Abstract
Objective
Loss of muscle strength is common and associated with a variety of adverse health outcomes in old age, but few studies have examined the association of muscle strength with the risk of Alzheimer’s disease (AD) or mild cognitive impairment (MCI). We tested the hypothesis that muscle strength is associated with incident AD and MCI.
Design
Prospective, observational cohort study.
Setting
Retirement communities across the Chicago metropolitan area.
Participants
More than 900 community-based older persons without dementia at the baseline evaluation and in whom strength was measured in nine muscle groups in both arms and legs as well as in the axial muscles and summarized into a composite measure of muscle strength.
Main Outcome Measures
Incident AD, MCI and rate of change in global cognitive function.
Results
During a mean follow-up of 3.6 years, 138 persons developed AD. In a proportional hazards model adjusted for age, sex, and education, each 1 unit increase in muscle strength at baseline was associated with about a 43% decrease in the risk of AD (HR, 0.57; 95% CI, 0.41,0.79). The association of muscle strength with AD persisted even after adjustment for several covariates, including body mass index, physical activity, pulmonary function, vascular risk factors, vascular diseases and apolipoprotein E4 status. Further, in a mixed-effects model adjusted for age, sex, education, and baseline level of global cognition, increased muscle strength was associated with a slower rate of decline in global cognitive function (p<0.001). Finally, muscle strength was associated with a decreased risk of MCI, the precursor to AD (HR, 0.67; 95% CI, 0.54, 0.84).
Conclusion
These findings suggest a link between muscle strength, AD and cognitive decline in older persons.
Introduction
Although Alzheimer’s disease (AD) is characterized clinically by a progressive deterioration in memory and other cognitive abilities, AD is associated with a variety of non-cognitive features including affective manifestations (e.g., depressive symptoms) and impaired motor function (e.g., gait impairment) [1–5]. Recent data suggest that these non-cognitive features may be early signs of AD, as they often predict the onset of clinical AD [6–10]. Although grip strength is related to risk of AD, few studies have examined grip strength and the more general association of muscle strength (measured in multiple body regions) with incident AD remains unknown [11–13]. Further, body mass index (BMI) and physical activity also are related to risk of AD [14–17], yet it is unclear whether the association of muscle strength with AD is independent of these important confounding variables.
We used data from the Rush Memory and Aging Project [18], a longitudinal study of aging, to examine the association of muscle strength with incident AD in more than 900 well-characterized persons initially free of dementia. Participants underwent structured evaluations of muscle strength, including strength testing of 9 muscle groups in the extremities and axial muscle strength based on maximal inspiratory (MIP) and expiratory pressures (MEP), and detailed annual cognitive evaluations. Further, since progressive cognitive decline is the hallmark of AD, we examined the relationship between muscle strength and cognitive decline. Finally, we examined the association of muscle strength with the risk of incident mild cognitive impairment (MCI), the earliest manifestation of AD [21].
Methods
Participants
Participants are from the Rush Memory and Aging Project [18], all of whom have agreed to annual clinical evaluations and organ donation. The study was approved by the Institutional Review Board of Rush University Medical Center.
Eligibility for these analyses required muscle strength testing, the absence of a clinical diagnosis of dementia at baseline, and at least one follow-up evaluation. At the time of these analyses, 1121 participants had completed baseline testing. 76 with dementia were excluded. Of the 1045 remaining, 75 had not yet reached or died before their first follow-up. This resulted in a final group of 970 participants (241 men and 729 women, 92% white) who completed at least 1 follow-up with an average of 3.6 follow-up years (SD=1.5, range: 1–6). Their mean age was 80.3 years (SD=7.5; range=54–100), education was 14.5 years (SD=3.0, range=3–28 years), and MMSE score was 28 (SD=2.1; range=18–30) [19]. Of these persons, 89% had 2 or more evaluations, 75% had 3 or more, and 55% had 4 or more.
Clinical diagnoses
Details of the clinical evaluation have been described [18]. Briefly, each participant underwent a uniform structured baseline evaluation, including medical history, neurological and neuropsychological examinations. Annual follow-ups were identical to the baseline evaluation. Cognitive function was assessed annually via 21 tests [20,21] and the data were reviewed by an experienced neuropsychologist who made a judgment regarding the presence of cognitive impairment. Participants were evaluated in person by a clinician who diagnosed dementia according to the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association [22], which require a history of cognitive decline and evidence of impairment in two or more domains of cognition; classification of AD, the primary outcome in this study, requires that memory is one of the domains affected [22]. Persons were classified as having MCI if they had cognitive impairment but did not meet criteria for dementia, as previously described [21].
Assessment of muscle strength
A composite measure of muscle strength derived from testing in 11 muscle groups was used in this study, as previously described [23, 24]. Appendicular muscle strength was measured using hand-held dynamometers (Lafayette Muscle Test System, Model 01163) in the upper extremities (both arms: abduction, flexion, extension) and the lower extremities (both legs: hip flexion, knee extension, plantar flexion, and ankle dorsiflexion). Grip and pinch strength were measured bilaterally using the Jamar® hydraulic hand and pinch dynamometers (Lafayette Instruments). Axial strength was measured using a hand-held device containing a pressure sensitive transducer to assess maximal pressures generated during inspiration (MIP) and expiration (MEP) [23,25–27]. Bilateral measures were averaged and the scores from each muscle group were converted to z scores using sex-specific means and standard deviations from the baseline evaluations. Finally, z scores of all muscles were averaged to yield a global measure of muscle strength.
Assessment of global cognition
Cognitive function was assessed at each evaluation via 21 tests [18,21]. MMSE scores were used to describe the cohort and Complex Ideational Material was used for diagnostic classification [18,21]. Scores on 19 tests were used to create a composite measure of global cognition: immediate and delayed recall of story A from Logical Memory, immediate and delayed recall of the East Boston Story, Word List Memory, Word List Recall, Word List Recognition, a 15-item Boston Naming Test, Verbal Fluency, a 15-item reading test, Digit Span Forward, Digit Span Backward, Digit Ordering, Symbol Digit Modalities Test, Number Comparison, two indices from the Stroop Test, a 15-item Judgment of Line Orientation and a 16-item Standard Progressive Matrices. To compute the composite, raw scores on each of the individual tests were converted to z-scores using the baseline mean and standard deviation of the entire cohort, and the z-scores of all 19 tests were averaged. Further psychometric information on this composite is contained in previous publications [18,21].
Assessment of Covariates
Age, education, gender, race and ethnicity were recorded at baseline. Weight and height were measured and recorded at each visit, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared [9]. Vascular diseases included stroke, myocardial infarction and claudication and vascular risk factors included diabetes, hypertension, and smoking; the number of diseases and risk factors present at baseline were used in analyses [21]. Pulmonary function was tested using a hand held spirometer which measured vital capacity, force expiratory volume and peak expiratory flow [23, 27]. Physical activity was evaluated with questions from the 1985 Health Interview Survey [18,23].
Data analysis
Pearson correlations were used to examine bivariate associations, and t-tests were used to compare men and women and those who did versus those who did not develop AD. A proportional hazards model [28] with time to AD as the outcome was used to examine the association of muscle strength with the risk of incident AD; this model controlled for age, sex, and education. We also examined the influence of important covariates, conducted a series of sensitivity analyses, and finally examined the association of strength with the risk of MCI.
Mixed-effects models [29] were used to examine the association of muscle strength with cognitive decline. Thus, we estimated the mean change in the group, conditional on covariates, as in standard fixed-effects repeated measures models, and the mixed-effects model included random coefficients which provided estimates of individual differences from the group. Each person was assumed to follow the average path of the group except for random effects that caused the baseline level of cognition to be lower or higher and the rate of change in cognition to be faster or slower. The variance-covariance matrix for the random coefficients was not assumed to be of a restricted form and we assumed that residual error was normally distributed and independent of the random effects. A major strength of this approach is the ability to model all data available for each person, regardless of length of follow-up, number and spacing of evaluations, or missing data at some evaluations.
The mixed-effects model controlled for age, sex, and education and included terms for time, time-squared, muscle strength, and the interaction of muscle strength with time. We also tested for non-linearity in the association of the strength measure with cognition, but since this was not significant, the term for time-squared × muscle strength was not retained in the final models. All models were validated graphically and analytically and programming was done in SAS®[30].
Results
Metric properties of the composite measure of muscle strength
Muscle strength ranged from −1.6 to 3.3 (mean=0.006, SD=0.66), with higher scores reflecting greater strength. Muscle strength was negatively associated with age (r=−0.35, p<0.001) and positively associated with global cognition (r=0.20, p<0.001).
Muscle strength and the risk of AD
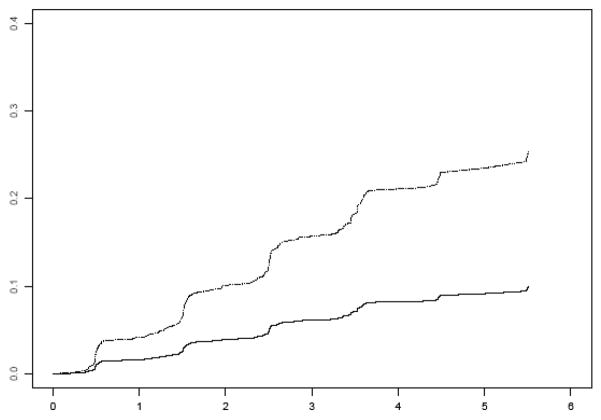
Over an average of 3.6 years of follow-up, 138 persons developed AD (15% of 970). Those who developed AD were older, had lower cognitive function, and decreased strength in several muscles compared to those who did not (Table 1). In the core proportional hazards model adjusted for age, sex, and education, muscle strength was associated with the risk of developing AD, such that each 1 unit increase in muscle strength (based on 11 muscle groups) at baseline was associated with about a 43% decrease in the risk of AD (HR, 0.57; 95% CI, 0.41,0.79). Figure 1 shows that a participant with a high level of muscle strength (90th percentile, score=0.85) has about a 61% decreased risk of developing AD compared to a participant with a low level of strength (10th percentile, score=−0.81).
Table 1.
Baseline characteristics of participants who did vs. did not develop AD.
| Characteristic | Developed AD (mean, SD) N=138 | Did not develop AD (mean, SD) N=832 | P-Value* |
|---|---|---|---|
| Age, years | 84.5, 6.0 | 79.8, 7.3 | < 0.0001 |
| Men, % | 19.48 | 80.52 | 0.03 |
| Educ, years | 14.5, 3.0 | 14.6, 7.2 | 0.9 |
| Arm abduction (lbs.) | 3.5, 2.4 | 4.0, 2.2 | 0.02 |
| Elbow flexion (lbs.) | 11.6, 4.7 | 12.9, 5.4 | 0.004 |
| Elbow extension (lbs.) | 9.6, 3.5 | 10.8, 3.9 | 0.001 |
| Grip (lbs.) | 43.2, 17.6 | 49.3, 18.0 | 0.0003 |
| Pinch (lbs.) | 9.9, 5.1 | 10.9, 5.0 | 0.03 |
| Hip flexion (lbs.) | 9.8, 4.1 | 10.6, 4.7 | 0.03 |
| Knee extension (lbs.) | 9.9, 3.8 | 10.8, 4.1 | 0.02 |
| Ankle Plantar flexion (lbs.) | 14.6, 4.5 | 15.2, 5.2 | 0.13 |
| Ankle dorsiflexion (lbs.) | 10.4, 4.0 | 11.7, 5.0 | 0.001 |
| Maximal expiratory pressure (mm H20) | 60.8, 25.2 | 68.1, 24.5 | 0.002 |
| Maximal inspiratory pressure (mm H20) | 33.8, 18.4 | 41.7, 20.8 | < 0.0001 |
| BMI (mean, sd) | 26.3, 4.1 | 27.5, 5.5 | 0.004 |
| Pulmonary function (mean, sd) | −0.09, 0.83 | 0.05, 0.91 | 0.1 |
| Physical activity (mean, sd) | 3.1, 3.8 | 3.1, 3.6 | 0.8 |
| Vascular risk factors (mean, sd) | 1.1, 1.0 | 1.2, 1.0 | 0.7 |
| Vascular diseases (mean, sd) | 0.4, 0.6 | 0.3, 0.6 | 0.4 |
| MMSE Score | 26.28, 2.8 | 28.27, 1.84 | < 0.0001 |
| Global cognition | −0.41, 0.49 | 0.22, 0.48 | <0.001 |
Mean values and standard deviations are presented unless otherwise noted and statistical significance is based on t-tests or Chi-Square tests, as appropriate.
Figure 1.
Cumulative hazard of developing AD for participants with low (10th percentile, dotted line) versus high muscle strength (90th percentile, solid line).
Next, because prior studies have shown that grip strength is related to the risk of AD and it is possible that our finding was driven by grip, we constructed a proportional hazards model to simultaneously examine the relative predictive influence of the components of strength (i.e., grip strength, all other measures of upper extremity strength, lower extremity strength, and axial muscle strength) with the risk of AD. In this model, grip strength was associated with AD (HR=0.61, 95% CI=0.47, 0.80); however, axial muscle strength was associated with risk of AD even after accounting for the effect of grip strength (HR=0.68, 95% CI=0.53, 0.87); by contrast, lower extremity function and upper extremity strength were not individually associated with the risk of AD.
Further, because there are several covariates that may account for the association of muscle strength with AD, we repeated the core model above after adding terms for the following covariates separately and together: physical activity, pulmonary function, vascular risk factors, vascular disease, body mass index, body mass index-squared (because both low and high BMI are associated with AD), and the presence of the apolipoprotein E4 allele. The addition of these covariates individually (results not shown) or together in a single model did not substantially affect the association between muscle strength and risk of AD (HR 0.59, 95% CI 0.41, 0.84).
Sensitivity analyses
We conducted a series of sensitivity analyses to address the possibility that the findings above were driven by the inclusion of persons with very early and undiagnosed AD or those with the lowest function at baseline. First, we repeated the core analysis after sequentially excluding persons who developed AD in the first year of follow-up (n=32) and then in the first or second year (n=74). In these analyses, the association of muscle strength with AD was not substantially changed (HR=0.59, 95% CI 0.40, 0.86, and HR=0.61, 95% CI 0.37, 1.03, respectively). Next, we re-ran the core analysis after excluding persons in the bottom 15% in terms of cognition at baseline and the finding persisted (HR=0.48, 95% CI=0.30. 0.77). Finally, we re-ran the core model after excluding persons in the bottom 15% in terms of muscle strength at baseline and the association of muscle strength with AD persisted (HR=0.57, 95% CI=0.35, 0.90).
Muscle strength and change in global cognitive function
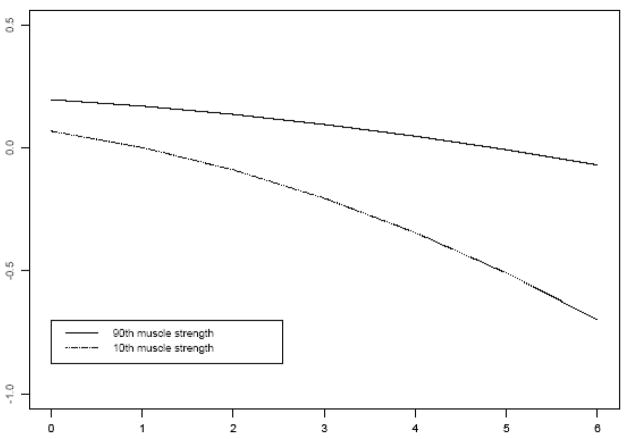
Because AD develops slowly over many years and its hallmark is change in cognitive function, we examined the association of muscle strength with cognitive decline. At baseline, scores on the composite measure of global cognition (based on 19 tests) ranged from −1.8 to 1.4 (mean=0.12, standard deviation=0.54), with higher scores indicating better performance. We constructed a mixed-effects model that controlled for age, sex, and education and included terms for time, time-squared, muscle strength, and the interaction of muscle strength with time to examine the association of strength with cognitive decline. Scores on the composite measure of global cognitive function showed both linear and non-linear decline (both p’s<0.05). Further, each one-unit increase in muscle strength at baseline was associated with about a 0.04 standard unit decrease in the rate of decline in global cognition (p<0.001). Figure 2 shows that the rate of cognitive decline for a participant with a high level of muscle strength (90th percentile, score=0.85) was considerably slower than that of a participant with a low level of strength (10th percentile, score=−0.81). The addition of covariates did not substantially affect the association between muscle strength and rate of cognitive decline (results not shown).
Figure 2.
Decline in global cognitive function for participants with low (10th percentile, dotted line) versus high muscle strength (90th percentile, solid line).
Muscle strength and the risk of MCI
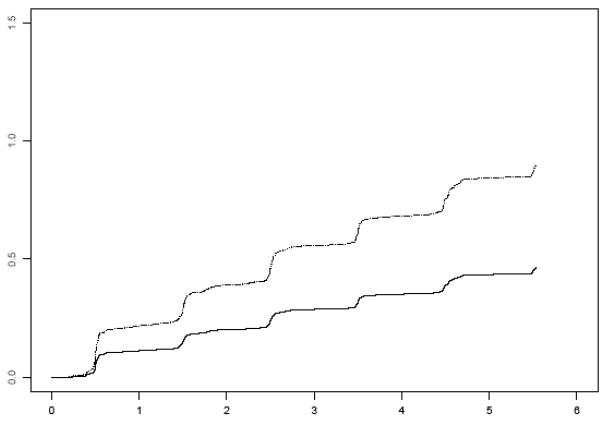
Finally, because it is now widely recognized that most persons who develop AD transition through an early stage of impairment referred to as MCI, we excluded persons with any evidence of cognitive impairment at baseline and constructed a proportional hazards model examining the association of muscle strength with incident MCI. Over an average of 3.6 years of follow-up, 275 persons developed MCI (40% of 694). In the proportional hazards model adjusted for age, sex and education, muscle strength was associated with the risk of developing MCI (HR, 0.67; 95% CI, 0.54, 0.84). Figure 3 shows that a participant with a high level of muscle strength (90th percentile, score=0.85) had about a 48% decreased risk of developing MCI compared to a participant with a low level of strength (10th percentile, score=−0.81). Further, muscle strength also was associated a decreased risk of persistent MCI (persistent meaning MCI followed by MCI, dementia or death at a subsequent evaluation; HR=0.55, 95% CI=0.38, 0.79).
Figure 3.
Risk of MCI for participants with low (10th percentile, dotted line) versus high muscle strength (90th percentile, solid line).
Discussion
In more than 900 well-characterized community-based older persons without dementia, we found that greater muscle strength was associated with a decreased risk of developing AD. This finding persisted in sensitivity analyses in which we excluded persons who developed AD in the early follow-up years and those with the lowest function at baseline and in models that controlled for BMI, physical activity, pulmonary function, vascular risk factors, vascular diseases and the apolipoprotein E4 allele. Further, muscle strength was associated with the rate of cognitive decline, such that persons with greater strength at baseline exhibited a considerably slower rate of decline. Finally, in an analysis excluding persons dementia or MCI at baseline, muscle strength was associated with the risk of developing MCI, the earliest manifestation of cognitive impairment. Overall, these data show that greater muscle strength is associated with a decreased risk of developing AD and MCI and suggest that a common pathogenesis may underlie loss of strength and cognition in aging.
Although the clinical hallmark of AD is declining cognition, motor signs that frequently accompany AD often precede and predict the clinical diagnosis of AD [1–12,32–34,36,47]. Loss of muscle strength and mass also are common in aging, and frailty and BMI are associated with risk of AD [6–12,36]. While measures of frailty and BMI can be obtained inexpensively, they do not inform on the role of muscle mass versus strength with the risk of AD, and recent data suggest that muscle strength is associated with cognition independent of muscle mass [14]. To date, data on muscle strength and AD is limited. One study reported that grip strength was predictive of cognitive decline in older Mexican Americans [35], but this study likely included persons with mild dementia at baseline. In another cohort of Catholic Clergy, we found that grip strength was associated with incident AD [17]. While these findings are important and motivated the current study, grip strength may not fully capture the association of muscle strength (measured more comprehensively) with the risk of AD. We quantified muscle strength in all four extremities as well as in the axial muscles in a large cohort of older persons and found that greater strength was associated with a reduced risk of AD. Further, in analyses of the components of muscle strength, axial muscle strength was associated with the risk of AD even after accounting for grip strength, suggesting that comprehensive assessments of strength may be useful for identifying persons at risk for cognitive impairment. Finally, muscle strength also was associated with a substantially decreased risk of MCI, suggesting a temporal relation whereby impaired strength precedes the development of cognitive impairment in aging. Assessment of muscle strength may have utility for identifying persons at risk for even the earliest manifestation of cognitive impairment and who may benefit most from intervention.
The basis of the association of muscle strength with AD is unknown. Although decreased muscle strength may represent a true risk factor for AD, it is more likely that loss of muscle strength is the result of an underlying disease process that also leads to cognitive decline and clinical AD. For example, muscle plays an important role in energy production and regulation; the mitochondrial theory of aging proposes that damaged mitochondria accumulate over time and these and related energy disruptions are in part responsible for loss of muscle strength and other signs of aging [44]. The discovery of mitochondrial diseases in aged organisms provides some support for this hypothesis; however, questions remain about its relevance to human aging. It is also important to consider that, while muscle is situated outside the blood brain barrier making it vulnerable to systemic diseases, muscle function is controlled by spinal motor neurons, which reflect supraspinal motor control systems [37,38]. Decreased strength may result from disorders of the central nervous system (e.g. stroke) that may also unmask subclinical AD [46]. Notably, in this study, the association between strength and AD was unchanged after controlling for vascular risk factors and diseases, suggesting that other factors are important. One such factor is AD pathology, which accumulates slowly over time (before the onset of clinical dementia) and may contribute to decreased strength and cognition. AD pathology frequently occurs in regions that subserve motor function [39–42]. Further, the link between executive cognition and movement may suggest that AD pathology even in cognitive regions may contribute to motor decline [45]. We previously reported an association between AD pathology in the cognitive regions and grip strength [43]. Future studies are needed to clarify the neurobiologic basis of the association of muscle strength with the risk of AD and the rate of cognitive decline.
Limitations of this study include the selected nature of the cohort, which included participants willing to provide organ donation. Replication of these results in a population-based study is important. Further, although our results suggest that decreased muscle strength precedes the development of cognitive impairment, observational studies cannot directly address the issue of causality. We also cannot rule out the possibility of residual confounding or that a latent variable underlies the association of muscle strength and cognition. However, the study has several strengths, including the use of a large cohort of well-characterized older persons and composite measures of strength and cognition, the uniform classification of AD and MCI, and the ability to examine several potential confounders of the association between strength and AD.
References
- 1.Scarmeas N, Albert M, Brandt J, Blacker D, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Wegesin D, Marder K, Bell K, Honig L, Stern Y. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Motor dysfunction in mildly demented AD individuals without extrapyramidal signs. Neurology. 1999;53:956–962. doi: 10.1212/wnl.53.5.956. [DOI] [PubMed] [Google Scholar]
- 3.Pettersson AD, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(5–6):299–304. doi: 10.1159/000084555. [DOI] [PubMed] [Google Scholar]
- 4.Shin IS, Carter M, Masterman D, Fairbanks L, Cummings Jl. Neuropsychiatric symptoms and quality of life in Alzhiemer’s disease. Am J Geriatr Psychiatry. 2005;13(6):469–74. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61(8):1102–1107. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–20. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 7.Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol. 2004;61:1273–6. doi: 10.1001/archneur.61.8.1273. [DOI] [PubMed] [Google Scholar]
- 8.Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J Neurol Sci. 2005;229–230:89–93. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60:539–44. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 10.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty Is Associated with Incident Alzheimer’s Disease and Cognitive Decline in the Elderly. Psychosom Med. 2007;69(5):483–9. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 11.Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61(8):859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milne JS, Maule MM. A longitudinal study of handgrip and dementia in older people. Age Aging. 1984;13:42–48. doi: 10.1093/ageing/13.1.42. [DOI] [PubMed] [Google Scholar]
- 13.Buchman, Boyle Wilson, Bienias, Bennett Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1–2):66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003 Jul 14;163(13):1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 15.Taafe DR, Irie F, Masaki KH, Abbott RD, Petrovitch H, Ross GW, White LR. Physical activity, physical function, and incident dementia in elderly men: the Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2008;63(5):529–351. doi: 10.1093/gerona/63.5.529. [DOI] [PubMed] [Google Scholar]
- 16.Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Wilson RS, Bienias JL, Shah R, Evans DA, Bennett DA. Change in body mass index (BMI) and risk of incident Alzheimer’s disease (AD) Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush memory and aging project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 21.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology. 2006;67(3):441–445. doi: 10.1212/01.wnl.0000228244.10416.20. \. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachmann D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease. Report of the NINCDS-ADRDA Work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Buchman AS, Boyle PA, Wilson RS, Gu L, Bienias J, Bennett Pulmonary function, muscle strength and mortality in old age. Mech Aging Dev. 2008;129(11):625–631. doi: 10.1016/j.mad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchman AS, Wilson RS, Boyle PA, Bienias J, Bennett DA. Change in motor function and risk of mortality in older persons. JAGS. 2007;55(1):11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Sapienza CM. Implications of expiratory muscle strength training for rehabilitation of the elderly: Tutorial. J Rehabil Res Dev. 2005;42:211–224. doi: 10.1682/jrrd.2004.07.0077. [DOI] [PubMed] [Google Scholar]
- 26.Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular health study research group. Am J Respir Crit Care Med. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AS, Boyle PA, Wilson RS, Leurgans SL, Shah R, Bennett DA. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008;31(3):174–178. doi: 10.1159/000154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life-tables [with discussion] J R Stat Soc (B) 1972;74:187–220. [Google Scholar]
- 29.Laird N, Ware J. Random effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 30.SAS Institute Inc. SAS/STAT User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 31.Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 years. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 32.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43:2184–2188. doi: 10.1212/wnl.43.11.2184. [DOI] [PubMed] [Google Scholar]
- 33.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs and incident dementia: follow-up analysis. Neurology. 1995;45:1942. doi: 10.1212/wnl.45.10.1942. [DOI] [PubMed] [Google Scholar]
- 34.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004 Sep 22;292(12):1447–53. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 35.Alfaro-Acha A, Al Snih S, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006 Aug;61(8):859–65. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded weight loss for at least a decade. Neurology. 2007;69(8):739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 37.Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. doi: 10.1002/(sici)1097-4598(199706)20:6<679::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Johannsen P, Christensen LO, Sinkjaer T, Nielsen JB. Cerebral functional anatomy of voluntary contractions of ankle muscles in man. J Physiol. 2001;535:397–406. doi: 10.1111/j.1469-7793.2001.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;2:217. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 40.Lehericy S, Bardinet E, Tremblay L, Van de Moortele P-F, Pochon J-B, Dormont D, Kim D-S, Yelnik J, Ugurbil K. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cerebral Cortex. 2006;16:149–61. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- 41.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–73. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 42.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64:1397–403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 43.Buchman AS, Schneider JA, Leurgans SL, Bennett DA. Frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71(7):499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston AP, De Lisio M, Parise G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl Physiol Nutr Metab. 2008;33(1):191–9. doi: 10.1139/H07-141. [DOI] [PubMed] [Google Scholar]
- 45.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disorders . 2008:329–42. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]