Abstract
Proton pump inhibitors profoundly affect the stomach and have been associated with carcinoid tumors in female rats. There is now sufficient experience with this class of drugs to allow reasonable estimation of their safety in terms of cancer development. Long term proton pump inhibitor use is associated with an increase in gastric inflammation and development of atrophy among those with active Helicobacter pylori infections. The actual risk is unknown but is clearly low. However, it can be markedly reduced or eliminated by H. pylori eradication leading to the recommendation that patients considered for long term proton pump inhibitor therapy be tested for H. pylori infection and if present, it should be eradicated. Oxyntic cell hyperplasia, glandular dilatations, and fundic gland polyps may develop in H. pylori-uninfected patients, but these changes are believed to be reversible and without significant cancer risk.
Introduction
The stomach is a tightly regulated organ that serves many functions, including that of a protective barrier to ingested pathogens. By the early 1900's gastric cancer was known to be closely related to gastritis and gastric atrophy. Much of the stomach's protective powers can be attributed to the secretion of acid and pepsin; it was commonly believed that the normal stomach was generally sterile, especially during fasting, and that this was a direct result of the presence of its low pH. In contrast, the inflamed and atrophic stomach was found to contain a wide variety of bacteria, including some species that were normal residents of the lower gut. This change was also associated with an increased bacterial load in the small intestine, which was thought to adversely affect its function (1). Correa proposed a role for the bacteria colonizing the atrophic stomach in the induction of carcinogens, thus providing a hypothesis that unified the structural and physiologic changes that created the background for gastric cancer (2). The potential role of bacteria and yeast in gastric carcinogenesis has remained an active area of investigation and continues to elicit interest as new potential mediators are being identified.
H2-receptor antagonists were introduced in the mid 1970's. Their ability to reliably reduce gastric acidity prompted inquiries regarding whether they might also promote gastric colonization and enhance the risk of gastric cancer (1). However, since this class of drugs is unable to continuously maintain the gastric pH at levels sufficiently low (e.g., above pH 4) to allow bacterial overgrowth, concerns regarding gastric carcinogenesis subsided. The question resurfaced less than a decade later following the introduction of the more potent proton pump inhibitors (PPIs) and remains incompletely answered to this day (1). The issue became even more complicated when it was discovered that in the majority of instances gastric inflammation and the resulting atrophy were one outcome of infection with the bacterial pathogen Helicobacter pylori (3). Even among those with H. pylori infection acid secretion varied in relation to the pattern of gastritis with high acid secretion and duodenal ulcer being related to an antrum-predominant gastritis, and gastric ulcer and gastric cancer being associated with inflammation involving both the antrum and corpus (pangastritis). The focus of therapy with anti-secretory agents also changed from duodenal ulcer to gastroesophageal reflux disease, which appears to be increasingly associated with H. pylori-uninfected stomachs.
To completely understand the potential role of PPIs in altering gastric physiology one must understand their effects on both the normal and the H. pylori-infected stomach, in which PPI effects vary with different patterns of gastric inflammation.
Effects of Proton-pump Inhibitors on the Gastric Mucosa
Oxyntic gland dilatation and fundic gland polyps
Experienced pathologists can generally determine whether a patient is receiving PPIs. Oxyntic glands, which are normally tight tubes with a virtual lumen lined by smooth parietal, mucous, and chief cells, become unevenly dilated and the lumen acquires a ragged lining caused by the protruding oxyntic cells, which are increased in height and project minuscule cytoplasmic protrusions into the lumen. In biopsy specimens from the corpus these changes may be observed in just a few glands or in the majority of them; consequently, the mucosa may acquire an oddly irregular aspect. Although it is usually more difficult to detect because of the inflammation, dilatation of oxyntic glands is also observed in H. pylori-infected mucosa.
Some of the dilated glands may acquire a cystic aspect, resulting in the histopathologic appearance of an empty cavity lined by flattened parietal and chief cells; these cavities may become large enough to become visible to the endoscopist, making the transition to a fundic gland polyp. Fundic gland polyps were described in the 1970s by the German pathologist Kurt Elster (4), who considered them to represent hamartomatous lesions. They are commonly present in patients with the familial adenomatous polyposis syndrome, where they may develop foci of dysplasia and acquire a prevalently adenomatous appearance with its inherent risk of malignant progression (5). In contrast, sporadic fundic gland polyps that occur in conjunction with PPI intake are considered generally benign lesion that typically regress if PPIs are discontinued (6-9). Interestingly, fundic gland polyps arise only exceptionally in H. pylori-infected stomachs. For example, in a study of more than 6,000 patients with fundic gland polyps we found concurrent H. pylori-gastritis in only 29 patients and H. pylori organisms were present on the polyp itself in only two patients (Lash and Genta, abstract submitted).
ECL-cell, G-cell hyperplasia, and hypergastrinemia
Enterochromaffine-cell-like cells (ECL cells) are distributed throughout the oxyntic mucosa where they participate in the regulation of acid production. Gastrin-producing cells (G-cells) are endocrine-type cells confined to the gastric antrum. In response to acid suppression G-cell increase their production of gastrin in an attempt to promote acid secretion. Gastrin stimulates acid secretion in two mechanisms: by direct action on the parietal cell's basolateral membrane CCKB receptors and indirectly by affecting the ECL cell's CCKB receptors with respond by releasing histamine which stimulates parietal cell acid secretion by binding to the parietal cell's basolateral histamine2 (H2) receptor. The increased stimulation of parietal cells results in the hypertrophy and hyperplasia described in the previous section; the stimulation of ECL cells induces their hyperplasia, the initial step of a sequence that may progress to the formation of aggregates of ECL-cells that, depending on their size and shape, are known as linear hyperplasia, micro-carcinoids, and carcinoids.
The normal range of serum gastrin levels depends on the method and laboratory, but is generally below 150 pg/mL. Very high levels of gastrin (e.g., >400 pg/mL) were considered indicative of the Zollinger-Ellison syndrome or end-stage atrophic gastritis, where the gastric corpus has lost most or all the parietal cells and antral G-cells secrete ever increasing quantities of gastrin in a futile attempt to stimulate acid secretion. Chronic use of H2-receptor antagonists or PPIs is typically associated with a slight increase in serum gastrin. However, about 20–25% of chronic PPI users develop modest degrees of hypergastrinemia (200–400 pg/mL) (10). Approximately 1% per year develop significant hypergastrinemia (>400 pg/mL) (vide infra). This significant hypergastrinemia is much more frequent in patients with H. pylori infection than in uninfected subjects with a previously normal stomach.
Effect of PPIs on the H. pylori-infected gastric mucosa
In the late 1980's it was noted that PPI use was associated with an improvement in H. pylori-associated antral inflammation. In 1989 Unge et al. examined both the antrum and corpus and observed that PPI use was associated with a reversible improvement in antral inflammation and H. pylori density, whereas H. pylori density in the corpus either increased or was unchanged (11). Subsequent histologic studies by Stolte and Bethke reported that H. pylori were not detected histologically in either the antrum or corpus in 34% of 154 PPI users, were absent from the antrum and decreased in the corpus in 31%, and decreased in both the antrum and corpus in 35%. In no instance were H. pylori found only in the antrum (12). Later studies in which histology has been combined with quantitative culture confirmed that PPIs use is associated with a change in the distribution of gastric inflammation with improvement in antral histology and worsening and deepening of the corpus inflammation to include the proliferative zone (13). These changes have been attributed to alteration in local pH as H. pylori are killed at pH's below 4 and above 8, are able to survive but not replicate at pH's between 4 and 6, and only replicate at pH's between 6 and 8 (14). The pH of acid within an actively secreting pit is below 1 making colonization of actively secreting pits difficult or impossible for H. pylori.
Overall, these results are consistent with the observations of Kuipers et al. suggesting that PPI use was associated with an increased risk of development of atrophic gastritis, the acknowledged primary risk factor for development of gastric cancer (15-18). Their landmark studies prompted a number of additional investigations; although the final word has yet to be written, most agree that PPI use in H. pylori-infected patients is associated with an increase in corpus inflammation and atrophy (eg, (17;19-23)).
Our approach to the estimation of risk for gastric cancer would be to determine the risk of developing severe atrophic gastritis. Marked hypergastrinemia in PPI users generally identifies patients who have developed gastric atrophy. While there are few studies, the prevalence of gastric atrophy seems to be directly related to the length of the study, suggesting an incidence of approximately 1% per year among those with H. pylori infection (24-29). Importantly, those without known active H. pylori also may develop gastric atrophy while taking PPIs, suggesting that in some patients either H. pylori was not detected or overgrowth by non-H. pylori organisms may on occasion lead to the same outcome. Since approximately a quarter of PPI users develop gastrin levels in the range of 200 to 400 pg/mL, it may be possible to identify those most at risk. Measuring gastrin levels may also provide a good biomarker for prospective studies as well as to identify patients who may benefit from a reduction of PPI dose or use of an alternate approach.
How Each of these Changes Could Theoretically Relate to Cancer
Each of the PPI-induced changes outlined above could be linked to the development of cancer in the stomach and elsewhere in the gastrointestinal tract. Below we present the current evidence for each of these possibilities.
Malignant transformation of fundic gland polyps
Although long-term PPI use is associated with an up to fourfold increase in the risk of fundic gland polyps, the risk of dysplasia is negligible. In a series of 107 chronic PPI users with fundic gland polyps from the Netherlands, only one polyp was believed to have low-grade dysplasia (6). In our own series of 6,065 patients with fundic gland polyps, a single polyp was initially interpreted by the original pathologist as possibly having low-grade dysplasia. Serial sectioning and re-evaluation in a consensus conference led to the diagnosis of reactive changes, most likely related to the healing of an erosion that had occurred on the surface epithelium lining the dilated glands. We conclude that outside of familial polyposis there is no evidence suggesting a malignant potential for fundic gland polyps.
Gastric carcinoids
Although PPI or H2-receptor antagonist-associated hypergastrinemia has been reported to cause gastric carcinoids in female rats, this has not been found in other species, including humans. After approximately 20 years of use and several billion prescriptions worldwide, gastric carcinoids have not been documented to arise in association with PPI use. In a series of 1326 patients receiving various doses of esomeprazole and followed for 6 to 12 months with serial gastric biopsies, simple, linear, or micronodular hyperplasia were detected on final biopsy in 5-12% of patients. However, there were no instances ECL cell dysplasia, carcinoids, or neoplasia (30). We conclude that the risk for carcinoids is low to absent.
Hypergastrinemia and colon cancer
It has been suggested, largely based on in vitro studies that hypergastrinemia may increase the risk of colon cancer. Recent studies have not supported that hypothesis and we conclude that this concern appears unwarranted (31;32).
Accelerated corpus atrophy
PPI use is clearly associated with a low risk of development of gastric atrophy. The risk of developing gastric cancer among those with gastric atrophy is in the range of 500 to 1000 per 100,000 per year (33). Thus, the maximum rate would be in the range of 1/10,000 users per year among H. pylori infected individual and would be difficult or impossible to separate from “naturally occurring” H. pylori-associated gastric cancer. Eradication of H. pylori would reduce the risk remarkably. Clearly, the risk of developing atrophy is very low to non-existent among those without H. pylori infections and can be reduced by H. pylori eradication among those with active H. pylori infections.
Conclusions
PPI use in those with H. pylori infection
Since inflammation of the oxyntic mucosa is believed to be not only a precursor of atrophy, but also an independent risk factor for gastric cancer, it is now generally agreed that H. pylori-positive subjects who are candidates for long-term acid suppression, H. pylori eradication is indicated. However, because, because PPI use is associated with a reduction in the H. pylori bacterial load and, therefore, the diagnosis may be difficult because testing by histology, rapid urease tests, culture, urea breath testing or stool antigen testing can all be negative. Histology is especially likely to be negative in the antrum, and it is important that the endoscopist take samples from the gastric angle and corpus and for the pathologist to be sensitive to the presence of gastric inflammation. One approach it to substitute an H2-receptor antagonist for the PPI as H2-receptor antagonists have no effect on H. pylori growth in the stomach and, therefore, testing would be accurate. A two week period of H2-receptor antagonist substitution should be sufficient. The alternative is to do serologic testing. Because of the high likelihood of a false positive when testing in a low pretest probability condition such as gastroesophageal reflux disease, positive results should be confirmed using a test for active infection (i.e., one of those listed above). False negatives in this instance would be rare and a negative value can be taken at face value.
For those without H. pylori infection
As noted above, the risk of developing gastric cancer in this group is believed to be extremely low. Those who have a marked response and develop consistently high intragastric pH could possibly develop bacterial overgrowth and eventually gastric atrophy. One way to prevent this would be to check serum gastrin levels at regular intervals (e.g., every 5 years). Chronic PPI use must also be considered in relation to other potential risks including risk of developing gastric atrophy, vitamin B12 deficiency, and possible an increase in the risk for hip fractures, community acquired pneumonia, and pseudomembraneous colitis (34-40). Clinically, the most common indications for long term PPI use are for the management of symptomatic gastroesophageal reflux disease and for prevention of NSAID-associated upper gastrointestinal complications. While strict compliance is critical for prevention of NSAID-associated injury (41), intermittent therapy is an effective and less costly strategy for management of uncomplicated gastroesophageal reflux disease, and should also prevent sustained gastric colonization with its possible consequences (42).
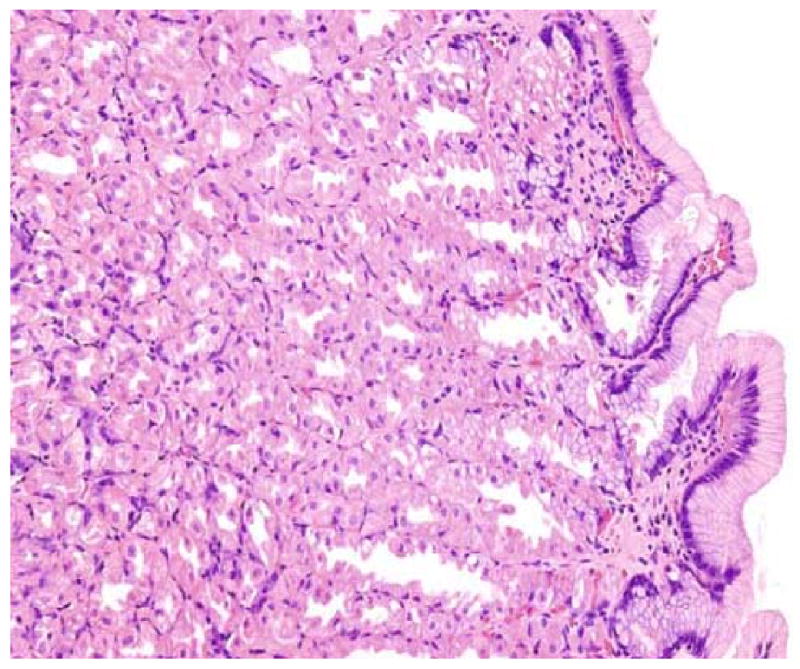
An experienced pathologist will have no difficulty recognizing this patient as a non-H. pylori infected chronic PPI user: the gastric corpus is completely devoid of inflammation, but parietal cells are hyperplastic and protrude into the lumen of dilated oxyntic glands. When these dilatations become more prominent (and, therefore, endoscopically visible) they are known as fundic polyps. Hematoxylin and eosin, original magnification 20×.
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH. In the last 3 years, Dr. Graham has received small amounts of grant support and/or free drugs or urea breath tests from Meretek, Jannsen/Eisai, and TAP, and BioHit for investigator initiated and completely investigator controlled research. Dr. Graham is a consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a also paid consultant for Otsuka Pharmaceuticals and until July 2007 was member of the Board of Directors of Meretek, Diagnostics, the manufacturer of the 13C-urea breath test. Dr. Graham also receives royalties on the Baylor College of Medicine patent covering materials related to 13C-urea breath test. Dr. Genta has been a consultant for AstraZeneca and TAP, and has received grants from Otsuka Pharmaceuticals. Currently he receives no support from any industry is an employee of the Office for Veterans Affairs and Caris Diagnostic, Irving, Texas.
Contributor Information
David Y. Graham, Professor of Medicine and Molecular Virology and Microbiology, Michael E. DeBakey VA Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. Houston, Texas 77030, dgraham@bcm.tmc.edu
Robert M. Genta, Clinical Professor of Pathology and Medicine (Gastroenterology), University of Texas Southwestern Medical Center, 8400 Esters Boulevard - Suite 190, Irving, TX 75063, robert.genta@utsouthwestern.edu
References
- 1.Yeomans ND, Brimblecombe RW, Elder J, et al. Effects of acid suppression on microbial flora of upper gut. Dig Dis Sci. 1995;40:81S–95S. doi: 10.1007/BF02214873. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Chronic gastritis as a cancer precursor. Scand J Gastroenterol Suppl. 1984;104:131–36. [PubMed] [Google Scholar]
- 3.Graham DY, Sung JY. Helicobacter pylori. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger & Fordtran's Gastrointestinal and liver disease Pathophysiology, diagnosis, management. 7th. Philadelphia: WB Saunders Co; 2006. pp. 1049–66. [Google Scholar]
- 4.Elster K. Histologic classification of gastric polyps. Curr Top Pathol. 1976;63:77–93. doi: 10.1007/978-3-642-66481-6_3. [DOI] [PubMed] [Google Scholar]
- 5.Jalving M, Koornstra JJ, Gotz JM, et al. High-grade dysplasia in sporadic fundic gland polyps: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:1229–33. doi: 10.1097/00042737-200311000-00013. [DOI] [PubMed] [Google Scholar]
- *6.Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341–48. doi: 10.1111/j.1365-2036.2006.03127.x. [DOI] [PubMed] [Google Scholar]; This is one of the largest studies showing that long-term proton pump inhibitor use is associated with an up to fourfold increase in the risk of fundic gland polyps and that the risk of dysplasia is negligible. The article also speculates on the genesis of these polyps, which would seem to arise because of parietal cell hyperplasia and parietal cell protrusions resulting from acid suppression.
- 7.Choudhry U, Boyce HW, Jr, Coppola D. Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol. 1998;110:615–21. doi: 10.1093/ajcp/110.5.615. [DOI] [PubMed] [Google Scholar]
- 8.Kazantsev GB, Schwesinger WH, Heim-Hall J. Spontaneous resolution of multiple fundic gland polyps after cessation of treatment with lansoprazole and Nissen fundoplication: a case report. Gastrointest Endosc. 2002;55:600–602. doi: 10.1067/mge.2002.122583. [DOI] [PubMed] [Google Scholar]
- 9.Stolte M. Fundic gland polyps: a rare, innocuous, and reversible disturbance. Gastroenterology. 1993;105:1590–1591. doi: 10.1016/0016-5085(93)90187-h. [DOI] [PubMed] [Google Scholar]
- *10.Orlando LA, Lenard L, Orlando RC. Chronic hypergastrinemia: causes and consequences. Dig Dis Sci. 2007;52:2482–89. doi: 10.1007/s10620-006-9419-3. [DOI] [PubMed] [Google Scholar]; This overview of the origin, causes, and potential risks of chronic hypergastrinemia discusses the two main roles of gastrin in gastrointestinal physiology, as a major factor in meal-stimulated gastric acid secretion and as a trophic hormone for epithelial and enterochromaffin cells. It also discusses the concerns about the potential risk of chronic hypergastrinemia in proton pump inhibitors users.
- 11.Unge P, Gad A, Gnarpe H, Olsson J. Does omeprazole improve antimicrobial therapy directed towards gastric Campylobacter pylori in patients with antral gastritis? A pilot study. Scand J Gastroenterol Suppl. 1989;167:49–54. doi: 10.3109/00365528909091311. [DOI] [PubMed] [Google Scholar]
- 12.Stolte M, Bethke B. Elimination of Helicobacter pylori under treatment with omeprazole. Z Gastroenterol. 1990;28:271–74. [PubMed] [Google Scholar]
- 13.Graham DY, Opekun AR, Yamaoka Y, Osato MS, El Zimaity HM. Early events in proton pump inhibitor-associated exacerbation of corpus gastritis. Aliment Pharmacol Ther. 2003;17:193–200. doi: 10.1046/j.1365-2036.2003.01400.x. [DOI] [PubMed] [Google Scholar]
- 14.Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43 1:S56–60. doi: 10.1136/gut.43.2008.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuipers EJ, Lee A, Klinkenberg-Knol EC, Meuwissen SG. Review article: the development of atrophic gastritis-- Helicobacter pylori and the effects of acid suppressive therapy. Aliment Pharmacol Ther. 1995;9:331–40. doi: 10.1111/j.1365-2036.1995.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 16.Kuipers EJ, Uyterlinde AM, Pena AS, et al. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–28. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 17.Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med. 1996;334:1018–22. doi: 10.1056/NEJM199604183341603. [DOI] [PubMed] [Google Scholar]
- *18.Kuipers EJ, Klinkenberg-Knol EC, Vandenbroucke-Grauls CM, et al. Role of Helicobacter pylori in the pathogenesis of atrophic gastritis. Scand J Gastroenterol Suppl. 1997;223:28–34. [PubMed] [Google Scholar]; This seminal study concluded that patients with reflux esophagitis and H. pylori infection who are treated with long-term acid inhibition have an increased risk of atrophic gastritis. As this term evokes associations with an increased risk of gastric cancer, the possibility was subsequently raised that anti-secretory maintenance therapy might increase the risk of cancer in H. pylori-positive patients and led to many additional studies.
- 19.Moayyedi P, Wason C, Peacock R, et al. Changing patterns of Helicobacter pylori gastritis in long-standing acid suppression. Helicobacter. 2000;5:206–14. doi: 10.1046/j.1523-5378.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- *20.Lundell L, Havu N, Miettinen P, et al. Changes of gastric mucosal architecture during long-term omeprazole therapy: results of a randomized clinical trial. Aliment Pharmacol Ther. 2006;23:639–47. doi: 10.1111/j.1365-2036.2006.02792.x. [DOI] [PubMed] [Google Scholar]; Results of a large prospective study of long term proton pump inhibitor therapy on the gastric mucosa in patients with and without H. pylori infections. This is the latest in a series of studies and had sufficient power and length to provide good recommendations regarding the outcome and the need for H. pylori eradication.
- 21.Larkin CJ, Watson RGP, Sloan JM, et al. Distribution of atrophy in Helicobacter pylori-infected subjects taking proton pump inhibitors. Scand J Gastroenterol. 2000;35:578–82. doi: 10.1080/003655200750023525. [DOI] [PubMed] [Google Scholar]
- 22.Eissele R, Brunner G, Simon B, Solcia E, Arnold R. Gastric mucosa during treatment with lansoprazole: Helicobacter pylori is a risk factor for argyrophil cell hyperplasia. Gastroenterology. 1997;112:707–17. doi: 10.1053/gast.1997.v112.pm9041231. [DOI] [PubMed] [Google Scholar]
- 23.Schenk BE, Kuipers EJ, Nelis GF, et al. Effect of Helicobacter pylori eradication on chronic gastritis during omeprazole therapy. Gut. 2000;46:615–21. doi: 10.1136/gut.46.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bateson MC. Hypergastrinaemia with long-term omeprazole treatment. Aliment Pharmacol Ther. 1999;13:440–441. doi: 10.1046/j.1365-2036.1999.0467d.x. [DOI] [PubMed] [Google Scholar]
- 25.Ligumsky M, Lysy J, Siguencia G, Friedlander Y. Effect of long-term, continuous versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J Clin Gastroenterol. 2001;33:32–35. doi: 10.1097/00004836-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Robinson M. Review article: current perspectives on hypergastrinaemia and enterochromaffin-like-cell hyperplasia. Aliment Pharmacol Ther. 1999;13 5:5–10. doi: 10.1046/j.1365-2036.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 27.Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, Eskes SA, Meuwissen SG. Helicobacter pylori and the efficacy of omeprazole therapy for gastroesophageal reflux disease. Am J Gastroenterol. 1999;94:884–87. doi: 10.1111/j.1572-0241.1999.982_e.x. [DOI] [PubMed] [Google Scholar]
- 28.Schenk BE, Kuipers EJ, Klinkenberg-Knol EC, et al. Hypergastrinaemia during long-term omeprazole therapy: influences of vagal nerve function, gastric emptying and Helicobacter pylori infection. Aliment Pharmacol Ther. 1998;12:605–12. doi: 10.1046/j.1365-2036.1998.00349.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamberts R, Brunner G, Solcia E. Effects of very long (up to 10 years) proton pump blockade on human gastric mucosa. Digestion. 2001;64:205–13. doi: 10.1159/000048863. [DOI] [PubMed] [Google Scholar]
- 30.Genta RM, Rindi G, Fiocca R, et al. Effects of 6-12 months of esomeprazole treatment on the gastric mucosa. Am J Gastroenterol. 2003;98:1257–65. doi: 10.1111/j.1572-0241.2003.07489.x. [DOI] [PubMed] [Google Scholar]
- *31.Robertson DJ, Larsson H, Friis S, et al. Proton pump inhibitor use and risk of colorectal cancer: a population-based, case-control study. Gastroenterology. 2007;133:755–60. doi: 10.1053/j.gastro.2007.06.014. [DOI] [PubMed] [Google Scholar]; Large population based study that addressed the issue of whether proton pump inhibitor use increase the risk for colon cancer. The conclusion was clearly no.
- 32.Yang YX, Hennessy S, Propert K, et al. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology. 2007;133:748–54. doi: 10.1053/j.gastro.2007.06.022. [DOI] [PubMed] [Google Scholar]
- *33.Graham DY, Uemura N. Natural history of gastric cancer after Helicobacter pylori eradication in Japan: after endoscopic resection, after treatment of the general population, and naturally. Helicobacter. 2006;11:139–43. doi: 10.1111/j.1523-5378.2006.00391.x. [DOI] [PubMed] [Google Scholar]; Review of the issues regarding the effect of H. pylori eradication on changing the incidence of gastric cancer. Includes a discuss of the pitfalls of many recent studies.
- 34.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57:422–28. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect. 2003;54:243–45. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 36.Hauben M, Horn S, Reich L, Younus M. Association between gastric acid suppressants and Clostridium difficile colitis and community-acquired pneumonia: analysis using pharmacovigilance tools. Int J Infect Dis. 2007;11:417–22. doi: 10.1016/j.ijid.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Cadle RM, Mansouri MD, Logan N, Kudva DR, Musher DM. Association of proton-pump inhibitors with outcomes in Clostridium difficile colitis. Am J Health Syst Pharm. 2007;64:2359–63. doi: 10.2146/ajhp060629. [DOI] [PubMed] [Google Scholar]
- 38.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 39.Mallow S, Rebuck JA, Osler T, Ahern J, Healey MA, Rogers FB. Do proton pump inhibitors increase the incidence of nosocomial pneumonia and related infectious complications when compared with histamine-2 receptor antagonists in critically ill trauma patients? Curr Surg. 2004;61:452–58. doi: 10.1016/j.cursur.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–53. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- *41.Graham DY, Chan FK. NSAIDs, risks, and gastroprotective strategies: current status and future. Gastroenterology. 2008;134:1240, 1246. doi: 10.1053/j.gastro.2008.02.007. [DOI] [PubMed] [Google Scholar]; Review of the strategies to prevent NSAID-associated gastrointestinal complications that included a review of the data that compliance with gastroprotective therapy was a critical issue in predicting outcome.
- *42.Raghunath AS, O'Morain C, McLoughlin RC. Review article: the long-term use of proton-pump inhibitors. Aliment Pharmacol Ther. 2005;22 1:55–63. doi: 10.1111/j.1365-2036.2005.02611.x. [DOI] [PubMed] [Google Scholar]; Excellent review of all aspects of the pro's and con's of long term proton pump inhibitor use in light of some of the associated risks. The authors conclude that on-demand therapy is more cost-effective than continuous therapy and should be considered wherever possible.


