Abstract
DNA polymerase ν (POLN or pol ν) is a newly discovered A family polymerase that generates a high error rate when incorporating nucleotides opposite dG; its translesion DNA synthesis (TLS) capability has only been demonstrated for high fidelity replication bypass of thymine glycol lesions. In the current investigation, we describe a novel TLS substrate specificity of pol ν, demonstrating that it is able to bypass exceptionally large DNA lesions whose linkages are through the DNA major groove. Specifically, pol ν catalyzed efficient and high fidelity TLS past peptides linked to N6-dA via a reduced Schiff base linkage with a γ-hydroxypropano-dA. Additionally, pol ν could bypass DNA interstrand cross-links with linkage between N6-dAs in complementary DNA strands. However, the chemically identical DNA−peptide and DNA interstrand cross-links completely blocked pol ν when they were located in the minor groove via a N2-dG linkage. Furthermore, we showed that pol ν incorporated a nucleotide opposite the 1,N6-etheno-dA (εdA) in an error-free manner and (+)-trans-anti-benzo[a]pyrene-7,8-dihydrodiol 9,10-epoxide-dA [(+)-BPDE-dA] in an error-prone manner, albeit with a greatly reduced capability. Collectively, these data suggest that although pol ν bypass capacity cannot be generalized to all major groove DNA adducts, this polymerase could be involved in TLS when genomic replication is blocked by extremely large major groove DNA lesions. In view of the recent observation that pol ν may have a role in cellular tolerance to DNA cross-linking agents, our findings provide biochemical evidence for the potential functioning of this polymerase in the bypass of some DNA−protein and DNA−DNA cross-links.
Introduction
Translesion DNA synthesis (TLS)1 is a significant DNA damage tolerance mechanism to overcome replication blocks caused by DNA lesions or to seal gaps opposite lesions (1,2). In the past decade, numerous DNA polymerases (pols) have been discovered that possess lesion bypass activity. Recently, a new human DNA pol has been identified, POLN or pol ν, and falls in the A-family of DNA pols (3). This family includes Escherichia coli (E. coli) pol I and human pol θ. Pol ν is a moderately processive enzyme and is able to carry out strand displacement DNA synthesis with much higher efficiency than the Klenow fragment, the pol-proficient portion of E. coli pol I. It also replicates nondamaged (ND) DNA with low fidelity; specifically, it frequently misincorporates dT opposite template dG with about half the efficiency of incorporation of the correct nucleotide dC opposite template dG (4,5). Because low fidelity of DNA synthesis is common for TLS pols and pol ν lacks 3′→5′ exonuclease proofreading activity, a role of pol ν in the bypass of specific DNA lesions has been proposed (4,5). Indeed, this pol can carry out TLS in vitro, being specifically proficient in accurate bypass of thymine glycols, major groove DNA lesions (5). However, it is completely blocked by a number of other DNA modifications, including a cisplatin-induced GpG intrastrand cross-link, an abasic site, a cyclobutane pyrimidine dimer, and a thymine−thymine 6-4 photoproduct (5).
A function of pol ν in DNA cross-link repair in mammalian cells has been proposed (6). In particular, the data suggested that pol ν is involved in homologous recombination in response to various DNA cross-linking agents and potentially interacts with multiple proteins in the Fanconi anemia pathway, which are relevant to DNA cross-link repair. However, a cellular role for pol ν in homologous recombination-independent, TLS-dependent repair of DNA cross-links has not yet been addressed. Additionally, as already mentioned, thymine glycol is the only lesion that pol ν has been reported to be able to bypass in vitro (5). To understand the cellular function of pol ν in TLS, it is crucial to identify types of DNA lesions that this pol can bypass. Because it has been previously established that both the exo+ and the exo− Klenow fragment can catalyze TLS past certain major groove DNA adducts (7,8), we hypothesized that in addition to thymine glycols, pol ν may catalyze replication bypass of additional major groove DNA lesions. This study was designed to test this hypothesis to facilitate insight into the cellular function of pol ν in TLS.
Experimental Procedures
Materials
[γ-32P]ATP was obtained from PerkinElmer Life Sciences (Waltham, MA). P-6 Bio-Spin columns were obtained from Bio-Rad (Hercules, CA). Uracil DNA glycosylase, T4 DNA ligase, and T4 polynucleotide kinase were purchased from New England BioLabs (Beverly, MA). Sodium cyanoborohydride was obtained from Sigma (St. Louis, MO). Slide-A-Lyzer dialysis cassettes with a molecular weight cutoff of 10000 were purchased from Thermo Scientific (Rockford, IL). Human pol ν was purified as described (5). Yeast pol δ and PCNA were purified as previously described (9) and were generous gifts from Dr. Peter M. J. Burgers (Washington University, St. Louis, MO). The Lys-Trp-Lys-Lys and Lys-Phe-His-Glu-Lys-His-His-Ser-His-Arg-Gly-Tyr peptides were obtained from Sigma-Genosys (St. Louis, MO).
Oligodeoxynucleotide Synthesis
(a) All ND oligodeoxynucleotides were synthesized by the Molecular Microbiology and Immunology Research Core Facility at Oregon Health & Science University (Portland, OR).
(b) Oligodeoxynucleotides containing γ-hydroxypropano-dA or γ-hydroxypropano-dG were synthesized as previously described (10). DNAs containing DNA−peptide cross-links (PCLs) (Figure 1a,c) were prepared by the reaction of the γ-hydroxypropano-dA- or γ-hydroxypropano-dG-containing oligodeoxynucleotides with either 20 nmol of Lys-Trp-Lys-Lys or 4 nmol of Lys-Phe-His-Glu-Lys-His-His-Ser-His-Arg-Gly-Tyr in the presence of 50 mM sodium cyanoborohydride according to the previously published procedure (11). The sequences of oligodeoxynucleotides containing a N6-dA PCL and a N2-dG PCL are shown in Table 1, column 2, rows 1 and 2, respectively.
Figure 1.
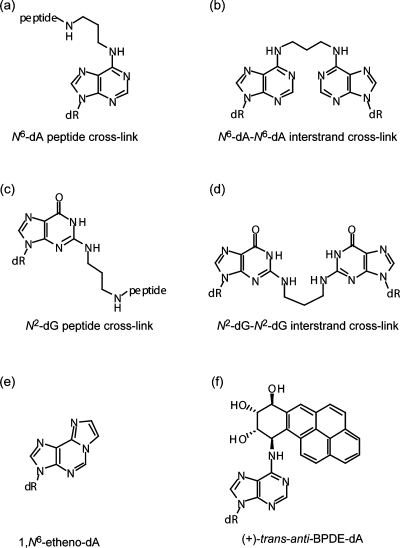
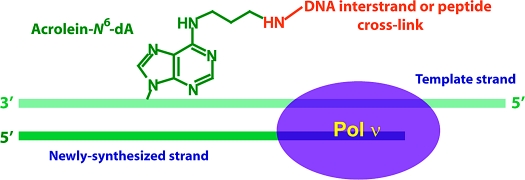
Structures of DNA adducts. The PCLs consist of either a tetra (Lys-Trp-Lys-Lys) or dodecyl (Lys-Phe-His-Glu-Lys-His-His-Ser-His-Arg-Gly-Tyr) peptide attached via an acrolein moiety at the N6 position of dA (a) or N2 position of dG (c). The resulting PCLs are referred to as N6-dA PCL4, N6-dA PCL12, N2-dG PCL4, and N2-dG PCL12. (b) Structure of N6-dA−N6-dA interstrand cross-link. (d) Structure of N2-dG−N2-dG interstrand cross-link. (e) Structure of 1,N6-etheno-dA. (f) Structure of (+)-trans-anti-BPDE-dA.
Table 1. Sequences of DNA Primers and Lesion-Containing Templatesa.
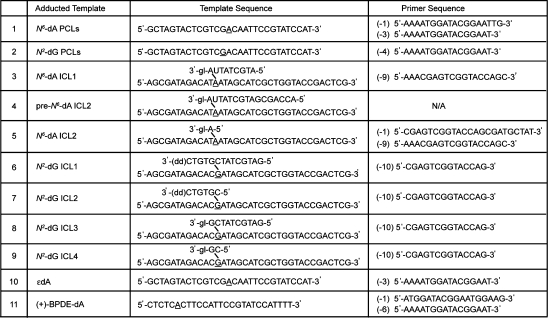 |
Modified bases are underlined. A 3′-glycerol unit (gl) or a dideoxycytidine (dd) were incorporated to prevent DNA synthesis off of the shorter strand of the ICL-containing oligodeoxynucleotides. The number in the parentheses in front of the primer sequence indicates the position of the primer 3′-OH.
(c) The synthesis of the oligodeoxynucleotides containing interstrand DNA cross-link (ICL) between N6-dAs of complementary strands (Figure 1b) is described in the Supporting Information. The sequences of a N6-dA ICL1 and a N6-dA ICL2 are shown in Table 1, column 2, rows 3 and 5, respectively. The N6-dA ICL2 oligodeoxynucleotide was generated from a precursor oligodeoxynucleotide (pre-N6-dA ICL2, Table 1, column 2, row 4) as described previously (12).
(d) Oligodeoxynucleotides containing an ICL between N2-dGs (Figure 1d) were synthesized as previously described (12−14), and the sequences of the oligodeoxynucleotides containing each N2-dG−N2-dG ICL (N2-dG ICL1, N2-dG ICL2, N2-dG ICL3, and N2-dG ICL4) are shown in Table 1, column 2, rows 6−9. A detailed description of each type of N2-dG ICLs has been reported previously (12).
(e) An oligodeoxynucleotide containing 1,N6-etheno-dA (εdA) (Figure 1e and Table 1, column 2, row 10) was synthesized by the Molecular Microbiology and Immunology Research Core Facility at Oregon Health & Science University (Portland, OR).
(f) An oligodeoxynucleotide (5′-CTCTCACTTCC-3′) containing (+)-trans-anti-benzo[a]pyrene-7,8-dihydrodiol 9,10-epoxide-dA [(+)-BPDE-dA] (Figure 1f) at the underlined nucleotide was synthesized as previously described (15) and was a generous gift from Dr. Nicholas E. Geacintov (New York University, NY). This oligodeoxynucleotide was ligated to 16-mer oligodeoxynucleotide (5′-ATTCCGTATCCATTTT-3′) using 800 units of T4 DNA ligase at 12 °C overnight in the presence of 23-mer scaffold oligodeoxynucleotide (5′-TGGATACGGAATGGAAGTGAGAG-3′). The ligation reaction was terminated by the addition of an equal volume of formamide dye solution [95% (v/v) formamide, 10 mM EDTA, 0.03% (w/v) xylene cyanol, and 0.03% (w/v) bromphenol blue], and the products were separated on a 10% acrylamide gel containing 8 M urea. DNA was eluted from the gel with 0.5 M ammonium acetate/10 mM magnesium acetate solution, dialyzed against 10 mM Tris-HCl (pH 7.4) buffer containing 1 mM EDTA in Slide-A-Lyzer dialysis cassette (MWCO of 10000), and further purified through a P-6 Bio-Spin column. The resulting 27-mer was used for DNA replication assays (Table 1, column 2, row 11).
DNA Replication Assays
For replication assays, primers were designed for reactions under a running or standing start condition. In the former case, the primer 3′-OH was positioned several nucleotides upstream of the lesion site. In latter case, the primer 3′-OH was positioned immediately prior to the lesion site (−1 primer). The sequences of primers are listed in Table 1, column 3. DNA replication assays were carried out in the following buffers: For pol ν, the reaction mixture contained 25 mM Tris-HCl (pH 7.5), 8 mM MgCl2, 10% glycerol, 100 μg/mL bovine serum albumin, and 5 mM dithiothreitol. For reaction with pol ν with N2-dG−N2-dG ICLs, Mg-acetate was used instead of MgCl2. For pol δ, the reaction mixture contained 40 mM Hepes-KOH (pH 6.8), 10% glycerol, 200 μg/mL bovine serum albumin, 1 mM dithiothreitol, and 8 mM MgCl2. All reactions were carried out at 37 °C. DNA substrate, pol, and dNTP(s) concentrations, as well as reaction times, are given in figure legends. Reaction products were resolved through 15% acrylamide gel containing 8 M urea and visualized using a PhosphorImager screen. The percentage of primer extended in extension reactions was determined by taking the ratio of extended primer beyond the adducted base to the total amount of primer (unextended primer + extended primer up to and opposite the lesion + extended primer beyond the lesion). The percentage of primer extended in single nucleotide incorporations was determined by taking the ratio of extended primer to the total amount of primer (unextended primer + extended primer). Quantifications were done using ImageQuant 5.2 software.
Results
Replication Bypass of N6-dA Peptide Cross-Links by Pol ν
To address the hypothesis that pol ν can catalyze replication bypass of additional major groove DNA lesions, we utilized N6-dA acrolein-mediated PCLs as a model for the bulky major groove DNA adducts (11). Specifically, tetra- and dodecylpeptide cross-links (Figure 1a, N6-dA PCL4 and N6-dA PCL12, respectively) were used. The molecular masses of these PCL-modified bases were 763 Da for N6-dA PCL4 and 1738 Da for N6-dA PCL12 versus 135 Da for unmodified adenine. These lesions were positioned 15 nucleotides from the 5′ end of the 30-mer template and annealed with a 16-mer radiolabeled primer in which the 3′-OH was three nucleotides upstream of the lesion site.
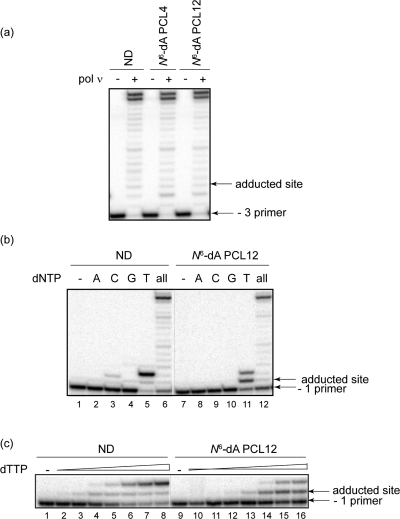
Data in Figure 2a revealed that pol ν could fully extend primers annealed to these adducted templates; under the conditions used, very minimal blockage to DNA synthesis was observed one nucleotide prior to the lesion. To assess the identity of the nucleotides incorporated opposite the lesion, single nucleotide incorporations were conducted. Because N6-dA PCL12 is bulkier than N6-dA PCL4, further assays used only substrate containing this lesion. Data showed that pol ν incorporated only the correct nucleotide, dT, opposite N6-dA PCL12 (Figure 2b), suggesting that it catalyzed error-free bypass of this adduct. It is of interest to note that two nucleotides were incorporated for both the ND and the N6-dA PCL12 substrates when only dTTP was present. It has been reported that pol ν catalyzes error-prone insertion of dT opposite dG (4,5). Because the templating nucleotide immediately downstream of the adducted base is a dG, this specific sequence context likely accounts for the observed incorporation of the second dT. To quantitatively evaluate the extent of the blockage posed by N6-dA PCL12 for the pol ν-catalyzed nucleotide insertion, dTTP titration assays (6.4 nM to 100 μM dTTP at 5-fold incrementally increased concentrations, left to right) were performed (Figure 2c). These results demonstrated that pol ν could incorporate approximately an equal amount of dT opposite the adducted base at concentrations only 5-fold higher relative to ND dA; specifically, ∼20% of primers were extended at 800 nM dTTP using ND substrate and at 4 μM dTTP using damaged substrate.
Figure 2.
Replication bypass of N6-dA peptide cross-links by human pol ν. (a) Primer extensions were catalyzed by 50 nM human pol ν under running start conditions (−3 primer) in the presence of 5 nM primer template containing ND, N6-dA PCL4, or N6-dA PCL12 for 30 min at 37 °C. (b) Single nucleotide incorporations and primer extensions were catalyzed by 5 nM human pol ν under standing start conditions (−1 primer) in the presence of 2 nM primer template containing ND or N6-dA PCL12 for 15 min at 37 °C. Reactions shown in lanes 1−6 and lanes 7−12 were conducted side-by-side, and products were separated on the same gel. The reactions shown in panels a and b were carried out in the presence of 100 μM individual or all dNTPs. (c) dTTP titration assays with 2 nM primer template were catalyzed by 5 nM human pol ν under standing start conditions (−1 primer) for 10 min at 37 °C using the same substrates as described in panel b. dTTP concentration ranges from 6.4 nM to 100 μM (5-fold incremental increase). Reactions shown in lanes 1−8 and lanes 9−16 were conducted side-by-side, and products were separated on the same gel.
Replication Bypass of N6-dA−N6-dA DNA Interstrand Cross-Links by Pol ν
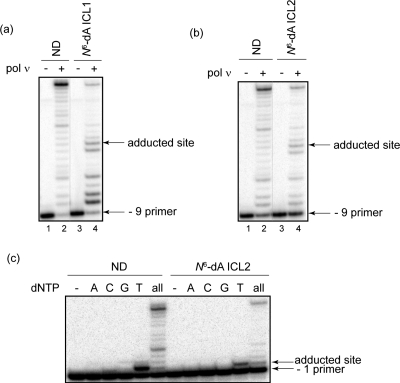
Having demonstrated that pol ν can readily bypass N6-dA PCLs, we hypothesized that it may also be able to carry out TLS past other N6-dA major groove adducts, including N6-dA−N6-dA ICLs (Figure 1b). Although ICLs had been previously thought to pose an insurmountable block to replication, our laboratory has recently demonstrated that human DNA pol κ and E. coli DNA pol IV can catalyze replication bypass of an ICL between the N2-positions of dGs, which models the ICL formed by acrolein in a CpG sequence context (12,16). Related ICLs were constructed between the N6-positions of dA placing the cross-link in the major groove (Figure 1b and Table 1, rows 3 and 5). An N6-dA ICL1 was designed to model for a potential intermediate of ICL processing that could be generated by exo/endonucleolytic removal of DNA patch 3′ to the ICL site. Even though N6-dA ICL1 contains an eight nucleotide duplex region 5′ to the ICL site, it was anticipated that pol ν would efficiently catalyze strand displacement DNA synthesis due to its intrinsic high strand displacement activity (5), a property that is useful for replicating this type of DNA lesion. A N6-dA ICL2 was constructed to model another potential intermediate of ICL processing; this could be generated by removal of DNA patches both 3′ and 5′ to the ICL site. When these major groove ICL-containing DNAs were used in primer extension reactions, pol ν could extend primers and generate full-length products (Figure 3a,b). For both types of ICLs, the amount of primers extended beyond the adducted site was less as compared to ND substrate; specifically, a 10- and 4-fold decrease was observed for N6-dA-ICL1 and N6-dA-ICL2, respectively. As expected, pol ν catalyzed strand displacement synthesis on the N6-dA ICL1 template (Figure 3a). When the identity of nucleotides incorporated opposite the cross-linked site was examined by single nucleotide incorporations using the N6-dA ICL2 template, the data indicated that pol ν preferentially incorporated the correct nucleotide, dT, opposite the N6-modified dA (Figure 3c).
Figure 3.
Replication bypass of N6-dA−N6-dA interstrand cross-links by human pol ν. Primer extensions were catalyzed by 10 nM human pol ν under running start conditions (−9 primer) using ND, N6-dA ICL1 (a), or N6-dA ICL2 (b). Reactions shown in lanes 1−2 and lanes 3−4 were conducted side-by-side, and products were separated on the same gel. (c) Single nucleotide incorporations and primer extensions were catalyzed by 5 nM human pol ν under standing start conditions (−1 primer) using ND and N6-dA ICL2. All reactions were carried out in the presence of 5 nM primer template and 100 μM individual or all dNTPs for 15 min at 37 °C.
Pol ν-Catalyzed Replication Is Blocked by N2-dG Peptide and DNA Interstrand Cross-Links
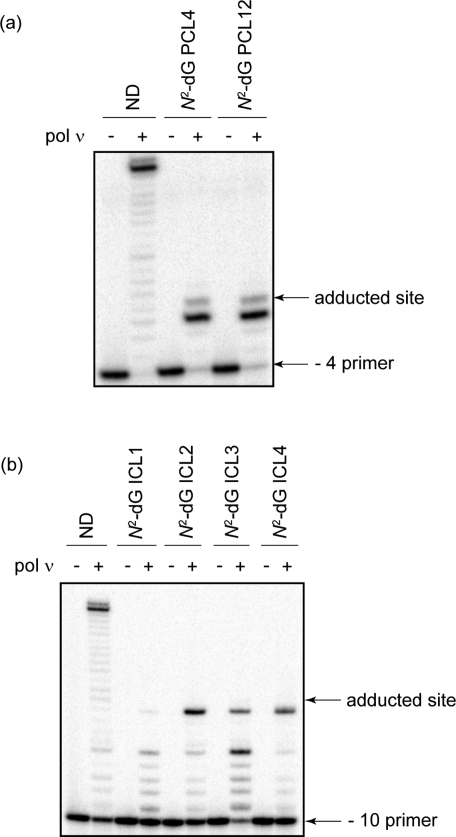
Given that pol ν was able to bypass the extremely bulky major groove N6-dA PCLs and ICLs, we tested whether it could replicate past chemically identical PCL and ICL lesions except situated in the minor groove through a linkage with the N2 position of dG (Figure 1c,d). Primer extensions using these N2-dG linked PCLs revealed that pol ν was strongly blocked one nucleotide prior to both PCLs and was completely blocked at the site opposite the lesion, with no further primer extension observed (Figure 4a). The identity of the nucleotide(s) incorporated opposite the PCLs was not determined due to the limited nucleotide incorporation. Even though pol ν could not replicate past the N2-dG PCLs, suggesting an inability to replicate past minor groove DNA adducts, we tested whether pol ν could catalyze TLS past N2-dG−N2-dG ICLs (Figure 1d and Table 1, rows 6−9). No incorporation opposite the lesion site was observed for these ICL-containing DNAs (Figure 4b). Thus, similar to the observations made using PCL-containing DNAs, pol ν has much higher tolerance for the major groove N6-dA ICLs than the minor groove N2-dG ICLs.
Figure 4.
Replication bypass of N2-dG peptide and interstrand cross-links by human pol ν. (a) Primer extensions were catalyzed by 10 nM human pol ν under running start conditions (−4 primer) in the presence of 5 nM primer template containing ND, N2-dG PCL4, or N2-dG PCL12 for 30 min at 37 °C. (b) Primer extensions were catalyzed by 25 nM human pol ν under running start conditions (−10 primer) in the presence of 7.5 nM primer template containing ND, N2-dG ICL1, N2-dG ICL2, N2-dG ICL3, or N2-dG ICL4 for 10 min at 37 °C. All reactions were carried out in the presence of 100 μM dNTPs.
Substrate Limitations of Pol ν-Catalyzed Bypass of Major Groove-Linked DNA Lesions
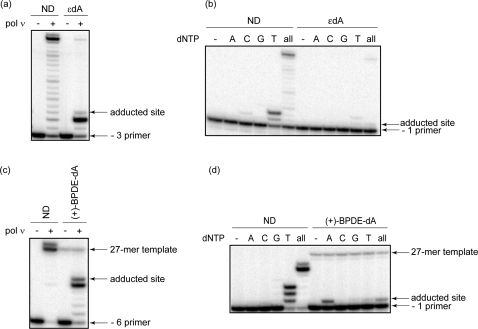
Because pol ν was able to accurately replicate past major groove N6-dA PCL and ICL lesions, we wanted to further explore the substrate range of pol ν and address the question concerning pol ν ability to bypass dA adducts in which both N1 and N6 sites are blocked by modification. Thus, the ability of pol ν to bypass an εdA (Figure 1e) was examined. εdA is a promutagenic lesion that can be formed by exposure to industrial toxicants such as vinyl chloride and by the reaction of DNA with bis-electrophiles produced by lipid peroxidation (17). The running start primer extensions demonstrated that pol ν was severely blocked one nucleotide prior to the εdA adduct, with only a very small amount of full-length product formed (Figure 5a). However, the nucleotide incorporation opposite the lesion appeared to be quite accurate. Under the conditions used, the primers were extended in the presence of dTTP but not other dNTPs (Figure 5b). On the basis of these data, we conclude that relatively nonbulky modifications at both N1 and N6 of dA are sufficient to inhibit pol ν-catalyzed TLS.
Figure 5.
Replication bypass of 1,N6-etheno-dA and (+)-trans-anti-BPDE-dA by human pol ν. (a) Primer extensions were catalyzed by 10 nM human pol ν under running start conditions (−3 primer) in the presence of 5 nM primer template containing ND or εdA. (b) Single nucleotide incorporations and primer extensions were catalyzed by 5 nM human pol ν under standing start conditions (−1 primer) in the presence of 5 nM primer template using the same substrates as described in panel a. (c) Primer extensions were catalyzed by 10 nM human pol ν under running start conditions (−6 primer) in the presence of 2 nM primer template containing ND or (+)-BPDE-dA. (d) Single nucleotide incorporations and primer extensions were catalyzed by 5 nM human pol ν under standing start conditions (−1 primer) in the presence of 2 nM primer template using the same substrates as described in panel c. All reactions were carried out in the presence of 100 μM individual or all dNTPs for 15 min at 37 °C.
We also investigated the ability of pol ν to carry out TLS past (+)-BPDE-dA (Figure 1f), one of the adducts formed by the active metabolite of the prevalent environmental pollutant, benzo[a]pyrene (18). To construct an oligodeoxynucleotide of sufficient length for TLS analyses, an 11-mer oligodeoxynucleotide containing the (+)-BPDE-dA was 32P-labeled, ligated to a 16-mer oligodeoxynucleotide, and gel-purified. Residual radioactive signals of the resulting 27-mer template are evident in all lanes [Figure 5c,d, (+)-BPDE-dA-27-mer template]. Primer extensions revealed that pol ν could not fully extend the −6 primer and was severely blocked one nucleotide prior to the adducted site (Figure 5c). In addition, pol ν displayed error-prone patterns of nucleotide incorporation opposite the lesion (Figure 5d); in the presence of dATP, ∼16% of primers were extended, while only ∼1% of primers were extended in the presence of the correct dTTP. A limited primer extension (<1%) was also observed in the presence of dGTP. Overall, pol ν-catalyzed TLS past (+)-BPDE-dA was inefficient, incomplete, and error-prone.
Discussion
Although very significant progress has been made in both structural analyses of many of the TLS pols and the range of damaged DNA substrates that these pols can bypass, in the majority of cases, the biological role of these enzymes can only be inferred by changes in efficiency and mutagenic rates of TLS in their absence. Little data have been gathered concerning the role of these enzymes in developmental biology, their tissue-specific expression and steady-state distribution in adult organisms, the mechanism(s) for recruitment to sites of DNA damage, and the processes that regulate their expressions in response to environmental toxicant exposures and/or endogenous stresses. In the case of pol ν, the initial characterizations have demonstrated a very limited repertoire of lesions that pol ν can efficiently bypass (5). Although a cellular role of pol ν in TLS has not yet been established, its high frequency of misincorporation of dT opposite dG suggests that its expression would be tightly regulated. Thus, understanding the potential substrate specificity of pol ν may help to guide future investigations of its biological role in TLS and circumstances for induction or activation. In this regard, we demonstrated that although pol ν could carry out TLS past bulky major groove DNA lesions such as N6-dA PCLs and N6-dA−N6-dA ICLs, it failed to replicate DNAs containing chemically similar minor groove DNA lesions. Thus, the location of the lesion within DNA is one important structural determinant that influences the ability of pol ν to bypass the lesion.
We also discovered that pol ν could not efficiently bypass εdA, and we speculate that this may be either due to pol ν requirement for the Watson−Crick edge for efficient dA templating or structural heterogeneity of the lesion within the active site. Regarding the former possibility, pol ν may fail to catalyze efficient TLS past εdA because the Watson−Crick edge of this adduct is blocked by the modification. The majority of DNA pols utilize the geometry of the canonical Watson−Crick pairs for the nucleotide selection (19), but some TLS pols utilize alternative mechanisms. For example, pol ι induces a syn conformation on template purines, which results in a Hoogsteen base pairing with the correctly matched incoming nucleotide and enables bypass of lesions that disrupt the Watson−Crick edge but not the Hoogsteen edge (20). The diminished capability of pol ν to bypass εdA strongly suggests that the accessibility of the Watson−Crick edge of the template dA is important for the efficient replication by this pol; thus, it is likely that pol ν-catalyzed polymerization involves the recognition of the Watson−Crick geometry.
With regard to structural heterogeneity of εdA adduct, NMR analyses have revealed that orientation of the base around the glycosydic bond is affected by identity of nucleotide opposite the lesion (21,22). When paired with dT, the εdA is in a normal anti conformation; however, the εdA base prefers the syn conformation when placed opposite a mispaired dG. Additionally, the cocrystal structure of DNA containing an εdA bound to pol ι also revealed that the lesion adopted a syn conformation (20). Thus, εdA can adopt a syn or anti conformation depending on the base it is paired with, as well as interactions with the pol. There is a possibility that similar to pot ι, the adducted base is in a syn conformation in the pol ν active site. However, it is unclear whether either orientation of the ε-modified base can be efficiently utilized for nucleotide insertion.
Intriguingly, pol ν also could not fully bypass a (+)-BPDE-dA. The NMR solution structure of this lesion opposite dT in duplex DNA showed complex conformational heterogeneity (23). Computational studies using an NMR structure of duplex DNA where dT was placed opposite the modified dA have revealed that the glycosydic bond of (+)-BPDE-dA was in syn-anti equilibrium; therefore, the modified dA has a diminished capability to participate in Watson−Crick base pairing (24). It is possible that the glycosydic bond of modified dA rotates and adopts a syn conformation at the primer-template junction and therefore cannot participate in Watson−Crick base pairing. An alternative explanation may be that the structure of the active site of pol ν is significantly distorted by bulky aromatic ring system of this lesion. It has been shown that the BPDE moiety of this lesion is intercalated into the double helix, on the 3′ side of the adducted dA, and thus causes significant structural distortions at the active site of T7 pol (25). Because the T7 pol is also a member of the A-family pols, the inability of pol ν to bypass this lesion may be due to the active site distortions, similar to those shown for T7 pol. With regard to the low fidelity synthesis past this lesion by pol ν, it has been shown that the adducted dA is displaced from the active site of T7 pol as a result of steric hindrance between the BPDE moiety and the primer template. Among the four dNTPs, dATP fits best into the dNTP binding pocket that is enlarged due to the displacement of adducted dA (25). This structural change provides a likely explanation for the predominant misincorporation of dA by pol ν.
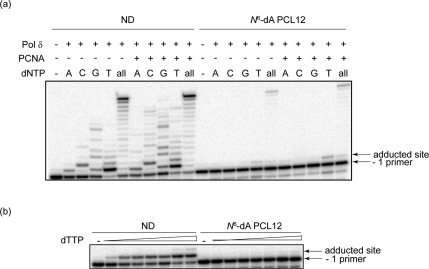
Recently, we reported a comparative study of the mutagenic consequences of identical lesions linked in the major groove via N6-dA versus the minor groove linkage via N2-dG during the replication of single-stranded pMS2 shuttle vectors in COS-7 cells (11); these lesions were the same site-specific PCL4s substrates used in this investigation. These data revealed that DNA containing either lesion could be replicated, with the N2-dG PCL4 being moderately mutagenic, while the N6-dA PCL4 showed very low mutagenic potential. Although it is anticipated that the extremely bulky nature of the N6-dA PCLs lesions would block replicative pols, we carried out in vitro replication assays using pol δ (Figure 6). The primer extensions and single nucleotide incorporations showed some limited bypass of N6-dA PCL12 at very high dNTP concentrations (100 μM) (Figure 6a), while the titration experiment with varying concentrations of dTTP (from 6.4 nM to 100 μM) revealed the severity of the blockage posed by this lesion for the pol δ-catalyzed nucleotide insertion (Figure 6b). Specifically, pol δ could incorporate dT opposite the ND dA at concentrations as low as 6.4 nM with ∼8% of primers being extended, while even at 100 μM dTTP, only ∼2% of primers were extended on the N6-dA PCL12-containing substrate. These results suggest that although in vitro conditions could be manipulated to observe bypass of N6-dA PCLs by pol δ, the block to replication is likely to occur when pol δ encounters such bulky lesions; therefore, specialized pols may be recruited to carry out TLS. The data presented herein suggest that pol ν is a likely candidate to perform TLS past a variety of major groove lesions. At present, the structural basis is unknown why pol ν could efficiently bypass PCL and ICL as large as 3025 Da but fails to bypass less bulky BPDE adduct. However, we speculate that the lesions that are readily bypassed by pol ν may have significant conformational flexibility and thus, can be accommodated without distorting DNA−enzyme interactions within the pol active site. Overall, our data suggest that since pol ν is capable of bypassing extremely large major (but not minor) groove lesions, it may play a role in TLS following toxicant challenges that result in the formation of such lesions in the cells.
Figure 6.
Replication bypass of N6-dA peptide cross-link by yeast pol δ. (a) Single nucleotide incorporations and primer extensions were catalyzed by 0.5 nM yeast pol δ with 2 nM primer template containing ND or N6-dA PCL12 under standing start conditions (−1 primer) in the presence or absence of 5 nM PCNA for 20 min at 37 °C. Reactions were carried out in the presence of 100 μM individual or all dNTPs. (b) dTTP titration assays with 2 nM primer template were catalyzed by 0.5 nM yeast pol δ under standing start conditions (−1 primer) in the absence of PCNA for 10 min at 37 °C using the same substrates as described in panel a. dTTP concentration ranges from 6.4 nM to 100 μM (5-fold incremental increase).
Acknowledgments
This work was supported by PO1 ES05355 (R.S.L. and C.J.R.), R01 CA106858 (R.S.L.), 2R01 CA099194 (Geacintov Laboratory), and the M. D. Anderson Research Trust (R.D.W. and K.T.). We appreciate Dr. Nicholas E. Geacintov for providing the oligodeoxynucleotide containing (+)-BPDE-dA. We also appreciate Dr. Peter M. J. Burgers for providing the yeast pol δ and yeast PCNA.
Supporting Information Available
Scheme and methods for the syntheses of oligodeoxynucleotides containing N6-dA−N6-dA interstrand cross-links. This material is available free of charge via the Internet at http://pubs.acs.org.
Footnotes
Abbreviations: TLS, translesion DNA synthesis; pol, polymerase; E. coli, Escherichia coli; ND, nondamaged; BPDE, benzo[a]pyrene-7,8-dihydrodiol 9,10-epoxide; PCL, DNA−peptide cross-link; ICL, interstrand DNA cross-link; εdA, 1,N6-etheno-dA.
Supplementary Material
References
- Guo C.; Kosarek-Stancel J. N.; Tang T. S.; Friedberg E. C. (2009) Y-family DNA polymerases in mammalian cells. Cell. Mol. Life Sci. 66, 2363–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. S.; Minesinger B. K.; Wiltrout M. E.; D’Souza S.; Woodruff R. V.; Walker G. C. (2009) Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol. Mol. Biol. Rev. 73, 134–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini F.; Kim N.; Schuffert A.; Wood R. D. (2003) POLN, a nuclear PolA family DNA polymerase homologous to the DNA cross-link sensitivity protein Mus308. J. Biol. Chem. 278, 32014–32019. [DOI] [PubMed] [Google Scholar]
- Arana M. E.; Takata K.; Garcia-Diaz M.; Wood R. D.; Kunkel T. A. (2007) A unique error signature for human DNA polymerase ν. DNA Repair 6, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata K.; Shimizu T.; Iwai S.; Wood R. D. (2006) Human DNA polymerase N (POLN) is a low fidelity enzyme capable of error-free bypass of 5S-thymine glycol. J. Biol. Chem. 281, 23445–23455. [DOI] [PubMed] [Google Scholar]
- Moldovan G. L., Madhavan M. V., Mirchandani K. D., McCaffrey R. M., Vinciguerra P., and D’Andrea A. D. (2009) DNA polymerase POLN participates in crosslink repair and homologous recombination. Mol. Cell. Biol. DOI: 10.1128/MCB.01124-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham G. J.; Harris C. M.; Harris T. M.; Lloyd R. S. (1995) The efficiency of translesion synthesis past single styrene oxide DNA adducts in vitro is polymerase-specific. Chem. Res. Toxicol. 8, 422–430. [DOI] [PubMed] [Google Scholar]
- Chary P.; Lloyd R. S. (1995) In vitro replication by prokaryotic and eukaryotic polymerases on DNA templates containing site-specific and stereospecific benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide adducts. Nucleic Acids Res. 23, 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder B. L.; Burgers P. M. (1991) Saccharomyces cerevisiae replication factor C. I. Purification and characterization of its ATPase activity. J. Biol. Chem. 266, 22689–22697. [PubMed] [Google Scholar]
- Nechev L. V.; Harris C. M.; Harris T. M. (2000) Synthesis of nucleosides and oligonucleotides containing adducts of acrolein and vinyl chloride. Chem. Res. Toxicol. 13, 421–429. [DOI] [PubMed] [Google Scholar]
- Minko I. G.; Kozekov I. D.; Kozekova A.; Harris T. M.; Rizzo C. J.; Lloyd R. S. (2008) Mutagenic potential of DNA-peptide crosslinks mediated by acrolein-derived DNA adducts. Mutat. Res. 637, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minko I. G.; Harbut M. B.; Kozekov I. D.; Kozekova A.; Jakobs P. M.; Olson S. B.; Moses R. E.; Harris T. M.; Rizzo C. J.; Lloyd R. S. (2008) Role for DNA polymerase κ in the processing of N2-N2-guanine interstrand cross-links. J. Biol. Chem. 283, 17075–17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley P. A.; Tsarouhtsis D.; Korbel G. A.; Nechev L. V.; Shearer J.; Zegar I. S.; Harris C. M.; Stone M. P.; Harris T. M. (2001) Structural studies of an oligodeoxynucleotide containing a trimethylene interstrand cross-link in a 5′-(CpG) motif: Model of a malondialdehyde cross-link. J. Am. Chem. Soc. 123, 1730–1739. [DOI] [PubMed] [Google Scholar]
- Dooley P. A.; Zhang M.; Korbel G. A.; Nechev L. V.; Harris C. M.; Stone M. P.; Harris T. M. (2003) NMR determination of the conformation of a trimethylene interstrand cross-link in an oligodeoxynucleotide duplex containing a 5′-d(GpC) motif. J. Am. Chem. Soc. 125, 62–72. [DOI] [PubMed] [Google Scholar]
- Buterin T.; Hess M. T.; Luneva N.; Geacintov N. E.; Amin S.; Kroth H.; Seidel A.; Naegeli H. (2000) Unrepaired fjord region polycyclic aromatic hydrocarbon-DNA adducts in ras codon 61 mutational hot spots. Cancer Res. 60, 1849–1856. [PubMed] [Google Scholar]
- Kumari A.; Minko I. G.; Harbut M. B.; Finkel S. E.; Goodman M. F.; Lloyd R. S. (2008) Replication bypass of interstrand cross-link intermediates by Escherichia coli DNA polymerase IV. J. Biol. Chem. 283, 27433–27437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J.; Barbin A.; Velic I.; Bartsch H. (1999) Etheno DNA-base adducts from endogenous reactive species. Mutat. Res. 424, 59–69. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E.; Cosman M.; Hingerty B. E.; Amin S.; Broyde S.; Patel D. J. (1997) NMR solution structures of stereoisometric covalent polycyclic aromatic carcinogen-DNA adduct: Principles, patterns, and diversity. Chem. Res. Toxicol. 10, 111–146. [DOI] [PubMed] [Google Scholar]
- McCulloch S. D.; Kunkel T. A. (2008) The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases. Cell Res. 18, 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D. T.; Johnson R. E.; Prakash L.; Prakash S.; Aggarwal A. K. (2006) Hoogsteen base pair formation promotes synthesis opposite the 1,N6-ethenodeoxyadenosine lesion by human DNA polymerase ι. Nat. Struct. Mol. Biol. 13, 619–625. [DOI] [PubMed] [Google Scholar]
- de los Santos C.; Kouchakdjian M.; Yarema K.; Basu A.; Essigmann J.; Patel D. J. (1991) NMR studies of the exocyclic 1,N6-ethenodeoxyadenosine adduct (εdA) opposite deoxyguanosine in a DNA duplex. εdA (syn)·dG(anti) pairing at the lesion site. Biochemistry 30, 1828–1835. [DOI] [PubMed] [Google Scholar]
- Kouchakdjian M.; Eisenberg M.; Yarema K.; Basu A.; Essigmann J.; Patel D. J. (1991) NMR studies of the exocyclic 1,N6-ethenodeoxyadenosine adduct (εdA) opposite thymidine in a DNA duplex. Nonplanar alignment of εdA(anti) and dT(anti) at the lesion site. Biochemistry 30, 1820–1828. [DOI] [PubMed] [Google Scholar]
- Yeh H. J.; Sayer J. M.; Liu X.; Altieri A. S.; Byrd R. A.; Lakshman M. K.; Yagi H.; Schurter E. J.; Gorenstein D. G.; Jerina D. M. (1995) NMR solution structure of a nonanucleotide duplex with a dG mismatch opposite a 10S adduct derived from trans addition of a deoxyadenosine N6-amino group to (+)-(7R,8S,9S,10R)-7,8-dihydroxy-9,10-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene: An unusual syn glycosidic torsion angle at the modified dA. Biochemistry 34, 13570–13581. [DOI] [PubMed] [Google Scholar]
- Yan S.; Shapiro R.; Geacintov N. E.; Broyde S. (2001) Stereochemical, structural, and thermodynamic origins of stability differences between stereoisomeric benzo[a]pyrene diol epoxide deoxyadenosine adducts in a DNA mutational hot spot sequence. J. Am. Chem. Soc. 123, 7054–7066. [DOI] [PubMed] [Google Scholar]
- Yan S. F.; Wu M.; Geacintov N. E.; Broyde S. (2004) Altering DNA polymerase incorporation fidelity by distorting the dNTP binding pocket with a bulky carcinogen-damaged template. Biochemistry 43, 7750–7765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.