Abstract
A novel screening method to identify selective Sox-based fluorescent probes for Ser/Thr kinases has been developed. Peptide libraries were exposed to a kinase of interest and the products of the timed reaction were analyzed by MALDI-TOF. To demonstrate the potential of this methodology, a selective substrate for Aurora-A kinase was identified that showed a 7-fold improvement in catalytic efficiency over the best substrate described to date in the literature.
Phosphorylation is a ubiquitous post-translational modification reaction that is responsible for regulation of protein activity in both eukaryotes and prokaryotes. By catalyzing the transfer of γ-phosphoryl group of ATP to the side chains of serine, threonine, and/or tyrosine (in eukaryotes), protein kinases play an important role in regulation of many aspects of cellular function, including proliferation, the cell cycle, metabolism, transcription, and apoptosis.i Protein kinases have also emerged as attractive targets for drug discovery, since many are associated with a wide variety of diseases, from cancer to inflammation.ii Thus, tools that allow for simple monitoring of kinase activity are in great demand in both pharmaceutical and academic settings. Recently, our laboratory developed highly versatile sulfonamido-oxine (Sox)-based fluorescent peptides for the continuous assay of Ser/Thr and Tyr kinases.iii The Sox-containing substrate is silent, but upon phosphorylation the chromophore can bind Mg2+ and undergoes chelation-enhanced fluorescence (CHEF). Such probes have been used to monitor various kinases both in vitro and in crude cell lysates.iv
The key challenge in the field of kinase analysis is specificity, particularly with peptide-based substrates, which lack the spatial and temporal control that cellular substrates such as proteins possess. As a result, much effort has been devoted to identification of kinase substrates. Traditional methods to elucidate specificity of kinases include solid-phase phosphorylation screening of either phage display librariesv or synthetic peptidesvi and the use of degenerate libraries of peptides oriented around the residue to be phophorylated.vii But, these techniques depend on laborious and time-consuming substrate peptide decoding procedures.6a,5c Furthermore, the detection of phosphate is based on the transfer of 32P from [γ-32P]ATP to target peptides or proteins,viii which is a risk to human health and the environment, or on antibodies directed against phosphorylated residues,ix which in some cases is problematic due to their low specificity.
Herein, we report the development of a new method for identification of Sox-based probes with improved specificity for serine/threonine kinases. A combinatorial peptide library is first exposed to the desired kinase. Upon chemical modification of the phosphopeptides in the peptide mixture, Matrix-Assisted Laser Desorption Ionization Time-of-Flight mass spectrometry (MALDI-TOF MS) is employed to identify the products. Using this approach, the best sequence for Protein Kinase A (PKA) was found to be the well-established and extensively studied Kemptide,x demonstrating that our method is reliable. Moreover, when applied to Aurora A (AurA, Aurora 2), a peptide sequence was identified that exhibited a 7-fold improvement in catalytic efficiency over the best literature substrate when incorporated into a Sox-based probe.
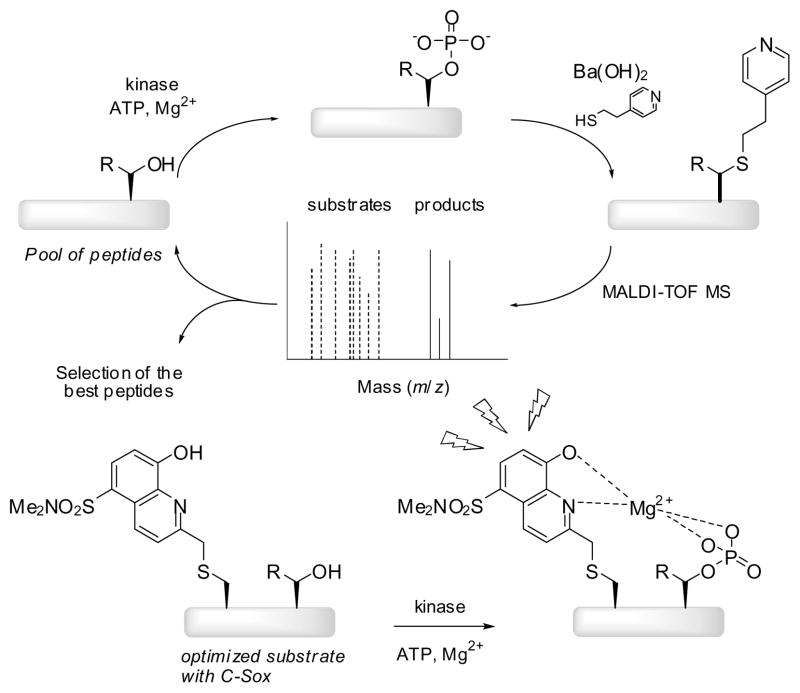
The method to identify probes is summarized in Figure 1. First, a library containing amino acid variations at the site of investigation was generated by Fmoc-based solid-phase peptide synthesis (SPPS). 2-Naphthyl alanine (2-Nal) was incorporated in place of C-Sox (generally placed in the +2 position) due to the tendency of the Sox chromophore to be partially eliminated under the MALDI conditions. The cleaved equimolar peptide mixture was incubated with the desired kinase for varying times. The direct detection of the phosphopeptide product by MALDI-TOF is usually poor due to the inefficient ionization of the negatively charged phosphate group and the tendency of phosphate to be eliminated under the MALDI conditions. Thus, to enhance signal intensities in the MALDI analysis, a previously reported method was employed.xi Briefly, the kinase reaction products were subjected to base [Ba(OH)2] which promoted β-elimination of the phosphate moiety, followed by Michael addition of 4-mercaptoethylpyridine (4-MEP). New peaks appearing in the MALDI spectrum (121 g/mol greater than the parent peptide) were interpreted as evidence of phosphorylation. The change in mass after the reaction was due to the loss of phosphate (98 g/mol) during the β-elimination and addition of 4-MEP (139 g/mol). This strategy enabled us to follow the progress of the reaction and to evaluate the kinase activity semiquantitatively by comparing intensities of derivatized peaks with those of the parent peptides in the same spectrum (Figure 2). Once the best residue was found for a particular position, it was fixed in that place and the method was applied to a different position in an iterative fashion until the best substrate was obtained. Finally, the optimized sequence was synthesized with C-Sox3a instead of 2-Nal, effectively turning the best substrate into a selective kinase reporter and simultaneously enabling determination of the kinetic parameters.
Figure 1.
Screening Method to Design Selective Substrates for Serine/Threonine Kinases.
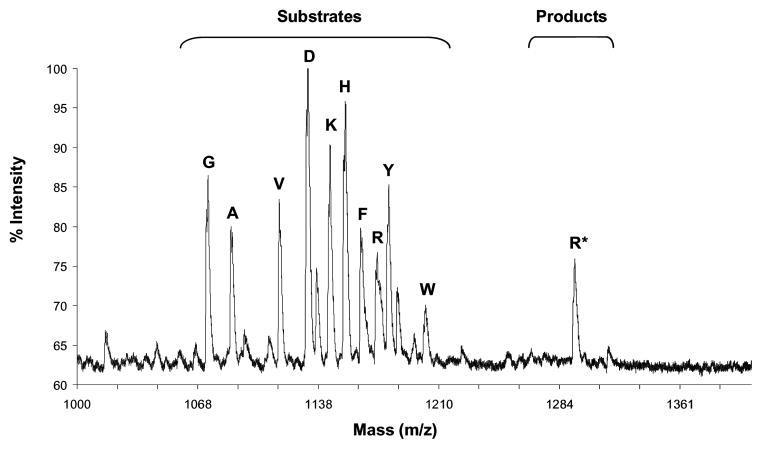
Figure 2.
MALDI-TOF spectrum of the peptide library at the −3 position after its incubation with PKA for 1h and chemical derivatization with Ba(OH)2/MEP.
In order to optimize and validate the screening method, we initially focused our efforts on PKA and the corresponding selective substrate, Kemptide (Ac-LRRASLG-CONH2).10 Two peptide libraries based on Kemptide were used to assess the preference of PKA for residues at the −1 and −3 positions (Table 1). In all screens, Ser, Thr, Cys and Met were excluded to avoid side reactions (such as oxidations). Additionally, since there are three groups of amino acids with similar masses (Asp, Leu, Ile, Asn = 131–133 g/mol; Lys, Glu, Gln = 146–147 g/mol; and Val, Pro = 115–117 g/mol), only one from each group was chosen in order to simplify the MALDI analysis. However, if necessary, it is also possible to use all amino acids in the screen by utilizing encoded peptide caps during synthesis, effectively producing a nondegenerate mass ladder for each peptide.xii A mixture of the following amino acids was selected: Asp, Lys, Val, Ala, Arg, Gly, His, Phe, Trp and Tyr. Libraries were synthesized on Fmoc-PAL-PEG-PS resin. For positions that were varied, isokinetic mixture of 10 amino acids was created by using a ratio of equivalents of amino acids based on their reported coupling rates.xiii The MALDI spectra of the peptide libraries before and after incubation with PKA for varying time periods (10 min, 30 min, 1 h, 2 h and 24 h) followed by chemical derivatization with 4-MEP, showed that there is a preference for Arg at the −3 position (Figure 2) and for small hydrophobic residues at the −1 position (Table 1). This result is in full agreement with the consensus sequence described for PKA.10,xiv
Table 1.
Peptide libraries for PKA.
| Entry | Substrate Sequencesa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | |||
| 1 | Ac | L | X | R | F | S | L | 2Nal | A | A | CONH2 |
| 2 | Ac | L | R | R | X | S | L | 2Nal | A | A | CONH2 |
| Result | L | R | R | G/A | S | L | 2Nal | A | A | ||
X = Asp, Lys, Val, Ala, Arg, Gly, His, Phe, Trp and Tyr.
To demonstrate the generality of the method, Aurora kinase A (Aurora 2, AurA) was selected. Aurora kinases (A, B and C) belong to the Ser/Thr protein kinase family and are involved in various aspects of mitosis.xv AurA, specifically, functions in centrosome maturation, mitotic spindle assembly and it plays a central role in cell cycle progression.xvi Additionally, the gene coding for AurA maps to a region frequently amplified in tumors and its overexpression has been detected in various cancers.xvii However, very little is presently known about its substrates and the mechanism of its activation/deactivation. This is particularly the case with peptide substrates for AurA. The only study so far conducted to determine the preferred residues in the recognition domain surrounding the phosphorylated residue,13 proposed the consensus sequence to be RRXSZ (where Z denotes any hydrophobic residue except for Pro and X is a small hydrophobic amino acid) and was based on peptides derived from an extended version of the Kemptide sequence (ALRRASLGAA). The best of these peptides has a KM of ca. 300 μM (Tables 3 and 4, entry 1). Thus, to make a fluorescent probe that can be used in complex environments to study AurA, it was imperative to first find substrates with enhanced selectivity.
Table 3.
Sequences of Sox-substrates for AurA.
| Entry | Substrate sequences |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | |||
| 1 | Ac | A | L | R | R | A | S | L | C-Sox | A | A | CONH2 |
| 2 | Ac | A | L | R | R | F | S | L | C-Sox | A | A | CONH2 |
| 3 | Ac | A | L | R | R | F | S | L | C-Sox | G | A | CONH2 |
| 4 | Ac | A | G | R | R | Y | S | L | C-Sox | DA | A | CONH2 |
Table 4.
Kinetics of Sox-substrates with AurA.
| Entrya | KM (μM)b | Vmax (μmol mg−1 min−1)b | catalytic efficiencyc |
|---|---|---|---|
| 1 | 297.8 ± 3.9 | 1.7 ± 0.3 | 1 |
| 2 | 152.3 ± 2.7 | 2.6 ± 0.2 | 3 |
| 3 | 57.0 ± 8.2 | 2.2 ± 0.1 | 7 |
| 4 | 65.0 ± 7.0 | 1.8 ± 0.1 | 5 |
Peptides from Table 3.
Kinetic parameters (KM and Vmax) were obtained from initial slopes and corrected appropriately for substrate and product fluorescence as described in Supporting Information. The values reported are the mean ± s.e.m. of duplicate experiments as calculated from a direct fit of [S]/ṿ vs. [S] plots.
Catalytic efficiency of each substrate was calculated as kcat/KM (min−1 μM−1).
We made six peptide libraries using equimolar mixtures of amino acids at the −1, −3, −4, +1, +3 and +4 positions based on the sequence of Kemptide (Table 2). The results of these libraries after incubation with AurA for varying time periods (10 min, 30 min, 1 h, 2 h and 24 h) followed by chemical derivatization with 4-MEP showed that, first, there was an elevated preference for an aromatic residue, Tyr or Phe, in the −1 position (Table 2, entry 3). For the remaining libraries, Phe was fixed in this position. Second, there was a high preference for Arg in the −3 position, which is in agreement with the consensus sequence described for AurA13 (entry 2). Third, in the +1 position, aromatic residues, such as Phe, were favored (entry 4). This result coincides with previous work done by Pinna et al.13 that identified Phe, Leu, and Ile in the −1 position (Leu and Ile were excluded from our screen). Fourth, small hydrophobic residues, mainly Gly, were selected for positions −4, +3 and +4 (entries 1, 5 and 6). Lastly, due to the preference for Gly at −4 and +3 positions, we made two additional peptide libraries using D-amino acids (entries 1 and 5). As in the case of the L-peptide libraries, AurA selected Gly in both positions and, interestingly, DAla in +3 position.
Table 2.
Peptide libraries for AurA.
| Entry | Substrate sequencesa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | |||
| 1 | Ac | X/Y | R | R | F | S | L | 2Nal | A | A | CONH2 |
| 2 | Ac | L | X | R | F | S | L | 2Nal | A | A | CONH2 |
| 3 | Ac | L | R | R | X | S | L | 2Nal | A | A | CONH2 |
| 4 | Ac | L | R | R | F | S | X | 2Nal | A | A | CONH2 |
| 5 | Ac | L | R | R | F | S | L | 2Nal | X/Y | A | CONH2 |
| 6 | Ac | L | R | R | F | S | L | 2Nal | A | X | CONH2 |
| Result | G/A | R | R | F | S | F | 2Nal | G/DA | G | ||
X = Asp, Lys, Val, Ala, Arg, Gly, His, Phe, Trp and Tyr; Y = DAsp, DLys, DVal, DAla, DArg, DGly, DHis, DPhe, DTrp and DTyr.
Several sequences that were selected by our screen were individually synthesized with C-Sox in place of 2-Nal and evaluated as probes for AurA. Tables 3 and 4 summarize the sequences and the kinetics parameters, respectively, of the best peptides. A 2-fold improved KM and a 3-fold improved catalytic efficiency were obtained by incorporating a Phe residue at the −1 position (Tables 3 and 4, entry 2). A 6-fold improved KM and a 5- to 7-fold improved catalytic efficiency compared to Kemptide was obtained with Gly (Tables 3 and 4, entry 3) or DAla (Tables 3 and 4, entry 4) at position +3. These peptides are the best substrates described so far for AurA.
Although chemical methods to detect phosphorylated products using mass spectrometry and fluorescence have been reported, none were able to obtain substrates with improved selectivity for the desired kinase.xviii Herein, we have presented a new screen that allows identification of substrates for serine/threonine kinases using a chemically modified combinatorial peptide library and MALDI-TOF MS. The strategy was first validated by obtaining Kemptide as the most selective PKA peptide, which is in full agreement with current literature. Moreover, the screen was applied to AurA resulting in a substrate with a 6-fold improvement in KM and a 7-fold rise in catalytic efficiency with respect to the best sequence described so far in the literature. Compared with the conventional approaches, this strategy is simple, easy to perform and it does not require complex instrumentation, the use of radioisotopes, or antibodies. The iterative nature of the method and its ability to incorporate unnatural elements (such as D-amino acids) should make searches for substrates of virtually any kinase possible. Lastly, the conversion of the most selective peptides into fluorescent Sox-containing probes should give a specific reporter for any kinase of choice.
Supplementary Material
Acknowledgments
This work was supported by the NIH Cell Migration Consortium (GM064346), the Invitrogen Corporation and the A. M. Escudero Foundation postdoctoral fellowship for J.A.G-V. We thank the Biophysical Instrumentation Facility for the Study of Complex Macromolecular Systems (NSF-0070319).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- i.Hunter T. Cell. 2000;100:113. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- ii.(a) Adams J. Chem Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]; (b) Manning GW, Martinez R, Hunter T, Sudarsanam S. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]; (c) Skorski T. Nat Rev Cancer. 2002;2:1–10. doi: 10.1038/nrc799. [DOI] [PubMed] [Google Scholar]; (d) Johnson LN, Lewis R. J Chem Rev. 2001;101:2209–2242. doi: 10.1021/cr000225s. [DOI] [PubMed] [Google Scholar]; (e) Yarden Y, Sliwkowski MX. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- iii.(a) Lukoviæ EE, González-Vera JA, Imperiali B. J Am Chem Soc. 2008;130:12821–12827. doi: 10.1021/ja8046188. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shults MD, Carrico-Moniz D, Imperiali B. Anal Biochem. 2006;352:198–207. doi: 10.1016/j.ab.2006.03.003. [DOI] [PubMed] [Google Scholar]; (c) Shults MD, Imperiali B. J Am Chem Soc. 2003;125:14248–14249. doi: 10.1021/ja0380502. [DOI] [PubMed] [Google Scholar]
- iv.Shults MD, Janes KA, Lauffenburger DA, Imperiali B. Nat Methods. 2005;2:277–283. doi: 10.1038/nmeth747. [DOI] [PubMed] [Google Scholar]
- v.(a) Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. J Biol Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]; (b) Fukunaga R, Hunter T. EMBO J. 1997;16:1921–1923. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jiang W, Jimenez G, Wells NJ, Hope TJ, Walh GM, Hunter T, Fukunaga R. Moll Cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- vi.(a) Hutti JE, Jarrell ET, Chang JD, Abbott DW, Storz P, Toker A, Cantley LC, Turk BE. Nat Methods. 2004;1:27–29. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]; (b) Uttamchandani M, Chan EW, Chen GY, Yao SQ. Bioorg Med Chem Lett. 2003;13:2997–3000. doi: 10.1016/s0960-894x(03)00633-4. [DOI] [PubMed] [Google Scholar]; (c) Wu J, Ma QN, Lam KS. Biochemistry. 1994;33:14825–14833. doi: 10.1021/bi00253a022. [DOI] [PubMed] [Google Scholar]; (d) Akita S, Umezawa N, Higuchi T. Org Lett. 2005;7:5565–5568. doi: 10.1021/ol052125k. [DOI] [PubMed] [Google Scholar]; (e) Slon-Usakiewicz JJ, Dai JR, Ng W, Foster JE, Deretey E, Toledo-Sherman L, Redden PR, Pasternak A, Reid N. Anal Chem. 2005;77:1268. doi: 10.1021/ac048716q. [DOI] [PubMed] [Google Scholar]; (f) Ahsen O, Bomer U. Chembiochem. 2005;6:481–490. doi: 10.1002/cbic.200400211. [DOI] [PubMed] [Google Scholar]
- vii.(a) Pearson RB, Kemp BE. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]; (b) Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- viii.MacBeath G, Schreiber SL. Science. 2000;289:1760–1763. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- ix.Kim YG, Shin DS, Kim E, Park HY, Lee CS, Kim JH, Lee BS, Lee YS, Kim BS. Angew Chem. 2007;46:5408–5411. doi: 10.1002/anie.200700195. [DOI] [PubMed] [Google Scholar]
- x.Moore MJ, Adams JA, Taylor SS. J Biol Chem. 2003;278:10613–10618. doi: 10.1074/jbc.M210807200. [DOI] [PubMed] [Google Scholar]
- xi.Arrigoni G, Resjo S, Levander F, Nilsson R, Degerman E, Quadroni M, Pinna LA, James P. Proteomics. 2006;6:757–766. doi: 10.1002/pmic.200500073. [DOI] [PubMed] [Google Scholar]
- xii.(a) Hoffmann C, Blechschmidt D, Krüger R, Karas M, Griesinger C. J Comb Chem. 2002;4:79–86. doi: 10.1021/cc010057x. [DOI] [PubMed] [Google Scholar]; (b) Nitz M, Franz KJ, Maglathlin RL, Imperiali B. ChemBioChem. 2003;4:272–276. doi: 10.1002/cbic.200390047. [DOI] [PubMed] [Google Scholar]
- xiii.Backes BJ, Harris JL, Leonetti F, Craik CS, Ellman JA. Nature Biotech. 2000;18:187–193. doi: 10.1038/72642. [DOI] [PubMed] [Google Scholar]
- xiv.Ferrari S, Marin O, Pagano MA, Meggio F, Hess D, El-Shemmerly M, Krystyniak A, Pinna L. Biochem J. 2005;390:293–302. doi: 10.1042/BJ20050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xv.Nigg EA. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- xvi.(a) Glover DM, Leibowitz MH, McLean DA, Parry H. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]; (b) Hannak E, Kirkham M, Hyman AA, Oegema KJ. Cell Biol. 2001;155:1109–1115. doi: 10.1083/jcb.200108051. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mendez R, Hake LE, Anderson T, Littlepage LE, Ruderman JV, Ritcher JD. Nature. 2000;404:302–307. doi: 10.1038/35005126. [DOI] [PubMed] [Google Scholar]
- xvii.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginter C. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xviii.(a) Akita S, Umezawa N, Higuchi T. Org Lett. 2005;7:5565–5568. doi: 10.1021/ol052125k. [DOI] [PubMed] [Google Scholar]; (b) Akita S, Umezawa N, Kato N, Higuchi T. Bioorg Med Chem. 2008;16:7788–7794. doi: 10.1016/j.bmc.2008.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.