Abstract
The goal of this study was to assess the ability of iloprost, an orally active prostacyclin analog, to inhibit transformed growth of human non-small cell lung cancer (NSCLC) and to define the mechanism of iloprost's tumor suppressive effects. In a panel of NSCLC cell lines, the ability of iloprost to inhibit transformed cell growth was not correlated with the expression of the cell surface receptor for prostacyclin, but instead was correlated with the presence of Frizzled 9 (Fzd 9) and the activation of peroxisome proliferator-activated receptor-γ (PPARγ). Silencing of Fzd 9 blocked PPARγ activation by iloprost, and expression of Fzd 9 in cells lacking the protein resulted in iloprost's activation of PPARγ and inhibition of transformed growth. Interestingly, soluble Frizzled-related protein-1, a well-known inhibitor of Wnt/Fzd signaling, also blocked the effects of iloprost and Fzd 9. Moreover, mice treated with iloprost had reduced lung tumors and increased Fzd 9 expression. These studies define a novel paradigm, linking the eicosanoid pathway and Wnt signaling. In addition, these data also suggest that prostacyclin analogs may represent a new class of therapeutic agents in the treatment of NSCLC where the restoration of noncanonical Wnt signaling maybe important for the inhibition of transformed cell growth.
Introduction
Lung cancer continues to be the leading cause of cancer death in both men and women in the United States [1]. Most lung cancers are diagnosed in former smokers, and to date, no effective treatment has been discovered for nonsurgical lung cancer. The abysmal 5-year lung cancer survival rates [2,3] underscore the need for better chemotheraputeic agents in the treatment of this disease.
Prostacyclin (PGI2) is a naturally occurring eicosanoid that possesses anti-inflammatory and antimetastatic properties [4]. Our laboratory has demonstrated that mice with targeted overexpression of prostacyclin synthase (PGIS) in the distal lung epithelia are protected against lung tumor formation in response to chemical carcinogens or cigarette smoke [5,6]. Supplementation with iloprost (a long-acting oral PGI2 analog) also protects against chemically induced lung carcinogenesis [7]. In fact, a recent phase 2 clinical trial provides evidence that shows iloprost to be a very effective chemopreventive agent in former smokers. However, the mechanism of iloprost's antitumorigenic effect is not well understood. Classically, the biological effects of PGI2 are mediated through binding to a specific G protein-coupled receptor (PTGIR), which signals through Gs resulting in the elevation of cyclic adenosine monophosphate (cAMP) [8]; however, mice overexpressing PGIS that lack PTGIR, the receptor for PGIS/iloprost, are still protected against lung tumorigenesis, suggesting signaling through other receptors [7]. In addition, the efficacy of prostacyclin analogs as chemotherapeutic agents in lung cancer has not been examined. The goal of this study was to determine the mechanism by which PGI2/iloprost affects the growth on human non-small cell lung cancer (NSCLC) cells independent of the PTGIR membrane receptor. We report that the ability of iloprost to inhibit transformed growth is dependent on the expression of the Frizzled 9 (Fzd 9) receptor. Interestingly, iloprost, similar to Wnt 7a [9], also activates peroxisome proliferator-activated receptor-γ (PPARγ) through an ERK5-dependent pathway, suggesting that iloprost is able to mimic the effects of Wnt 7a signaling and directly signals through Fzd 9. In addition, this novel study is the first to identify an association between the Wnt 7a and prostacyclin signaling pathways in NSCLC.
Materials and Methods
Materials, Cell Culture, and Retrovirus-Mediated Gene Transfer
NSCLC lines of H358, A549, H661, H1793, H2009, H157, H460, H1334, H1703, and H2122 were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in an humidified 5% CO2 incubator. RL-65, a spontaneously immortalized, nontransformed lung epithelial cell, was cultured as previously described by Bren-Mattison [10]. BEAS-2B, another immortalized, but non-transformed lung epithelial cell line, was cultured in RPMI 1640 medium supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator. Wi-38 was cultured in minimum essential medium with Earle salts, nonessential amino acids, glutamine, and 1 mM sodium pyruvate, supplemented with 10% FBS at 37°C in a humidified 5% CO2 incubator. The H157-LNCX-LPCX and H157-Fzd-9 stable clones have been previously described [11,12]. H157-PTGIR stable clones were also created. The chemicals 10 µM iloprost (Cayman Chem, Ann Arbor, MI), 10 µM ciglitazone (Cayman Chem), 10 µM PGE2 (Sigma, St. Louis, MO), 5 µM T0070907 (Cayman Chem), 25 µM PD98059 (Sigma), and 5 µM PD184352 (a generous gift from Dr. Philip Cohen, University of Dundee, Scotland) were all applied daily for each cell line used in our studies.
Quantitative Polymerase Chain Reaction
RNA was extracted from cultured cells with the RNeasy mini kit (Qiagen, Inc, Valencia, CA). Total RNA that had been extracted from the various cell lines. Aliquots of the RNA (10 µg) were converted to complementary DNA with Superscript II (Invitrogen Corp, Carlsbad, CA) and random hexamers according to the manufacturer's specifications. Primer sets for the quantitative reverse transcription-polymerase chain reaction (RT-PCR) of human Fzd 9, human and rat PTGIR, human PPARγ, human E-cadherin, and GAPDH were designed. Rat IP/PTGIR: For 5′ TGT CAC ATG TAC CGC CAA CAG AGA 3′, Rev 5′ ACC AGA ACT TGA GGC GTT GGA AGA 3′, Human IP/PTGIR: For 5′ GCC CTC CCC CTC TAC CAA 3′, Rev 5′ TTT TCC AAT AAC TGT GGT TTT TGT G 3′, Human PPARγ: For 5′ CAT AAT GCC ATC AGG TTT GG 3′, Rev 5′ TCA GCG GAC TCT GGATTC AG 3′, Human FZD9: For 5′ AAT TTT CAT GTC ACT GGT GGT GG 3′, Rev 5′ TGC GGT AGC ACA GGG TCT G 3′, Human E-cadherin: For 5′CGG GAA TGC AGT TGA GGATC 3′, Rev 5′ AGGATG GTG TAA GCG ATG GC3′, Human GAPDH: For 5′ GGT GTC GCT GTT GAA GTC AGA G 3′, Rev 5′ GCC AAA TAT GAT GAC ATC AAG AAG G 3′, Mouse GAPDH: For 5′ CGT GGA GTC TAC TGG CGT CTT CAC 3′, Rev 5′ CGG AGA TGA CCC TTT TGG C 3′. The relative quantification of real-time PCR data was determined by ΔΔCt. P ≤ .05 was considered statistically significant by Student's t-test.
Transfections and Reporter Luciferase Assays
The reporter plasmids (PPAR-RE, PDRE, PPARα, PPARγ-gal-4, E-cadherin, Topflash, and MEF2C-gal 4), effector plasmids (Wnt 7a, Fzd 9, wild-type PPARγ, wild-type PPARδ, wild-type PPARα, soluble Frizzled-related protein-1 [sFRP1], β-catenin, PGIS, DN-MEK5, and MKK5-alpha DD), and CMV-β-gal control plasmids were transfected into cells using LipofectAmine reagent (Life Technologies, Carlsbad, CA) as previously described [12]. Small interfering RNA (siRNA) against Fzd 9 were purchased from Qiagen and used at concentrations between 50 and 75 nM. All cells were transfected with a negative control siRNA tagged with a fluorescent marker (Qiagen), which allows for an assessment of transfection efficiency.
Immunoblot Analysis
For immunoblot analysis of phospho-ERK5 (1:500; Cell Signaling, Danvers, MA), total ERK5 (1:500; Cell Signaling), phospho-p44/42 mitogen-activated protein kinase (1:1000; Cell Signaling), total p44/42 MAP kinase (1:1000; Cell Signaling), E-cadherin (1:500; BD Transduction Laboratories, San Jose, CA), β-actin (1:2000; Abcam, Cambridge, MA), β-catenin (1:1000; BD Transduction Laboratories), and Fzd 9 (1:400; Aviva Systems Biology, San Diego, CA), cell extracts were prepared in MAP kinase lysis buffer as previously described [13].
Soft Agar Colony Formation
Measurement of anchorage-independent cell growth was performed as previously described [13].
Cell Lines and cAMP Determination
Freshly isolated cell lines were resuspended in medium containing 10% fetal calf serum with 100 µM isobutylmethylxanthine (Sigma) ± 5 µM iloprost. The cells were incubated at 37°C for 45 minutes and then washed three times quickly with cold phosphate-buffered saline. The cell pellet was then lysed in cold 0.1 M HCl. Cellular cAMP content was measured using the direct cAMP kit from Assay Designs (Ann Arbor, MI). Results are reported as picomole of cAMP per milligram of protein.
Carcinogenesis Protocol and Iloprost Treatment of Mice
FVB/N mice 8 to 12 weeks of age were subjected to a single intraperitoneal injection of ethyl carbamate (Sigma) at a dose of 1 mg/g mouse weight dissolved in normal saline. To determine the ability of iloprost to prevent tumor formation, mice were fed antioxidant-free chow (AIN-76A; Test Diet, Richmond, IN) containing 3% ground iloprost tablets as described previously [5]. Iloprost or control chow was maintained for 5 weeks after the initial urethane treatment. Animals were killed 20 weeks later through pentobarbital overdose. Lungs were removed, and tumors were counted. Whole lungs from control or iloprost-fed mice were snap-frozen, and whole-cell lysates were analyzed by Western analysis for Fzd 9 expression, as described previously.
Statistical Analysis
Experimental results are represented in graphs as means ± SEM. Data were analyzed by Student's t-test for comparison of two data sets using GraphPad Prism (San Diego, CA) with statistical significance at P < .05 and is indicated by an asterisk (*).
Results
Anchorage-Independent Growth Is Inhibited in Iloprost-Sensitive NSCLC
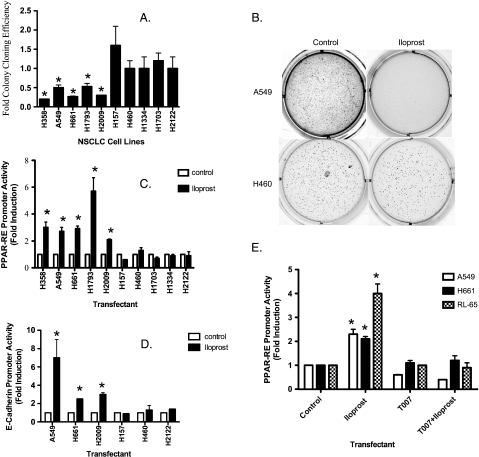
We assessed the effects of iloprost on anchorage-independent growth in a panel of NSCLC cell lines. Measurement of soft agar colony formation showed that iloprost was inhibitory by greater than 50% reduction in colony formation in some of the NSCLC tested (H358, A549,H661, H1793,H2009) but not in others (H157, H460, H1334, H1703, H2122; Figure 1, A and B). These results suggest that the cell lines that responded to iloprost express a receptor that the nonresponding cell lines lack. Because several studies have demonstrated frequent loss of both PGIS and PTGIR in lung cancer cell lines and primary tumors [14–16], we measured PTGIR expression by quantitative RT-PCR. Levels of PTGIR messenger RNA (mRNA) were low or undetectable in all of the NSCLC cell lines tested compared with several nontransformed lung epithelial cell lines (RL-65 and BEAS2B) and Wi-38, which was used as a positive control for PTGIR (Figure W1A). The NSCLC cell lines were also evaluated for their level of PPARγ expression and response to the specific PPARγ agonist ciglitazone. All the cell lines had detectable PPARγ expression as determined by quantitative RT-PCR (data not shown) and were responsive to ciglitazone as demonstrated by ciglitazone's ability to activate PPAR-RE (data not shown). To confirm the lack of functional PTGIR expression, NSCLCs were stimulated with iloprost and increases in cAMP were determined [7,17]. Iloprost failed to increase cAMP in any of the NSCLC lines examined (Figure W2B). As a positive control, iloprost increased cAMP levels in H157 cells stably expressing PTGIR.
Figure 1.
Iloprost inhibits anchorage-independent growth in some but not all NSCLC. (A) The indicated NSCLC cell lines were grown in soft agar in the absence or presence of 10 µM iloprost. The colonies were incubated for several weeks and subsequently counted as described in the Materials and Methods. Data are shown as mean of three independent experiments with the SEM indicated. (B) The cell line A549 is shown to be iloprost-sensitive by soft agar, whereas H460 is seen to be an iloprost-insensitive cell line. (C) Activation of PPARγ and E-cadherin is induced by iloprost. The indicated NSCLC cell lines were transiently transfected with PPAR-RE, along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. Extracts were prepared and promoter activity was determined as luciferase units normalized to CMV-β-gal. Results represent the mean of three independent experiments with the SEM indicated. (D) The indicated NSCLC cell lines were transiently transfected with E-cadherin reporter, along with CMV-β-gal to normalize for transfection efficiency and exposed for 48 hours with 10 µM iloprost. The PPARγ antagonist compound T0070907 reduces PPAR-RE activity induced by iloprost/Fzd 9. (E) The indicated cell lines were transiently transfected with PPAR-RE, or empty vector, along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost and/or 5 µM T0070907.
Iloprost Drives PPARγ Activation in a Subset of NSCLC and Correlates to Anchorage-Independent Growth in NSCLC Cell Lines
Data from other groups have shown that prostacylin analogs can signal through PPARs [6,18,19]. We examined the ability of iloprost to activate PPARα, PPARδ, or PPARγ in a panel of NSCLC cell lines using a PPARα-, δ-, or γ-specific reporter linked to firefly luciferase. Iloprost increased PPAR-RE activity in H358, A549, H661, H1793, and H2009, but gave no significant stimulation in H157, H460, H1334, H1703, or H2122 (Figure 1C). Interestingly, neither PPARα nor PPARδ was activated by iloprost in the iloprost-sensitive cell lines A549, H661, and H2009 (Figure W1C). We also examined E-cadherin, a downstream target of PPARγ [9,11], and discovered that iloprost similarly increased both E-cadherin reporter activity and protein expression (Figures 1D and W1B). Interestingly, iloprost increased PPAR-RE activity in several cell lines, specifically A549, H661, and RL-65, a nontransformed lung epithelial cell; this increase was completely blocked by the specific PPARγ inhibitor, T0070907 (Figure 1E). Thus, activation of PPARγ and E-cadherin by iloprost correlated with the inhibition of anchorage-independent growth. Iloprost did not stimulate the activation or reduction of the canonical β-catenin pathway in any of the cell lines tested (data not shown).
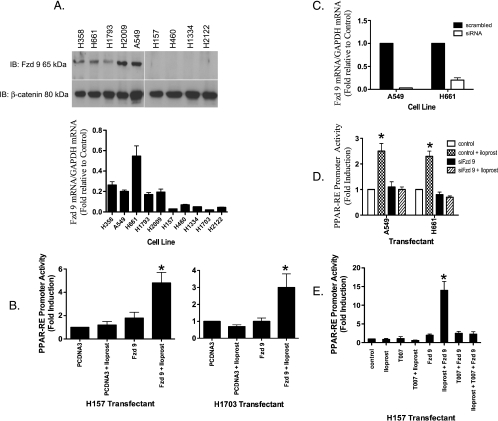
The Presence of Fzd 9 Is Critical for Activation of PPARγ by Iloprost
Given that most of the NSCLC cell lines lacked PTGIR (Figure W1A) and that iloprost did not increase PPARγ activity through cAMP (Figure W2B), we hypothesized that iloprost-mediated activation of PPARγ might be mediated by another seven-transmembrane spanning G protein-coupled receptor. We identified Fzd 9 as being a potential candidate receptor mediating the effects of iloprost activation of PPARγ. Quantitative RT-PCR and immunoblot of Fzd 9 mRNA and protein were detected in all cell lines in which iloprost activated PPAR-RE (H358, A549, H661, H1793, and H2009) but was absent in cells that were unresponsive to iloprost (H157, H460, H1334, H1703, and H2122; Figure 2A). Furthermore, quantitative RT-PCR of these NSCLC cell lines showed that the H358, A549, H661, H1793, and H2009 cells all expressed endogenous Fzd 9 but no Wnt 7a [9,12]. H460, H1334, and H2122 expressed endogenous Wnt 7a, but no Fzd 9 [9,12], and H157 and H1703 did not express either Wnt 7a or Fzd 9 [9,12]. RL-65, a spontaneously immortalized, nontransformed lung epithelial cell, expressed both Wnt 7a and Fzd 9. The expression of Fzd 9 or iloprost alone in both the H157 and H1703 cells (these cells lacked endogenous Wnt 7a, Fzd 9, and PTGIR) did not affect basal PPAR-RE activity, but iloprost increased PPAR-RE activity by approximately three-fold in the context of transient expression of Fzd 9 (Figure 2B).
Figure 2.
RNAi knockdown of Fzd 9 results in reduced PPARγ activity expression. (A) Total RNA purified from the indicated NSCLC cell lines were submitted to quantitative RT-PCR using primers specific for Fzd 9 as described under Materials and Methods. The relative mRNA abundance for Fzd 9 in the different samples was normalized to human GAPDH measured by RT-PCR in the same samples. Aliquots of extracts containing equal protein as measured by the Bradford assay from the indicated cells were resolved by SDS-PAGE and immunoblotted with antibodies to Fzd 9 (100 kDa; Aviva Systems Biology). The filters were stripped and reimmunoblotted for β-catenin (80 kDa; BD Transduction Laboratories) as a loading control. (B) The cell lines H157 and H1703 were transiently transfected with or without Fzd 9 and PPAR-RE along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (C) siRNA knockdown of Fzd 9 was performed as described in Materials and Methods on the indicated cell lines. Total RNA purified from these cells were then submitted to quantitative RT-PCR using primers specific for Fzd 9 as previously described. The relative mRNA abundance for Fzd 9 in the different samples was normalized to human GAPDH. (D) The indicated cell lines were transiently transfected with PPAR-RE, along with CMV-β-gal to normalize for transfection efficiency. In addition, Fzd 9 was knocked down by transient siRNA expression as indicated previously. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (E) The indicated cell line was transiently transfected with PPAR-RE, empty vector, or Fzd 9 along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost and/or 5 µM T0070907.
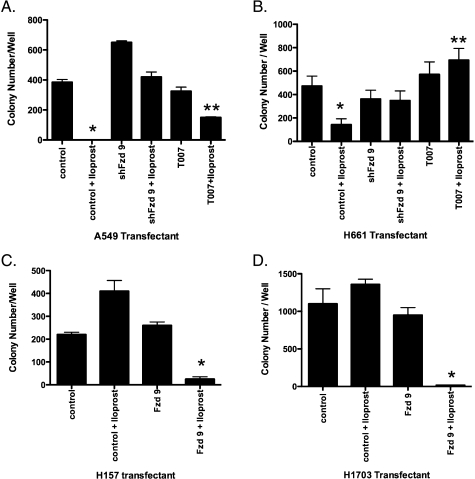
We then examined the effect of silencing Fzd 9 in iloprost-sensitive cell lines. Several NSCLC lines that expressed endogenous Fzd 9 but lacked Wnt 7a and PTGIR (A549 and H661) were transfected with a siRNA to Fzd 9, resulting in significantly decreased Fzd 9 expression (Figures 2C and W1B). Silencing of Fzd 9 inhibited iloprost-mediated activation of PPARγ in all cell lines tested (Figure 2D). Furthermore, whereas iloprost was associated with decreased colony formation in the cell lines with endogenous Fzd 9 expression (Figure 1A), siRNA knockdown of Fzd 9 in theses cells resulted in no reduction of colony number on soft agar assay in the presence of iloprost (Figure 3, A and B). Conversely, we stably expressed Fzd 9 in the H157 and H1703 cell lines that lacked endogenous Fzd 9, Wnt 7a, and PTGIR proteins. We then examined the effects of the stable expression of Fzd 9 on colony formation in soft agar in the H157 and H1703 cells. In the control cells not expressing Fzd 9, iloprost did not reduce colony formation (Figure 3, C and D). Consistent with previous findings [12], stable expression of Fzd 9 alone in the H157 and H1703 cells did not affect colony formation (Figure 3, C and D). However, iloprost inhibited colony formation by approximately 90% in both the H157 and H1703 cells stably expressing Fzd 9 (Figure 3, C and D). These data indicate that iloprost and Fzd 9 have the ability to reverse anchorage-independent growth and suggest a potential tumor suppressive effect in NSCLC where iloprost can engage the Fzd 9 receptor.
Figure 3.
Fzd 9 is necessary for the effects of iloprost reversal of anchorage independent growth. (A and B) A549 and H661 cells were grown in soft agar exposed to siRNA knockdown of Fzd 9 or the PPARγ antagonist compound T0070907 at a concentration of 5 µM in the absence or presence of 10 µM iloprost. The colonies were incubated for several weeks and subsequently counted as previously described in Materials and Methods. (C and D) The H157 and H1703 cell lines encoding stable empty vector LPCX/LNCX or Fzd 9 was grown in soft agar in the absence or presence of 10 µM iloprost. The colonies were incubated for several weeks and subsequently counted through Metamorph (Downingtown, PA).
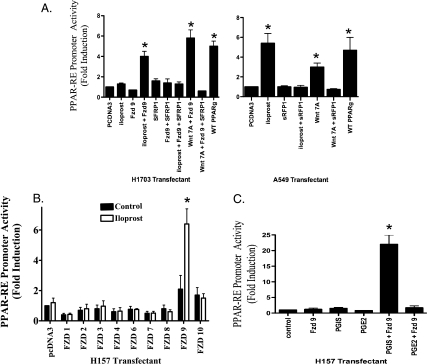
Furthermore, the PPARγ antagonist compound T0070907 blocked PPAR-RE activity in H157 cells expressing Fzd 9 exposed to iloprost (Figure 2E). This effect was specific for Fzd 9 because overexpression of other Fzd receptors in H157 cells (1, 2, 3, 4, 6, 7, 8, and 10) failed to increase PPARγ activity (Figure 4B). Interestingly, the expression of Wnt 7a in the H157 cell line did not result in further stimulation of PPAR-RE in the presence of iloprost (Figure W2A), supporting the idea that both iloprost and Wnt 7a interact with Fzd 9 in a similar fashion. To confirm that iloprost is mimicking the effects of PGI2, activation of PPAR-RE was examined in H157 cells overexpressing PGIS. Overexpression of PGIS alone had no effect on PPAR-RE activity; however, transient expression of Fzd 9 significantly increased PPAR-RE activity in cells overexpressing PGIS (Figure 4C). Expression of PGE2, a known protumorigenic prostacyclin family member, in the context of Fzd 9 had no effect on PPAR-RE activity (Figure 4C), indicating that this was not a generalized response to prostaglandins.
Figure 4.
Iloprost and Fzd 9 are specific in its activation of PPAR-RE and is not a generalized response to all prostaglandins, the effects are also blocked by sFRP1. (A) Iloprost and Fzd 9 activate PPARγ. The A549 and H1703 cell lines were transiently transfected with the PPAR-RE, sFRP1, Fzd 9, Wnt 7a, along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (B) The H157 cell line was transiently transfected with PPAR-RE, Fzds (1–10), along with CMV-β-gal to normalize for transfection efficiency. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (C) The cell line H157 was transiently transfected with the reporter plasmid PPAR-RE and the effector plasmid PGIS, or Fzd 9, or exposed to 10 µM PGE2. Of note, CMV-β-gal was used to normalize for transfection efficiency. The cells were incubated for 48 hours, and luciferase and β-galactosidase activities were measured. Data are presented as relative light units/milliunit of β-galactosidase activity. The results showed that the coexpression of PGIS and Fzd 9 stimulated PPAR-RE activity but not the sole expression of PGIS, Fzd 9, or PGE2. Furthermore, the coexpression of PGE2 and Fzd 9 similarly did not stimulate PPAR-RE activity.
Expression of sFRP1 Blocks the Effect of Iloprost and Fzd 9 Activation of PPARγ
Soluble Frizzled-related proteins (sFRP) are known to block Wnt-Fzd interaction, thereby turning off the Wnt signaling pathway [20]. We investigated whether sFRP1 could also block iloprost interaction with Fzd 9. As a positive control, we coexpressed sFRP1 in the context of NSCLC cell lines expressing Wnt 7a/Fzd 9 and, as predicted, Wnt 7a/Fzd 9 was shown to have significantly reduced PPAR-RE activity in those cells exposed to sFRP1 (Figure 4A). More interesting, however, was that the expression of sFRP1 also blocked iloprost ability to activate PPAR-RE in cells expressing Fzd 9 (Figure 4A). These results suggest that there is a significant interaction between iloprost and Fzd 9.
Iloprost Activation of PPARγ Is Mediated through Activation of ERK5
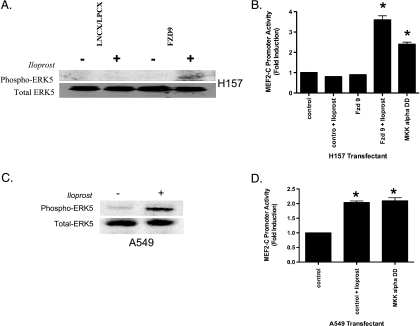
We sought to determine whether iloprost activation of PPARγ involved ERK5 activation in NSCLC. Iloprost increased the MEF2C reporter activity, a marker of MEK5 activation, and the phospho-ERK5 levels five-fold in H157 cells stably expressing Fzd 9 but failed to increase phospho-ERK5 in empty vector controls (Figure 5, A and B); levels of total ERK5 were not changed (Figure 5A, bottom panels). Similarly, A549 cells expressing endogenous Fzd 9 exposed to iloprost showed a several fold increase in p-ERK5 expression relative to the empty vector control cell lines by immunoblot and had increased induction of the MEF2C reporter activity (Figure 5C). In all of these cells, no significant changes in phospho-ERK1/2 levels were observed (data not shown).
Figure 5.
Iloprost and Fzd 9 induce phospho-ERK5 and MEF2C activity in NSCLC and is associated with PPARγ activity. (A) Retroviruses encoding stable empty LNCX/LPCX or Fzd 9 were used to transduce the H157 NSCLC cell line as described previously in Materials and Methods. Extracts were prepared from pooled G418 and puromycin-resistant cultures with MAPK lysis buffer, and aliquots containing 100 µg of protein were resolved on 10% polyacrylamide SDS gels, transferred to nitrocellulose, and probed with an antibody to phospho-ERK5 (115 kDa; Cell Signaling). The filters were stripped and reimmunoblotted for total ERK5 (115 kDa; Cell Signaling), which was used as a loading control protein. (B) The H157 cell line was transiently transfected with the MEF2C reporter, along with CMV-β-gal to normalize for transfection efficiency. MKK5 alpha DD was used as a positive control plasmid. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost. (C) Retroviruses encoding empty LNCX were used to transduce the A549 NSCLC cell line as described previously in Materials and Methods. Extracts were prepared from pooled G418-resistant cultures with MAPK lysis buffer and aliquots containing 100 µg of protein were resolved on 10% polyacrylamide SDS gels, transferred to nitrocellulose, and probed with an antibody to phospho-ERK5 (115 kDa; Cell Signaling). The filters were stripped and reimmunoblotted for total ERK5 (115 kDa; Cell Signaling), which was used as a loading control protein. (D) The A549 cell line was transiently transfected with the MEF2C reporter, along with CMV-β-gal to normalize for transfection efficiency. MKK5 alpha DD was used as a positive control plasmid. After an overnight incubation, cells were exposed for 48 hours with 10 µM iloprost.
To test the functional role of ERK5 in activating PPARγ, cells were treated with PD 98059, which selectively inhibits MEK1/2 and MEK5. Exposure of H157 cells to 25 µM PD98059 blocked the iloprost-mediated increase of PPAR-RE activity in cells expressing Fzd 9 (Figure W2A). Treatment of H157 expressing Fzd 9 with PD 184352, which selectively inhibits MEK1/2 at the concentrations used, had no effect on iloprost stimulation of PPAR-RE activity (Figure W2A). In addition, expression of DN-MEK5 had no effect on basal PPAR-RE activity but inhibited the activation with iloprost in the setting of Fzd 9 expression (data not shown).
Iloprost-Treated Mice Had Reduced Tumor Number and Their Sensitivity Was Associated with the Presence of Fzd 9
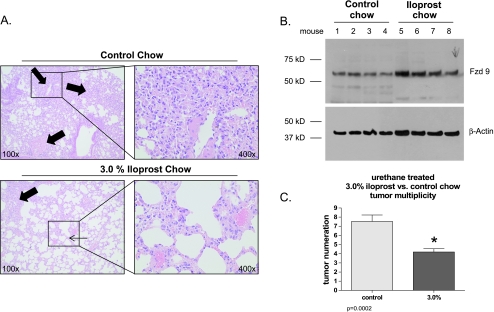
To determine the effect of iloprost in an in vivo model, FVB/N mice were maintained on control or iloprost chow for 5 weeks after urethane injection with carcinogen ethyl carbamate (urethane). The mice treated with 3% iloprost had decreased tumor numbers (3.4 ± 0.6 vs 7.8 ± 1.4 tumors/mouse, P < .0002) compared with the control mice (Figure 6, A and C). The mice receiving 3% iloprost also had a trend toward smaller tumors compared with the untreated mice.
Figure 6.
Iloprost treatment reduces tumor number and increases whole lung expression of Fzd 9. (A) Representative images of hematoxylin and eosin-stained urethane-treated lung tissue showing reduced tumor numbers and size in lungs from iloprost-fed mice (bottom) compared with control chow-fed mice (top). The thick arrows indicate tumor(s) in the lung and the thin arrow points out the more normal lung architecture in the mice receiving 3% iloprost chow. The right panels are higher-magnification images from the insets on the left, demonstrating the effect of iloprost on individual adenoma size. Magnifications: x100 (left) and x400 (right). (B) Whole lung tissue from urethane-treated mice fed normal or iloprost-impregnated chow (n = 4 each group) was snap-frozen, and whole-cell lysates were analyzed by Western blot analysis for Fzd 9 expression. β-Actin was used as a loading control. (C) Lungs from mice described in panel A were removed and tumors were counted. *P = .0002, different from control chow-fed mice.
Interestingly, the iloprost-treated mice had increased expression of Fzd 9 as detected by immunoblot (Figure 6B) compared with the untreated mice. Our in vitro experiments showed that the NSCLC cell lines that did not express Fzd 9 did not seem to have increased levels of Fzd 9 in response to iloprost (data not shown). Of note, there was no difference in the protein expression level of Wnt 7a between the treated versus untreated mice (data not shown). These findings suggest that the effects of iloprost's ability to reduce tumor number depend on Fzd 9 being present. The levels of PTGIR were determined to be low in these mice after urethane exposure.
Discussion
The arachidonic acid pathway and its metabolites play a central role in inflammation, pulmonary diseases, and cancer [21–23]. Elevated levels of PGE2 have been observed in a variety of cancer, including NSCLC, leading to the use of nonsteroidal anti-inflammatory drugs as chemotherapeutic agents. In contrast, our data, as well as others, demonstrate that the levels of PGIS, PGI2, and PTGIR are low in most NSCLC [14–16], suggesting that both PGIS and its receptor are downregulated. Our laboratory has demonstrated that increasing PGI2 levels through targeted overexpression of PGIS or by administration of prostacyclin analogs is chemoprotective against lung tumor formation in mice [5]. The results from a large multicenter clinical trial using iloprost in patients at risk for lung cancer seems to suggest a very favorable outcome with the use of iloprost as a chemopreventive agent in smokers. However, the chemotherapeutic potential of prostacyclin has not been investigated. Previous data have indicated that although expression of PGIS is low in most established human lung tumors [14–16], a subset of patients with detectable expression had a marked survival advantage [16]. Understanding the mechanisms whereby PGI2 and its analogs inhibit cancer growth and progression and developing biomarkers to identify patients who may respond to PGI2 therapy are therefore of critical importance.
From this study, we conclude that iloprost can inhibit transformed growth of a subset of NSCLC through the activation of PPARγ through an independent membrane receptor mechanism involving the Fzd 9 pathway. Work from our laboratory and by other investigators have demonstrated that PPARγ activation in NSCLC leads to growth inhibition, promotion of apoptosis, inhibition of invasiveness, and metastasis [10,11]. Moreover, PPARδ has been shown to enhance tumorigenesis in lung epithelial cells [24]. Therefore, defining the factors that are necessary for iloprost to activate PPARγ will be critical in developing this agent as a chemotherapeutic drug. Our data indicate that the expression of the G protein-coupled receptor, Fzd 9 [25–29], determines the ability of iloprost to activate PPARγ and inhibit cancer growth in NSCLC cells. Silencing of Fzd 9 expression in NSCLC cell lines that express the protein blocks the ability of iloprost to activate PPARγ and renders the cells insensitive to growth inhibition by iloprost (Figure 3, A and B). Conversely, expressing Fzd 9 in NSCLC cell lines lacking endogenous Fzd 9 seemed to sensitize the cells to growth inhibition by iloprost through a PPARγ-dependent pathway (Figure 3, C and D). Although Fzd 9 has been shown to be a receptor for Wnt 7a, and the engagement of this pathway inhibits transformed growth through ERK5-dependent activation of PPARγ [9,12], there is no existing soluble Wnt 7a protein that could be used for clinical trial. A recent study has demonstrated that prostacyclin analogs activate PPARγ through the prostacyclin receptor PTGIR [30]. Our data, however, do not support a role for PTGIR in mediating the effects of iloprost in NSCLC. All of the NSCLCs we have examined have very low to undetectable levels of PTGIR mRNA, and administration of iloprost failed to increase cAMP, an effector pathway for PTGIR (Figures W1A and W2B). Although we have not directly demonstrated iloprost binding to Fzd 9 in this current study, our data strongly suggest that this may indeed be the case as shown by sFRP1's ability to block iloprost/Fzd 9 signaling. This study is the first to demonstrate such an association between the Wnt and prostacyclin pathways. Iloprost stimulation in Fzd 9-positive cells results in the activation of ERK5 (Figure 5), as occurs with Wnt 7a stimulation, and this activation is critical for subsequent PPARγ activation.
Our data as well as others' raise the possibility that activators of PPARγ may represent an effective treatment of patients with lung cancer [9,31,32]. Thiazolinediones have been shown to reduce the risk of lung cancer in clinical trials [33], and activation of PPARγ in NSCLC cell lines has been shown to reverse the transformed phenotype in several studies [10,11,34–40]. Engagement of Fzd 9 by its ligand Wnt 7a inhibits growth of NSCLC through activation of PPARγ [9]. Whereas Wnt 7a recombinant therapy seems attractive, there is no recombinant Wnt 7a protein available for clinical use at this time. Studies using epidermal growth factor receptor inhibitors are focused on developing biomarkers to predict which patients will best respond to these agents. This study indicates that a similar strategy can be used to predict responders to iloprost by assessing their Fzd 9 status. Future clinical chemoprevention trials should determine if the expression of Fzd 9 is associated with the effects of iloprost on progression of dysplasias. However, our data indicate that Fzd 9 status may also define the utility of prostacyclin analogs as chemotherapeutic agents. Clinical trials to examine this seem to be warranted.
Supplementary Material
Acknowledgment
The authors thank Rebecca E. Schweppe for her gifts DN-MEK5, MKK5-alpha DD, and MEF2C-Luciferase reporter.
Footnotes
This work was supported by a VA Merit Award and the National Institutes of Health (NCI K22 CA113700).
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Michaud CM, Murray CJ, Bloom BR. Burden of disease—implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981;212:1270–1272. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- 5.Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, Malkinson AM, Golpon HA, Nemenoff RA, Geraci MW. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–740. [PubMed] [Google Scholar]
- 6.Keith RL, Miller YE, Hudish TM, Girod CE, Sotto-Santiago S, Franklin WA, Nemenoff RA, March TH, Nana-Sinkam SP, Geraci MW. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64:5897–5904. doi: 10.1158/0008-5472.CAN-04-1070. [DOI] [PubMed] [Google Scholar]
- 7.Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, Stearman RS, Winn RA, Weiser-Evans M, Geraci MW, et al. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator-activated receptor gamma. Cancer Prev Res (Phila Pa) 2008;1:349–356. doi: 10.1158/1940-6207.CAPR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 9.Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Jr, Heasley LE, Nemenoff RA. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor γ. J Biol Chem. 2006;281:26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- 10.Bren-Mattison Y, Van Putten V, Chan D, Winn R, Geraci MW, Nemenoff RA. Peroxisome proliferator-activated receptor-γ (PPARγ) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC) Oncogene. 2005;24:1412–1422. doi: 10.1038/sj.onc.1208333. [DOI] [PubMed] [Google Scholar]
- 11.Wick M, Hurteau G, Dessev C, Chan D, Geraci MW, Winn RA, Heasley LE, Nemenoff RA. Peroxisome proliferator-activated receptor-γ is a target of nonsteroidal anti-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol Pharmacol. 2002;62:1207–1214. doi: 10.1124/mol.62.5.1207. [DOI] [PubMed] [Google Scholar]
- 12.Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–19634. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- 13.Winn RA, Bremnes RM, Bemis L, Franklin WA, Miller YE, Cool C, Heasley LE. γ-Catenin expression is reduced or absent in a subset of human lung cancers and re-expression inhibits transformed cell growth. Oncogene. 2002;21:7497–7506. doi: 10.1038/sj.onc.1205963. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard WC, Alley MC, Gray GN, Green KC, McLemore TL, Boyd MR. Evidence for prostanoid biosynthesis as a biochemical feature of certain subclasses of non-small cell carcinomas of the lung as determined in established cell lines derived from human lung tumors. Cancer Res. 1989;49:826–832. [PubMed] [Google Scholar]
- 15.Kreutzer M, Fauti T, Kaddatz K, Seifart C, Neubauer A, Schweer H, Komhoff M, Muller-Brusselbach S, Muller R. Specific components of prostanoid-signaling pathways are present in non-small cell lung cancer cells. Oncol Rep. 2007;18:497–501. [PubMed] [Google Scholar]
- 16.Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA, Jr, Johnson GL, Hirsch FR, Merrick DT, Franklin WA, et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol. 2005;167:1763–1775. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiriyama M, Ushikubi F, Kobayashi T, Hirata M, Sugimoto Y, Narumiya S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br J Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci USA. 2000;97:13275–13280. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keshamouni VG, Reddy RC, Arenberg DA, Joel B, Thannickal VJ, Kalemkerian GP, Standiford TJ. Peroxisome proliferator-activated receptor-γ activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004;23:100–108. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 20.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 21.Claria J. Cyclooxygenase-2 biology. Curr Pharm Des. 2003;9:2177–2190. doi: 10.2174/1381612033454054. [DOI] [PubMed] [Google Scholar]
- 22.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 23.Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–222. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 24.Pedchenko TV, Gonzalez AL, Wang D, DuBois RN, Massion PP. Peroxisome proliferator-activated receptor beta/delta expression and activation in lung cancer. Am J Respir Cell Mol Biol. 2008;39:689–696. doi: 10.1165/rcmb.2007-0426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Malbon CC. Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front Biosci. 2004;9:1048–1058. doi: 10.2741/1308. [DOI] [PubMed] [Google Scholar]
- 27.Wang HY, Liu T, Malbon CC. Structure-function analysis of Frizzleds. Cell Signal. 2006;18:934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang HY, Malbon CC. Wnt-frizzled signaling to G-protein-coupled effectors. Cell Mol Life Sci. 2004;61:69–75. doi: 10.1007/s00018-003-3165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falcetti E, Flavell DM, Staels B, Tinker A, Haworth SG, Clapp LH. IP receptor-dependent activation of PPARγ by stable prostacyclin analogues. Biochem Biophys Res Commun. 2007;360:821–827. doi: 10.1016/j.bbrc.2007.06.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ. PPAR-γ activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005;7:294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy RC, Srirangam A, Reddy K, Chen J, Gangireddy S, Kalemkerian GP, Standiford TJ, Keshamouni VG. Chemotherapeutic drugs induce PPAR-γ expression and show sequence-specific synergy with PPAR-γ ligands in inhibition of non-small cell lung cancer. Neoplasia. 2008;10:597–603. doi: 10.1593/neo.08134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 34. Betz MJ, Shapiro I, Fassnacht M, Hahner S, Reincke M, Beuschlein F. Peroxisome proliferator-activated receptor-γ agonists suppress adrenocortical tumor cell proliferation and induce differentiation. J Clin Endocrinol Metab. 2005;90:3886–3896. doi: 10.1210/jc.2004-1267. [DOI] [PubMed] [Google Scholar]
- 35.Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor γ in non-small cell lung cancer. Cancer Res. 2000;60:1129–1138. [PubMed] [Google Scholar]
- 36.Han S, Rivera HN, Roman J. Peroxisome proliferator-activated receptor-γ ligands inhibit α5 integrin gene transcription in non-small cell lung carcinoma cells. Am J Respir Cell Mol Biol. 2005;32:350–359. doi: 10.1165/rcmb.2004-0345OC. [DOI] [PubMed] [Google Scholar]
- 37.Han S, Sidell N, Fisher PB, Roman J. Up-regulation of p21 gene expression by peroxisome proliferator-activated receptor γ in human lung carcinoma cells. Clin Cancer Res. 2004;10:1911–1919. doi: 10.1158/1078-0432.ccr-03-0985. [DOI] [PubMed] [Google Scholar]
- 38.Seargent JM, Yates EA, Gill JH. GW9662, a potent antagonist of PPARγ, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARγ agonist rosiglitazone, independently of PPARγ activation. Br J Pharmacol. 2004;143:933–937. doi: 10.1038/sj.bjp.0705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Ca inhibits the human stress-responsive p38 and JNKMAPK pathways. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, Inoue K, Hla T, Kondo M. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-γ agonists through induction of apoptosis. Biochem Biophys Res Commun. 2000;270:400–405. doi: 10.1006/bbrc.2000.2436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.