Abstract
We previously reported the activation profiles of the human kallikrein-related peptidases (KLKs) as determined from a KLK pro-peptide fusion-protein system. That report described the activity profiles of 12 of the 15 mature KLKs versus the 15 different pro-KLK sequences. The missing profiles in the prior report, involving KLK9, 10, and 15, are now described. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, mass spectrometry, and N-terminal sequence analyses show that KLK9 and 10 exhibit low hydrolytic activities towards all of the 15 pro-KLK sequences, while KLK15 exhibits significant activity towards both Arg- and Lys-containing KLK prosequences. The ability of KLK15 to activate pro-KLK8, 12, and 14 is confirmed using recombinant pro-KLK proteins, and shown to be significant for activation of pro-KLK8 and 14, but not 12. These additional data for KLK9, 10, and 15 now permit a completed KLK activome profile, using a KLK pro-peptide fusion-protein system, to be described. The results suggest that KLK15, once activated, can potentially feed back into additional pro-KLK activation pathways. Conversely, KLK9 and 10, once activated, are unlikely to participate in further pro-KLK activation pathways, although similar to KLK1 they may activate other bioactive peptides.
Keywords: activation cascade, activome, kallikrein, kallikrein-related peptidases (KLKs), protease
The 15 KLK (Lundwall et al., 2006) members (KLK1–15), comprising the ‘classic’ (KLK1–3) and ‘neo’ KLKs (KLK4–15), represent the largest cluster of S1 (or chymotrypsin-homologous) serine proteases within the human genome (Yousef and Diamandis, 2001). Considerable interest has been generated in the KLKs due to their potential use as disease biomarkers; particularly in relation to prostate (Stamey et al., 1987; Luderer et al., 1995; Catalona et al., 1997; Darson et al., 1999; Recker et al., 2000; Fuessel et al., 2003) and other types of cancer (Clements, 1989; Diamandis et al., 2000; Diamandis and Yousef, 2001; Clements et al., 2004; Pampalakis and Sotiropoulou, 2007; Ramsay et al., 2008; Singh et al., 2008). KLKs have been shown to participate in the regulation of skin desquamation (Lundstrom and Egelrud, 1991; Brattsand and Egelrud, 1999; Brattsand et al., 2005), inflammatory demyelination (Blaber et al., 2004; Scarisbrick et al., 2006), neurodegeneration (Scarisbrick et al., 2008), and semen liquefaction (Kumar et al., 1997; Vaisanen et al., 1999; Malm et al., 2000; Michael et al., 2006). These activities highlight the emerging importance of KLKs in the diagnosis and treatment of serious human diseases.
KLKs are secreted as inactive pro-forms, and a key regulatory step is their extracellular activation via proteolytic removal of an amino-terminal pro-peptide. Known regulatory cascades of other protease families suggests that the KLK family might similarly interact in activation cascades that regulate their function (Lovgren et al., 1997; Takayama et al., 1997; Sotiropoulou et al., 2003; Brattsand et al., 2005; Michael et al., 2006). For example, Lovgren and coworkers showed that KLK2 is able to cleave the pro-form of KLK3 to yield mature active KLK3 (Lovgren et al., 1997) which promotes semen liquefaction. A more extensive activation cascade involving KLKs 5, 7, and 14 has been elucidated in skin desquamation (Lundstrom and Egelrud, 1991; Yousef et al., 2003; Caubet et al., 2004; Brattsand et al., 2005; Michael et al., 2005). Recent studies have expanded considerably the number of relevant KLK pair-wise activation relationships predicted from the hydrolytic profiling of pro-KLK peptide-fusion proteins (Yoon et al., 2007) or soluble pro-KLK peptides (Emami and Diamandis, 2008). Such studies in combination with knowledge of tissue-specific expression of the KLKs permits hypotheses to be developed and tested regarding physiologically relevant KLK activation cascades (Yoon et al., 2007; Emami and Diamandis, 2008).
We previously reported the activation profiles of the human kallikrein-related peptidases (KLKs) as determined from a KLK pro-peptide fusion-protein system (Yoon et al., 2007). That report described the activity pro374 files of 12 of the 15 mature KLKs versus the 15 different pro-KLK sequences; the missing profiles in the prior report, involving KLK9, 10, and 15, are now presented. These additional data for KLK9, 10, and 15 permit a completed KLK activome profile, characterized using a KLK pro-peptide fusion-protein system, to be described. Under the conditions evaluated (100:1 molar ratio pro- KLK fusion protein:mature KLK, pH 6.0 or 7.4, and for either 1 or 24 h incubations at 37°C) KLK9 and KLK10 exhibited minimal specificity towards hydrolysis of the 15 pro-KLK fusion proteins; however, KLK15 exhibited the ability to hydrolyze several pro-KLK sequences (Figure 1). A summary of the hydrolysis densitometry data for KLK9, 10, and 15 hydrolyses of the 15 different pro-KLK fusion proteins is given in Table 1 and a complete listing of hydrolysis, mass spectrometry, and N-terminal sequencing data are provided as supplementary data.
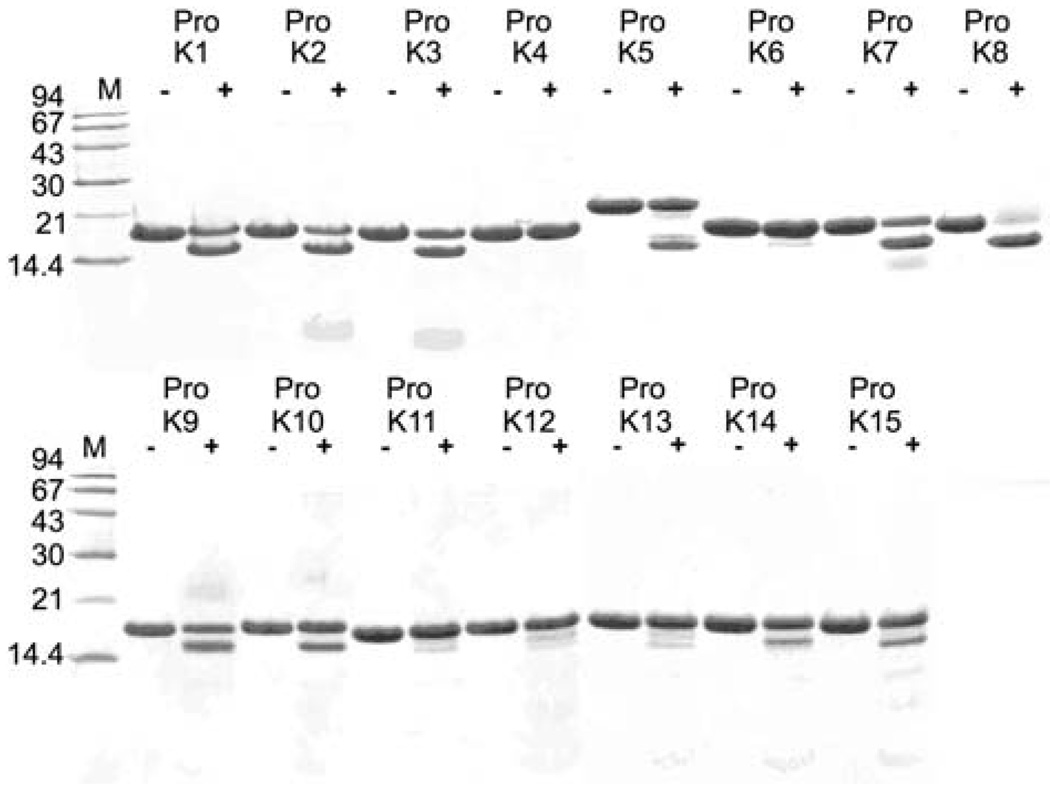
Figure 1. Gel electrophoretic analysis of pro-KLK1–15 fusion protein cleavage.
Coomassie Brilliant Blue-stained 16.5% Tricine sodium dodecyl sulfate-polyacrylamide gel after separation of the products of 100:1 molar ratio incubation of pro-KLK1–15 fusion proteins (abbreviated as ‘Pro K’; final concentration of 40 µm) with KLK15 for 24 h at pH 7.4.
Table 1.
Percent hydrolysis of pro-KLK(1–15) fusion proteins for 100:1 molar ratio incubation with mature KLK9, 10, and 15 at pH 6.0/7.4 for 1 h and 24 h at 37°C.
| P1 | KLK9 | KLK10 | KLK15 | ||||
|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | 1 h | 24 h | ||
| pro-KLK1 | Arg | – | – | – | 0/5 | 1/9 | 34/62 |
| pro-KLK2 | Arg | – | 5/13 | – | 1/9 | 1/11 | 34/72 |
| pro-KLK3 | Arg | – | 4/8 | – | 8/3 | 0/8 | 32/77 |
| pro-KLK4 | Gln | – | – | – | – | – | – |
| pro-KLK5 | Arg | – | – | – | – | 0/4 | 16/47 |
| pro-KLK6 | Lys | – | – | – | – | – | – |
| pro-KLK7 | Lys | – | – | – | 3/14 | 3/10 | 39/77 |
| pro-KLK8 | Lys | – | – | – | 2/11 | 3/16 | 43/86 |
| pro-KLK9 | Arg | – | –/14 | – | 0/7 | 3/10 | 27/64 |
| pro-KLK10 | Arg | – | – | – | 0/6 | 0/0 | 3/24 |
| pro-KLK11 | Arg | – | –/20 | – | – | 0/4 | 18/71 |
| pro-KLK12 | Lys | – | – | – | – | – | 1/12 |
| pro-KLK13 | Lys | – | –/21 | – | – | 0/2 | 10/30 |
| pro-KLK14 | Lys | – | – | – | 0/7 | 0/5 | 14/54 |
| pro-KLK15 | Lys | – | – | – | 2/10 | 6/16 | 46/91 |
Mature KLK10 and KLK15 proteins were expressed from an Escherichia coli host, refolded and purified as previously described (Debela et al., 2006). Mature KLK9 was expressed as recombinant protein from HEK293 human embryonic kidney epithelial cells as the expression host, as previously described for pro-KLK6 (Yoon et al., 2008). The pro-KLK9 was activated to mature KLK9 by incubation with enterokinase, as described previously for activation of pro-KLK6 (Blaber et al., 2007). Assay conditions utilized 40 mm of pro-KLK fusion proteins as previously described (Yoon et al., 2007).
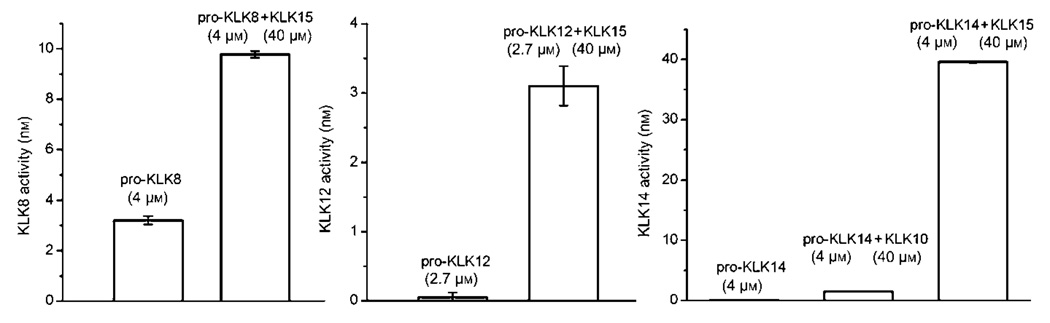
The hydrolysis results for pro-KLK fusion proteins indicate that after 24 h incubation at pH 7.4 with KLK15, greater than 50% hydrolysis was achieved against pro- KLK1–3, 7–9, 11, and 14–15; a significant sub-set of the pro-KLKs. No hydrolysis was observed for pro-KLK4 and 6 fusion proteins, and hydrolysis of pro-KLK12 resulted in only 12% hydrolysis under these conditions (Table 1). Based upon an available set of expressed and purified recombinant pro-KLK proteins, the ability of KLK15 to activate pro-KLK8, pro-KLK12, and pro-KLK14 was evaluated; the ability of KLK10 to activate pro-KLK14 was also evaluated. A summary of these activation assays is shown in Figure 2. The results confirm that KLK15 is capable of activating pro-KLK8, pro-KLK12, and pro-KLK14, and with a relative ranking of activation efficiency being pro-KLK14>pro-KLK8>pro-KLK12. Weak activation of pro-KLK12 by KLK15 was predicted from hydrolysis of the pro-KLK fusion protein data, and with the actual pro-KLK12 protein evidence of activation by KLK15 required extended (24 h) incubation (Figure 2). Although the pro-KLK8 fusion protein was hydrolyzed by KLK15 with greater apparent efficiency than the pro-KLK14 fusion protein, this activity is reversed in the hydrolysis of the actual pro-KLK proteins (Figure 2). This result suggests the potential for an exosite interaction between KLK15 and pro-KLK14 that is not present in the pro-KLK fusion protein. The results also indicate that KLK10 exhibits minimally detectable hydrolytic activity versus pro-KLK14.
Figure 2. Activation assay results.
Raw data for the activation assay of pro-KLK8 (4.0 µm, 1 h; left panel) with KLK15, pro-KLK12 (2.7 µm, 24 h; center panel) with KLK15 and pro-KLK14 (4.0 µm, 1 h; right panel) with KLK10 and KLK15 are given. Assay of pro-KLK12 at 2.7 µm was due to solubility concerns. Measured KLK activity has been normalized to account for enzyme and substrate controls as previously described (Yoon et al., 2007, 2008).
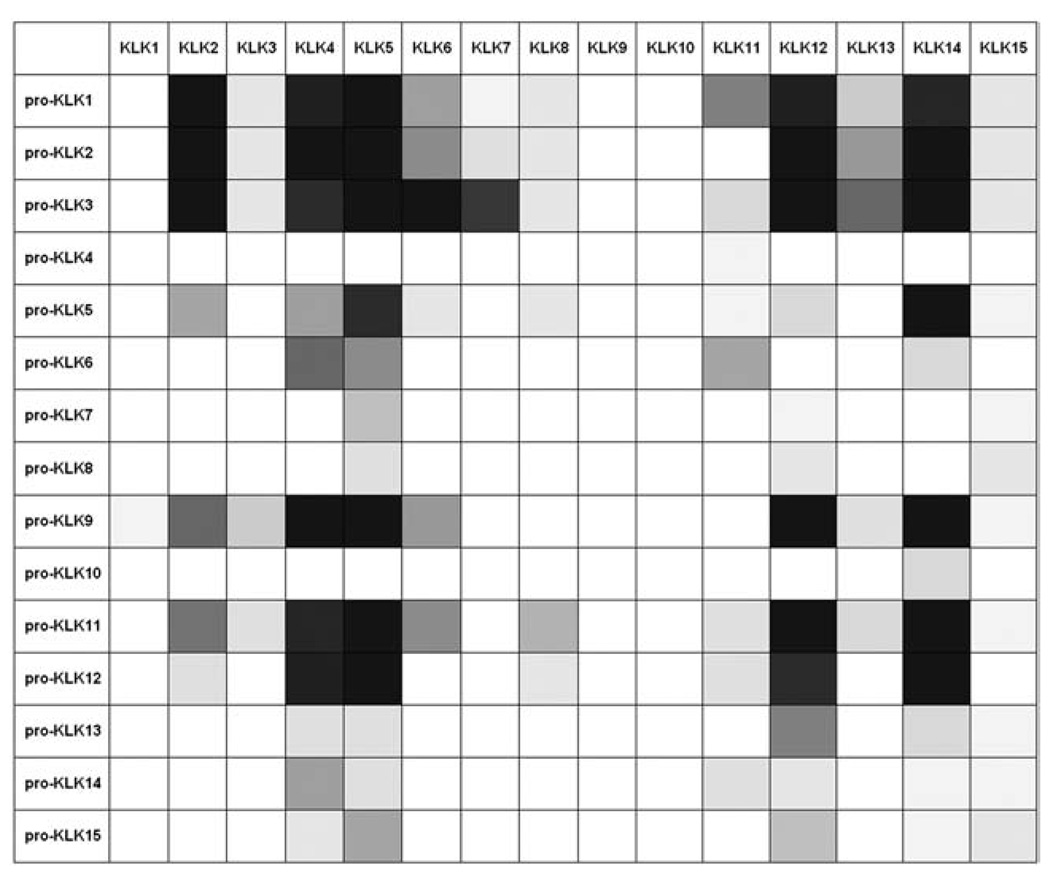
Combining the results presented here with data from our prior study allows a completed ‘activome’ description of relative hydrolysis rates for the KLK family (Figure 3). This now completed view groups KLK9 and KLK10 with KLK1, in that these KLKs once activated, appear unlikely to feed back into any further KLK activation cascade. As with KLK1, which hydrolyzes the bioactive kinins, KLK9 and 10 may recognize separate bioactive peptide substrates distinct from any pro-KLK family member. In this regard, KLK9 contains an atypical Gly residue at position 189 (Yousef and Diamandis, 2000), located in the base of the substrate S1 pocket. This residue is characteristically Asp in proteases with trypsin-like specificity, including most of the KLKs, and provides an electrostatic selectivity for the basic residues Arg or Lys in the substrate P1 position (present in the majority of KLK prosequences). Thus, Gly189 is likely responsible for the inability of KLK9 to hydrolyze the pro-KLK sequences; the specificity of KLK9 remains undetermined, but does not include the KLK pro-sequences.
Figure 3. A completed KLK activome.
Summary of pro-KLK fusion protein hydrolysis [pH 7.4 for 1 h at 37°C; represented in gray scale (whites0%, blacks100% hydrolysis)] by KLK9, 10, and 15 and combined with previous results for similar hydrolyses by the remaining 15 KLKs (Yoon et al., 2007) yielding a completed KLK ‘activome’ analysis.
KLK10 is unique among the KLK family in that it contains a Ser residue at position 193, which is present as a characteristically conserved Gly residue in serine proteases (Liu et al., 1996). The main chain amide of Gly193 participates in stabilization of the oxyanion intermediate in the hydrolytic reaction of peptide substrates. Schmidt and coworkers studied the catalytic effects of a Glu mutation at position 193 in coagulation factor XI, identified from a genetically inherited deficiency in factor XI activity (Schmidt et al., 2004). Their results showed that substitution of Gly193 by Glu reduced kcat by several orders of magnitude, and likely distorted the critical stereochemistry of the oxyanion binding pocket. Ser193 in KLK10 may similarly distort the oxyanion binding pocket, resulting in significant reduction in overall catalytic activity. Thus, KLK10 may therefore be an essentially inactive protease, or may have an as-yet unidentified substrate.
KLK15 is unique among the KLKs in having a Glu residue at position 189 in the base of the substrate S1 pocket (Yousef et al., 2001). Evnin and coworkers have reported that Arg/Lys selectivity in trypsin can be shifted 35-fold in favor of Lys by an Asp→Glu mutation at position 189 (Evnin et al., 1990). KLK15 exhibits the highest efficiency of hydrolysis against its own pro-peptide sequence (and is therefore predicted to be subject to autocatalytic activation), followed by KLK8 and KLK7 (Table 1). These KLK pro-peptides all contain a Lys in the P1 position, and the results indicate that the Glu at position 189 influences the Arg/Lys preference in favor of recognition of Lys, as predicted by the results of Evnin and coworkers (although KLK15 retains the capability of activating Arg-containing pro-KLK sequences). Thus, KLK15 is hypothesized to participate in activation cascades involving other members of the KLK family, particularly those with Lys-containing pro-peptides, with the notable exception of pro-KLK6, and weak activation potential against pro-KLK12. The ‘P’ and ‘P-prime’ residue positions of the pro-KLKs play a key role in their recognition and hydrolysis and are uniquely different. In this regard, pro-KLK6 is distinct in containing a His residue in the P3′ position; similarly, pro-KLK12 contains a unique sequence in the P4-P2 positions. The stereochemical complementarities of these positions in the corresponding KLK15 ‘S’ and ‘S-prime’ sites appear energetically unfavorable for recognition and hydrolysis. KLK15 is robustly expressed in the thyroid, but is also detectable in the prostate, salivary glands, adrenal glands, colon, testis, and kidney (Yousef et al., 2001). Other members of the KLK family expressed in thyroid include KLK2–5, 7, 11–14 (Yousef and Diamandis, 2001); of these, pro-KLK7 and pro-KLK12–15 contain a Lys at the activation P1 position, and (with the exception of KLK12) are hydrolyzed most efficiently by KLK15 (Table 1). The presence of KLK4, 5, and 12 in thyroid provide pathways to activate pro-KLK15 (in addition to the autolytic activation potential of KLK15). Thus, activating cascades involving these members of the KLK family appears likely in thyroid, as well as other tissues, including prostate.
Acknowledgments
This work was supported by NIH grant 1R15NS057771-01 (M.B.), and National Multiple Sclerosis Society grants PP1113 (M.B.) and RG3367 (I.A.S).
References
- Blaber SI, Ciric B, Christophi GP, Bernett M, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6 proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;18:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Yoon H, Scarisbrick IA, Juliano MA, Blaber M. The autolytic regulation of human kallikrein-related peptidase 6. Biochemistry. 2007;46:5209–5217. doi: 10.1021/bi6025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J. Biol. Chem. 1999;274:30033–30040. doi: 10.1074/jbc.274.42.30033. [DOI] [PubMed] [Google Scholar]
- Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J. Invest. Dermatol. 2005;124:198–203. doi: 10.1111/j.0022-202X.2004.23547.x. [DOI] [PubMed] [Google Scholar]
- Catalona WJ, Besser JA, Smith DS. Serum free prostate specific antigen and prostate specific antigen measurements for predicting cancer in men with prior negative prostatic biopsies. J. Urol. 1997;158:2162–2167. doi: 10.1016/s0022-5347(01)68187-4. [DOI] [PubMed] [Google Scholar]
- Caubet C, Jonca N, Brattsand M, Guerrin M, Bernard D, Schmidt R, Egelrud T, Simon M, Serre G. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- Clements JA. The glandular kallikrein family of enzymes: tissue-specific expression and hormonal regulation. Endocr. Rev. 1989;10:393–419. doi: 10.1210/edrv-10-4-393. [DOI] [PubMed] [Google Scholar]
- Clements JA, Willemsen NM, Myers SA, Dong Y. The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit. Rev. Clin. Lab. Sci. 2004;41:265–312. doi: 10.1080/10408360490471931. [DOI] [PubMed] [Google Scholar]
- Darson MF, Pacelli A, Roche P, Rittenhouse HG, Wolfert RL, Saeid MS, Young CY, Klee GG, Tindall DJ, Bostwick DG. Human glandular kallikrein 2 expression in prostate adenocarcinoma and lymph node metastases. Urology. 1999;53:939–944. doi: 10.1016/s0090-4295(98)00637-2. [DOI] [PubMed] [Google Scholar]
- Debela M, Magdolen V, Schechter N, Valachova M, Lottspeich F, Craik CS, Choe Y, Bode W, Goettig P. Specificity profiling of seven human tissue kallikreins reveals individual subsite preferences. J. Biol. Chem. 2006;281:25678–25688. doi: 10.1074/jbc.M602372200. [DOI] [PubMed] [Google Scholar]
- Diamandis EP, Yousef GM. Human tissue kallikrein gene family: a rich source of novel disease biomarkers. Expert Rev. Mol. Diagn. 2001;1:182–190. doi: 10.1586/14737159.1.2.182. [DOI] [PubMed] [Google Scholar]
- Diamandis EP, Yousef GM, Luo LY, Magklara A, Obiezu CV. The new human kallikrein gene family: implications in carcinogenesis. Trends Endocrinol. Metab. 2000;11:54–60. doi: 10.1016/s1043-2760(99)00225-8. [DOI] [PubMed] [Google Scholar]
- Emami N, Diamandis EP. Human kallikrein-related peptidase 14 (KLK14) is a new activator component of the KLK proteolytic cascade. J. Biol. Chem. 2008;283:3031–3041. doi: 10.1074/jbc.M707253200. [DOI] [PubMed] [Google Scholar]
- Evnin LB, Vasquez JR, Craik CS. Substrate specificity of trypsin investigated by using a genetic selection. Proc. Natl. Acad. Sci. USA. 1990;87:6659–6663. doi: 10.1073/pnas.87.17.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuessel S, Sickert D, Meye A, Klenk U, Schmidt U, Schmitz M, Rost AK, Eweigle B, Kiessling A, Wirth MP. Multiple tumor marker analyses (PSA, HK2, PSCA, tryp-p8) in primary prostate cancers using quantitative RT-PCR. Int. J. Oncol. 2003;23:221–228. [PubMed] [Google Scholar]
- Kumar A, Mikolajczyk SD, Goel AS, Millar LS, Saedi MS. Expression of pro-form of prostate-specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res. 1997;57:3111–3114. [PubMed] [Google Scholar]
- Liu X-L, Wazer DE, Watanabe K, Band V. Identification of a novel serine protease-like gene, the expression of which is down-regulated during breast cancer progression. Cancer Res. 1996;56:3371–3379. [PubMed] [Google Scholar]
- Lovgren J, Rajakoski K, Karp M, Lundwall A, Lilja H. Activation of the zymogen form of prostate-specific antigen by human glandular kallikrein 2. Biochem. Biophys. Res. Commun. 1997;238:549–555. doi: 10.1006/bbrc.1997.7333. [DOI] [PubMed] [Google Scholar]
- Luderer A, Chen Y-T, Soraino TF, Kramp WJ, Carlson G, Cuny C, Sharp T, Smith W, Petteway J, Brawer KM, Thiel R. Measurement of the proportion of free to total prostate specific antigen improves diagnostic performance of prostate-specific antigen in the diagnostic gray zone of the total prostate specific antigen. Urology. 1995;46:187–194. doi: 10.1016/s0090-4295(99)80192-7. [DOI] [PubMed] [Google Scholar]
- Lundstrom A, Egelrud T. Stratum corneum chymotryptic enzyme: a proteinase which may be generally present in the stratum corneum and with a possible involvement in desquamation. Acta Derm. Venereol. 1991;71:471–474. [PubMed] [Google Scholar]
- Lundwall A, Band V, Blaber M, Clements JA, Courty Y, Diamandis EP, Fritz H, Lilja H, Malm J, Maltais LJ, Olsson AY, et al. A comprehensive nomenclature for serine proteases with homology to tissue kallikreins. Biol. Chem. 2006;387:637–641. doi: 10.1515/BC.2006.082. [DOI] [PubMed] [Google Scholar]
- Malm J, Hellman J, Hogg P, Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): substrate specificity and regulation by Zn2+, a tight-binding inhibitor. Prostate. 2000;45:132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Michael IP, Sotiropoulou G, Pampalakis G, Magklara A, Ghosh M, Wasney GA, Diamandis EP. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J. Biol. Chem. 2005;280:14628–14635. doi: 10.1074/jbc.M408132200. [DOI] [PubMed] [Google Scholar]
- Michael IP, Pampalakis G, Mikolajczyk SD, Malm J, Sotiropoulou G, Diamandis EP. Human tissue kallikrein 5 (hK5) is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J. Biol. Chem. 2006;281:12743–12750. doi: 10.1074/jbc.M600326200. [DOI] [PubMed] [Google Scholar]
- Pampalakis G, Sotiropoulou G. Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim. Biophys. Acta. 2007;1776:22–31. doi: 10.1016/j.bbcan.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ, Dong Y, Hunt ML, Linn M, Samaratunga H, Clements JA, Hooper JD. Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are coexpressed during prostate cancer progression. J. Biol. Chem. 2008;283:12293–12304. doi: 10.1074/jbc.M709493200. [DOI] [PubMed] [Google Scholar]
- Recker F, Kwiatkowski MK, Piironen T, Pettersson K, Huber A, Lummen G, Tscholl R. Human glandular kallikrein as a tool to improve discrimination of poorly A completed KLK activome differentiated and non-organ-confined prostate cancer compared with prostate-specific antigen. Urology. 2000;55:481–485. doi: 10.1016/s0090-4295(99)00611-1. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Blaber SI, Tingling JT, Rodriguez M, Blaber M, Christophi GP. Potential scope of action of tissue kallikreins in CNS immune-mediated disease. J. Neuroimmunol. 2006;178:167–176. doi: 10.1016/j.jneuroim.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Linbo R, Vandell AG, Keegan M, Blaber SI, Blaber M, Sneve D, Lucchinetti CF, Rodriguez M, Diamandis EP. Tissue kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biol. Chem. 2008;389:739–745. doi: 10.1515/BC.2008.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AE, Ogawa T, Gailani D, Bajaj SP. Structural role of Gly193 in serine proteases. J. Biol. Chem. 2004;279:29485–29492. doi: 10.1074/jbc.M402971200. [DOI] [PubMed] [Google Scholar]
- Singh J, Naran A, Misso NL, Rigby PJ, Thompson PJ, Bhoola KD. Expression of kallikrein-related peptidases (KRP/hK5, 7, 6, 8) in subtypes of human lung carcinoma. Int. Immunopharmacol. 2008;8:300–306. doi: 10.1016/j.intimp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G, Rogakos V, Tsetsenis T, Pampalakis G, Zafiropoulos N, Simillides G, Yiotakis A, Diamandis EP. Emerging interest in the kallikrein gene family for understanding and diagnosing cancer. Oncol. Res. 2003;13:381–391. doi: 10.3727/096504003108748393. [DOI] [PubMed] [Google Scholar]
- Stamey TA, Yang N, Hay AR, McNal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987;15:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- Takayama TK, Fujikawa K, Davie EW. Characterization of the precursor of prostate-specific antigen. Activation by trypsin and by human glandular kallikrein. J. Biol. Chem. 1997;272:21582–21588. doi: 10.1074/jbc.272.34.21582. [DOI] [PubMed] [Google Scholar]
- Vaisanen V, Lovgren J, Hellman J, Piironen T, Lilja H, Pettersson K. Characterization and processing of prostate specific antigen (hK3) and human glandular kallikrein (hK2) secreted by LNCaP cells. Prostate Cancer Prostatic Dis. 1999;2:91–97. doi: 10.1038/sj.pcan.4500289. [DOI] [PubMed] [Google Scholar]
- Yoon H, Laxmikanthan G, Lee J, Blaber SI, Rodriguez A, Kogot JM, Scarisbrick IA, Blaber M. Activation profiles and regulatory cascades of the human kallikrein-related proteases. J. Biol. Chem. 2007;282:31852–31864. doi: 10.1074/jbc.M705190200. [DOI] [PubMed] [Google Scholar]
- Yoon H, Blaber SI, Evans DM, Trim J, Juliano MA, Scarisbrick IA, Blaber M. Activation profiles of human kallikrein-related peptidases by proteases of the thrombostasis axis. Protein Sci. 2008;17:1998–2007. doi: 10.1110/ps.036715.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef G, Diamandis EP. The expanded human kallikrein gene family: locus characterization and molecular cloning of a new member, KLK-L3 (KLK9) Genomics. 2000;65:184–194. doi: 10.1006/geno.2000.6159. [DOI] [PubMed] [Google Scholar]
- Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr. Rev. 2001;22:184–204. doi: 10.1210/edrv.22.2.0424. [DOI] [PubMed] [Google Scholar]
- Yousef GM, Scorilas A, Jung K, Ashworth LK, Diamandis EP. Molecular cloning of the human kallikrein 15 gene (KLK15). Up-regulation in prostate cancer. J. Biol. Chem. 2001;276:53–61. doi: 10.1074/jbc.M005432200. [DOI] [PubMed] [Google Scholar]
- Yousef GM, Kapadia C, Polymeris ME, Borgono C, Hutchinson S, Wasney GA, Soosaipillai A, Diamandis EP. The human kallikrein protein 5 (hK5) is enzymatically active, glycosylated and forms complexes with two protease inhibitors in ovarian cancer fluids. Biochim. Biophys. Acta. 2003;1628:88–96. doi: 10.1016/s0167-4781(03)00116-7. [DOI] [PubMed] [Google Scholar]