Abstract
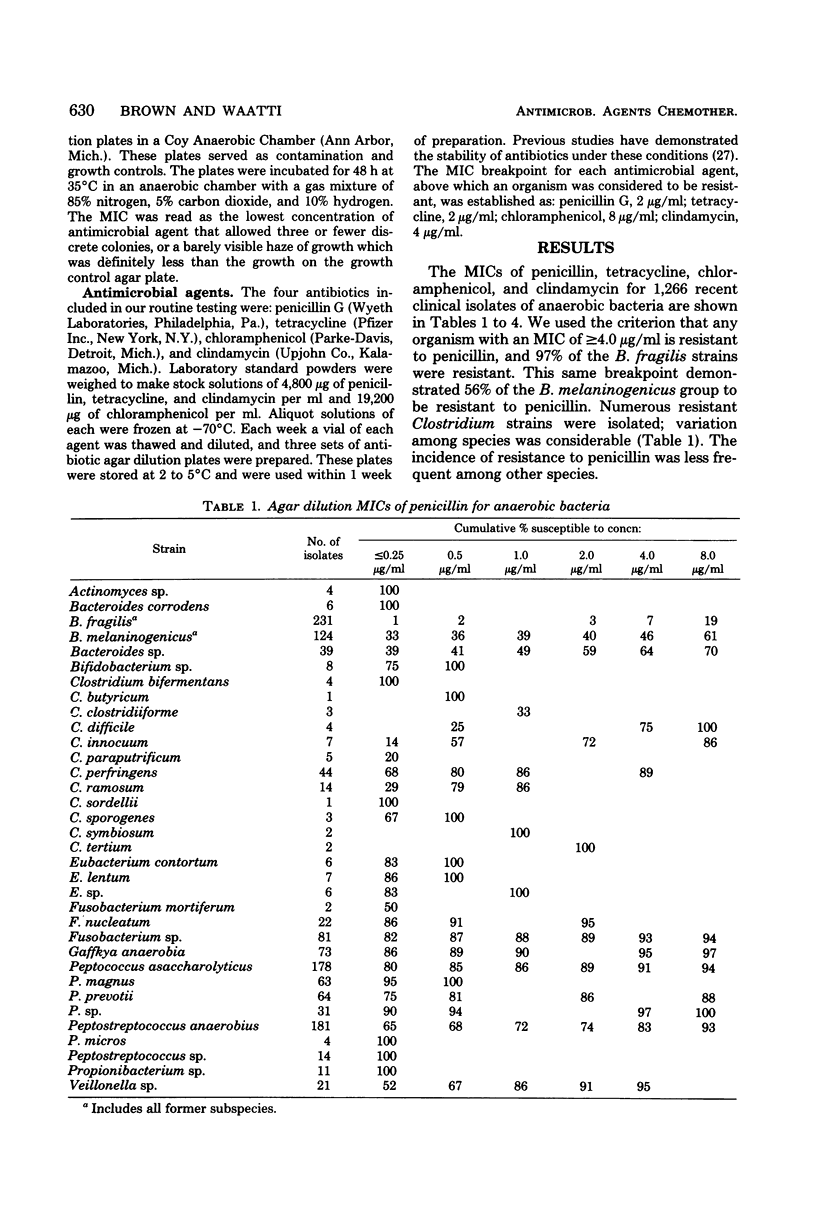
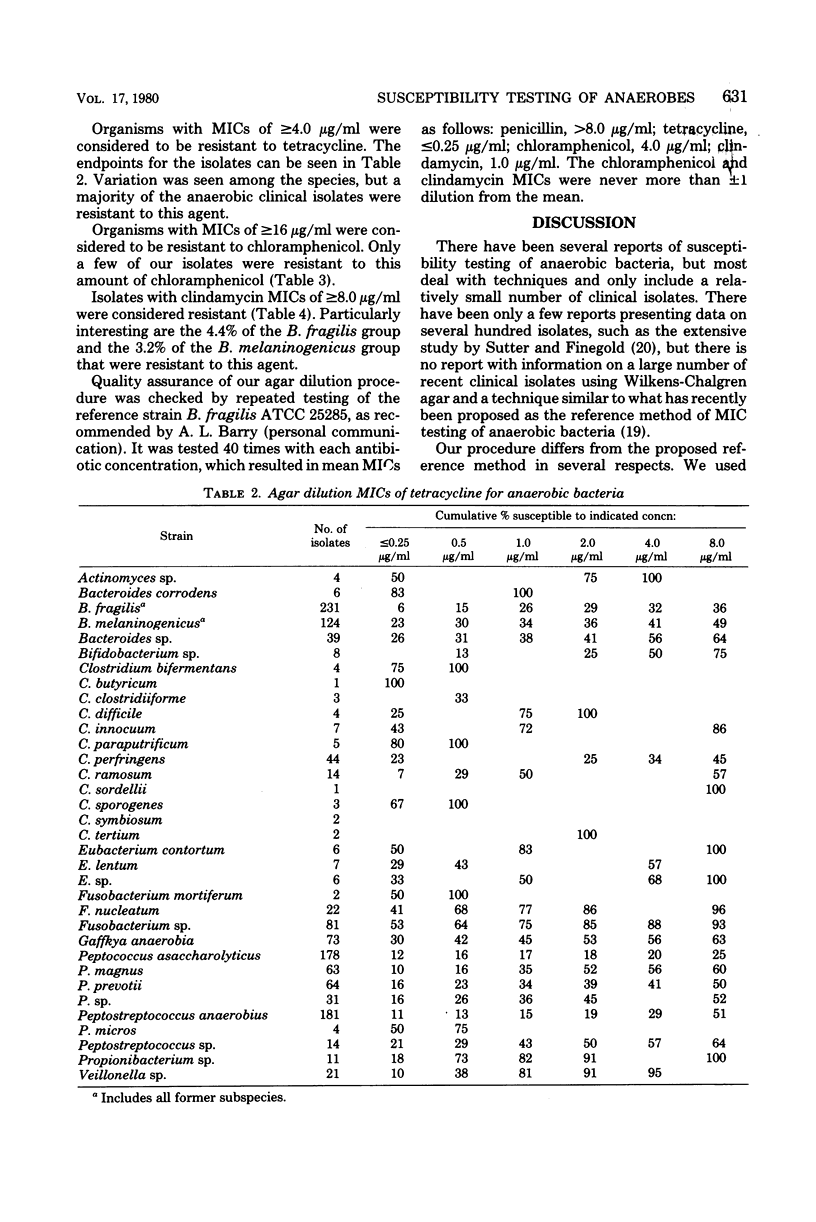
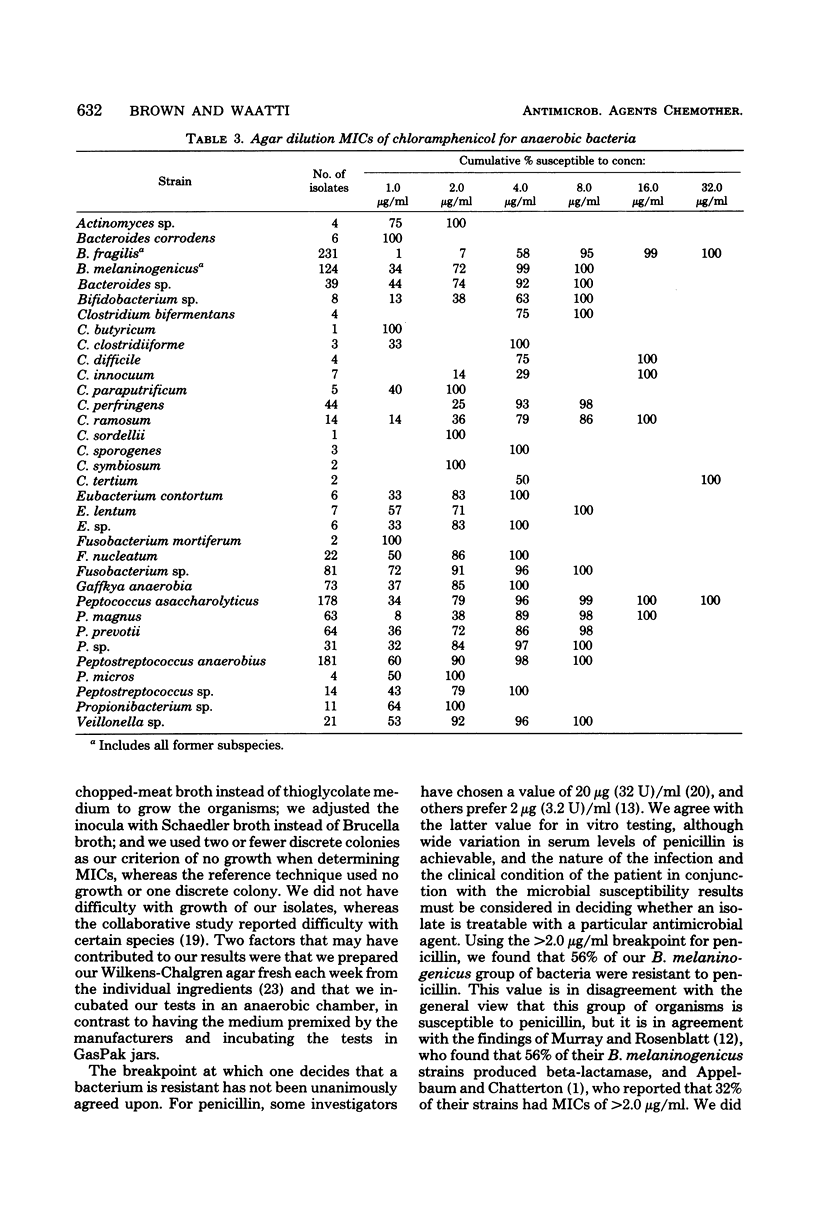
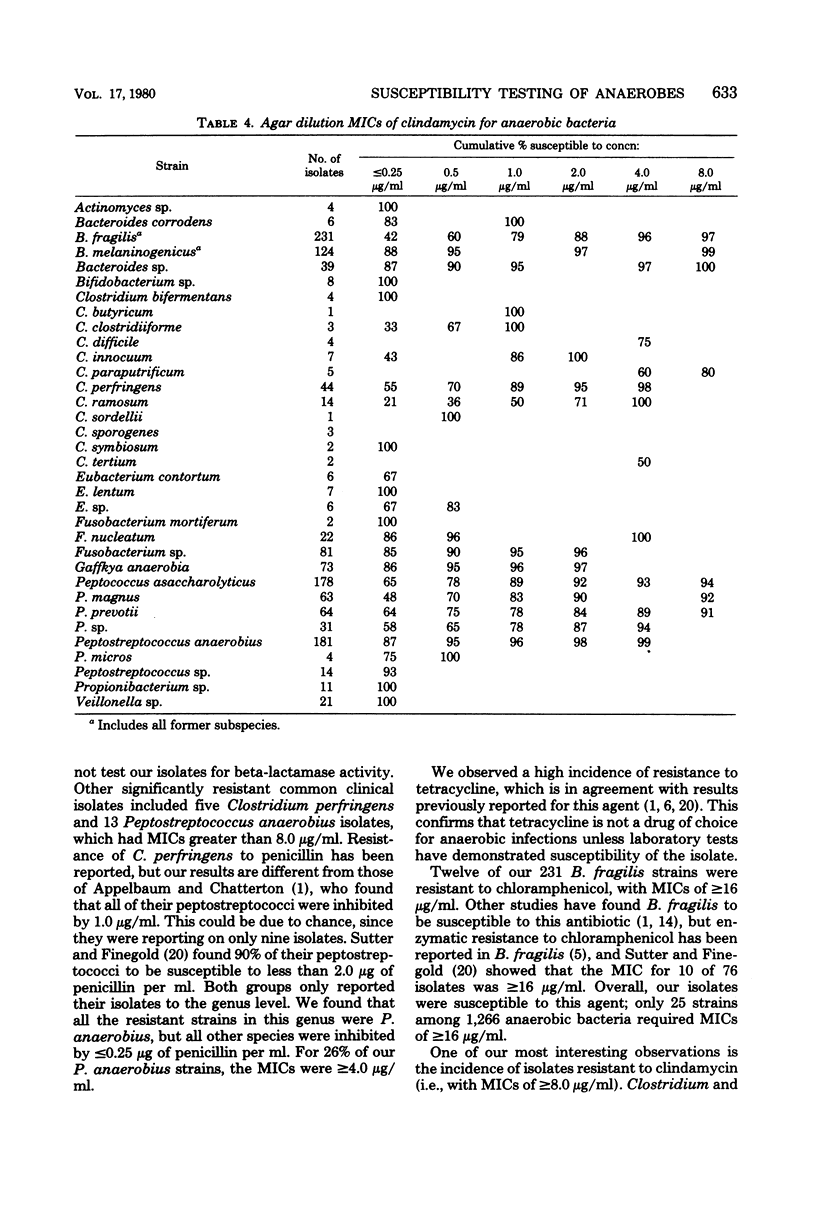
Agar dilution minimal inhibitory concentrations (MICs) of penicillin, tetracycline, chloramphenicol, and clindamycin were determined using Wilkens-Chalgren agar for 1,266 clinical isolates of anaerobic bacteria. In addition, a reference strain of Bacteroides fragilis was repeatedly tested and demonstrated the precision of the technique. Fifty-six percent of our Bacteroides melaninogenicus strains were resistant (MIC greater than or equal to 4.0 microgram/ml) to penicillin. Resistance to this antibiotic was also seen among other anaerobes, but the results are more in accord with previous reports. Resistance to tetracycline (MIC greater than or equal to 4.0 microgram/ml) was found in 60% of our isolates. Chloramphenicol proved to be the most effective agent in vitro with only 2.0% of strains resistant (MIC less than or equal to 16 microgram/ml). Only 5% of strains were resistant to clindamycin (MIC greater than or equal to 8.0 microgram/ml), and this included 10 isolates of B. fragilis and 4 of B. melaninogenicus. The incidence of resistance of anaerobic bacteria to these frequently used antibiotics is greater than previous reports and indicates the need for reliable susceptibility testing of anaerobic bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum P. C., Chatterton S. A. Susceptibility of anaerobic bacteria to ten antimicrobial agents. Antimicrob Agents Chemother. 1978 Sep;14(3):371–376. doi: 10.1128/aac.14.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawdon R. E., Rozmiej E., Palchaudhuri S., Krakowiak J. Variability in the susceptibility pattern of Bacteroides fragilis in four Detroit area hospitals. Antimicrob Agents Chemother. 1979 Nov;16(5):664–666. doi: 10.1128/aac.16.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazevic D. J. Antibiotic susceptibility of the subspecies of Bacteroides fragilis. Antimicrob Agents Chemother. 1976 Mar;9(3):481–484. doi: 10.1128/aac.9.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazevic D. J. Evaluation of the modified broth-disk method for determining antibiotic susceptibilities of anaerobic bacteria. Antimicrob Agents Chemother. 1975 Jun;7(6):721–723. doi: 10.1128/aac.7.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz M. L., Wilkinson R. G. Chloramphenicol acetyltransferase of Bacteroides fragilis. Antimicrob Agents Chemother. 1978 Jul;14(1):105–111. doi: 10.1128/aac.14.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Patten V., Guze L. B. Comparative susceptibility of anaerobic bacteria to minocycline, doxycycline, and tetracycline. Antimicrob Agents Chemother. 1975 Jan;7(1):46–49. doi: 10.1128/aac.7.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzynski T. A., Yrios J. W., Helstad A. G., Field C. R. Aerobically incubated thioglycolate broth disk method for antibiotic susceptibility testing of anaerobes. Antimicrob Agents Chemother. 1976 Oct;10(4):727–732. doi: 10.1128/aac.10.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok Y. Y., Tally F. P., Sutter V. L., Finegold S. M. Disk susceptibility testing of slow-growing anaerobic bacteria. Antimicrob Agents Chemother. 1975 Jan;7(1):1–7. doi: 10.1128/aac.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Rosenblatt J. E. Penicillin resistance and penicillinase production in clinical isolates of Bacteroides melaninogenicus. Antimicrob Agents Chemother. 1977 Apr;11(4):605–608. doi: 10.1128/aac.11.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E., Murray P. R., Sonnenwirth A. C., Joyce J. L. Comparison of anaerobic susceptibility results obtained by different methods. Antimicrob Agents Chemother. 1979 Mar;15(3):351–355. doi: 10.1128/aac.15.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotilie C. A., Fass R. J., Prior R. B., Perkins R. L. Microdilution technique for antimicrobial susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1975 Mar;7(3):311–315. doi: 10.1128/aac.7.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaki J. S., Black R., Tally F. P., Kislak J. W. Bacteroides fragilis resistant to the administration of clindamycin. Am J Med. 1976 Mar;60(3):426–428. doi: 10.1016/0002-9343(76)90759-2. [DOI] [PubMed] [Google Scholar]
- Stalons D. R., Thornsberry C. Broth-dilution method for determining the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1975 Jan;7(1):15–21. doi: 10.1128/aac.7.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Washington J. A., 2nd Antimicrobial susceptibilities of anaerobic bacteria: recent clinical isolates. Antimicrob Agents Chemother. 1974 Sep;6(3):311–315. doi: 10.1128/aac.6.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Barry A. L., Wilkins T. D., Zabransky R. J. Collaborative evaluation of a proposed reference dilution method of susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1979 Oct;16(4):495–502. doi: 10.1128/aac.16.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Susceptibility of anaerobic bacteria to 23 antimicrobial agents. Antimicrob Agents Chemother. 1976 Oct;10(4):736–752. doi: 10.1128/aac.10.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L. In vitro susceptibility of anaerobes: comparison of clindamycin and other antimicrobial agents. J Infect Dis. 1977 Mar;135 (Suppl):S7–12. doi: 10.1093/infdis/135.supplement.s7. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikins T. D., Holdeman L. V., Abramson I. J., Moore W. E. Standardized single-disc method for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1972 Jun;1(6):451–459. doi: 10.1128/aac.1.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1976 Dec;10(6):926–928. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother. 1973 Mar;3(3):350–356. doi: 10.1128/aac.3.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins T. D., Thiel T. Resistance of some species of Clostridium to clindamycin. Antimicrob Agents Chemother. 1973 Jan;3(1):136–137. doi: 10.1128/aac.3.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J., Hauser K. J. Stability of antibiotics in Wilkins-Chalgren anaerobic susceptibility testing medium after prolonged storage. Antimicrob Agents Chemother. 1977 Sep;12(3):440–441. doi: 10.1128/aac.12.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]



