Abstract
Profound neuronal dysfunction in the entorhinal cortex contributes to early loss of short-term memory in Alzheimer’s disease1–3. Here we show broad neuroprotective effects of entorhinal brain-derived neurotrophic factor (BDNF) administration in several animal models of Alzheimer’s disease, with extension of therapeutic benefits into the degenerating hippocampus. In amyloid-transgenic mice, BDNF gene delivery, when administered after disease onset, reverses synapse loss, partially normalizes aberrant gene expression, improves cell signaling and restores learning and memory. These outcomes occur independently of effects on amyloid plaque load. In aged rats, BDNF infusion reverses cognitive decline, improves age-related perturbations in gene expression and restores cell signaling. In adult rats and primates, BDNF prevents lesion-induced death of entorhinal cortical neurons. In aged primates, BDNF reverses neuronal atrophy and ameliorates age-related cognitive impairment. Collectively, these findings indicate that BDNF exerts substantial protective effects on crucial neuronal circuitry involved in Alzheimer’s disease, acting through amyloid-independent mechanisms. BDNF therapeutic delivery merits exploration as a potential therapy for Alzheimer’s disease.
The entorhinal cortex is a primary source of inputs to the hippocampus, exerting a key influence in learning and memory4. The most common age-related neurodegenerative disorder, Alzheimer’s disease, affects the entorhinal cortex profoundly and early in the disease, contributing to cardinal symptoms of short-term memory loss1–3. BDNF and its receptor, tyrosine receptor kinase B (TrkB), are widely expressed in the entorhinal cortex, and BDNF is anterogradely trafficked into the hippocampus5, where it is implicated in plasticity mechanisms hypothesized to underlie learning6. Furthermore, amounts of BDNF protein are reduced in the entorhinal cortex and hippocampus in Alzheimer’s disease7–9. We explored the hypothesis that therapeutic BDNF gene delivery to the entorhinal region can ameliorate entorhinal cortex and hippocampus degeneration in diverse models related to Alzheimer’s disease.
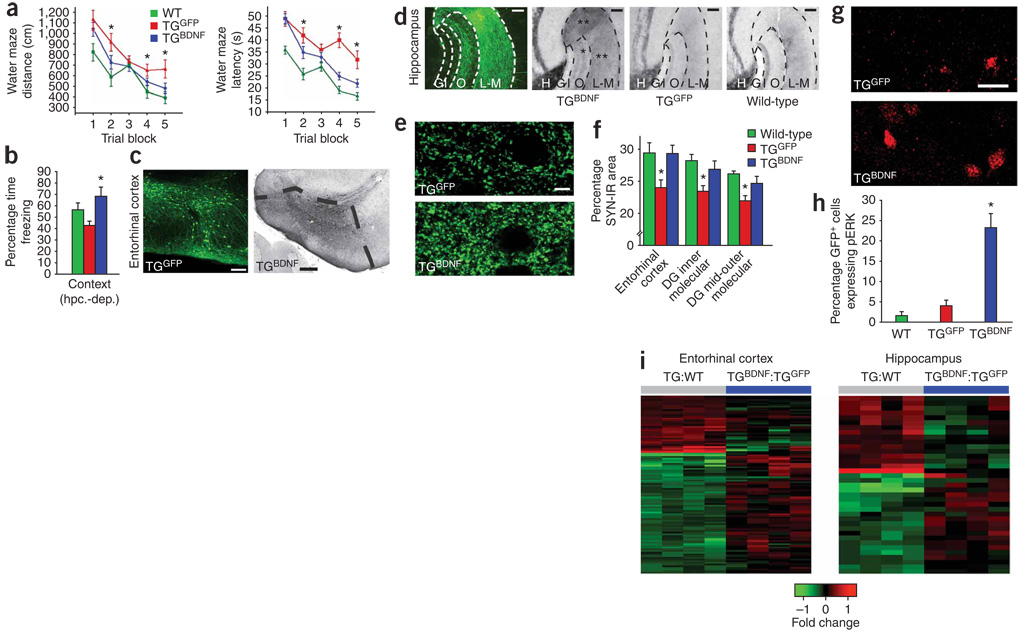
We first examined whether BDNF influences neuronal degeneration in a transgenic mouse model directly related to Alzheimer’s disease pathology in humans. J20 transgenic mice express the human amyloid precursor protein (APP) transgene bearing both the Swedish and the Indiana APP mutations10. Beginning at age 2–3 months, these mice show cortical plaques and progressive cell loss in the entorhinal cortex and show cognitive decline by 6–7 months of age10,11. Lentiviral vectors constitutively expressing BDNF-GFP under control of the CMV–β-actin hybrid promoter (TGBDNF, n = 8) or GFP alone (TGGFP, n = 8) were injected into the entorhinal cortices of transgenic mice bilaterally at age 6 months, when neuropathological degeneration and cell loss are established10,11. Age-matched wild-type (WT) littermates underwent sham surgery (n = 4) or injection of lentivirus expressing GFP (lenti-GFP) into the entorhinal cortex (n = 4). One month later, spatial memory was tested in the Morris water maze. The performance of sham-operated and GFP-injected WT mice did not differ, and these two groups were combined for subsequent statistical analysis (Fig. 1a). Over five successive daily blocks of trials, significant amelioration of spatial memory deficits occurred in TGBDNF mice compared to TGGFP mice (P < 0.01 by analysis of variance (ANOVA); P < 0.05 by post hoc Fisher’s test comparing TGBDNF to TGGFP; Fig. 1a). The performance of TGBDNF mice did not differ significantly from WT littermate controls (Fig. 1a). Transgenic mice were also tested on a fear-conditioning paradigm that measured both hippocampal-dependent (context) and hippocampal-independent (cue) learning (Fig. 1b and Supplementary Fig. 1a online). In the hippocampal-dependent aspect of the task, TGGFP mice showed trends toward impaired performance compared to WT mice (P < 0.05 by ANOVA; P = 0.12 by post hoc Fisher’s test comparing TGGFP versus WT mice). TGBDNF mice performed significantly better than TGGFP mice on this task (P < 0.05; Fig. 1b). Hippocampal-independent fear conditioning was not impaired in either transgenic mouse group (Supplementary Fig. 1a), as expected. Weight loss, a potential consequence of disseminated growth factor spread in the central nervous system, did not occur (6.0% gain in TGBDNF and 6.6% gain in TGGFP).
Figure 1. BDNF effects in APP-transgenic mice.
(a) BDNF gene delivery effect on spatial memory in the Morris water maze in mutant APP-transgenic mice (TG) on distance (left) and latency (right) measures (P < 0.01 by ANOVA, *P < 0.05 by post hoc Fisher’s test, comparing TGBDNF to TGGFP). (b) BDNF effect on hippocampal-dependent (hpc.-dep.) contextual fear conditioning (P < 0.05). (c) Lentiviral gene delivery effect on gene expression (GFP reporter, left) and dense BDNF immunolabeling (black reaction product, right) in the entorhinal cortex of TGBDNF mice. (d) Axon terminals of entorhinal cortex neurons projecting into the hippocampus identified by GFP immunolabeling after entorhinal lenti-GFP injection in APP-transgenic mice (left panel, TGGFP); injection of lenti-BDNF into entorhinal neurons increases BDNF expression in the hippocampal dentate gyrus (TGBDNF), stratum lacunosum-moleculare (L–M) (**) and outer molecular layer (O, *), compared to TGGFP controls and WT mice. H, hilar region; G, granule cell layer; I, inner molecular layer (BDNF immunolabel). (e) Synaptophysin labeling in entorhinal cortex of TGBDNF mice compared to TGGFP mice. (f) Quantification of the effect of entorhinal cortex delivery of lenti-BDNF on synaptophysin immunoreactivity (SYN-IR) in both entorhinal cortex and hippocampus of APP-transgenic mice (P < 0.01 by ANOVA, *P < 0.05 by post hoc Fisher’s test comparing TGBDNF and TGGFP). (g,h) pErk immunolabeling in entorhinal cortex of TGBDNF mice compared to TGGFP mice (P < 0.001 by ANOVA, P < 0.001 by post hoc Fisher’s test comparing TGBDNF to TGGFP or WT). (i) Heat maps depicting fold changes of APP-related genes before and after treatment with BDNF. 107 probe sets are differentially expressed in entorhinal cortex of TG mice compared to WT (TG:WT), and 37 probe sets are differentially expressed in hippocampus (gray bar columns, P < 0.005 by Bayesian t-test, see Supplementary Data 1). Upregulated genes are shown in red, and downregulated genes are shown in green; color intensity corresponds to fold change in expression (Supplementary Fig. 2 and Supplementary Data 1). BDNF treatment in APP-transgenic mice (blue columns) shifts gene expression toward wild type patterns in entorhinal cortex and hippocampus. Genes are clustered by similarity; individual columns represent array samples from individual mice compared to expression in WT mice. Error bars indicate means ± s.e.m. Scale bars: c, 100 µm (left) and 200 µm (right); d, 50 µm; e, 10 µm; g, 10 µm.
Immunolabeling confirmed high amounts of BDNF in the entorhinal cortices of TGBDNF mice (Fig. 1c). Furthermore, substantial trafficking of BDNF into hippocampal regions CA1–3 and dentate gyrus occurred (Fig. 1d). BDNF induced significant recovery of synaptic markers in both entorhinal cortex and dentate gyrus of transgenic mice (P < 0.05; Fig. 1e,f). BDNF treatment also significantly increased extracellular signal–related kinase (Erk) phosphorylation, thereby augmenting cell signaling related to the functional state of neurons (P < 0.001; Fig. 1g,h). Notably, BDNF treatment initiated after disease onset affected neither neuronal number nor amyloid plaque density (Supplementary Fig. 1b–d), indicating that the ameliorative effects of BDNF in this model are exerted independently of direct modulation of insoluble β-amyloid (Aβ; amyloid oligomers were not measured). Gene expression in the entorhinal cortex and hippocampus were compared with mouse whole-genome arrays in WT mice after sham surgery (n = 3), in WT mice receiving injections of lenti-GFP (n = 2), in TGGFP mice (n = 4) and in TGBDNF mice (n = 4) 4 weeks after gene delivery (Fig. 1i and Supplementary Fig. 2 and Supplementary Data 1 online). Overexpression of mutant APP perturbed normal patterns of entorhinal cortex and hippocampal gene expression, with dysregulation of canonical pathways related to oxidative stress and lipid metabolism, among other pathways (Supplementary Fig. 2 and Supplementary Data 1). Many of the genes whose expression changed in transgenic mice have been previously related to Alzheimer’s disease and neurodegeneration (for example, Mfge8 (ref. 12), Galk2 (ref. 13) and St13 (ref. 14), related to phagocytosis of Aβ peptide12, post-translational modifications of APP13 and heat shock protein interactions14, respectively). Notably, entorhinal BDNF gene delivery improved APP-related alterations in gene expression in 55% of dysregulated genes in transgenic mice (Fig. 1i, Supplementary Fig. 2 and Supplementary Data 1). The restorative effects of BDNF on array profiles were also observed in hippocampus (Fig. 1i and Supplementary Fig. 2). Thus, BDNF, acting independently of direct effects on insoluble Aβ, improved genetic, molecular, synaptic and behavioral parameters of APP-transgenic mice when treatment was initiated after disease onset.
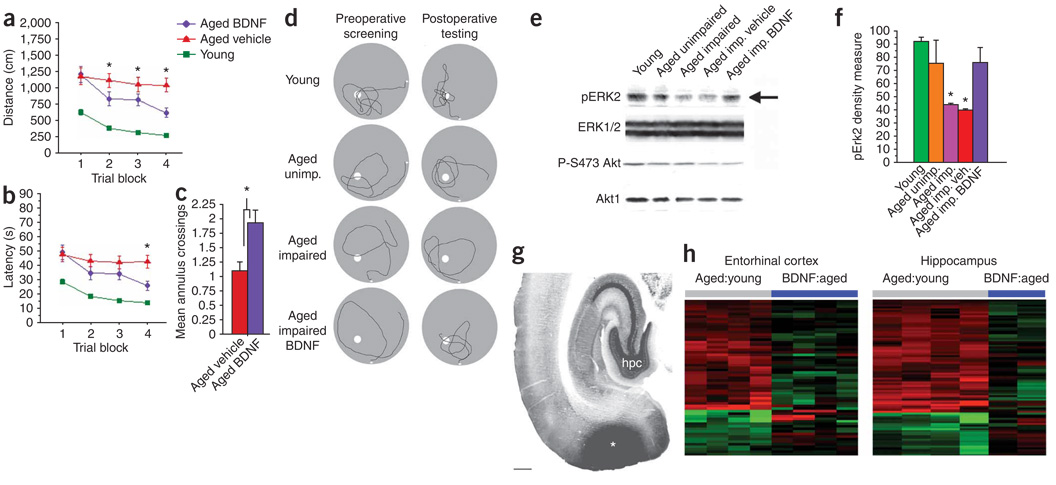
We next tested whether BDNF would enhance the function of entorhinal cortex neurons in a second model of neuronal degeneration, aging (Fig. 2). Aging is accompanied by marked declines in cognitive function associated with the entorhinal cortex15; cell death is not a mechanism underlying this decline16,17. Spatial learning and memory were assessed in 36 young (5-month-old) and 37 aged (24-month-old) male Fischer rats in the Morris water maze16. Eighty-one percent of aged rats showed baseline cognitive impairment and then received bilateral, 28-d infusions of either recombinant BDNF protein (120 ng per day per side) or vehicle (artificial cerebrospinal fluid) into the medial entorhinal cortex. BDNF infusions in aged cognitively impaired rats significantly improved spatial learning and memory on all three measures of water maze performance compared to vehicle-infused rats—distance and latency improved on test trials, and platform crossings increased on probe trials (P < 0.05 by ANOVA; P < 0.05 by post hoc Fisher’s test comparing aged rats given BDNF to aged rats given vehicle; Fig. 2a–c). Before treatment, young and aged cognitively unimpaired rats concentrated searches for the escape platform in the quadrant normally containing the escape platform, whereas aged cognitively impaired rats had poorly focused search strategies (Fig. 2d). After BDNF treatment, aged cognitively impaired rats showed marked improvement in search strategy, with greater swimming around the platform location (Fig. 2d). Aged rats did not lose weight after BDNF infusion (3.1% gain in aged rats given BDNF, 2.7% gain in aged rats given vehicle). Functional impairment with aging was associated with significant reductions in entorhinal concentrations of phosphorylated Erk (P < 0.05 by ANOVA; *P < 0.05 by post hoc Fisher’s test for aged cognitively impaired rats versus aged cognitively unimpaired rats and young rats); infusion of BDNF restored Erk phosphorylation to levels not differing from those in young and aged cognitively unimpaired rats (Fig. 2e,f). Alterations in the cell survival phosphoinositide-3 kinase–Akt pathway were not associated with age-related impairment in the water maze (Fig. 2e), an observation consistent with the fact that cell loss in the entorhinal cortex is not observed with aging16,17. Deficiencies in canonical signaling through Erk–mitogen-activated protein kinase kinase, but not Akt, would be predicted in a model of cell dysfunction but not death6; our findings in aged rats are consistent with this prediction, as BDNF ameliorates age-related deficiencies in Erk without detectably perturbing Akt. Gene expression patterns were examined in additional aged rats 1 month after entorhinal cortex administration of BDNF or GFP lentivirus; viral injections of BDNF were used instead of protein infusions for gene array studies to avoid confounding effects of chronic low-level damage from continuous infusions (n = 4 aged rats given BDNF, n = 4 aged rats given GFP, n = 4 control aged rats not receiving virus and n = 4 young rats; Fig. 2h). Aging was associated with altered patterns of gene expression compared to young rats in both the entorhinal cortex (61 probe sets) and hippocampus (181 probe sets) (Fig. 2h; P < 0.005 by Bayesian t-test, see also Supplementary Fig. 3 and Supplementary Data 2 online), consistent with previous reports18. Oxidative stress (as seen in the APP model) and lipid and glutathione functional pathways were altered (Supplementary Fig. 3). Entorhinal cortex BDNF delivery induced at least a 30% change toward normalization in 43% of entorhinal age-related genes and 42% of hippocampal genes (P < 0.005, Fig. 2h and Supplementary Data 2). Thus, entorhinal cortex BDNF administration compensates for genetic, molecular and behavioral parameters of age-related neural dysfunction.
Figure 2. BDNF effects in aged rats.
(a, b) Effect of BDNF infusion into entorhinal cortex of aged rats on spatial memory in Morris water maze determined by distance (a) and latency (b) measures (P < 0.05 by ANOVA, *P < 0.05 by post hoc Fisher’s test comparing BDNF-treated to aged controls). (c) BDNF effects on search strategy in water maze after platform removal (probe trials) (P < 0.001). (d) Representative samples of search strategy during probe trials in individual rats. (e) Western blot of Erk phosphorylation and Akt phosphorylation (on Ser473, P-S473Akt) in entorhinal cortex of aged cognitively impaired rats compared to young and aged cognitively unimpaired rats; arrow indicates effect of BDNF treatment. Protein loading was standardized to Erk1/2, an abundant cellular protein. (f) Quantification of western blots (P < 0.05 by ANOVA, *P < 0.05 by post hoc Fisher’s test comparing aged cognitively impaired rats and aged cognitively impaired rats treated with vehicle to all other groups). (g) BDNF immunolabeling showing accurate targeting of infusion to entorhinal cortex (*). hpc, hippocampus. Scale bar, 1 mm. (h) Heat maps depicting fold changes of aging-related genes before and after treatment with BDNF. Genes and samples are clustered by similarity; individual columns represent array samples from individual rats compared to young rats.
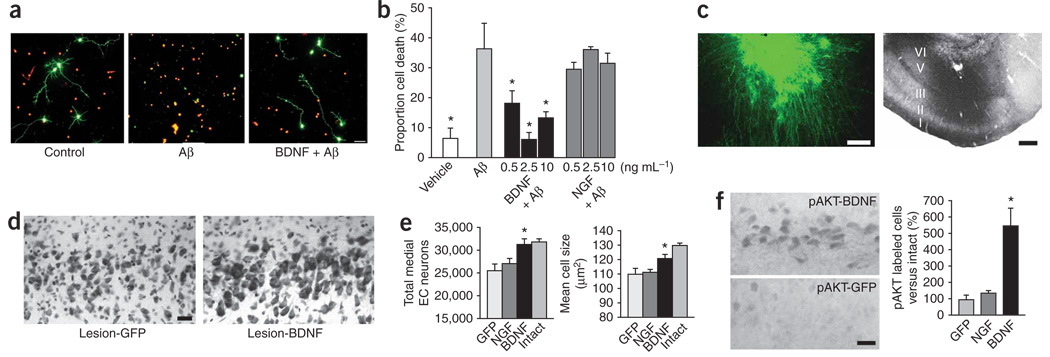
The preceding models show the protective and restorative effects of BDNF in models of neuronal atrophy and synapse loss. Next, we determined whether BDNF can also prevent the death of entorhinal neurons. First, primary entorhinal cortex neurons from postnatal day 3 rats were exposed to the toxic Aβ1–42 peptide in vitro, resulting in cell death within 24 h (Fig. 3a,b). BDNF treatment concurrent with Aβ1–42 exposure prevented neuron death (P < 0.05; Fig. 3a,b). Nerve growth factor (NGF) had no effect (Fig. 3b; cortical neurons do not express the NGF receptor TrkA19). BDNF specifically prevented Aβ1–42–induced cell death, because BDNF did not increase neuron number in naive entorhinal cultures (Supplementary Fig. 4 online). To determine whether BDNF also prevents entorhinal cortex neuronal death in vivo, we subjected rats to perforant path transections that typically induce death in 20% of neurons in layer II of the entorhinal cortex20. Although the cell death in this model occurs independently of Alzheimer’s disease–related Aβ toxicity and neurofibrillary degeneration, the final mechanisms of caspase activation and mitochondrial calcium release are common to apoptotic and necrotic cell death21. Indeed, growth factor–mediated prevention of neuronal loss in transection models has predicted protection from disease-specific mechanisms in other models22–25. Rats received injections into the right entorhinal cortex of lentiviral vectors expressing either BDNF (n = 8 rats), GFP (n = 6) or NGF (n = 8, a negative control). Two weeks after the lesions were made, control GFP- and NGF-treated rats showed a significant 20% loss of stereologically quantified neurons in layer II of the entorhinal cortex compared to the contralateral side of the brain (P < 0.05 by ANOVA; P < 0.05 by post hoc Fisher’s test comparing intact to lesioned control rats; Fig. 3c–e). BDNF prevented cell death (Fig. 3d; P < 0.01) and ameliorated cell atrophy (Fig. 3e; P < 0.05). BDNF also significantly increased expression of phospho-AKT, a marker of canonical cell survival pathways6, in entorhinal cortex neurons (Fig. 3f).
Figure 3. BDNF effects on cell survival.
(a) Fluorescent images of living cells (green, calcein label) and dead cells (red, ethidium homodimer-1 label) in cultures of postnatal day 3 entorhinal cortex neurons in control condition (no Aβ exposure), after addition of Aβ1–42 peptide (Aβ), and after addition of Aβ peptide plus 2.5 ng ml−1 BDNF. Scale bar, 50 µm. (b) Quantification of Aβ42-induced cell death after 24 h in vitro. P < 0.05 by ANOVA, * indicates the difference between the vehicle- and BDNF-treated groups and the Aβ group, P < 0.001 by Fisher’s post hoc test. (c) Perforant path lesion model: GFP immunolabeling in entorhinal cortex at site of lenti-GFP vector injection (left) and BDNF protein production (region of dark immunolabeling) in the same region (right). I–VI indicates cortical laminae. Scale bar, 150 µm. (d,e) Perforant path lesion causes significant loss of Nissl-stained neurons in layer II of the entorhinal cortex (EC) in GFP-injected controls (P < 0.01 versus intact). Nissl stain of layer II entorhinal cortex after perforant path lesions in GFP-injected controls (Lesion-GFP) and BDNF-treated rats (Lesion-BDNF) is shown in d. Stereological quantification of cell number and size after perforant path lesions is shown in e (left, P < 0.05 by ANOVA, *P < 0.01 BDNF versus GFP and NGF by Fisher’s post hoc test; right, P < 0.01 by ANOVA, *P < 0.05 BDNF versus GFP and NGF by post hoc Fisher’s test). Scale bar, 25 µm. (f) pAKT label in entorhinal cortex of treated versus control perforant path–lesioned rats, with stereological quantification at right. Scale bar, 10 µm (*P < 0.001).
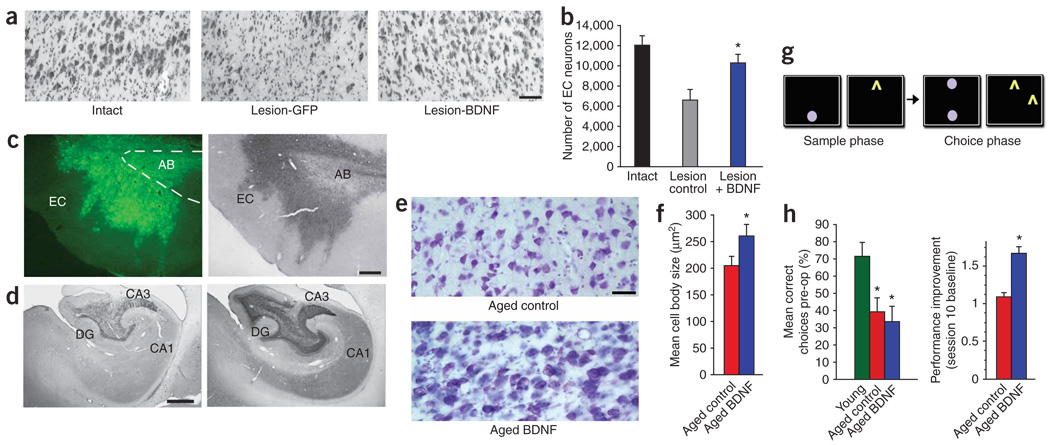
Next, we determined whether the restorative effects of BDNF are scalable to nonhuman primates. First, we established a nonhuman primate model of entorhinal cortex neuronal death. Radiofrequency lesions of the perforant path were performed bilaterally, and lentiviral vectors expressing BDNF were injected into the right entorhinal cortex (n = 6 monkeys). Lenti-GFP (n = 4 monkeys) or no virus (n = 2 monkeys) was injected into the left entorhinal cortex (Fig. 4a,b). Stereological quantification revealed that perforant path lesions without BDNF treatment resulted in the loss of 45.9 ± 8.5% of neurons in layer II of the entorhinal cortex in the lateral region relative to those in intact monkeys (Fig. 4a,b). BDNF significantly prevented lesion-induced entorhinal neuronal death (P < 0.01), maintaining 85.4 ± 7.1% of neurons on the lesioned side compared to intact subjects (Fig. 4b). (Because axonal regeneration and reinnervation of hippocampal targets does not occur in this model, no effort was made to assess cognitive performance after perforant path lesions.)
Figure 4. BDNF effects in primates.
(a) Nissl stain of layer II entorhinal cortex neuronal death induced by perforant path lesion and neuroprotection by lenti-BDNF injection. Scale bar, 65 µm. (b) Stereological quantification showing loss of layer II entorhinal cortex neurons after perforant path lesions and protection of neurons by injection of lenti-BDNF (P < 0.01 by ANOVA, *P < 0.01 by Fisher’s post hoc test compared to lesion controls). (c) GFP immunolabeling in entorhinal cortex (EC, left) and BDNF protein expression (right, BDNF immunolabel) following injection of lenti-BDNF vector in aged, cognitively impaired monkeys. AB, angular bundle (dashed lines). Scale bar, 75 µm. (d) BDNF immunolabeling in hippocampal dentate gyrus (DG) and hippocampal regions CA3 and CA1 in control aged subjects injected with lenti-GFP in entorhinal cortex (left); BDNF increases after lenti-BDNF injection (right). Scale bar, 250 µm. (e) Nissl stain showing hypertrophy of neurons in aged monkey entorhinal cortex after BDNF gene delivery. Scale bar, 35 µm. (f) Quantification of neuronal size in aged monkeys (*P < 0.01 by t-test). (g) Visuospatial discrimination task used to test cognitive function in aged monkeys27 (details in text). (h) Quantification of preoperative choice accuracy in aged and young monkeys before treatment (left; P < 0.01 by ANOVA, *P < 0.01 both aged groups compared to young) and proportional improvement in performance after lenti-GFP or lenti-BDNF gene delivery to entorhinal cortex (right; *P < 0.05, two-tailed t-test).
Finally, we examined the effects of BDNF gene delivery to the entorhinal cortex in aged monkeys (Fig. 4c–h). The aged primate is the best available model of age-related neurodegeneration in humans, showing cognitive decline with aging and reductions in neuronal function15. Extensive cell death does not occur in the aged primate entorhinal cortex with aging17,26, consistent with findings in aged rodents16. Aged monkeys (n = 9; seven males and two females, mean age = 24.5 ± 1.2 years) were preoperatively characterized on a visuospatial discrimination task sensitive to temporal lobe function27,28. After learning the procedural component of the task (identification of a previously presented test stimulus on the touch screen after a delay)27, the monkeys underwent testing on a two-choice version of the task (Fig. 4g), undergoing 20 trials per session over 20 preoperative test sessions. All aged monkeys showed significantly impaired visuospatial learning compared to young monkeys (P < 0.01; Fig. 4g,h). Aged monkeys then received bilateral injections of lentiviral vectors expressing either BDNF (n = 4) or GFP (n = 5) into four locations distributed over the rostral-to-caudal extent of the medial temporal cortex, targeting entorhinal cortex neurons projecting to the hippocampus (Fig. 4c,d). After treatment, monkeys were retested on the visuospatial discrimination task. BDNF-treated aged monkeys showed a significant improvement in performance compared to control aged monkeys by the tenth postoperative session (P < 0.05, Fig. 4h). Both BDNF-treated and control aged monkeys showed slight but nonsignificant reductions in weight over the experimental period (5.6% and 3.4% relative to preoperative baseline, respectively). Analysis of brain sections showed accurate targeting of lentiviral vectors to the entorhinal cortex and elevated BDNF protein distribution in the hippocampus, suggesting anterograde transport (Fig. 4c,d). Furthermore, mean entorhinal neuronal size significantly increased in BDNF-treated monkeys compared to monkeys receiving lenti-GFP (P < 0.01; Fig. 4e,f).
BDNF is produced in the entorhinal cortex throughout life and it undergoes anterograde trafficking into the hippocampus5. BDNF influences neuronal plasticity by facilitating neurotransmitter release29, increasing vesicular docking, enhancing glutamate-evoked post-synaptic responses30 and facilitating hippocampal and cortical long-term potentiation31. Notably, the concentrations of BDNF decline in the entorhinal cortex and hippocampus in Alzheimer’s disease7–9, leading us to hypothesize that therapeutic application of BDNF would influence neurodegeneration. This study demonstrates the extensive trophic actions of BDNF on neurons of the entorhinal cortex, and, via anterograde transport, the hippocampus. BDNF administration in these models prevents cell death, reverses neuronal atrophy and ameliorates behavioral deficits. The mechanisms underlying these actions include normalization of gene expression patterns in models of disease and aging, augmentation of intracellular signaling related to the functional state of the cell and enhancement of synaptic marker expression. The therapeutic effects of BDNF extend across multiple animal models of Alzheimer’s disease, including mutant amyloid mice, aged rats and adult perforant path–lesioned rats. Furthermore, these effects extend to primate models, which show amelioration of cell death, enhancement of cell size and improvement in age-related cognitive decline in response to BDNF. Taken together, these results establish a rationale for BDNF delivery to the entorhinal cortex as a means for treating entorhinal and hippocampal degeneration in Alzheimer’s disease. This approach acts independently of direct modulation of amyloid, providing a key alternative to, and potential synergy with, amyloid-modifying treatments.
A lack of methods for effectively and safely delivering growth factors to the nervous system over sustained time periods has been a major factor limiting their translational testing32. Intraventricular infusions of growth factors typically fail to reach most neurons33, and hence recent clinical trials used intraparenchymal infusions or gene delivery34–37. Our experiment demonstrated efficacy using both treatment methods. These approaches could be scaled up to deliver BDNF to a target the size of the human entorhinal cortex, ~8 cm3 in volume, thereby broadly affecting neuronal activity and function throughout the hippocampus as a consequence of BDNF transport5. The potency of BDNF in the preclinical models presented here, including its validation in primate models, provides a rationale for exploring clinical translation.
METHODS
Transgenic mice
BL/6 transgenic mice expressed mutant APP under control of the Thy1 promoter10. We injected 2 µl lentivirus expressing BDNF or enhanced GFP into two sites spanning the midportion of the medial entorhinal cortex. Four weeks later, we tested the mice for 5 consecutive d in the Morris water maze (four morning and four afternoon trials daily; probe testing was not done after the last test trial).We tested visual and motoric sufficiency in a subsequent cued version of the water maze (visible platform), resulting in the exclusion of one sham subject. One week later, we assessed contextual fear conditioning with a 20-s tone that terminated with a 3-s footpad shock (0.45 mA). Twenty-four hours later, we reexposed each mouse to the same chamber and recorded freezing over 3 min. Two hours later, we placed the mice into novel chambers (new shape, odor and color) and recorded freezing for 3 min before and during each of three conditioned stimulus tone presentations (cued conditioning). We tracked the movements by infrared monitors. See the Supplementary Methods online for a description of stereological analyses and immunolabeling procedures. Experimental manipulations and data analysis in this and all subsequent studies were conducted in a blinded fashion. The University of California–San Diego Institutional Animal Care and Use Committee (IACUC) gave approval for mouse studies.
Aged rats
We gave aged cognitively impaired rats (Harlan, National Institute of Aging colony) one of the following treatments: BDNF protein infusion (n = 10), vehicle (artificial cerebrospinal fluid) infusion (n = 10) or sham surgery (n = 10). Seven aged and cognitively unimpaired rats and 36 young rats did not receive infusions. We performed Morris water maze testing over four preoperative testing blocks, each consisting of six total trials (three trials per day, of a maximum duration of 90 s per trial). The last trial of each block consisted of a probe trial. Thirty of 37 aged rats were cognitively impaired, which we identified as inferior performance compared to the worst-scoring young rat on latency and distance measures during the fourth block. We then gave aged cognitively impaired rats continuous 4-week infusions (Alzet mini-pumps) of either 120 ng BDNF per day in artificial cerebrospinal fluid or artificial cerebrospinal fluid alone. Postoperative testing began 2 weeks later; we utilized four testing blocks of six trials per block. We used visible platform testing to assess visual and motoric adequacy, which showed no significant differences across groups (latency, P = 0.68; distance, P = 0.35).We performed western blotting for Erk, phosphorylated Erk (pErk), Akt and pAKT on entorhinal cortices from four BDNF-infused aged rats, four vehicle-infused aged rats, four nonoperated aged cognitively impaired rats, four nonoperated aged cognitively unimpaired rats and eight young rats. We normalized protein loading to Erk levels. Details of western blotting are in the Supplementary Methods. We used Affymetrix Mouse Genome 430_2 and Rat Genome 230_2 arrays per manufacturer instructions for microarray studies. We assessed quality by inter-array Pearson correlation and RNA degradation plotting. We performed contrast analysis of differential expression with the Linear Models of Microarray (LIMMA) software38.We analyzed pathway and gene ontology with Ingenuity. The Veterans Administration San Diego Healthcare System IACUC provided approval for the aging rat study and the perforant path rat study.
In vitro β-amyloid toxicity studies
We cultured primary entorhinal cortex neurons from rats on postnatal day 3. We treated the cultured neurons with Aβ1–42 peptide (5 or 10 µM (gift from C. Glabe)) dissolved in DMSO. Control cultures received DMSO alone; scrambled Aβ had no cell toxic effect. We added BDNF or NGF (0, 0.5, 2.5 or 10 ng ml−1) concurrently with Aβ, and we assessed cell viability 24 h later with the LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes).
Rat perforant path lesions
We injected 5 µl of lentivirus-BDNF-GFP (1.25 × 108 infectious units per ml) into three sites evenly distributed over the rostral-to-caudal extent of the right entorhinal cortex in adult Fischer rats (Harlan; n = 8); we injected lenti-GFP (n = 6) or lentivirus encoding NGF (n = 8) as controls. Four days later, we made right perforant path lesions. Two weeks later, we performed stereological counts of neurons in layer II of the medial entorhinal cortex.
Primate perforant path lesions
We gave six adult rhesus monkeys (California National Primate Research Center) perforant path lesions with a Radionics probe placed into the angular bundle, which we identified on preoperative magnetic resonance images. We then gave the monkeys injections of lentiviral vectors into the entorhinal cortex over four rostral-to-caudal sites expressing either BDNF (n = 6) on one side of the brain and GFP (n = 4) or no lentiviral injections (n = 2) on the opposite side of the brain. We injected 20 µl of vector (5 × 108 infectious units per ml) per site at a rate of 1 µl min−1. One month later, we quantified the cells in layer II of the lateral entorhinal cortex on Nissl-stained sections with StereoInvestigator (Microbrightfield) software (optical sampling frame 10%, z axis inclusion-exclusion zone 5% at section top and bottom). The University of California–Davis IACUC gave approval for the primate studies.
Aged primates
Before gene transfer, we trained all monkeys to criterion performance on a computerized visuospatial learning task39. Briefly, we showed the monkeys a stimulus, the computer screen became blank and, after a 2-s delay, three choice objects were presented. The monkey was required to choose the original object. 20 trials/session were presented over 20 consecutive sessions. Four aged monkeys then underwent lenti-BDNF injection into the entorhinal cortex at the same locations and doses used in the perforant path lesion study, and five monkeys received lenti-GFP. One month later, we reassessed cognitive performance daily over ten sessions. We compared each monkey’s change in performance to the postoperative session 1 baseline. We quantified entorhinal cortex layer II neuronal size by stereological methods.
Statistical analyses
We used Student’s t-tests to compare two groups, whereas we used analysis of variance with Fischer post hoc tests for multigroup comparisons. For gene array studies, we calculated a Bayesian estimate of differential expression after linear model fitting with a threshold for significance of P < 0.005 (Bayesian modified t-test). Graphs are expressed as means ± s.e.m., unless noted otherwise.
Additional methods
Detailed methodology is described in Supplementary Methods.
Supplementary Material
ACKNOWLEDGMENTS
We thank C. Glabe (University of California, Irvine) for Aβ1–42 peptide. We thank D. Amaral and P. Lavenex for assistance with the primate perforant path model, K. Loew, T. Mead, R. Torres and M. Mateling for technical support, and F. Gao for data analysis. This work was supported by the US National Institutes of Health (AG10435), the California Regional Primate Research Center Base Grant, the Veterans Administration, the Alzheimer’s Association, the State of California (04-35530), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and The Shiley Family Foundation.
Footnotes
Accession codes. GSE14522 (GEO).
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
A.H.N. contributed surgery, data collection, analysis and manuscript writing for all studies; D.A.M. contributed data collection, analysis and manuscript writing for aging studies; G.C. and D.G. contributed to array studies and analysis; S.T. contributed to in vitro Aβ toxicity studies; B.E.S., G.M.S. and E.K. contributed to Aβ in vitro toxicity studies and breeding and behavior of APP mice; L.W. contributed stereology of APP mice; A.B. generated gene expression vectors; A.K. and M.V.C. contributed western blot analysis in aged rats; J.M.C. contributed ELISA and biochemical analyses; E.R. contributed surgery of APP mice; E.M. contributed synaptophysin quantification and surgery in APP mice; A.A.C. contributed surgery and behavioral analysis in aged rats and manuscript writing; M.H.T. contributed experimental design, surgery in rats and primates, data analysis and manuscript writing.
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Gomez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordower JH, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- 3.Price JL. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR, Kandel ER. Memory. New York: Scientific American Library; 2000. Chapter 5. [Google Scholar]
- 5.Yan Q, et al. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 1997;378:135–137. [PubMed] [Google Scholar]
- 6.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 7.Narisawa-Saito M, Wakabayashi K, Tsuji S, Takahashi H, Nawa H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport. 1996;7:2925–2928. doi: 10.1097/00001756-199611250-00024. [DOI] [PubMed] [Google Scholar]
- 8.Connor B, et al. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res. Mol. Brain Res. 1997;49:71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- 9.Hock C, Heese K, Hulette C, Rosenberg C, Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- 10.Mucke L, et al. High-level neuronal expression of aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palop JJ, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc. Natl. Acad. Sci. USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boddaert J, et al. Evidence of a role for lactadherin in Alzheimer’s disease. Am. J. Pathol. 2007;170:921–929. doi: 10.2353/ajpath.2007.060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy PH, et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum. Mol. Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 14.Scherzer CR, et al. Molecular markers of early Parkinson’s disease based on gene expression in blood. Proc. Natl. Acad. Sci. USA. 2007;104:955–960. doi: 10.1073/pnas.0610204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 16.Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J. Comp. Neurol. 2001;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- 17.Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III and V/VI of aged primates. J. Comp. Neurol. 2000;422:396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Rowe WB, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtzman DM, et al. TrkA expression in the CNS: evidence for the existence of several novel NGF-responsive CNS neurons. J. Neurosci. 1995;15:1567–1576. doi: 10.1523/JNEUROSCI.15-02-01567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson DA, Lucidi-Phillipi CA, Eagle KL, Gage FH. Perforant path damage results in progressive neuronal death and somal atrophy in layer II of entorhinal cortex and functional impairment with increasing postdamage age. J. Neurosci. 1994;14:6872–6885. doi: 10.1523/JNEUROSCI.14-11-06872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohn TT, Rissman RA, Head E, Cotman CW. Caspase activation in the Alzheimer’s disease brain: tortuous and torturous. Drug News Perspect. 2002;15:549–557. doi: 10.1358/dnp.2002.15.9.740233. [DOI] [PubMed] [Google Scholar]
- 22.Tomac A, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 23.Winkler C, Sauer H, Lee CS, Bjorklund A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 1996;16:7206–7215. doi: 10.1523/JNEUROSCI.16-22-07206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gash DM, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 25.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 26.Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol. Aging. 1997;18:549–553. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 27.Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Modeling a task that is sensitive to dementia of the Alzheimer’s type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav. Brain Res. 2004;149:123–133. doi: 10.1016/s0166-4328(03)00214-6. [DOI] [PubMed] [Google Scholar]
- 28.Gould RL, Brown RG, Owen AM, Fytche DH, Howard RJ. fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage. 2003;20:1006–1019. doi: 10.1016/S1053-8119(03)00365-3. [DOI] [PubMed] [Google Scholar]
- 29.Stoop R, Poo MM. Synaptic modulation by neurotrophic factors. Prog. Brain Res. 1996;109:359–364. doi: 10.1016/s0079-6123(08)62118-4. [DOI] [PubMed] [Google Scholar]
- 30.Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc. Natl. Acad. Sci. USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 32.Thoenen H, Sendtmer M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002;5:1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 33.Kordower JH, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann. Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 34.Gill SS, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 35.Tuszynski MH, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 36.Marks WJ, Jr, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 37.Kaplitt MG, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 38.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 39.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.