyzed in mice fed for 2 weeks resveratrol-supplemented diet. Using LC-MS/MS in multiple reaction monitoring mode, resveratrol was detected in deconjugated brain extracts of treated mouse brain but not in control animals (Fig. 6D). Strikingly, in these animals administered with a resveratrol-supplemented diet, we observed an increase in the phosphorylation of both AMPK and ACC in the brain as compared with control mice (Fig. 6, E and F).
FIGURE 7.
Resveratrol lowers Aβ accumulation and deposition in vivo in mice. A–K, from 15 to 30 weeks of age, male APP/PS1 mice were fed a diet supplemented (RSV) or not (CTRL) with 0.35% resveratrol. Mouse weight (A) and resveratrol (RSV) intake (B) were monitored weekly. C and D, shown are ELISA measurements of soluble (SDS Fraction) and insoluble (formic acid, FA Fraction) Aβ1–40 and Aβ1–42 levels in total mouse brain. E, brain extracts were analyzed by WB for APP, APP-CTFs, and actin. F–K, shown are amyloid deposition assessments in the cortex (F–H) and hippocampus (I–K) of control (CTRL) and RSV-fed mice by immunohistochemistry staining using 6E10 anti-Aβ antibody. Graphs show the number of plaques per section (F and I) and the percent area occupied with positive staining (G and J). Graphs indicate the mean ± S.E., n = 9. *, p ≤ 0.05; **, p < 0.02; ***, p < 0.005 (Student's t test).
The APP/PS1 mice fed the diet supplemented with resveratrol were in apparent good health at the end of the 15-week period. The mice gained weight slightly during the treatment period, with no significant difference between the controls and the resveratrol-fed mice (Fig. 7A). By ELISA and WB analyses, the levels of soluble and insoluble Aβ1–40 and Aβ1–42 from brain homogenates sequentially extracted with SDS and formic acid, respectively, were analyzed in resveratrol-fed and control mice (Fig. 7, C and D). The hippocampal and cortical regions were also analyzed by immunohistochemistry using 6E10 anti-Aβ antibody (Fig. 7, F–K). We found a significant decrease in both soluble and insoluble Aβ1–40 (30%, p = 0.018 and 25%, p = 0.05, respectively) and of insoluble Aβ1–42 (25%, p = 0.012) in total brain homogenates in resveratrol-fed mice, as compared with control mice (Fig. 7, C and D). A trend of decrease in soluble Aβ1–42 was also observed in resveratrol-treated mice (17%, p = 0.36; Fig. 7C), whereas APP and APP-CTF levels remained unchanged between treated and untreated mice (Fig. 7E). Although no significant effect on amyloid deposition in the hippocampus was found (Fig. 7, I–K), a significant reduction of amyloid deposition in the cerebral cortex was observed in resveratrol-fed mice, as determined by measurements of both plaque numbers (42% decrease, p = 0.005) and amyloid deposition area (34% decrease, p = 0.042; Fig. 7, F–H). Thus, resveratrol administered in the diet reached the brain where it significantly activated AMPK and reduced Aβ levels and amyloid deposition in the cerebral cortex.
DISCUSSION
Using an unbiased approach, we showed that AMPK signaling activation is a central event in the anti-amyloidogenic effect of resveratrol in vitro and in vivo. Specifically, we demonstrated that resveratrol turned on AMPK activity both in cell cultures and in vivo in mouse brain after oral dosing and that AMPK inhibition significantly impaired the effect of resveratrol on Aβ accumulation in cell lines and in primary neurons. The observation that resveratrol activates AMPK is in line with previous studies using cell line cultures and animal models (42, 45–49). For instance, it was shown that resveratrol administered orally through the diet can activate AMPK at the periphery in mouse liver (42). Resveratrol was also found to activate AMPK in the brain when administered by intraperitoneal injections (46). Using MS separation, we demonstrate in this study that resveratrol administered orally by gavage or by chronic supplementation of the diet led to the accumulation of this polyphenol in the brain. Consequently, we observed a robust activation of cerebral AMPK and a significant reduction of Aβ levels and deposition in the mouse cortex. These results not only revealed that resveratrol is bioavailable and bioactive in the brain after oral dosing but also demonstrated the anti-amyloidogenic potential of this polyphenol in vivo.
The direct target of resveratrol in vitro and in vivo and the exact mechanism by which AMPK is activated is not firmly established. Resveratrol binds in vitro to SIRT1 and activates the deacetylase activity of this enzyme (50, 51). SIRT1 may also represent a main target of resveratrol metabolic functions in muscle tissue (43). It was proposed that SIRT1 activation by resveratrol can lead to LKB1 and AMPK activation in HepG2 hepatocytes and HEK293T cells (52, 53). However, recent studies showed that resveratrol can also activate AMPK independently of SIRT1. Indeed, resveratrol can activate AMPK in SIRT1-deficient mouse embryonic fibroblasts (13) or in the presence of SIRT1 inhibitors in neurons (46).
The present work provides strong evidence that resveratrol activated AMPK by increasing cytosolic calcium levels and by activating CaMKKβ-dependent phosphorylation of AMPK. Our data indicate that resveratrol promoted both extracellular calcium entry into the cells and ER calcium depletion. These consequences of resveratrol treatments could both contribute to the observed elevation of cytosolic calcium levels. ER calcium release, which leads to a transient depletion in ER calcium levels, is coupled to the mechanism of calcium entry called store-operated calcium entry (54). Interestingly, a similar effect of resveratrol on ER calcium release (55, 56) and store-operated calcium entry (55) has also been reported in vascular myocyte cultures and breast cancer cells. Additional studies will be required to determine whether resveratrol increased cytosolic calcium by directly triggering ER calcium release to facilitate the resulting calcium influx via store-operated calcium entry.
Recently, it was shown that the anti-diabetic drug metformin increases Aβ levels by up-regulating BACE1 expression (57). The authors proposed that the effect of the drug on Aβ levels is dependent on AMPK activation (57). The exact mechanism of action of metformin in vivo is not known. The drug activates AMPK in several cell lines and requires the AMPK-activating kinase LKB1 to control glucose homeostasis in mice (58). However, AMPK-independent molecular targets of metformin were also identified in vivo (59). In this report we employed pharmacological and genetic approaches to demonstrate that the direct activation of AMPK led to a robust inhibition of Aβ accumulation. We showed that the AMP analog AICAR lowered Aβ levels at concentrations promoting AMPK activation. We also found that expression of a constitutively active form of AMPK resulted in a marked reduction of extracellular Aβ levels. These results clearly demonstrate that AMPK activation directly correlated with a decrease of Aβ levels (see also Ref. 74). Therefore, it is likely that the effect of metformin on Aβ levels and BACE1 expression is controlled in part by AMPK-independent pathways.
Strong evidence indicates that autophagy is deregulated in AD brain and participates in intracellular Aβ degradation in cell lines and in vivo in animal models (37, 38). Our previous work proposed that resveratrol did not affect APP processing but instead promoted Aβ degradation (8). Here, we show that resveratrol activated autophagy and intracellular clearance of Aβ by the lysosomal pathway. Indeed, resveratrol was found to potently inhibit the activity of mTOR, an AMPK target critically involved in autophagy repression. This inhibition of mTOR by resveratrol resulted in LC3 conversion and in the formation of LC3 positive vesicles, two key markers of autophagy induction. Autophagy is coupled to lysosomal degradation, and evidence is increasing that the effect of autophagy on Aβ is due to coupling to the lysosomal system (38, 60). We found that neutralization of lysosomal degradation by the use of lysosomotropic drugs resulted in a significant increase of intracellular Aβ accumulation in the presence of resveratrol. To the best of our knowledge, this study is the first to reveal that resveratrol can induce autophagy to facilitate intracellular degradation of Aβ by the lysosomal system.
The anti-amyloidogenic effect of resveratrol (8) was just confirmed in vivo in mice (61). In this study, which occurred as our study was completed, the mice received an identical diet as the one used in our report (AIN-93G) supplemented with 0.2% resveratrol. Although in our study the diet was supplemented with a higher amount of resveratrol (0.35%), the estimated daily dosage was comparable between the two studies, between 300 and 350 mg/kg (see Fig. 7B and Ref. 61). In agreement with our data, the authors found that resveratrol intake lowered both plaque numbers and plaque area in different brain regions, with the exception of the hippocampal region where no statistically significant changes were noted in both studies (see Fig. 7 and Ref. 61).
Concordant epidemiological data suggest that moderate consumption of red wine is associated with a lower incidence of dementia and AD (62–65). Studies in AD mouse models have also shown that red wine intake attenuates cerebral amyloid deposition and Aβ-associated cognitive deterioration (66, 67). Resveratrol is found in abundance in grape skin and red wine and has potential antioxidant, anti-amyloidogenic, and neuroprotective properties (6, 7, 9). Indeed, resveratrol delays Aβ-induced toxicity in different neuronal cell culture models (68–70) and exerts anti-aggregation and anti-fibrillogenic effects on Aβ in vitro (71, 72). Resveratrol, delivered by intracerebroventricular injections, was also found to reduce neurodegeneration in p25 transgenic mice, a model for AD and tauopathies (73). Together with the present work demonstrating the anti-amyloidogenic effect of resveratrol in vivo, these data indicate that resveratrol could explain in part the beneficial effects of wine consumption in AD (7, 8).
In summary, this work shows that resveratrol lowered Aβ accumulation by activating AMPK signaling in cell lines and in primary neuronal cultures. Resveratrol activated AMPK by increasing cytosolic calcium levels and by promoting CaMKKβ-dependent phosphorylation of AMPK. In addition, resveratrol reduced Aβ accumulation by activating autophagy and by facilitating the lysosomal degradation of Aβ. We further report that orally administered resveratrol in mice crossed the blood-brain barrier, activated brain AMPK, and reduced Aβ levels and deposition in the cerebral cortex. Evidence is emerging to support the potential of resveratrol against neurodegenerative disorders. However, no clear neuroprotective mechanism has been proposed so far. This study identifies AMPK as a key neuroprotective kinase against Aβ accumulation. This work provides a rationale for exploring the therapeutic potential of resveratrol and AMPK activation in AD (7).
Supplementary Material
Acknowledgments
We thank Dr. Gopal Thinakaran (University of Chicago, Chicago, IL) for kindly providing us with APP695-N2a cells, Dr. Luciano D'Adamio (Albert Einstein College of Medicine, Bronx, NY) for APP695-HEK293 cells, Dr. David Carling (MRC Clinical Sciences Centre, Imperial College, London, UK) for T172D-AMPK and T172A-AMPK cDNAs, Dr. Pankaj D. Mehta (Institute for Basic Research in Developmental Disabilities, Staten Island, NY) for R1 antibody, and Dr. Amanda Chan (The Feinstein Institute for Medical Research, Manhasset, NY) for assistance with microscopy studies.
This work was supported, in whole or in part, by National Institutes of Health Grant PO1 AT004511 (NCCAM Project 2; to P. M.). This work was also supported in part by the Alzheimer's Association (to P. M.) and the Institute for the Study of Aging (Alzheimer's Drug Discovery Foundation (to P. M.)).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods” and Fig. S1.
- AD
- Alzheimer disease
- Aβ
- amyloid-β
- APP
- amyloid-β precursor protein
- AMPK
- AMP-activated protein kinase
- CaMKKβ
- calcium/calmodulin-dependent protein kinase kinase-β
- ACC
- acetyl-CoA carboxylase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-riboside
- CA
- constitutively active
- DN
- dominant negative
- HPLC
- high performance liquid chromatography
- LC3
- light chain 3
- CREB
- cAMP-response element-binding protein
- ELISA
- enzyme-linked immunosorbent assay
- WB
- Western blot
- LC
- liquid chromatography
- MS
- mass spectrometry
- ER
- endoplasmic reticulum.
REFERENCES
- 1.Selkoe D. J. (2001) Physiol. Rev. 81, 741–766 [DOI] [PubMed] [Google Scholar]
- 2.Wilquet V., De Strooper B. (2004) Curr. Opin. Neurobiol. 14, 582–588 [DOI] [PubMed] [Google Scholar]
- 3.Haass C. (2004) EMBO J. 23, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marambaud P., Robakis N. K. (2005) Genes Brain Behav. 4, 134–146 [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger J. A., Mayeux R. (2004) Lancet Neurol. 3, 579–587 [DOI] [PubMed] [Google Scholar]
- 6.Ramassamy C. (2006) Eur. J. Pharmacol. 545, 51–64 [DOI] [PubMed] [Google Scholar]
- 7.Vingtdeux V., Dreses-Werringloer U., Zhao H., Davies P., Marambaud P. (2008) BMC Neurosci. 9, Suppl. 2, S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marambaud P., Zhao H., Davies P. (2005) J. Biol. Chem. 280, 37377–37382 [DOI] [PubMed] [Google Scholar]
- 9.Baur J. A., Sinclair D. A. (2006) Nat. Rev. Drug Discov. 5, 493–506 [DOI] [PubMed] [Google Scholar]
- 10.Greer E. L., Brunet A. (2009) Aging Cell 8, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearson K. J., Baur J. A., Lewis K. N., Peshkin L., Price N. L., Labinskyy N., Swindell W. R., Kamara D., Minor R. K., Perez E., Jamieson H. A., Zhang Y., Dunn S. R., Sharma K., Pleshko N., Woollett L. A., Csiszar A., Ikeno Y., Le Couteur D., Elliott P. J., Becker K. G., Navas P., Ingram D. K., Wolf N. S., Ungvari Z., Sinclair D. A., de Cabo R. (2008) Cell Metab. 8, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantó C., Auwerx J. (2009) Curr. Opin. Lipidol. 20, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um J. H., Park S. J., Kang H., Yang S., Foretz M., McBurney M. W., Kim M. K., Viollet B., Chung J. H. (2009) Diabetes, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carling D., Sanders M. J., Woods A. (2008) Int. J. Obes. 32, Suppl 4, S55—S59 [DOI] [PubMed] [Google Scholar]
- 15.Hardie D. G. (2007) Nat. Rev. Mol. Cell Biol. 8, 774–785 [DOI] [PubMed] [Google Scholar]
- 16.Høyer-Hansen M., Bastholm L., Szyniarowski P., Campanella M., Szabadkai G., Farkas T., Bianchi K., Fehrenbacher N., Elling F., Rizzuto R., Mathiasen I. S., Jäättelä M. (2007) Mol. Cell 25, 193–205 [DOI] [PubMed] [Google Scholar]
- 17.Craft S. (2009) Arch. Neurol. 66, 300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., Dreses-Werringloer U., Davies P., Marambaud P. (2008) BMC Res. Notes 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawarabayashi T., Younkin L. H., Saido T. C., Shoji M., Ashe K. H., Younkin S. G. (2001) J. Neurosci. 21, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giliberto L., Borghi R., Piccini A., Mangerini R., Sorbi S., Cirmena G., Garuti A., Ghetti B., Tagliavini F., Mughal M. R., Mattson M. P., Zhu X., Wang X., Guglielmotto M., Tamagno E., Tabaton M. (2009) J. Biol. Chem. 284, 9027–9038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreses-Werringloer U., Lambert J. C., Vingtdeux V., Zhao H., Vais H., Siebert A., Jain A., Koppel J., Rovelet-Lecrux A., Hannequin D., Pasquier F., Galimberti D., Scarpini E., Mann D., Lendon C., Campion D., Amouyel P., Davies P., Foskett J. K., Campagne F., Marambaud P. (2008) Cell 133, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferruzzi M. G., Lobo J. K., Janle E. M., Cooper B., Simon J. E., Wu Q. L., Welch C., Ho L., Weaver C., Pasinetti G. M. (2009) J. Alzheimers Dis. 18, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roura E., Andrés-Lacueva C., Jáuregui O., Badia E., Estruch R., Izquierdo-Pulido M., Lamuela-Raventós R. M. (2005) J. Agric. Food Chem. 53, 6190–6194 [DOI] [PubMed] [Google Scholar]
- 24.Kim E. K., Miller I., Aja S., Landree L. E., Pinn M., McFadden J., Kuhajda F. P., Moran T. H., Ronnett G. V. (2004) J. Biol. Chem. 279, 19970–19976 [DOI] [PubMed] [Google Scholar]
- 25.Thomson D. M., Herway S. T., Fillmore N., Kim H., Brown J. D., Barrow J. R., Winder W. W. (2008) J. Appl. Physiol. 104, 429–438 [DOI] [PubMed] [Google Scholar]
- 26.Tyagi A., Singh R. P., Agarwal C., Siriwardana S., Sclafani R. A., Agarwal R. (2005) Carcinogenesis 26, 1978–1987 [DOI] [PubMed] [Google Scholar]
- 27.Marambaud P., Wen P. H., Dutt A., Shioi J., Takashima A., Siman R., Robakis N. K. (2003) Cell 114, 635–645 [DOI] [PubMed] [Google Scholar]
- 28.Ahn S., Olive M., Aggarwal S., Krylov D., Ginty D. D., Vinson C. (1998) Mol. Cell. Biol. 18, 967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz Y., Toledo R. T. (2004) J. Agric. Food Chem. 52, 255–260 [DOI] [PubMed] [Google Scholar]
- 30.Zheng J., Ramirez V. D. (2000) Br. J. Pharmacol. 130, 1115–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein S. C., Woods A., Jones N. A., Davison M. D., Carling D. (2000) Biochem. J. 345, 437–443 [PMC free article] [PubMed] [Google Scholar]
- 32.Turnley A. M., Stapleton D., Mann R. J., Witters L. A., Kemp B. E., Bartlett P. F. (1999) J. Neurochem. 72, 1707–1716 [DOI] [PubMed] [Google Scholar]
- 33.Culmsee C., Monnig J., Kemp B. E., Mattson M. P. (2001) J. Mol. Neurosci. 17, 45–58 [DOI] [PubMed] [Google Scholar]
- 34.Claret M., Smith M. A., Batterham R. L., Selman C., Choudhury A. I., Fryer L. G., Clements M., Al-Qassab H., Heffron H., Xu A. W., Speakman J. R., Barsh G. S., Viollet B., Vaulont S., Ashford M. L., Carling D., Withers D. J. (2007) J. Clin. Invest. 117, 2325–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minokoshi Y., Alquier T., Furukawa N., Kim Y. B., Lee A., Xue B., Mu J., Foufelle F., Ferré P., Birnbaum M. J., Stuck B. J., Kahn B. B. (2004) Nature 428, 569–574 [DOI] [PubMed] [Google Scholar]
- 36.Maiuri M. C., Zalckvar E., Kimchi A., Kroemer G. (2007) Nat. Rev. Mol. Cell Biol. 8, 741–752 [DOI] [PubMed] [Google Scholar]
- 37.Jaeger P. A., Wyss-Coray T. (2009) Mol. Neurodegener. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nixon R. A. (2007) J. Cell Sci. 120, 4081–4091 [DOI] [PubMed] [Google Scholar]
- 39.Meijer A. J., Codogno P. (2007) Autophagy 3, 238–240 [DOI] [PubMed] [Google Scholar]
- 40.Opipari A. W., Jr., Tan L., Boitano A. E., Sorenson D. R., Aurora A., Liu J. R. (2004) Cancer Res. 64, 696–703 [DOI] [PubMed] [Google Scholar]
- 41.Jankowsky J. L., Fadale D. J., Anderson J., Xu G. M., Gonzales V., Jenkins N. A., Copeland N. G., Lee M. K., Younkin L. H., Wagner S. L., Younkin S. G., Borchelt D. R. (2004) Hum. Mol. Genet. 13, 159–170 [DOI] [PubMed] [Google Scholar]
- 42.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Nature 444, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 44.Bertelli A. A., Gozzini A., Stradi R., Stella S., Bertelli A. (1998) Drugs Exp. Clin. Res. 24, 207–211 [PubMed] [Google Scholar]
- 45.Feige J. N., Lagouge M., Canto C., Strehle A., Houten S. M., Milne J. C., Lambert P. D., Mataki C., Elliott P. J., Auwerx J. (2008) Cell Metab. 8, 347–358 [DOI] [PubMed] [Google Scholar]
- 46.Dasgupta B., Milbrandt J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang J. T., Kwak D. W., Lin S. K., Kim H. M., Kim Y. M., Park O. J. (2007) Ann. N.Y. Acad. Sci. 1095, 441–448 [DOI] [PubMed] [Google Scholar]
- 48.Park C. E., Kim M. J., Lee J. H., Min B. I., Bae H., Choe W., Kim S. S., Ha J. (2007) Exp. Mol. Med. 39, 222–229 [DOI] [PubMed] [Google Scholar]
- 49.Zang M., Xu S., Maitland-Toolan K. A., Zuccollo A., Hou X., Jiang B., Wierzbicki M., Verbeuren T. J., Cohen R. A. (2006) Diabetes 55, 2180–2191 [DOI] [PubMed] [Google Scholar]
- 50.Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., Scherer B., Sinclair D. A. (2003) Nature 425, 191–196 [DOI] [PubMed] [Google Scholar]
- 51.Borra M. T., Smith B. C., Denu J. M. (2005) J. Biol. Chem. 280, 17187–17195 [DOI] [PubMed] [Google Scholar]
- 52.Hou X., Xu S., Maitland-Toolan K. A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T. J., Cohen R. A., Zang M. (2008) J. Biol. Chem. 283, 20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lan F., Cacicedo J. M., Ruderman N., Ido Y. (2008) J. Biol. Chem. 283, 27628–27635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 55.Campos-Toimil M., Elíes J., Alvarez E., Verde I., Orallo F. (2007) Eur. J. Pharmacol. 577, 91–99 [DOI] [PubMed] [Google Scholar]
- 56.Sareen D., Darjatmoko S. R., Albert D. M., Polans A. S. (2007) Mol. Pharmacol. 72, 1466–1475 [DOI] [PubMed] [Google Scholar]
- 57.Chen Y., Zhou K., Wang R., Liu Y., Kwak Y. D., Ma T., Thompson R. C., Zhao Y., Smith L., Gasparini L., Luo Z., Xu H., Liao F. F. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3907–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saeedi R., Parsons H. L., Wambolt R. B., Paulson K., Sharma V., Dyck J. R., Brownsey R. W., Allard M. F. (2008) Am. J. Physiol. Heart Circ. Physiol. 294, H2497–H506 [DOI] [PubMed] [Google Scholar]
- 60.Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., Small S., Spencer B., Rockenstein E., Levine B., Wyss-Coray T. (2008) J. Clin. Invest. 118, 2190–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karuppagounder S. S., Pinto J. T., Xu H., Chen H. L., Beal M. F., Gibson G. E. (2009) Neurochem. Int. 54, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luchsinger J. A., Tang M. X., Siddiqui M., Shea S., Mayeux R. (2004) J. Am. Geriatr. Soc. 52, 540–546 [DOI] [PubMed] [Google Scholar]
- 63.Truelsen T., Thudium D., Grønbaek M. (2002) Neurology 59, 1313–1319 [DOI] [PubMed] [Google Scholar]
- 64.Orgogozo J. M., Dartigues J. F., Lafont S., Letenneur L., Commenges D., Salamon R., Renaud S., Breteler M. B. (1997) Rev. Neurol. 153, 185–192 [PubMed] [Google Scholar]
- 65.Lindsay J., Laurin D., Verreault R., Hébert R., Helliwell B., Hill G. B., McDowell I. (2002) Am. J. Epidemiol. 156, 445–453 [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Ho L., Zhao Z., Seror I., Humala N., Dickstein D. L., Thiyagarajan M., Percival S. S., Talcott S. T., Pasinetti G. M. (2006) FASEB J. 20, 2313–2320 [DOI] [PubMed] [Google Scholar]
- 67.Ho L., Chen L. H., Wang J., Zhao W., Talcott S. T., Ono K., Teplow D., Humala N., Cheng A., Percival S. S., Ferruzzi M., Janle E., Dickstein D. L., Pasinetti G. M. (2009) J. Alzheimer's Dis. 16, 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han Y. S., Zheng W. H., Bastianetto S., Chabot J. G., Quirion R. (2004) Br. J. Pharmacol. 141, 997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang J. H., Surh Y. J. (2003) Free Radic. Biol. Med. 34, 1100–1110 [DOI] [PubMed] [Google Scholar]
- 70.Savaskan E., Olivieri G., Meier F., Seifritz E., Wirz-Justice A., Müller-Spahn F. (2003) Gerontology 49, 380–383 [DOI] [PubMed] [Google Scholar]
- 71.Ono K., Yoshiike Y., Takashima A., Hasegawa K., Naiki H., Yamada M. (2003) J. Neurochem. 87, 172–181 [DOI] [PubMed] [Google Scholar]
- 72.Rivière C., Richard T., Quentin L., Krisa S., Mérillon J. M., Monti J. P. (2007) Bioorg. Med. Chem. 15, 1160–1167 [DOI] [PubMed] [Google Scholar]
- 73.Kim D., Nguyen M. D., Dobbin M. M., Fischer A., Sananbenesi F., Rodgers J. T., Delalle I., Baur J. A., Sui G., Armour S. M., Puigserver P., Sinclair D. A., Tsai L. H. (2007) EMBO J. 26, 3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greco S. J., Sarkar S., Johnston J. M., Tezapsidis N. (2009) Biochem. Biophys. Res. Commun. 380, 98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.