Abstract
The objective of this study was to detect statistically significant racial disparities in lung cancer mortality at the U.S. congressional district level. We applied absolute disparity statistics to mortality data from the National Center for Health Statistics (NCHS) for 1990–2001, mapped significant lung cancer mortality disparities by race and gender within U.S. congressional districts, and uncovered previously unreported disparities. The disparity statistics comparing black and white females revealed higher mortality rates for black females in the Midwestern U.S., and higher mortality rates for white females in the South-eastern U.S. Our methodology provides a spatial tool for guiding public health cancer control practices to monitor, target and reduce disparities.
Keywords: Cancer, Congressional districts, Disparities, Lung, Mortality, Race
1. Introduction
The elimination of health disparities defined by differences in health outcomes by gender and race is a major goal of Healthy People 2010 (USDHHS, 2000). Public health methods that identify systematic and avoidable health differences are essential to developing effective policy interventions. Public Law 106-525 (MHHDREA, 2000, p. 2498) defines a population impacted by health disparities as one with “significant disparity in the overall rate of disease incidence, prevalence, morbidity, mortality or survival rates in the population as compared to the health status of the general population.” Disparities may result from determinants of health associated with avoidable outcomes in vulnerable groups, such as the poor, or socially disadvantaged minorities (Braveman, 2006). By identifying subpopulations defined by statistically significant health disparities, public health officials can target policy interventions to reduce these disparities. This paper examines disparities in lung cancer mortality, the leading cause of cancer deaths in the U.S.
Racial disparities in lung cancer mortality rates are well established at the national level. During the period from 1998 to 2002, the age-adjusted lung cancer mortality rate for African-American men was 101.3 per 100,000 population, compared to 75.2 for white men and 38.7 for Hispanic–Latino men (Jemal et al., 2006). Moreover, this gap has persisted historically; from 1969 through 2004 lung cancer mortality rates for black males have consistently exceeded those of white males (SEER, 2007).
Previous research has documented variations in lung cancer mortality by race and geographic area within the U.S. using age-adjusted mortality rates. Devesa et al. (1999) found that, during the period from 1990 to 1994, lung cancer mortality rates were highest for white males in the south, and for white females, along the Pacific and Atlantic coasts; whereas, for black men and women, rates were highest in the northern U.S. Hao et al. (2006) developed a methodology to estimate cancer death rates by race/ethnicity at the congressional district level, and reported similar findings. These studies mapped geographic patterns of cancer death rates for each racial/ethnic group; however, the identification of racial disparities was subjective because health disparities were not statistically analyzed. Recently, Goovaerts and colleagues (2007) conducted a simulation study to identify appropriate rate difference and rate ratio statistics for detecting and mapping disparities in cancer mortality between racial/ethnic groups, and illustrated their application at the county-level. To address the “small number problem” that results in unreliable extreme rate estimates in small populations, i.e., minority populations (Waller and Gotway, 2004), population sizes were incorporated into the computation of these absolute and relative measures.
The choice of geographical unit for visualizing and analyzing data can be important for reasons of statistical validity (Goodchild and Quattrochi, 1997; Grubesic and Matisziw, 2006; Krieger et al., 2002; Meliker et al., 2001; Openshaw and Taylor, 1981) and for purposes of communicating findings (Bell et al., 2006; Eisen and Eisen, 2007). Further, the unit of geographical analysis can be politically important for addressing public health. Navarro and colleagues found that politics and policy are linked; that is, political ideologies of governing parties influence public health outcomes (Navarro et al., 2006). Thus, there is an opportunity to select a geo-political unit of analysis to represent constituent health. Congressional districts (CDs) are key; within CDs, voters elect representatives to the U.S. Congress to represent their needs and interests. Examining disparities in CDs may shed light on local factors responsible for disparities, and can provide a tool for guiding public health cancer control practices to reduce these disparities. The identification and effective communication of significant health disparities to voters and their elected officials is essential for the establishment and implementation of effective policy interventions.
To our knowledge, ours is the first study to quantify statistically significant racial disparities in lung cancer mortality in congressional districts. We built on American Cancer Society methodology to estimate cancer death rates by CD (Hao et al., 2006) and the methodology developed by Goovaerts et al. (2007) to detect statistically significant differences between rates. Both rate difference and rate ratio statistics were computed on CD-specific mortality rates and their significance was tested to highlight areas with significant racial disparities.
2. Methods
Compilation of mortality data at the U.S. congressional district (CD) level has previously been described in detail (Hao et al., 2006), and is summarized here. The majority of CDs intersect county boundaries; therefore CD death rates cannot be computed by simply aggregating CDs to counties. Further, counties are the smallest geographic areas for which mortality data are available from the National Center for Health Statistics. County mortality data were used to estimate block group mortality rates, which were then aggregated into CDs. Census block populations by sex and race were determined from the 2000 U.S. census. Age-adjusted death rates by sex and race were calculated for cancer of the lung and bronchus from 1990 to 2001 for each county using SEER*Stat, and estimated for census blocks. Death rates based upon less than 20 deaths were interpreted as unreliable and excluded (Hao et al., 2006). The direct, rather than indirect, age-adjustment method was used because it is the more statistically correct method for comparing rates (Pickle and White, 1995). The resulting direct age-adjusted death rates represent the rate each congressional district would have if it had the age–sex–race distribution of the U.S. in 2000. Race/ethnic groups were broken down into two mutually exclusive subgroups, non-Hispanic white and non-Hispanic black, as well as a third overlapping subgroup, Hispanic.
We first computed the absolute disparity statistic to quantify gender-specific racial/ethnic disparities in cancer mortality (Goovaerts et al., 2007). This statistic is the same as the two-sample test for proportions and, in this study, was assumed to follow a normal distribution, based upon interpretation of the number of deaths as a random variable with a binomial distribution (Goovaerts et al., 2007). Goovaerts et al. (2007) assessed the statistical performance of six test statistics, both those with and without multiple comparison corrections. The best results, i.e., fewest false positives without compromised power, were obtained for the absolute disparity statistic uncorrected for multiple testing, which is used in this study. In simulation analyses of this absolute disparity statistic, fewer than 5% false positives were observed, supporting the use of a 5% alpha level without adjustment for multiple testing (Goovaerts et al., 2007). The absolute disparity statistic subtracts the mortality rate of the reference population from that of the target population, and adjusts for the population-weighted average of mortality rates.
where R1 is the mortality rate of the target population, R2 is the mortality rate of the reference population, N1 is the target population, N2 is the reference population, and W is the population-weighted average of rates = [(N1) * (R1) + (N2) * (R2)]/(N1 + N2). Minorities comprised the target populations and were compared to white reference populations. This disparity analysis was conducted in every CD for all pairwise comparisons.
We also calculated relative disparity, defined as the rate for the target population divided by the rate for the reference population. The corresponding test statistic incorporates the population sizes; see Goovaerts et al. (2007) for more details. As with the absolute disparity statistic, statistical significance was calculated at the 5% level. Both types of statistics produced highly similar results; only the absolute disparity results will be presented and discussed in this paper.
3. Findings
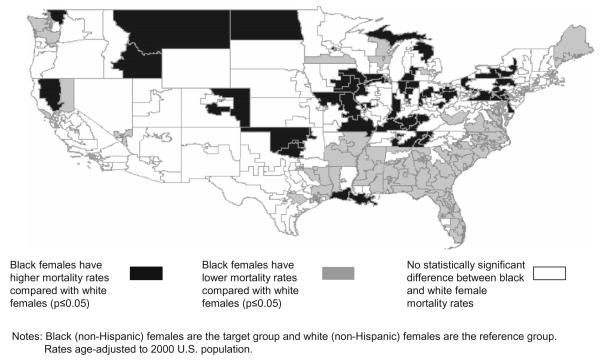
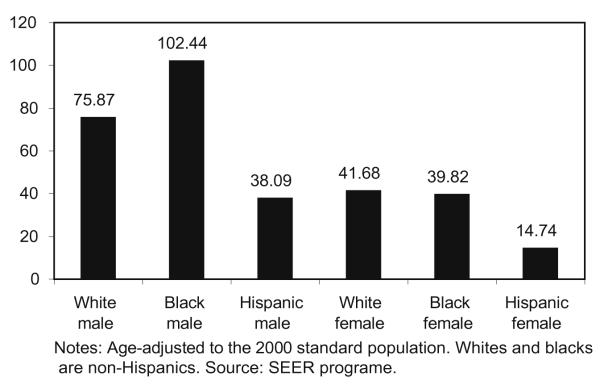
Key findings are summarized in Table 1; the distribution of significant (p ≤ 0.05) disparity statistics in CDs across the entire U.S. is summarized using medians and ranges, and regions of significance are highlighted. The greatest median disparity statistics were evident among black males relative to Hispanic males (disparity statistic = 8.39; range = 1.98, 24.72), and among Hispanic males relative to white males (disparity statistic = −7.41; range = −2.00, −19.50). The analysis of black females compared to white females revealed divergent disparity statistics for the Midwest (median disparity statistic = 3.27; range = 1.98, 65.77) and for the Southeast (median disparity statistic = −3.69; range = −1.96, −9.59), displayed in Fig. 1. Fifteen percent of CDs showed a significant positive absolute disparity, representing a statistically significant greater mortality rate for black women compared to white women; most notably in the Midwestern U.S. Twenty six percent of CDs showed a significant negative absolute disparity, representing a statistically significant lower mortality rate for black women compared to white women; most notably in the Southeastern U.S. These divergent findings represent a departure from the national data, which show relatively comparable lung cancer mortality rates between black and white females (Fig. 2).
Table 1.
Racial and ethnic disparities in lung cancer mortality in U.S. congressional districts: key findings using absolute disparity statisticsa, 1990–2001.
| Key findings | Mediana disparity statistic | Rangea of disparity statistics | Region of significance |
|---|---|---|---|
| Black females have higher lung cancer mortality rates compared with white females |
3.27 | 1.98, 65.77 | Midwest |
| Black females have lower lung cancer mortality rates compared with white females |
−3.69 | −1.96, −9.59 | Southeast |
| Black males have higher lung cancer mortality rates compared with white males |
5.36 | 1.99, 32.72 | Most of the U.S. |
| Hispanic females have lower lung cancer mortality rates compared with white females |
−6.46 | −2.03, −19.10 | Most of the U.S. |
| Hispanic males have lower lung cancer mortality rates compared with white males |
−7.41 | −2.00, −19.50 | Most of the U.S. |
| Black females have higher lung cancer mortality rates compared with Hispanic females |
5.22 | 2.01, 20.32 | Most of the U.S. |
| Black males have higher lung cancer mortality rates compared with Hispanic males |
8.39 | 1.98, 24.72 | Most of the U.S. |
Median and range are calculated to characterize the distribution of significant (α ≤ 0.05) disparity statistics in CDs across the U.S.
Fig. 1.
Racial disparities in lung cancer mortality rates: black females compared to white females, U.S. congressional districts, 1990–2001.
Fig. 2.
Age-adjusted total U.S. mortality rates for lung and bronchus cancer, all ages for 1995–2004 by race and sex.
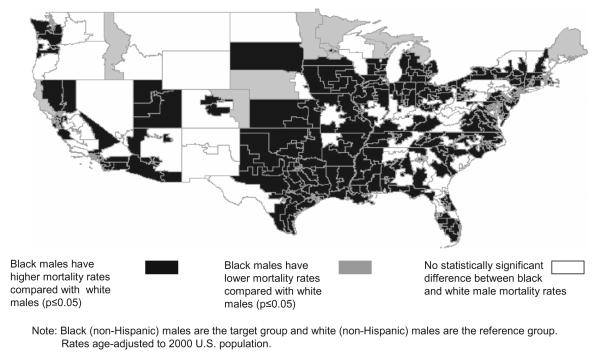
Mortality rate disparities between black and white men did not appear to diverge from national findings. Seventy three percent of CDs showed a significant positive absolute disparity, representing a statistically greater mortality rate for black men compared to white men (Fig. 3). Areas not found to be significant tended to have insufficient male African-American population to reach significance.
Fig. 3.
Racial disparities in lung cancer mortality rates: black males compared to white males, U.S. congressional districts, 1990–2001.
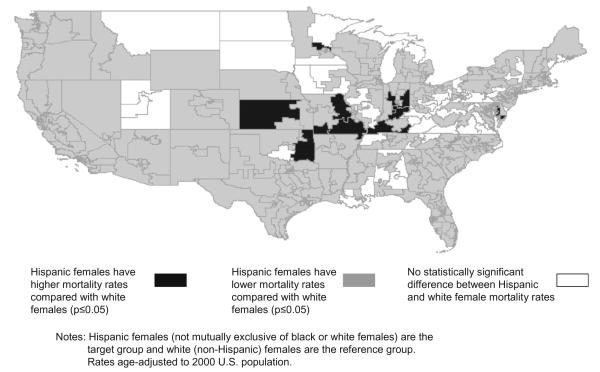
Our analyses of disparities for Hispanic populations were also largely consistent with national data on lung cancer mortality. Comparing Hispanic females to white females (Fig. 4), 87% of CDs show a significant negative absolute disparity, representing a statistically lower mortality rate for Hispanic women compared to white women. This disparity was evident across most regions of the country with sufficient Hispanic population. Comparing Hispanic males to white males, 86% of CDs showed a negative absolute disparity, representing a statistically lower mortality rate for Hispanic men compared to white men. This pattern held for comparisons between blacks and Hispanics, as well. Among black and Hispanic females, we found a significant positive absolute disparity in 82% of CDs, representing a statistically greater mortality rate for black women compared to Hispanic women. Consistent with the preceding findings, comparing black and Hispanic males, 88% of CDs showed a significant positive absolute disparity, representing a statistically greater mortality rate for black men compared to Hispanic men.
Fig. 4.
Racial Disparities in lung cancer mortality rates: Hispanic females compared to white females, U.S. congressional districts, 1990–2001.
4. Discussion
Our findings revealed previously unreported disparities within CDs, particularly a divergence from national lung cancer mortality data comparing black and white females such that, in the Midwestern U.S., black females have higher lung cancer mortality rates, but in the Southeastern U.S., white females have higher lung cancer mortality rates. These novel findings illustrate how the quantification and evaluation of the statistical significance of health disparities in local populations may be applied to congressional districts. Smoking prevalence may explain much of this disparity. In the U.S., smoking is directly responsible for about 90% of lung cancer deaths in men and almost 80% of lung cancer deaths in women (CDC, 2008). Lung cancer mortality rates during the most recent time period likely reflect historical patterns of smoking decades earlier. In the Southeastern U.S., from 1992 to 1993, the proportion of white females who smoked was 24.3% compared to 20.9% for black women (Shopland et al., 1996); this might explain the pattern of higher lung cancer mortality rates among white women compared to black women in southern CDs. In the Midwestern U.S., the percentage of black women who smoked was higher (26.5%) than that of white women (23.7%), and might explain the pattern of higher lung cancer mortality rates among black women compared to white women in Midwestern CDs. Smoking is the most preventable cause of premature death (CDC, 2006), and our analysis is expected to support the formulation of policy interventions designed to reduce smoking in specific sub-populations defined by race and gender within congressional districts.
Previous geographic analyses identified areas of high mortality within particular racial/ethnic/sex groupings. For example, among African-American women, highest lung cancer mortality rates were seen in the Midwest (Hao et al., 2006). Among white women, highest lung cancer mortality rates were in the west and coastal south (Hao et al., 2006). Hao and colleagues (2006) also reported higher mortality rates among white men in congressional districts in Appalachia and the south; and higher rates for black men in the Midwest and the south. These results are similar to our findings in some regions; however, our explicit consideration of disparities enables identification of differences between groups.
Smoking prevalence may also explain, in large part, why black males almost always have higher lung cancer mortality rates than other groups. Nationally, the highest smoking prevalence during 1992/1993 was reported by African-American males (31.3%), followed by white males (26.4%), then by Hispanic males (25%) (Shopland et al., 1996). Additionally, higher rates of lung cancer mortality among blacks may be attributed to socio-economic factors. Blacks are more likely to be uninsured than whites and to lose their health insurance following the diagnosis of cancer (Flenaugh and Henriques-Forsythe, 2006). Greenwald et al. (1998) reported lower lung cancer survival in the African-American population attributable to lower incomes. A limitation of this study is that we did not adjust for socio-economic status (SES). On the other hand, SES alone does not account for all health disparities by race. Krieger and colleagues (2003) related measures of economic deprivation to socio-economic gradients in health by race within small geographic areas, and reported the steepest gradients among the white population and shal-lowest among the black population; however, in areas of less economic deprivation, absolute rates were greater among black compared to white Americans (Krieger et al., 2003). The authors interpreted these findings to demonstrate why analyses of socio-economic disparities in health need to address racial disparities.
Flenaugh and Henriques-Forsythe (2006) present a review of the literature that discusses these determinants of lung cancer mortality discrepancies: treatment offered, accepted and available. Earle et al. (2002) reported that blacks were less likely to be referred to a cancer specialist for chemotherapy. McCann and colleagues (2005) found that African-Americans were offered surgical resection at a similar rate to whites (35%), but only 58% of blacks accepted treatment compared to 74% of whites. Differences in understanding the surgical risks, distrust of the health care system, or the belief that surgery leads to metastasis were cited as explanations by the researchers. Neighbors and colleagues (2007) found that blacks had lower odds of obtaining lung resection at a high-volume hospital compared to whites, and were more likely to die in the low-volume hospitals where they did receive care. The role of these different cultural factors, as well as access to and utilization of quality cancer care, needs to be explored to understand disparity patterns within CDs.
Racial disparities in smoking prevalence reported in the literature were also consistent with our findings regarding Hispanics, who had lower lung cancer mortality rates compared to blacks and whites. Hispanics had lower smoking prevalence than whites and blacks (Shopland et al., 1996). Lung cancer is the leading cause of cancer death among Hispanic men and the second leading cause of cancer death among Hispanic women. However, because of a traditionally lower rate of cigarette smoking among Hispanics, lung cancer mortality is about 50% lower than in non-Hispanic whites (O’Brien et al., 2003). For example, current smoking prevalence during 2002–2004 was about 20% among Hispanic men compared to 25% among non-Hispanic whites. The corresponding prevalence for Hispanic women and non-Hispanic white women were 10.6% and 22%, respectively (NCHS, 2006). It has been noted that a lack of sufficient data to calculate Hispanic mortality rates at the CD level across much of the country limits interpretation of regional variations (Hao et al., 2006). Additionally, Howe and colleagues (2006) suggest that anecdotal reports that Hispanics return to their country of birth after being diagnosed with cancer may underestimate the Hispanic mortality rate. This hypothesis has also been referred to as the “salmon-bias” effect; a metaphor for return migration to one’s birthplace to die (Abraido-Lanza et al., 1999). However, Palloni and Arias (2004) and Abraido-Lanza et al. (1999) found that this migratory hypothesis did not consistently explain the lower mortality among Latino subgroups of different national origin.
Our study’s limitations entail those associated with ecological studies, most significantly, the inability to generalize findings to individuals. Additionally, our study did not address determinants of health that may warrant closer scrutiny at the local level, including, socio-economic and health insurance status, variations in access to and utilization of quality cancer care, proximity to air pollution sources, cultural influences, race/ethnic subgroupings, population mobility, among others. Further, we found similar results using absolute and relative disparity statistics, but did not evaluate changes in mortality rates over time. Trend studies that report both absolute and relative disparity statistics may shed additional insights, particularly when divergent findings are revealed (Keppel et al., 2005).
In conclusion, application of the absolute disparity statistic to lung cancer mortality rates within congressional districts demonstrated how tests of statistical significance might be used to reveal previously unrecognized racial disparities at the local geo-political level. Specifically, within congressional districts located in the Southeastern U.S., white women showed higher lung cancer mortality compared to black women. On the other hand, within Midwestern congressional districts, black women showed higher lung cancer mortality rates compared to white women. These statistically significant differences represent departures from national lung cancer mortality data, and, among smokers, may be attributable to racial differences in smoking across geographic regions. Our statistical findings suggest that these variations are not random, but instead, are systematic differences resulting from health determinants associated with a largely preventable outcome: smoking-induced lung cancer. Small area geographic analyses of racial/ethnic disparities within CDs can help generate hypotheses to stem the tide of disparate health outcomes.
Acknowledgements
The research by the second author was funded by Grant R44-CA105819-02 from the National Cancer Institute. The views stated in this publication are those of the authors and do not necessarily represent the official views of the NCI.
References
- Abraido-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. Am J Public Health. 1999;89(10):1543–8. doi: 10.2105/ajph.89.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BS, Hoskins RE, Pickle LW, Wartenberg D. Current practices in spatial analysis of cancer data: mapping health statistics to inform policymakers and the public. Int J Health Geogr. 2006;5:49. doi: 10.1186/1476-072X-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health. 2006;27:167–94. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) [[accessed 23.03.08]];Smoking & tobacco use: fact sheet: cigarette smoking-related mortality. 2006 Available from: http://www.cdc.gov/tobacco/data_statistics/Factsheets/cig_smoking_ mort.htm.
- CDC (Centers for Disease Control and Prevention) [[accessed 23.03.08]];Smoking & tobacco use: fact sheet: health effects of cigarette smoking. 2008 Available from: http://www.cdc.gov/tabacco/data_statistics/Factsheets/health_effects.htm.
- Devesa SS, Grauman DJ, Blot WJ, Fraumeni JF. Cancer surveillance series: changing geographic patterns of lung cancer mortality in the United States, 1950 through 1994. J Natl Cancer Inst. 1999;91(12):1040–50. doi: 10.1093/jnci/91.12.1040. [DOI] [PubMed] [Google Scholar]
- Earle CC, Neumann PJ, Gelber RD, et al. Impact of referral patterns on the use of chemotherapy for lung cancer. J Clin Oncol. 2002;20(7):1786–92. doi: 10.1200/JCO.2002.07.142. [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ. Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerg Inf Dis. 2007;13(12):1816–20. doi: 10.3201/eid1312.070211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flenaugh EL, Henriques-Forsythe MN. Lung cancer disparities in African Americans: health vs. health care. Clin Chest Med. 2006;27:431–9. doi: 10.1016/j.ccm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Goodchild MF, Quattrochi DA. Scale, multiscaling, remote sensing, and GIS. In: Quattrochi DA, Goodchild MF, editors. Scale in remote sensing and GIS. CRC Press LLC; Boca Raton, FL: 1997. pp. 1–11. [Google Scholar]
- Goovaerts P, Meliker JR, Jacquez GM. A comparative analysis of aspatial statistics for detecting racial disparities in cancer mortality rates. Int J Health Geogr. 2007;6:32. doi: 10.1186/1476-072X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald HP, Polissar NL, Borgatta EF, McCorkle R, Goodman G. Social factors, treatment, and survival in early-state non-small cell lung cancer. Am J Public Health. 1998;88:1681–4. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubesic TH, Matisziw TC. On the use of ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int J Health Geogr. 2006;5:58. doi: 10.1186/1476-072X-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Ward EM, Jemal A, Pickle LW, Thun MJ. U.S. congressional district cancer death rates. Int J Health Geogr. 2006;5:28. doi: 10.1186/1476-072X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–42. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Keppel K, Pamuk E, Lynch J, et al. Methodological issues in measuring health disparities. National Center for Health Statistics. Vital Health Stat. 2005;2(141) [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures – the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93(10):1655–71. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Soobader MJ, Subraminian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–82. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- McCann J, Artinian V, Duhaime L, Lewis JW, Kvale PA, DiGiovine B. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–6. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- Meliker JR, Nriagu JO, Hammad AS, Savoie KL, Jamil H, Devries JM. Spatial clustering of emergency department visits by asthmatic children in an urban area: south-western Detroit, Michigan. Ambul Child Health. 2001;7:297–312. [Google Scholar]
- MHHDREA (Minority Health and Health Disparities Research and Education Act) United States Public Law 106-525. 2000:2498. [Google Scholar]
- NCHS (National Center for Health Statistics) Health, United States, 2006, with Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2006. Available from: http://www.cdc.gov/nchs/data/hus/hus06.pdf. [04.09.08] [PubMed] [Google Scholar]
- Navarro V, Muntaner C, Borrell C, Benach J, Quiroga A, Rodriguez-Sanz M, et al. Politics and health outcomes. Lancet. 2006;368(9540):1033–7. doi: 10.1016/S0140-6736(06)69341-0. [DOI] [PubMed] [Google Scholar]
- Neighbors CJ, Rogers ML, Shenassa ED, Sciamanna CN, Clark MA, Novak SP. Ethnic/racial disparities in hospital procedure volume for lung resection for lung cancer. Med Care. 2007;45(7):655–63. doi: 10.1097/MLR.0b013e3180326110. [DOI] [PubMed] [Google Scholar]
- O’Brien K, Cokkinides V, Jemal A, Cardinez CJ, Murray T, Samuels A, et al. Cancer statistics for Hispanics, 2003. CA Cancer J Clin. 2003;53(5):314. doi: 10.3322/canjclin.53.4.208. [DOI] [PubMed] [Google Scholar]
- Openshaw S, Taylor PJ. The modifiable areal unit problem. In: Wrigley N, Bennett R, editors. Quantitative geography: a British view. Routledge and Kegan Paul; London: 1981. pp. 60–9. [Google Scholar]
- Palloni A, Arias E. Paradox lost: explaining the Hispanic adult mortality advantage. Demography. 2004;41(3):385–415. doi: 10.1353/dem.2004.0024. [DOI] [PubMed] [Google Scholar]
- Pickle LW, White AA. Effects of the choice of age-adjustment method on maps of death rates. Stat Med I. 1995;4(5–7):615–27. doi: 10.1002/sim.4780140519. [cited by Hao et al. 2006] [DOI] [PubMed] [Google Scholar]
- Shopland DR, Hartman AM, Gibson JT, Mueller MD, Kessler LG, Lynn WR. Cigarette smoking among US adults by state and region: estimates from the current population survey. J Natl Cancer Inst. 1996;88(23):1748–58. doi: 10.1093/jnci/88.23.1748. [DOI] [PubMed] [Google Scholar]
- SEER (Surveillance, Epidemiology, and End Results) Program . SEER*Stat Database: Mortality – all COD, Public-Use With State, Total U.S. (1969–2004) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [released April 2007]. 2007. Available from: www.seer.cancer.gov. Underlying mortality data provided by NCHS www.cdc.gov/nchs. [Google Scholar]
- USDHHS (U.S. Department of Health and Human Services) U.S. Government Printing Office; Washington, DC: Healthy people 2010: understanding and improving health. (2nd ed) 2000 November; Available from: http://www.healthypeople.gov/About/ 2000 [11.09.08]
- Waller LA, Gotway CA. Applied spatial statistics for public health data. John Wiley and Sons; New Jersey: 2004. [Google Scholar]