Abstract
Epithelial ovarian cancer (EOC) comprises approximately 90% of ovarian cancers and arises from the surface epithelium. Typical treatment of EOC involves cytoreductive surgery combined with chemotherapy. More recent therapies have targeted the tumor vasculature using antiangiogenic compounds such as thrombospondin-1 (TSP-1). TSP-1 mimetic peptides such as ABT-510 have been created and have been in various clinical trials. We have previously shown that ABT-510 reduces abnormal vasculature associated with tumor tissue and increases the presence of mature blood vessels. It has been hypothesized that treatment with antiangiogenic compounds would allow increased delivery of cytotoxic agents and enhance treatment. In this study, we evaluated the potential role of ABT-510 and various chemotherapeutics (cisplatin and paclitaxel) on tumor progression, angiogenesis, and the benefits of combinational treatments on tissue uptake and perfusion using an orthotopic syngeneic mouse model of EOC. Animals were treated with ABT-510 (100 mg/kg per day) alone or in combination with cisplatin (2 mg/kg per 3 days) or paclitaxel (10 mg/kg per 2 days) at 60 days after tumor induction. Radiolabeled and fluorescently labeled paclitaxel demonstrated a significant increase in tumor uptake after ABT-510 treatment. Combined treatment with ABT-510 and cisplatin or paclitaxel resulted in a significant increase in tumor cell and tumor endothelial cell apoptosis and a resultant decrease in ovarian tumor size. Combined treatment also regressed secondary lesions and eliminated the presence of abdominal ascites. The results from this study show that through vessel normalization, ABT-510 increases uptake of chemotherapy drugs and can induce regression of advanced ovarian cancer.
Introduction
Ovarian cancer represents the most lethal gynecologic malignancy. With an estimated 22,000 new cases and 15,000 deaths annually, ovarian cancer is the fifth leading cause of cancer deaths among US women [1]. Tumors of the ovary are classified and named according to the cell type from which they originate and whether they are benign or cancerous. There are various subtypes of EOC according to the World Health Organization based on their histopathology [2]. Epithelial ovarian cancer (EOC) comprises approximately 90% of ovarian cancers and is thought to arise from the surface epithelium of the ovary [3]. Of these tumors, the most prevalent subtype of EOC is serous adenocarcinoma, constituting approximately 68% of EOCs. If diagnosed at early stages, the 5-year survival rate is 98% compared with only 25% at later stages of the disease [4]. Of particular concern with this disease is that the obscure clinical signs associated with EOC generally preclude early detection or treatment. Conventional treatment of ovarian cancer typically involves cytoreductive surgery (debulking) combined with platinum- or taxol-based chemotherapy [5]. Although this approach generally results in primary tumor shrinkage, tumor recurrence often occurs, and the incidence of EOC chemoresistance is very high [6]. To combat this chemoresistance and disease recurrence, numerous alternative therapies have been investigated including those designed to focus on ovarian tumor vasculature.
The concept of antiangiogenic therapy was proposed decades ago, and it was thought that inhibiting the tumor vasculature would induce dormancy and tumor regression. Various compounds have since been identified for their ability to inhibit angiogenesis [7]. Thrombospondin-1 (TSP-1) was the first protein recognized as an endogenous inhibitor of angiogenesis and has been investigated for its efficacy in the treatment of various cancers [8]. For therapeutic interventions, small TSP-1 mimetic peptides have been developed. The mimetic ABT-510 exploits the antiangiogenic domain (second type 1 repeat) of TSP-1, activating expression of FasL, inhibiting expression of proangiogenic factors vascular endothelial growth factor (VEGF) and basic fibroblast growth factor, and inducing tumor cell apoptosis [9].
ABT-510 has been tested clinically for the treatment of various cancers alone or in combination with other cytotoxic agents, with variable results [10–13]. In studies where ABT-510 was used as combination treatment, it was found that ABT-510 was well tolerated and that any adverse effects observed were consistent with those of chemotherapeutics on their own [14–16].
In this study, we evaluated the antiangiogenic mimetic peptide ABT-510 at various stages of disease and in combination with chemotherapeutics to determine the effect on epithelial ovarian tumors in a mouse model of the disease. Single-agent therapies have limited success in cancer treatment, and we hypothesize that combinational treatments of ABT-510 with cytotoxic agents may be more effective. Animals in this study were treated with ABT-510 alone or in combination with cisplatin or paclitaxel to determine the potential clinical effectiveness of these agents.
Materials and Methods
Cell Line
Murine surface epithelial cells were generously donated by Drs. Roby and Terranova (Kansas State University, KS) [17]. The ID8 cell line was cultured in Dulbecco's modified Eagle medium (Gibco, Burlington, Ontario, Canada) supplemented with 10% FBS and 1% antibiotic-antimycotic (Gibco) and used for subsequent in vivo experiments.
Animal Model
We have previously generated an orthotopic, syngeneic mouse model of EOC that closely replicates ovarian serous adenocarcinoma in women [18]. Briefly, 1.0 x 106 ID8 cells in 5 µl of PBS are injected directly under the ovarian bursa of C57Bl6 mice. Because the ID8 cells were derived from a C57Bl6 mouse, this syngeneic model allows for an intact immune system and orthotopic placement of the epithelial cells in their normal microenvironment. In this model, mice develop large primary tumors, numerous secondary peritoneal lesions, and abdominal ascites approximately 90 days after tumor induction. Primary tumors were collected and measured for subsequent analysis, and secondary tumors were assessed based on a lesion scoring system as previously described [18].
Drugs
ABT-510 was obtained from Abbott Laboratories (Abbott Park, IL). Cisplatin (Calbiochem, Gibbstown, NJ) and paclitaxel (Sigma-Aldrich, Oakville, Ontario, Canada) were purchased commercially.
Tumor induction was performed as described previously [18], and tumors were allowed to grow for 60 days before ABT-510, cisplatin and paclitaxel individual or combination treatments began. Animals received intraperitoneal (IP) injections of vehicle (D5W; 200 µl) alone or containing drug (ABT-510 100 mg/kg daily, cisplatin 2 mg/kg every 3 days, paclitaxel 10 m/kg every 2 days). For quantification of abdominal ascites and secondary lesions, mice were treated with a lower dosage of paclitaxel (5 mg/kg every 2 days) alone or in combination with paclitaxel. Cisplatin and paclitaxel were reconstituted in D5W or 50% cremophor and 50% ethanol, respectively, just before injections. The animals continued to receive IP injections of the various agents until the control mice became moribund, which occurred approximately 90 days after tumor induction. All animals were euthanized, and serum, ascites fluid, and ovarian tissue were collected and processed.
VEGF ELISA
Serum was collected (BD Vacutainers, Franklin Lakes, NJ) at the time of euthanasia from ABT-510-treated animals, and the expression of VEGF was determined using sandwich enzyme-linked immunosorbent assays (R&D Systems, Inc, Minneapolis, MN) according to the manufacturer's instructions. Briefly, serum samples were diluted 1:100 and added to the precoated wells. Unbound substrate was washed away, and an enzyme-linked polyclonal antibody specific for VEGF was added to the wells followed by subsequent washes and addition of color substrate. Individual well absorbance was measured at 450 nm using an EL 800 Universal Microplate Reader (Bio-Tek Instruments, Winooski, VT).
Immunoblot Analysis
Flash-frozen primary ovarian tumors were homogenized in RIPA buffer with protease and phosphatase inhibitors, and protein concentrations were determined using a DC protein assay (Bio-Rad Laboratories, Hercules, CA). All Western blots were performed using an XCell II BlotModule System (Invitrogen, Burlington, Ontario, Canada). Samples (40 µg of total protein) were reduced and subjected to SDS-PAGE using either 8% or 15% resolving gels. Proteins were transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories) and blocked at room temperature for 1 hour in 5% skim milk with Tris-buffered Saline Tween 20. Membranes were probed for overnight at 4°C for VEGF and VEGFR2 (Santa Cruz, Burlingame, CA). After washing with Tris-buffered Saline Tween 20, membranes were incubated for 1 hour at room temperature with antirabbit immunoglobulin G HRP-linked secondary antibodies (Cell Signaling Technology, Inc., Beverly, MA). The expressions of VEGF and its receptor were detected using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer BioSignal, Inc, Montreal, Quebec, Canada) and visualized using medical x-ray film (Konica Minolta Medical Imaging, Inc, Wayne, NJ). To ensure equal loading of samples, β-actin (Cell Signaling Technology) was probed for 1 hour at room temperature followed by antirabbit immunoglobulin G secondary antibody for 1 hour at room temperature. Computer-assisted densitometry was performed using AlphaEase FC software (AlphaInnotech, San Leandro, CA), and results were quantified and reported as integrated densitometry values relative to β-actin.
Immunohistochemistry
Ovarian tissue was formalin-fixed and processed using a paraffin tissue processor (Ventana Medical Systems, Tuscon, AZ), and 5-µm sections were cut and mounted onto slides. Sections were deparaffinized in xylene and rehydrated in graded alcohol solutions. Immunohistochemistry was performed to determine localization and expression of the endothelial cell marker CD31 within the ovarian tissue. Endogenous peroxidase activity was inhibited using 1% hydrogen peroxide for 10 minutes at room temperature. Antigen retrieval was achieved by immersing slides in 10 mM citrate buffer at 90°C for 12 minutes. Tissues were blocked using 5% BSA for 10 minutes at room temperature and incubated with anti-CD31 primary antibody (BD Biosciences Pharmingen, San Diego, CA) overnight at 4°C in a humidity chamber. The following day, sections were incubated with antimouse biotinylated secondary antibody (Sigma-Aldrich Canada Ltd) for 2 hours at room temperature. Tissues were then exposed to ExtrAvidin (Sigma-Aldrich Canada Ltd) for 1 hour at room temperature, and antibodies were visualized using DAB (Sigma-Aldrich Canada Ltd). Tissue was counterstained with Carazzi's hematoxylin, dehydrated, and mounted on coverslips. Slides were imaged using bright field microscopy.
Evaluation of Microvessel Density and Vessel Maturity
To evaluate tumor vessel area and microvessel density, tumor sections immunostained for CD31 were imaged at a magnification of x200, and microvessel density and blood vessel area were quantified using Metamorph integrated morphometry software (Molecular Devices, Downingtown, PA). For the determination of vessel density, a minimum of four fields of view per tissue section were used, with n = 6 per group. To evaluate blood vessel maturity, tissue sections were subjected to immunofluorescence colocalization of CD31 and α-smooth muscle actin (SMA). Briefly, slides were processed as above, and the CD31 antibody was incubated for 1 hour at room temperature. After washing in PBS, antimouse secondary antibody conjugated to Alexa Fluor 594 (Invitrogen) was applied for 1 hour at room temperature. Tissue sections were then rinsed in PBS and incubated for 1 hour at room temperature with anti-SMA primary antibody (Fitzgerald Industries International, Concord, MA). Secondary antibody, conjugated to Alexa Fluor 488 (Invitrogen), was applied for 1 hour. Tissue sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted with Prolong Gold antifade (Invitrogen) and allowed to dry overnight before image analysis with integrated morphometry software (Metamorph, Burlingame, CA).Mature blood vessels were characterized as having pericyte coverage (SMA-positive vessels) and were quantified as a percentage of total CD31-positive vessels.
Paclitaxel Incorporation and Localization
To determine if treatment with ABT-510 would increase the uptake of chemotherapeutic drugs, animals were injected with ID8 cells (as outlined in our animal model) and given daily IP injections of ABT-510 (or D5W the vehicle control) for 21 days starting at 60 days after tumor induction. After the ABT-510 treatment, animals were injected IP with tritiated paclitaxel (40 µCi; Moravek Biochemicals and Radiochemicals, Brea, CA) and were euthanized 12, 24, and 48 hours later. Ovaries were harvested from all animals and homogenized in scintillation fluid, and radioisotope was quantified with a Tri-carb Liquid Scintillation Counter (Perkin Elmer, Waltham, MA). To localize chemotherapy uptake, control and ABT-510-treated animals (n = 6 per group) received an IP injection of paclitaxel conjugated to Oregon Green 488 (Invitrogen) and were euthanized 24 hours later. The ovaries were formalin-fixed and sectioned, and immunofluorescence colocalization was performed to visualize blood vessels (using an anti- CD31 antibody) and to localize paclitaxel uptake. Slides were mounted and images were captured using an epifluorescent microscope.
Cisplatin Uptake
Accumulation of tissue platinum (Pt) was quantified using flameless atomic absorption spectroscopy [19]. Frozen control and ABT-510-treated tumor samples collected at 90 days after tumor induction (after 30 days of IP cisplatin treatment) were homogenized in deionized water and evaporated at 70°C for 2 hours. Tumor tissue was then digested with 200 µl of nitric acid (65%) for 2 hours at 75°C. Samples were diluted 10x in water before injecting into the spectrometer.
Tumor and Endothelial Cell Death
To visualize and quantify tumor cell death, TUNEL assay was performed using an In Situ Cell Death Detection Kit (Roche, Laval, Quebec, Canada) according to the manufacturer's instructions. Animals received vehicle or ABT-510 treatment alone or in combination with chemotherapy drugs cisplatin and paclitaxel as described previously. After treatment, tissues were extracted, fixed in 10% buffered formalin, and processed and sectioned onto glass slides. After membrane permeabilization, cells were washed with PBS and incubated with the TUNEL reaction mixture (label solution and enzyme solution) for 60 minutes at 37°C in the dark. Slides were rinsed with PBS, nuclei were stained with DAPI, and coverslips were mounted onto slides as previously described. Negative and positive controls were generated according to the kit instructions. All slides from immunofluorescence experiments were visualized using a fluorescent microscope, and quantification of the percent immunopositive cells was performed using integrated morphometry software (Metamorph). For the evaluation of endothelial cell apoptosis, CD31 immunofluorescence colocalization was performed on tumor sections as described previously. After addition of the Alexa Fluor 594-conjugated secondary antibody, slides were washed and subjected to the TUNEL protocol according to manufacturer's instructions. Tissue sections were counterstained with DAPI, and slides were mounted. The percentage of apoptotic endothelial cells was determined by colocalization with CD31 and tunnel staining and was expressed as a percentage of total endothelial cells.
Cancer-Associated Morbidity
At euthanasia after ABT-510 and chemotherapy drug treatment, animals were evaluated for the presence of ascites fluid and the number of secondary lesions was scored. For secondary lesions, animals were given a score of 0 for no lesions within the peritoneal cavity, a score of 1 if there were 1 to 2 lesions, a score of 2 if there were 3 to 10 secondary peritoneal lesions, and a score of 3 if there were greater than 10 lesions in the peritoneum. For ascites fluid, animals were scored based on the presence or absence of abdominal ascites.
Statistical Analysis
All in vitro and in vivo experiments contained three replicates and animals in each treatment group, respectively. Results from immunoblots, immunohistochemistry, MTT assays, ovarian tumor weights, and blood vessel density were analyzed using Student's t-test and analysis of variance with a Fisher post hoc test.
Results
ABT-510 Decreases Local and Systemic Levels of VEGF and VEGFR-2
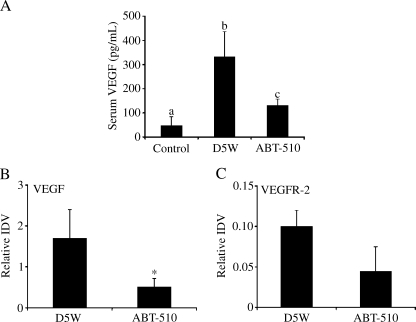
At 60 days after tumor induction, mice received daily IP treatment with vehicle or 100 mg/kg ABT-510 for 30 days. Western blot analysis showed that ABT-510 treatment significantly (P < .05) reduced the levels of VEGF in ovarian tumor homogenate lysates (Figure 1). Levels of VEGFR2 were also decreased within the tumors; however, this was not reported to be significant. ELISAs were performed on serum samples from control mice without tumors and from tumor-bearing mice after 30 days of treatment with vehicle or ABT-510. Vehicle-treated mice had a three-fold increase in circulating VEGF levels (P < .05; Figure 1). Treatment with 100 mg/kg ABT-510 significantly decreased VEGF serum levels compared with vehicle-treated animals (P < .05; Figure 1).
Figure 1.
Effect of ABT-510 on VEGF and VEGFR-2 levels. Serum and tumor tissue were collected from mice treated with vehicle (D5W) or ABT-510 and subjected to ELISA or Western blot analysis, respectively. (A) D5W-treated mice had increased circulating VEGF compared with non-tumor-bearing mice (control), whereas ABT-510 decreased VEGF levels compared with vehicle controls. (B) ABT-510 treatment at 60 days after tumor induction caused a decreased expression of VEGF protein in ovarian tumors. Bars with different letters are statistically different, and bars with asterisks are statistically different from D5W controls.
ABT-510 Alters Ovarian Tumor Vascular Morphology
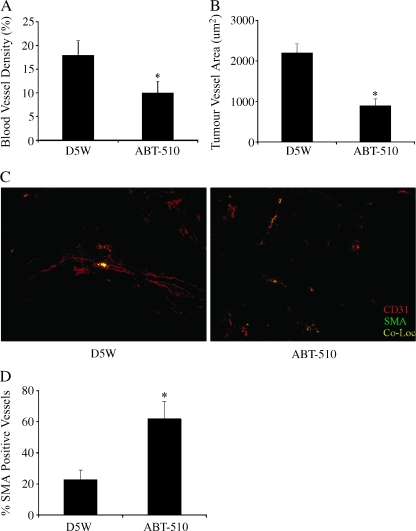
Ovarian tumor tissue collected from mice treated with ABT-510 had significantly (P < .05) reduced microvessel density and tumor blood vessel area compared with vehicle-treated controls (Figure 2, A and B). To determine whether there was a change in vessel maturity, tumor sections were costained for CD31 and SMA. ABT-510 treatment caused a significant (P < .01) increase in the percentage of pericyte-covered mature blood vessels compared with vehicle-treated controls (Figure 2, C and D).
Figure 2.
ABT-510 alters tumor vasculature. Ovarian tumors were collected from mice treated with vehicle or ABT-510 60 days after tumor induction. ABT-510 caused a significant decrease in blood vessel density (A) and tumor vessel area (B). (C) ABT-510 treatment resulted in more pericyte covered blood vessels, as evidenced by positive staining for CD31 and SMA. (D) Quantification of the percentage of mature, pericyte-covered blood vessels. Bars with asterisks are statistically different (P < .05) compared with D5W-treated controls.
ABT-510 Facilitates Vascular Uptake of Paclitaxel and Cisplatin in Ovarian Tumors
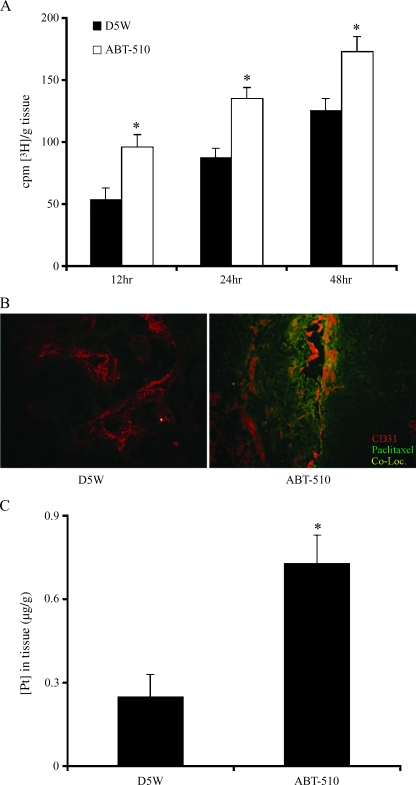
Mice being treated IP with either vehicle or 100 mg/kg ABT-510 for 21 days received an IP injection of [3H] paclitaxel, and ovarian tumor tissue was harvested 12, 24, and 48 hours later. Tumors from mice treated with ABT-510 had a significant (P < .05) increase in paclitaxel incorporation at all time points compared with vehicle-treated controls (Figure 3A). To localize the uptake of paclitaxel, mice received an IP injection of paclitaxel conjugated to Oregon green, and immunofluorescence colocalization for blood vessels (CD31-positive staining) and conjugated paclitaxel was performed. Mice treated with ABT-510 had a greater uptake of paclitaxel compared with controls (Figure 3B). Most of the conjugated paclitaxel was localized near the blood vessels and appeared to diffuse into the tumor tissue from the vessels (Figure 3B). Mice being treated with both ABT-510 and cisplatin had significantly increased (P < .05) incorporation of platinum of ovarian tumor tissue compared with mice treated with cisplatin and D5W (Figure 3C).
Figure 3.
Treatment with ABT-510 increases the uptake of paclitaxel and cisplatin in ovarian tumors. (A) Mice were injected IP with tritiated paclitaxel and tumors were harvested 12, 24, and 48 hours later. Tumors from mice treated with ABT-510 (white bars) had significantly increased drug uptake at all time points compared with D5W-treated controls (black bars). Bars with asterisks are statistically different (P < .05) from vehicle controls. (B) Mice were also injected with fluorochrome-conjugated paclitaxel, and immunofluorescence colocalization was performed on tumor tissue. Immunofluorescence colocalization showed that there was increased paclitaxel incorporation (green staining) that was localized near blood vessels (red staining for endothelial cells). (C) After combined treatment with cisplatin and ABT-510, platinum accumulation in tumor tissue was significantly increased (P < .5) compared with D5W-treated controls.
ABT-510 Increases the Tumor and Endothelial Cell Apoptotic Effects of Cisplatin and Paclitaxel
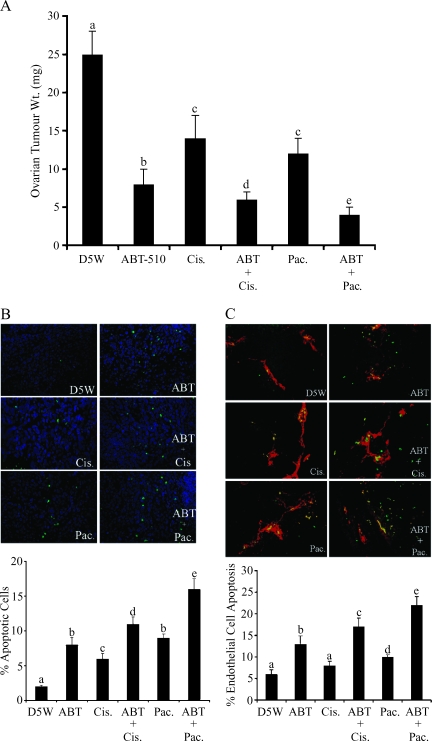
After 60 days of ovarian tumor growth, mice were treated IP with ABT-510, cisplatin, and paclitaxel, alone or in combination. Individual treatment with ABT-510, cisplatin, and paclitaxel caused a significant (P < .05) reduction in ovarian tumor weight compared with vehicle-treated control mice (Figure 4A). When ABT-510 was combined with either cisplatin or paclitaxel, there was a further reduction (P < .05) in ovarian tumor weight, which was greater than each compound alone (Figure 4A). TUNEL assay was performed to quantify tumor cell apoptosis in response to individual or combination IP treatments of ABT-510, cisplatin, and paclitaxel. ABT-510 and both chemotherapy drugs caused a significant (P < .05) increase in tumor cell apoptosis, and there was a further significant (P < .05) increase in tumor cell apoptosis when ABT-510 was combined with either drug (Figure 4B). To identify whether these compounds induced endothelial cell death, immunofluorescence colocalization of CD31 to visualize endothelial cells and TUNEL was performed. ABT-510, cisplatin, and paclitaxel treatment each significantly increased ovarian tumor endothelial cell death (Figure 4C). ABT-510, when combined with either cisplatin or paclitaxel, had an additive effect, with significantly (P < .05) increased endothelial cell apoptosis compared with controls or to treatment with individual compounds (Figure 4C).
Figure 4.
ABT-510 in combination with cisplatin and paclitaxel reduces tumor size and induces tumor and endothelial cell apoptosis. (A) After 60 days of tumor growth, mice were treated with ABT-510, cisplatin and paclitaxel, alone and in combination. All three drugs reduced tumor size compared with D5W-treated controls. When used in combination, the drugs had an effect greater than either alone. (B) Tumor cell apoptosis was significantly increased when ABT-510 was combined with either cisplatin or paclitaxel, as indicated by the percentage of TUNEL (green stain)-positive cells. (C) Endothelial cell apoptosis (as indicated by CD31- [red] and TUNEL- [green] costained cells) was significantly higher in mice treated with ABT-510 and cisplatin or paclitaxel. For all graphs, bars with different letters are statistically different (P < .05).
ABT-510, Cisplatin, and Paclitaxel Alter Tumor-Associated Morbidity
Mice receiving IP therapy of ABT-510, cisplatin, and paclitaxel alone or in combination were scored for the presence of secondary lesions within the peritoneal cavity and also for the presence of abdominal ascites. Vehicle-treated controls universally had greater than 10 peritoneal secondary lesions at the time of euthanasia (Table 1). ABT-510 treatment reduced the number of secondary lesions, as did cisplatin and paclitaxel treatment. When ABT-510 was combined with either cisplatin or paclitaxel, there was a complete absence of peritoneal lesions (Table 1). Six of seven mice in D5W-treated controls and five of six in ABT-510-treated mice exhibited abdominal ascites. However, when ABT-510 was combined with either cisplatin or paclitaxel, none of the mice had ascites fluid in the abdomen.
Table 1.
Presence of Secondary Lesions and Abdominal Ascites in Mice Treated with ABT-510, Cisplatin, and Paclitaxel Alone or in Combination, 60 Days After Tumor Induction.
| Lesion Score | D5W | ABT-510 | Cisplatin | ABT/CIS | Paclitaxel | ABT/PAC |
| 0 | None | ** | * | ****** | * | ****** |
| 1 | None | ** | * | None | ** | None |
| 2 | None | * | *** | None | ** | None |
| 3 | *** | None | * | None | * | None |
| No. Animals with Ascites | 6/7 | 1/6 | 1/6 | 0/6 | 1/6 | 0/6 |
Corresponds to individual mouse within each group.
ABT indicates ABT-510; CIS, cisplatin; PAC, paclitaxel.
Discussion
The concept of antiangiogenic therapy was first proposed in the 1970s when Judah Folkman [20] highlighted the necessity of this process for tumor growth. He hypothesized that if angiogenesis was essential for the growth of solid tumors then diminishing this supply might be an effective therapy. Since then, numerous agents have been developed and tested in various cancers, which either inhibit proangiogenic factors or upregulate antiangiogenic factors. Many studies have focused on decreasing proangiogenic factors such as VEGF using monoclonal antibodies. With respect to ovarian cancer, studies have shown prolonged survival after antiangiogenic treatment [21]; however, serious toxicities have also been reported with angiogenic and cytotoxic combinations [22–24]. More recently, compounds that increase the expression of antiangiogenic compounds such as TSP-1 have been developed. Specific TSP-1 mimetic peptides have been designed to exploit the antiangiogenic type 1 repeats within the gene. To date, various clinical studies have used these peptides as a single-agent therapy [10–13]. In our studies, we evaluated the use of the TSP-1 mimetic ABT-510 in combination with chemotherapeutic drugs cisplatin and paclitaxel for the treatment of EOC. The potential for ABT-510 as an antiangiogenic therapy has been previously documented in our laboratory in which animals were treated daily with ABT-510 immediately after tumor induction, and the results demonstrated a significant decrease in ovarian tumors, ascites fluid production, and secondary lesion formation [9]. In the current study, we evaluated the effects of ABT-510 alone or in combination with chemotherapeutics at a late stage of EOC. For these experiments, we initiated treatment at 60 days after tumor induction, at which time an established ovarian tumor has developed, and the animals have started to produce secondary lesions and ascites fluid. The morbidity seen at 60 days after tumor induction replicates stage III of the disease in women, according to the International Federation of Obstetricians and Gynaecologists classification. It is at this stage of disease where most women are diagnosed and treatment is initiated [25]. Animals were treated for 30 days with ABT-510 alone or in combination with cisplatin or paclitaxel to evaluate the effects of the peptide on established tumors, which is a clinically relevant stage at which women would receive treatment.
We have previously shown that if mice are treated with ABT-510 at the time of tumor induction, ovarian tumor growth is significantly reduced, and there is a reduction in expression of cytoprotective and angiogenic factors such as VEGF and VEGFR-2 [9].
Results from some initial antiangiogenic trials have led some to hypothesize that these compounds prune back the immature, disorganized tumor vasculature, although leaving the normal vasculature intact. In our study, treatment with ABT-510 reduced the tumor blood vessel density but increased the proportion of mature, functional blood vessels. As a result, the tumors were better perfused and allowed for an increased uptake of chemotherapy drug. The mechanism by which ABT-510 induced this vessel normalization was through direct apoptotic effects on endothelial cells of immature blood vessels. This apoptotic effect, coupled with the induction of apoptosis on tumor cells, was likely responsible for the decreased tumor size in mice treated with the compound. The vessel normalization and increased tumor perfusion have led some researchers to postulate that antiangiogenic therapy would be most effective when used in combination with chemotherapeutics [26–28]. Indeed, in our study, the effects of either cisplatin or paclitaxel were significantly increased when combined with ABT-510 treatment.
It seems from the results of this study that the additive effectiveness of ABT-510 and the chemotherapeutics was due to increased uptake of the drugs. Animals were treated for 3 weeks with ABT-510 daily before they were injected with radiolabeled paclitaxel. There was a significant increase in paclitaxel incorporation within the ovaries at all time points compared with animals that did not receive the mimetic. Similarly, ABT-510 also facilitated an increased uptake of cisplatin in ovarian tumor tissue. We also injected a group of animals with fluorescently labeled paclitaxel to localize the cytotoxic agent in the ovarian tumors. We found that animals pretreated with ABT-510 had increased paclitaxel uptake in the perivascular areas of the tumor. This localization suggests that the chemotherapy drug uptake was facilitated by the normalized vasculature resulting from ABT-510 treatment. In in vitro experiments in our laboratory, we found that treatment of ID8 cells by ABT-510 and the chemotherapy drugs alone or in combination did not affect the apoptotic effects of the compounds (data not shown). These data suggest that the enhanced antitumor effects of ABT-510 and the chemotherapy drugs were due to increased uptake rather than by additive apoptotic effects on tumor cells directly. The anti-VEGF antiangiogenic drug bevacizumab has been shown to reduce overall blood vessel density while simultaneously decreasing interstitial pressure, increasing tumor tissue perfusion, and increasing the effectiveness of radiation and chemotherapy in patients with rectal cancer [29]. Of considerable interest is the ability of TSP-1 mimetics to increase tissue perfusion and facilitate chemotherapy drug uptake. Intuitively, this would suggest that by increasing the efficiency of drug delivery, patients may require significantly lower dosages of chemotherapy drug to receive the same or improved effects. Also, increased delivery of chemotherapeutic drugs to tumor cells may increase the proapoptotic effects of these drugs, resulting in a more efficient chemical debulking of the tumor and decreased drug resistance. Because approximately 90% of patients with metastatic disease succumb to the disease for issues related to drug resistance [30], any strategy to reduce chemotherapy drug resistance could have tremendous benefit.
The size of the primary tumors was significantly reduced in animals treated with ABT-510 and even more reduced when combined with either cisplatin or paclitaxel. An important observation as well was the complete absence of secondary metastatic peritoneal lesions within the peritoneal space as well as the lack of ascites fluid when mice were treated with the combination of ABT-510 and cisplatin or paclitaxel. These data are significant because treatment with these compounds was not initiated until mice were already suffering from metastatic disease, indicating that ABT-510 treatment combined with chemotherapy caused regression of the disease. The clinical importance of these data is highlighted by the fact that most (approximately 75%) women are not diagnosed with the disease until the tumor has developed and spread within the abdomen [31].
TSP-1 seems to have important effects both on the tumor vasculature as well as direct apoptotic effects on tumor cells themselves. Trastuzumab is an effective antiangiogenic molecule that has been reported to normalize tumor vasculature. Interestingly, the reported mechanism by which trastuzumab decreases proangiogenic factor expression and induces tumor cell apoptosis is through the up-regulation of TSP-1 [32]. Low-dose metronomic chemotherapy is known to elicit a strong antiangiogenic influence [33,34], and increased expression of TSP-1 is an important mediator of this effect [35].
In conclusion, we have shown that the TSP-1 mimetic peptide ABT-510 causes tumor and endothelial cell apoptosis. The pruning of abnormal tumor vasculature seems to leave healthier, normalized blood vessels that facilitate uptake of chemotherapeutic drugs and increase their effectiveness. These results suggest that combined antiangiogenic treatment with TSP-1 mimetics and chemotherapy drugs may significantly improve the outcome of patients with EOC.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Scully RE. World Health Organization classification and nomenclature of ovarian cancer. Natl Cancer Inst Monogr. 1975;42:5–7. [PubMed] [Google Scholar]
- 3.Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U. Carcinoma of the ovary. FIGO 6th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;9:S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 5.Morrison J, Swanton A, Collins S, Kehoe S. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD005343.pub2. D005343. [DOI] [PubMed] [Google Scholar]
- 6.Ozols RF, Rubin SC, Thomas G, Robboy S. Epithelial ovarian cancer. In: Hoskins WJ, Perez CA, Young RC, editors. Principles and Practice of Gynecologic Oncology. 2nd ed. Philadelphia, PA: Lippincott-Raven; 1997. pp. 919–986. [Google Scholar]
- 7.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 8.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenaway J, Henkin J, Lawler J, Moorehead R, Petrik J. ABT-510 induces tumor cell apoptosis and inhibits ovarian tumor growth in an orthotopic, syngeneic model of epithelial ovarian cancer. Mol Cancer Ther. 2009;8:64–74. doi: 10.1158/1535-7163.MCT-08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markovic SN, Suman VJ, Rao RA, Ingle JN, Kaur JS, Erickson LA, Pitot HC, Croghan GA, McWilliams RR, Merchan J, et al. A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma. Am J Clin Oncol. 2007;30:303–309. doi: 10.1097/01.coc.0000256104.80089.35. [DOI] [PubMed] [Google Scholar]
- 11.Baker LH, Rowinsky EK, Mendelson D, Humerickhouse RA, Knight RA, Qian J, Carr RA, Gordon GB, Demetri GD. Randomized, phase II study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced soft tissue sarcoma. J Clin Oncol. 2008;26:5583–5588. doi: 10.1200/JCO.2008.17.4706. [DOI] [PubMed] [Google Scholar]
- 12.Gordon MS, Mendelson D, Carr R, Knight RA, Humerichouse RA, Iannone M, Stopeck AT. A phase 1 trial of 2 dose schedules of ABT-510, an antiangiogenic, thrombospondin-1-mimetic peptide, in patients with advanced cancer. Cancer. 2008;113:3420–3429. doi: 10.1002/cncr.23953. [DOI] [PubMed] [Google Scholar]
- 13.Ebbinghaus S, Hussain M, Tannir N, Gordon M, Desai AA, Knight RA, Humerickhouse RA, Qian J, Gordon GB, Figlin R. Phase 2 study of ABT-510 in patients with previously untreated advanced renal cell carcinoma. Clin Cancer Res. 2007;13:6689–6695. doi: 10.1158/1078-0432.CCR-07-1477. [DOI] [PubMed] [Google Scholar]
- 14.Hoekstra R, de Vos FY, Eskens FA, Gietema JA, van der Gaast A, Groen HJ, Knight RA, Carr RA, Humerickhouse RA, Verweij J, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 in patients with advanced cancer. J Clin Oncol. 2005;23:5188–5197. doi: 10.1200/JCO.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Gietema JA, Hoekstra R, de Vos FY, Uges DR, van der GA, Groen HJ, Loos WJ, Knight RA, Carr RA, Humerickhouse RA, et al. A phase I study assessing the safety and pharmacokinetics of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with gemcitabine and cisplatin in patients with solid tumors. Ann Oncol. 2006;17:1320–1327. doi: 10.1093/annonc/mdl102. [DOI] [PubMed] [Google Scholar]
- 16.Hoekstra R, de Vos FY, Eskens FA, de Vries EG, Uges DR, Knight R, Carr RA, Humerickhouse R, Verweij J, Gietema JA. Phase I study of the thrombospondin-1-mimetic angiogenesis inhibitor ABT-510 with 5-fluorouracil and leucovorin: a safe combination. Eur J Cancer. 2006;42:467–472. doi: 10.1016/j.ejca.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Roby KF, Niu F, Rajewski RA, Decedue C, Subramaniam B, Terranova PF. Syngeneic mouse model of epithelial ovarian cancer: effects of nanoparticulate paclitaxel, Nanotax. Adv Exp Med Biol. 2008;622:169–181. doi: 10.1007/978-0-387-68969-2_14. [DOI] [PubMed] [Google Scholar]
- 18.Greenaway J, Moorehead R, Shaw P, Petrik J. Epithelial-stromal interaction increases cell proliferation, survival and tumorigenicity in a mouse model of human epithelial ovarian cancer. Gynecol Oncol. 2008;108:385–394. doi: 10.1016/j.ygyno.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Straathof CSM, van den Bent MJ, Ma J, Schmitz PIM, Kros JM, Stoter G, Vecht ChJ, Schellens JHM. The effect of dexamethasone on the uptake of cisplatin in 9L glioma and the area of brain around tumor. J Neurooncol. 1998;37:1–8. doi: 10.1023/a:1005835212246. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mabuchi S, Terai Y, Morishige K, Tanabe-Kimura A, Sasaki H, Kanemura M, Tsunetoh S, Tanaka Y, Sakata M, Burger RA, et al. Maintenance treatment with bevacizumab prolongs survival in an in vivo ovarian cancer model. Clin Cancer Res. 2008;14:7781–7789. doi: 10.1158/1078-0432.CCR-08-0243. [DOI] [PubMed] [Google Scholar]
- 22.Richardson DL, Backes FJ, Seamon LG, Zanagnolo V, O'Malley DM, Cohn DE, Fowler JM, Copeland LJ. Combination gemcitabine, platinum, and bevacizumab for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2008;111:461–466. doi: 10.1016/j.ygyno.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, Minasian L, Sarosy G, Kotz HL, Premkumar A, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nimeiri HS, Oza AM, Morgan RJ, Friberg G, Kasza K, Faoro L, Salgia R, Stadler WM, Vokes EE, Fleming GF. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II consortia. Gynecol Oncol. 2008;110:49–55. doi: 10.1016/j.ygyno.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, Woolas R, Jeyarahjah AR, Sibley K, Lowe DG, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207–1210. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Waxman DJ. Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther. 2008;7:3670–3684. doi: 10.1158/1535-7163.MCT-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 28.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 29.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 31.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33:3–11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology—herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 33.Bocci G, Falcone A, Fioravanti A, Orlandi P, DiPaolo A, Fanelli G, Viacava P, Naccarato AG, Kerbel RS, Danesi R, et al. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer. 2008;98:1619–1629. doi: 10.1038/sj.bjc.6604352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamat AA, Kim TJ, Landen CN, Jr, Lu C, Han LY, Lin YG, Merritt WM, Thaker PH, Gershenson DM, Bischoff FZ, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2008;67:281–288. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 35.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin-1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–12922.. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]