Abstract
EEGs were examined in data collected from 348 1-week, 1-month and 3-month-old infants of depressed and non-depressed mothers across several studies. Both the percentage of infants exhibiting spectral peaks and the frequency in Hz at which those peaks were exhibited increased with age. Consistent with previous studies, infants of depressed mothers exhibited greater left frontal EEG power, suggesting greater relative right frontal EEG activity than infants of non-depressed mothers. This profile was apparent across a narrow frequency range, which shifted from 3–9Hz at one week of age to 4–9Hz by three months of age.
EEG in 1-Week, 1-Month and 3-Month-Old Infants of Depressed and Non-Depressed Mothers
Maternal depression has been noted to negatively affect infant development (see Field, Diego & Hernandez-Reif, 2006 for a review). Prior to birth, fetuses of depressed mothers exhibit distinct neurobehavioral profiles (Allister, Lester, Carr & Liu, 2001; Monk, Sloan, Myers et al., 2004) and shortly after birth, neonates of depressed mothers show neurobehavioral dysregulation (Field, Diego, Dieter, et al., 2004; Hernandez-Reif, Field, Diego & Ruddock, 2006), that persists throughout infancy (Jones, Field, Davalos &, Pickens 1997a; Jones, Field, Fox, Lundy & Davalos, 1997b) and may predispose these infants to develop cognitive, motor and emotional problems (Patel et al., 2003; Silverstein, Augustyn, Cabral & Zuckerman, 2006) and psychopathology (Luoma, Tamminen, Kaukonen et al., 2001; Warner, Weissman, Mufson, & Wickramaratne, 1999).
Infants of depressed mothers are also more likely to exhibit a particular pattern of resting brain electrical activity marked by greater left than right frontal EEG power suggesting relative right frontal EEG activity (Table 1). This pattern is evident as early as one week of age (Diego, Field, Hernandez-Reif, Cullen, Schanberg & Kuhn, 2004; Jones, Field, Fox, Davalos, Lundy & Hart, 1998) and has been shown to remain stable from one to three months (Jones et al., 1997b) and from three months to three years (Jones et al., 1997a). Furthermore, the pattern of EEG asymmetry exhibited by these infants is consistent with their behavioral affective style and is similar to the EEG pattern exhibited by their mothers (Field et al., 2004; Jones et al., 1998) and other depressed adults (see Davidson, 2000 for a review) even when these individuals show a remission in their behavioral symptoms (Henriques & Davidson, 1990).
Table 1.
Summary of findings for all studies examining differences in resting frontal EEG asymmetries between infants of depressed and non-depressed mothers.
❍ND ●D = Infants of depressed mothers exhibited greater right frontal EEG asymmetry (greater left than right frontal EEG power) than infants of non-depressed mothers.
❍I ●W = Infants of depressed withdrawn mothers exhibited greater right frontal EEG asymmetry (greater left than right frontal EEG power) than infants intrusive depressed mothers.
●I ● W = Both infants of depressed intrusive and withdrawn mothers exhibited greater right frontal EEG asymmetry (greater left than right frontal EEG power) than infants intrusive depressed mothers.
Reference locations: Cz = vertex, LM = Linked Mastoids.
Frequency Bands
In most adult studies, EEG asymmetries have been assessed on 8–13Hz- EEG power, as activity within this frequency band is thought to be inversely related to the degree of cortical activity occurring at a given electrode site (Davidson, 2000). In the waking infant, the EEG is primarily composed of low frequency activity (1–4Hz) which is widely preponderant across the scalp from birth to three months of age, and becomes more stable and increases in frequency to 3–5Hz by five months (Mizuno, Yamauchi, Watanabe, Komatsushiro, Takagi, Iinuma & Arakawa, 1970), and 7–8Hz by the end of the first year (Marshall, Bar-Haim & Fox, 2002; Stroganova, et al., 1999). Because the spectral properties of the human EEG change across development, it is not appropriate to use the adult frequency bands in the study of infant EEG (Pivik et al., 1993). As such, it has been proposed that researchers use either a wide frequency band that encompasses all frequencies in which there is a substantial amount of power (i.e. 3–12Hz), or use narrow frequency bands centered around the peaks in the spectrum (i.e. 6–9Hz) (Pivik et al., 1993).
Studies examining frontal EEG asymmetries in infants of depressed mothers have employed a wide range of frequency bands from the 3–12Hz and 3–13Hz bands on one week-olds to the 3–12Hz, the 2–6Hz and 6–9Hz bands on three to six month olds, and the 3–5Hz, 6–9Hz and 10–12Hz bands in older infants (Table 1). The use of distinct frequency bands creates a problem when comparing findings across studies. For example, while studies using the 2–6Hz and 3–12 Hz bands have found differences between infants of depressed and non-depressed mothers at three months (Jones et al., 1997; Field et al., 1995), studies using the 6–9Hz band, have yielded inconsistent results (Field et al., 1998; Jones et al., 1997). Furthermore, at least one study, which examined 3–5Hz, 6–9Hz and 10–12Hz band asymmetries, found that only the 6–9Hz band discriminated thirteen to fifteen month-old infants of depressed mothers from infants of non-depressed mothers (Dawson, Frey, Self et al., 1999). Because power within distinct frequency bands might reflect distinct components across development, the variation in the use of frequency bands due to a lack of clear understanding highlights the need for determining frequency bands that best discriminate infants of depressed from those of non-depressed mothers across early infant development.
Even though numerous studies have examined EEG asymmetries in infants of depressed mothers, none have critically evaluated the optimal methodological approach for deriving frontal EEG asymmetries in this population. As such, the present study examined the spectral properties of EEG records of one week, one month and three month-old infants of depressed and non-depressed mothers, and critically evaluated the efficacy of distinct methodological approaches currently used in the study of infant EEG asymmetries. Based on previous findings, we hypothesized that infants of depressed mothers would exhibit greater left than right frontal EEG power than infants of depressed mothers suggesting greater relative right frontal EEG activity. EEG asymmetries computed at certain frequencies were expected to more effectively discriminate infants of depressed mothers from those of non-depressed mothers.
Method
Participants
The sample consisted of the records of 348 infants obtained from databases on the effects of maternal depression on infant development including demographic information, infant EEG records and self-report measures of maternal depression previously reported by Field, Jones, Diego and their colleagues (Field, Diego, Dieter et al., 2004; Diego, Field, Jones, et al., 2004; Jones, Field, Fox, Lundy & Davalos, 1997; Jones, Field, Fox, Davalos, Lundy & Hart, 1998; Jones & Field, 1998; Jones, McFall & Diego, 2004). This cross-sectional sample consisted of the records from 100, one-week-old infants, 144, one-month-old infants and 104, three-month-old infants born to mothers with and without symptoms of depression (Total: Depressed n = 149; Non-Depressed n = 199; 1-Week: Depressed n = 45; Non-Depressed n = 55; 1-Month: Depressed n = 66; Non-Depressed n = 78; 3-Months: Depressed n = 38; Non-Depressed n = 66). The mothers had been recruited from prenatal clinics and newborn nurseries and were on average 25-years-old, predominantly middle SES on the Hollingshead index (Hollingshead, 1975) and distributed 36% African-American, 33% Hispanic, 29% Caucasian and 2% Asian. Records were only compiled for right-handed mothers who: a) had uncomplicated pregnancies and deliveries; b) did not smoke cigarettes, drink alcohol or use recreational drugs during pregnancy; c) had not been prescribed medications (including antidepressants or other psychotropic medications) other than vitamins; and d) had healthy, full-term infants who did not exhibit any abnormalities during pregnancy, delivery or the postpartum period. Only records with at least 15 valid windows of infant EEG were included.
Assessments
A Demographic Questionnaire was administered to the mothers after they signed an informed consent. The questionnaire included the mothers’ age, ethnicity, occupation and education. Answers to the occupation and education questions were then used to compute socioeconomic status (SES) based on the Hollingshead (1975) Four Factor Index of Social Status.
The Center for Epidemiological Studies-Depression scale (CES-D) (Radloff, 1977) is a 20-item scale, with scores ranging between 0 and 60. The respondents rate the frequency (within the last week) of 20 symptoms. The symptoms include depressed mood, feelings of helplessness and hopelessness, feelings of guilt and worthlessness, loss of energy, and problems with sleep and appetite. Despite the fact that the CES-D does not allow for a clinical diagnosis of depression, studies have shown that a score of 16 or greater can differentiate clinically depressed and non-depressed participants with only a 6% false positive and 36% false negative rate (Radloff, 1977). In addition, this scale has been shown to be reliable and valid for diverse demographic groups (Radloff, 1977). Test-retest reliability for the CES-D indicates satisfactory test-retest reliability ranging from .51 to .67 over a 2–8 week period and from .32 to .54 over a 3- to 12-month period (Radloff, 1977).
Although depression symptoms assessed via self-report scales such as the CES-D do not provide a clinical diagnosis for depression, they have been correlated with diagnosed depression based on clinical evaluations (Radloff & Teri, 1986) and with well established structured clinical interviews such as the DIS (Weissman, Prusoff & Newberry, 1975; Wilcox, Field, Promodromis & Scafidi, 1998). Furthermore, research on both adults and infants reveals that mothers who have scores above the cut off on self-report scales such as the CES-D and Beck Depression Inventory (BDI), exhibit a pattern of electrophysiological (Diego, Field & Hernandez-Reif, 2001; Henriques & Davidson, 1991; Shaffer, Davidson, & Saron, 1983) and biochemical dysregulation (Field, Diego, Dieter, Hernandez-Reif, Schanberg, Kuhn, Yando & Bendell, 2004) that is mimicked by their infants (Field et al., 1990; Field et al., 2001) and is not different when a clinical diagnosis for depression is used (Dawson, Frey, Panagiotides, Osterling, & Hessl, 1999; Jones et al., 1998; Henriques & Davidson, 1990). The mother’s CES-D was administered during the same visit that the infant’s EEG was recorded.
EEG
The infant’s EEG was recorded for three-minutes from four (F3, F4, P3 & P4) scalp locations referenced to the vertex (Cz). The vertex-referencing scheme was used, as it is the least invasive location for young infants and has been used in previous research examining EEG asymmetry in this age range. EEG recordings were conducted using lycra stretchable caps (manufactured by Electro-Cap, Inc.) that were positioned on the participant’s head using anatomical landmarks (Bloom & Anneveldt, 1982). Omni-prep gel and electrode gel were inserted into each site in order to gently abrade and provide good conductance. Impedances were less than 5K ohms or the site was re-abraded until optimal impedances were obtained. EOG was also obtained from the outer canthus and the supra-orbit position of one eye using Beckman mini-electrodes. 1-week and 1-month old infants were swaddled and rested in bassinette, while 3-month old infants sat in an infant car seat were during the EEG recordings. The infants mothers as well as the research team were instructed to remain quiet and out of the infant’s field of vision during these recordings to avoid any influence on the infants emotional state during these EEG baseline recordings.
The EEG signals were amplified using Grass Model 12 Neurodata Acquisition System amplifiers with filters set at 1Hz high pass and 100Hz low pass and a gain of 20,000, 60Hz notch filter on. Prior to data collection, the signal for each channel was calibrated using a 10Hz sine wave. The analogue output from the amplifiers was then digitized at a sampling rate of 512 samples per second and digitized using 12-bit conversion (Analog Devices RTI-815 A/D board). The data were then streamed to a computer screen and saved to a hard disk using data acquisition software (Snapstream, v. 3.21, HEM Data Corp. 1991).
Data Reduction
The digitized EEG data were graphically displayed and the EOG channels were used as cues, the data containing eye and motor movement artifact were underscored and eliminated from all channels. Artifact free data were submitted to a discrete Fourier Transform using a 1-second Hanning Window with 50% overlap using EEGEDIT software (James Long Company, Caroga Lake, NY). These analyses produced average power density values for each participant at each site single-hertz (Hz) frequency bins from 3–12Hz in picowatt ohms (one microvolt squared) for each channel. The mean number of artifact free windows across all infants was 93 (SD = 47). The mean number of artifact free windows for infants of depressed mothers was 89.5 (SD = 45.8) and 96.0 (SD = 48.5) for infants of non-depressed mothers.
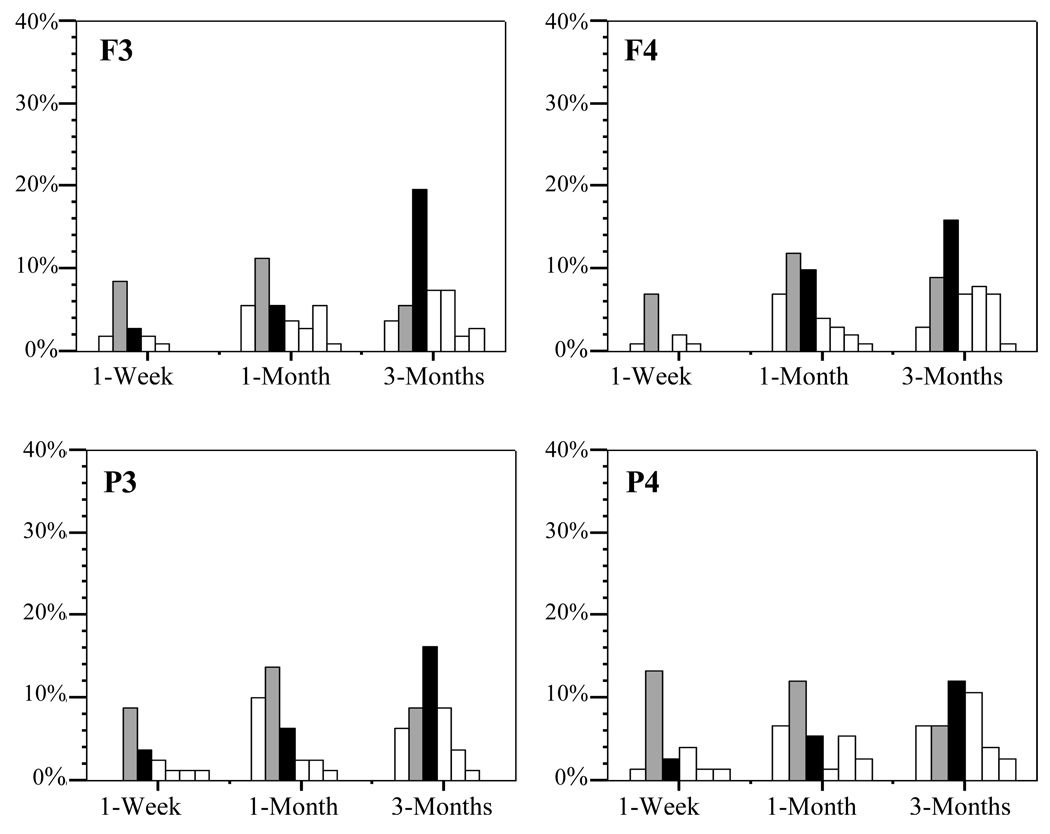
Individual spectral plots (for each infant at each recording site) were then visually inspected for either the absence of, or the presence of single or multiple visually detectable peaks in amplitude occurring within the 3–12Hz boundaries. Cumulative percentages of power occurring within the 3–12Hz bands revealed that approximately 55 to 63% of the EEG activity at both frontal and parietal sites for 1-week, 1-month and 3-month old infants occurred between 3 and 4Hz, 90% occurred between 3–8Hz and 95% occurred below 10Hz. 20% of 1-week infants, 27% of 1-month infants and 50% of 3-month infants exhibited at least one spectral peak over frontal sites and 19% of 1-week infants, 20% of 1-month infants and 36% of 3-month infants showing at least one spectral peak over parietal sites. Frequencies computed on the peak amplitude frequency across age groups revealed a mode peak frequency of 4Hz for the 1-week and 1-month old infants and a mode peak frequency of 5Hz for the 3-month old infants across all sites.
Inasmuch as power values were positively skewed and leptokurtotic, the natural logarithms of the absolute power values were used to compute log power values. EEG asymmetry scores were then computed on the log power values for each homologous electrode pair site (Ln(F3) − Ln(F4) for frontal asymmetry & Ln(P3) − Ln(P4) for parietal asymmetry) for each of the 3–12Hz frequency bins and for the 3–6Hz, 6–9Hz, 9–12Hz and 3–12Hz bands. Negative scores reflect greater left than right EEG power, and positive scores reflect greater right than left EEG power1.
Results
Demographics
Analyses of Variance and Chi Square analyses on Maternal Depression Group and Age Group differences (see Table 2) revealed that the groups (across both age and depression status) did not differ on maternal age, socioeconomic status (SES), ethnicity, or infant gender. The age at testing did not differ for infants of Depressed and Non-Depressed mothers within the one week, one month or three month-old groups. Similarly, the mothers’ depression scores did not differ for infants of depressed mothers in the one-week, one-month or three month-old groups or for infants of non-depressed mothers in the one-week, one-month or three-month-old groups.
Table 2.
Demographic distribution across age and depression group^
| 1-Week | 1-Month | 3-Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| D | ND | D | ND | D | ND | Age | Depression | Age × Dep. | |
| Infant Age | 6.89 (2.06) |
6.55 (2.64) |
36.64 (2.48) |
36.91 (3.57) |
95.44 (3.78) |
94.44 (5.77) |
F(2,342) = 14583, p <.001 |
F(1,342) = 0.78 p = .378 |
F(2,348) = 0.88 p = .417 |
| Maternal Age | 25.64 (5.32) |
24.91 (6.12) |
24.93 (5.78) |
26.47 (6.39) |
25.84 (4.42) |
26.83 (7.45) |
F(2,342) = 0.74 p = .478 |
F(1,342) = 0.77 p = .379 |
F(2,348) = 1.03 p = .357 |
| SES | 3.53 (1.50) |
3.29 (1.27) |
3.26 (1.22) |
2.99 (1.25) |
3.18 (1.39) |
3.05 (1.29) |
F(2,342) = 1.74 p = .177 |
F(1,342) = 2.21 p = .138 |
F(2,348) = 0.09 p = .918 |
| CES-D | 24.98 (8.14) |
8.13 (4.34) |
23.98 (8.15) |
6.83 (3.73) |
23.74 (8.06) |
5.76 (3.73) |
F(2,342) = 2.23 p = .109 |
F(1,342) = 665 p <.001 |
F(2,348) = 0.23 p = .795 |
| Gender | χ2(2) = 0.48 | χ2(1) = 0.20 | |||||||
| Male | 49% | 51% | 47% | 49% | 42% | 47% | p = .788 | p = .653 | |
| Female | 51% | 49% | 53% | 51% | 58% | 53% | |||
| Race | χ2(6) = 11.20 | χ2(3) = 6.37 | |||||||
| Caucasian | 20% | 18% | 24% | 37% | 21% | 44% | p = .082 | p = .095 | |
| Hispanic | 31% | 40% | 35% | 26% | 42% | 32% | |||
| African Am. | 44% | 42% | 39% | 36% | 34% | 21% | |||
| Asian | 4% | 0% | 2% | 1% | 3% | 3% | |||
Standard deviations in parentheses under means; SES = Hollingshead Index for Socio Economic Status; CES-D = Center for Epidemiological Studies Depression Scale, D = Infants of depressed mothers; ND= Infants of depressed of non-depressed mothers;
EEG Spectral Properties
Cumulative percentages of power occurring within the 3–12Hz bands revealed that approximately 55% to 63% of the EEG activity at both frontal and parietal sites for 1-week, 1-month and 3-month old infants occurred between 3 and 4Hz, 90% occurred between 3–8Hz and 95% occurred below 10Hz. These cumulative percentages of power were similar for infants of depressed and non-depressed mothers.
Chi square tests revealed that a presence of at least one spectral peak increased with age 20% of 1-week infants, 27% of 1-month infants and 50% of 3-month infants exhibited at least one spectral peak over frontal sites and 19% of 1-week infants, 20% of 1-month infants and 36% of 3-month infants showing at least one spectral peak over parietal sites (p < .001). Chi square tests failed to reveal any differences between infants of depressed and non-depressed mothers.
The peak amplitude frequency across age groups revealed a mode peak frequency of 4Hz for the 1-week and 1-month old infants and a mode peak frequency of 5Hz for the 3-month old infants across all sites (Figure 1). Mode peak frequencies for each age group were identical for infants of depressed and non-depressed mothers.
Figure 1.
Distribution of the peak frequency in absolute power within the 2–13Hz range for each electrode site (F3 thru P4). Bars indicate the percentage of infants within an age group whose frequency fell within each 1-Hz bin. Gray bars indicate 4Hz bin, Black bars indicate the 5Hz bin.
Frequency Band Analyses
An Age group (1-week/1-month/3-month) by Depression group (Depressed/Non-Depressed) by Frequency bin (3Hz, 4Hz…12Hz) by Hemisphere (Left/Right) by Region (Frontal/Parietal) ANOVA was conducted on log power values. This analysis revealed significant Hemisphere by Frequency by Age by Group, F (18, 3078) = 2.63, p = .021, ε = .281, a Hemisphere by Region by Frequency by Group, F (9, 3078) = 5.12, p = .003, ε = .296 interaction effects. The Age by Frequency interaction was followed by the examination of the mean relative power plots and Games-Howell post hoc comparisons comparing mean relative power values between each age group at each frequency. These analyses revealed that there was a steady decrease in amplitude from the lowest frequency bins to the highest frequency bins. Further, while there were not any significant differences between 1-week and 1-month olds, 3-month old infants had significantly less 3Hz, p = .011 relative power than 1-week old infants and significantly less 4Hz, p = .011, relative power than 4-month old infants. Further, 3-month old infants showed significantly greater 6Hz and 7Hz relative power than both 1-week olds (p < .001, p = .001) and 3-month olds (p < .001, p < .001 respectively).
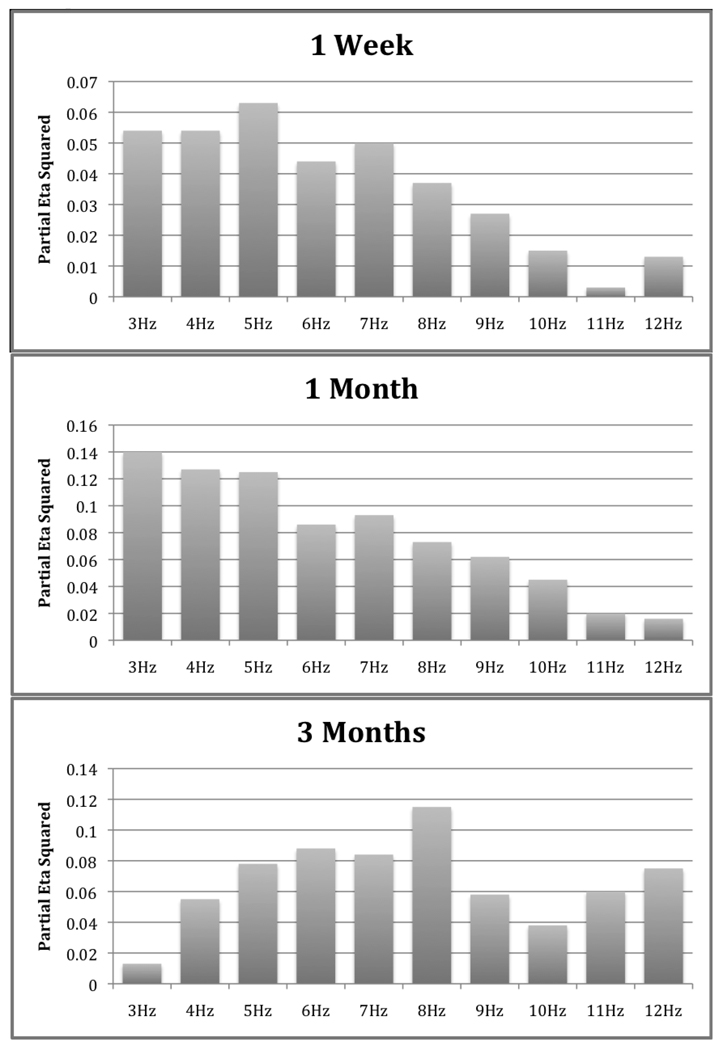
Group by Hemisphere by Age Group ANOVAs were then conducted for the frontal region at each frequency. These analyses revealed significant Group by Hemisphere interactions across all frequencies (3Hz–12Hz), but only a significant Group by Hemisphere by Age Group interaction for the 3Hz frequency bin, F (2, 3078) = 4.98, p = .008, suggesting that frontal EEG asymmetry differences between infants of depressed and non-depressed mothers were not consistent across the three age groups studied. Inasmuch as these analyses were conducted to test for differences in hemispheric activity between infants of depressed and non-depressed mothers, these significant four-way interactions were followed by Group by Hemisphere ANOVAs conducted separately for the frontal and parietal regions at each frequency (Figure 2). The conservativeGames-Howell procedure was used to control for alpha inflation, unequal group sizes and heterogeneous variances. These analyses revealed the following: 1) one week old infants of depressed mothers exhibited greater left than right frontal EEG power from 3Hz to 7Hz; 2) one month old infants of depressed mothers exhibited greater left than right frontal EEG power from 3Hz to 10Hz; and 3) three month old infants of depressed mothers exhibited greater left than right frontal EEG power from 4Hz to 12Hz.
Figure 2.
Partial Eta Square values for the Group by Hemisphere ANOVAs computed on log- power values at frontal (F3 & F4) sites for 1-Week, 1-Month and 3-Month old infants of depressed and non-depressed mothers.
To determine which frequency bands discriminated infants of depressed and non-depressed mothers at each age, discriminant function analyses were then computed for each age group separately with maternal depression group (Non-Depressed vs. Depressed) entered as the grouping variable and EEG asymmetry scores for single Hz- bins from 3–12Hz entered as the dependent variables. These analyses were followed by the examination of the discriminant function loadings and the F values from Group by Asymmetry ANOVAs to determine the frequency band with the greatest discriminant validity. In order to evaluate this band, subsequent ANOVAs and discriminant function analyses were then conducted to compare the discriminant validity of this frequency band to the broad frequency band (3–12Hz).
The classification ability of these bands was then evaluated using proportional chance criterion analyses. This analysis uses Z-scores to evaluate if the proportion of cases correctly classified within a group is significantly better than chance. These analyses revealed the following (Table 3): 1) Frontal EEG asymmetry within the 3–9Hz and the 3–12Hz frequency bands discriminated infants of depressed from infants of non-depressed mothers; 2) Frontal EEG Asymmetry within the 3–9Hz and the 3–12Hz frequency bands discriminated infants of depressed from infants of non-depressed mothers at one week of age; 3) Frontal EEG asymmetry within the 3–9Hz frequency bands discriminated infants of depressed from infants of non-depressed mothers at one month of age; 4) Frontal EEG asymmetry within the 4–9Hz frequency band discriminated infants of depressed from infants of non-depressed mothers at three months of age.
Table 3.
Cross validated classification results for the discriminant function analyses on frontal EEG asymmetry for each age group.
| Whole Sample | ||||||||
| Wide Frequency Band Model (3–12Hz) | Narrow Frequency Band Model (3–9Hz) | |||||||
| F (1, 346), 36.13** | F (1, 346), 36.87** | |||||||
| λ = .905; χ2 (1) =34.31** | λ = .904; χ2 (1) =34.98** | |||||||
| Hits | IC | Z | Hits | IC | Z | |||
| D | 55.03% | 22.54% | 3.18 | 55.70% | 22.54% | 3.18 | ||
| ND | 71.36% | 34.28% | 4.18 | 70.85% | 31.93% | 3.90 | ||
| 1-Week | ||||||||
| Wide Frequency Band Model 3–12Hz | Wide Frequency Band Model 3–9Hz | |||||||
| λ = .933; χ2 (1) =6.76, p =.009 | λ = .931; χ2 (1) =6.99, p =.008 | |||||||
| Hits | IC | Z | Hits | IC | Z | |||
| D | 64.44% | 35.35% | 2.62 | D | 64.44% | 35.35% | 2.62 | |
| ND | 69.09% | 31.31% | 2.10 | ND | 69.09% | 31.31% | 2.10 | |
| 1-Month | ||||||||
| Wide Frequency Band Model 3–12Hz | Narrow Frequency Band Model 3–9Hz | |||||||
| λ = .859; χ2 (1) =21.54, p <.001 | λ = .854; χ2 (1) = 22.31, p <.001 | |||||||
| Hits | IC | Z | Hits | IC | Z | |||
| D | 62.12% | 30.07% | 2.66 | D | 63.64% | 32.87% | 2.90 | |
| ND | 71.79% | 38.46% | 3.12 | ND | 71.79% | 38.46% | 3.12 | |
| 3-Months | ||||||||
| Wide Frequency Band Model 3–12Hz | Narrow Frequency Band Model 4–9Hz | |||||||
| λ = .936; χ2 (1) =6.69, p =.010 | λ = .913; χ2 (1) =9.29, p =.002 | |||||||
| Hits | IC | Z | Hits | IC | Z | |||
| D | 36.84% | 0.48% | 0.04 | D | 42.11% | 8.77% | 0.71 | |
| ND | 78.79% | 41.95% | 2.59 | ND | 80.30% | 46.09% | 2.84 | |
D = Infants of depressed mothers; ND= Infants of non-depressed mothers
Hits = Percent correctly classified; IC = Percent improvement over chance
Frontal EEG Asymmetry Variability
Inasmuch as the distributions of frontal EEG asymmetry values for infants of depressed and non-depressed mothers might be different, a series of Levene’s tests for equality of variances were conducted on frontal EEG asymmetry difference values at each age (Table 4). These analyses revealed that frontal EEG asymmetry difference variances did not differ for infants of depressed and non-depressed mothers at one week and at one month, however, at three month-old infants of depressed mothers show marginally greater variability than infants of non-depressed mothers at 3–9 Hz (F = 6.46, p = .06).
Table 4.
Between groups variance differences computed for frontal EEG asymmetry at each age group. p values are Bonferroni corrected for multiple comparisons.
| 3–9 Hz | 4–9 Hz | |
|---|---|---|
| 1-Wk | F = 0.71, p = N.S. | F = 1.31, p = N.S. |
| 1-Mo | F = 2.70, p = N.S. | F = 2.59, p = N.S. |
| 3-Mo | F = 6.46, p = .06 | F = 4.25, p = N.S. |
Discussion
Analyses of the individual spectral distribution plots revealed that older infants were more likely to exhibit a clearly discernable peak in frequency within the 3–12Hz range than younger infants. This is consistent with previous findings indicating that the proportion of infants who show a clearly discernable spectral peak within the 3–12Hz range significantly increases with age (Hagne, 1972; Marshall et al., 2002; Mizuno et al., 1970; Stroganova et al., 1999). Of those infants that showed a clearly discernable peak, the majority of 1-week and 1-month olds showed a peak at 4Hz and the majority of 3-month olds showed a peak at 5Hz (Figure 1). These findings are consistent with previous research indicating the presence of a 3–5Hz rhythm at about 3-months of age (Hagne, 1972; Lindsley, 1944, Mizuno et al., 1970; Smith, 1939). The shift in frequency from 4Hz at 1-week and 1-month to 5Hz at 3 months is also consistent with findings from studies showing development changes in the frequency distribution of these spectral peaks (Hagne, 1972; Marshall et al., 2002; Stroganova et al., 1999).
Infants of depressed mothers exhibited greater left than right frontal EEG power suggesting greater relative right frontal EEG activity than infants of non-depressed mothers, but they did not exhibit any hemispheric differences in parietal power. These findings are in accord with the greater left than right frontal (but not parietal) EEG power observed in infants of depressed mothers and infants exhibiting negative affective styles (see Table 1). They are also consistent with findings from adult studies indicating that depressed individuals exhibit greater left than right frontal EEG power (see Davidson, 1995; 2000 for reviews). However, even though this frontal asymmetry pattern was evident across all ages, the frequency bins on which these differences were exhibited differed with age. One-week old infants could only be differentiated at 3 to 7Hz, one-month old infants could only be differentiated at 3 to 10Hz, and three-month old infants exhibited significant differences at 4 to 12Hz (Figure 2).
Similarly, when all three age groups were pooled, the 3–9Hz bins yielded the greatest discriminant functions and F values. Frontal EEG asymmetry scores computed using both the wide 3–12Hz and the narrow 3–9Hz frequency bands, significantly classified infants of depressed and non-depressed mothers. Inasmuch as 95% of EEG activity occurs below 10Hz in young infants, it would be advisable to use the 3–9Hz frequency bin in EEG asymmetry studies conducted on young infants.
Discriminant function analyses conducted for each age group to evaluate the frequency at which frontal EEG asymmetries best discriminated infants of depressed from infants of non-depressed mothers, revealed that the 3–9Hz bins yielded the greatest discriminant functions and F values for one week and one month-old infants. For one week and one month-old infants, the discrimination obtained with the 3–9Hz band was almost identical to the discrimination obtained with the use of the wider 3–12Hz frequency band. In both cases, frontal EEG asymmetry scores were able to significantly classify infants of depressed and non-depressed mothers. The almost identical classification results obtained with the 3–12Hz and the 3–9Hz band for one week and one month-old infants suggest that resting EEG asymmetry values obtained using the wide 3–12Hz frequency band can be compared to findings obtained using discrete frequencies within the 3–9Hz range. This is encouraging as most of the frontal EEG asymmetry studies conducted on infants this age have examined the wide 3–12Hz band or frequencies between 3–9Hz (see Table 1).
Unlike younger infants, the 4–9Hz bins yielded the greatest discriminant functions and F values for three month-old infants, suggesting that the 4–9Hz band is better able to discriminate infants of depressed from infants of non-depressed mothers at this age. Three month-old infants’ frontal EEG asymmetry within the 3–12Hz band yielded less accurate classifications than those obtained with the narrow 4–9Hz frequency band. The majority of studies examining differences in frontal EEG asymmetry in infants older than three months have used the 6–9Hz band. Given our findings, it is not surprising that these studies have consistently found differences between infants of depressed and infants of non-depressed mothers (see table 1).
It was surprising that three-month old infants’ frontal EEG asymmetry scores were better able to classify infants of non-depressed mothers than infants of depressed mothers. This could be attributed to any one of a number of possible factors. For example, this could simply be an artifact of the group sizes analyzed at each age, while the group sizes for the depressed and non-depressed groups were similar for one-week (n=45, D; n=55, ND) and one-month old infants (n=66, D; n=78, ND), there were substantially fewer three-month old infants in the depressed group (n=38) than in the non-depressed group (n=66). The lower discriminant ability of frontal EEG scores in 3 month-old infants of depressed mothers might have resulted from their exposure to different social environments. By virtue of their age, three month-old infants have had a chance to more extensively interact with their mothers than younger infants. Depressed mothers may exhibit withdrawn or intrusive interaction styles when interacting with their infants (Cohn, Matias, Tronick, Connell, & Lyons-Ruth, 1986; Tronick & Field, 1986) and these styles affect the development of infant frontal EEG asymmetry differently, with infants of withdrawn depressed mothers showing a shift towards greater right frontal EEG activity infants of intrusive depressed mothers showing a shift towards greater left frontal EEG activity (Diego, et al., 2005; Jones, et al., 1997). Unfortunately, we were not able to assess maternal interaction styles in these mothers to determine if maternal interaction style was related to the greater EEG asymmetry variability exhibited by these infants. The heterogeneity of maternal depression is a potential limitation of these findings, as differences in maternal depression can differentially affect the infant's physiological patterns. Future studies should assess maternal depression subtypes including withdrawn and intrusive interaction styles.
In conclusion, the greater left frontal EEG power profile exhibited by infants of depressed mothers was pronounced across a narrow frequency range, which shifted from 3–9Hz in one-week and one month-old infants to 4–9Hz in three month-old infants. These narrow frequency band ranges were better able to discriminate infants of depressed from infants of non-depressed mothers than the 3–12Hz wide band frequency range.
The developmental change in the frequency band that best differentiated infants of depressed from infants of non-depressed mothers from 3–9Hz to 4–9Hz is consistent with developmental findings and suggests that during the first month of life, the 3–9Hz frequency band manifests regional cortical activity levels that may reflect affective style. By three months of age, the activity tapped by the 3–9Hz band during the first month shows a shift in its frequency distribution and becomes more apparent over the 4–9Hz frequency band. One potential limitation of these findings is the cross-sectional design used in the current study. Future studies should replicate and extend these findings by using a longitudinal design to assess the development of the EEG within this age range.
Acknowledgments
This manuscript is based on a Masters thesis by Miguel A. Diego. We would like to thank the mothers and infants who participated in these studies and the many research associates who assisted with these studies. This research was supported by an NIMH research Grant (#MH61888) to Nancy Aaron Jones and an NIMH Senior Research Scientist Award (MH #00331), an NCCAM Senior Research Scientist Award (#AT01585), and an NIMH merit award (MH #46586) to Tiffany Field, Ph.D., and funding from Johnson and Johnson Pediatric Institutes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Pearson’s r correlations computed on EEG asymmetry difference and EEG ratio scores (defined as [Ln(R) − Ln(L)]/ [Ln(R) − Ln(L)]) revealed that both of these indices were highly correlated at both 3–9 Hz (r = .98) and 4–9Hz (r = .96) frequency bands.
References
- Allister L, Lester BM, Carr S, Liu J. The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation. Developmental Neuropsychology. 2001;20:639–651. doi: 10.1207/S15326942DN2003_6. [DOI] [PubMed] [Google Scholar]
- Cohn JF, Matias R, Tronick EZ, Connell D, Lyons-Ruth K. Face-to-face interactions of depressed mothers and their infants. In: Tronick EZ, Field T, editors. Maternal Depression and Infant Disturbance. San Francisco: Jossey-Bass; 1986. pp. 31–45. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral Asymmetry, Emotion, and Affective Style. In: Davidson K RJH, editor. Brain asymmetry. Cambridge: MIT Press; 1995. [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35:607–614. doi: 10.1017/s0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. American Psychol. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Current Opinion in Neurobiology. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants' response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Schaffer CE, Saron C. Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophysiology. 1985;22:353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Slagter HA. Probing emotion in the developing brain: functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:166–170. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral asymmetry and emotion: Development and individual differences. In: Molfese SSD, editor. Developmental implications of brain lateralization. New York: Guilford; 1988. pp. 191–206. [Google Scholar]
- Dawson G, Ashman SB, Hessl D, Spieker S, Frey K, Panagiotides H, Embry L. Autonomic and Brain Electrical Activity in Securely- and Insecurely-Attached Infants of Depressed Mothers. Infant Behavior and Development. 2001;24:135–149. [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, Hessl D. Infants of depressed mothers exhibit atypical frontal brain activity: a replication and extension of previous findings. Journal of Child Psychology and Psychiatry. 1997;38:179–186. doi: 10.1111/j.1469-7610.1997.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Yamada E, Hessl D, Osterling J. Infants of depressed mothers exhibit atypical frontal electrical brain activity during interactions with mother and with a familiar, nondepressed adult. Child Development. 1999;70:1058–1066. doi: 10.1111/1467-8624.00078. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Self J, Panagiotides H, Hessl D, Yamada E, Rinaldi J. Frontal brain electrical activity in infants of depressed and nondepressed mothers: relation to variations in infant behavior. Developmental Psychopathology. 1999;11:589–605. doi: 10.1017/s0954579499002229. [DOI] [PubMed] [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Hill D, Spieker S. Frontal lobe activity and affective behavior of infants of mothers with depressive symptoms. Child Development. 1992;63:725–737. [PubMed] [Google Scholar]
- Diego MA, Field T, Hart S, Hernandez-Reif M, Jones N, Cullen C, Schanberg S, Kuhn C. Facial expressions and EEG in infants of intrusive and withdrawn mothers with depressive symptoms. Depression and Anxiety. 2002;15:10–17. doi: 10.1002/da.1079. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67:63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Jones N-A, Hernandez-Reif M. Withdrawn and intrusive maternal interaction style and infant frontal EEG asymmetry shifts in infants of depressed and non-depressed mothers. Infant Behavior and Development. 2006 doi: 10.1016/j.infbeh.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego MA, Field T, Jones NA, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. EEG responses to mock facial expressions by infants of depressed mothers. Infant Behavior & Development. 2004;27:150–162. [Google Scholar]
- Dieter J, Emory EK, Ansari Z. Maternal depression effects on vibratory stimulation response in late-term fetuses: Preliminary findings; Paper presented at the International Society on Infant Studies; Toronto, Canada. 2002. [Google Scholar]
- Dieter JNI, Field T, Hernandez-Reif M, Jones NA, Lecanuet JP, Salman FA, Redzepi M. Maternal depression and increased fetal activity. Journal of Obstetrics and Gynaecology. 2001;21:468–473. doi: 10.1080/01443610120072009. [DOI] [PubMed] [Google Scholar]
- Field T, Cohen D, Garcia R, Greenberg R. Mother stranger face discrimination by the newborn. Infant Behavior and Development. 1984;7:19–27. [Google Scholar]
- Field T. Models for reactive and chronic depression in infancy. New Directions in Child Development. 1986:47–60. doi: 10.1002/cd.23219863406. [DOI] [PubMed] [Google Scholar]
- Field T. The effects of mother's physical and emotional unavailability on emotion regulation. Monographs of the Society for Research in Child Devopment. 1994;59:208–227. [PubMed] [Google Scholar]
- Field T. Infants of depressed mothers. Infant Behavior and Development. 1995;18:1–13. doi: 10.1016/j.infbeh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Field T. Early interventions for infants of depressed mothers. Pediatrics. 1998;102:1305–1310. [PubMed] [Google Scholar]
- Field T. Maternal depression effects on infants and early interventions. Preventive Medicine. 1998;27:200–203. doi: 10.1006/pmed.1998.0293. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behavior and Development. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Prenatal depression effects on the foetus and neonate in different ethnic and socio-economic status groups. Journal of Reproductive & Infant Psychology. 2000;20:149–157. [Google Scholar]
- Field T, Diego MA, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Depressed withdrawn and intrusive mothers' effects on their fetuses and neonates. Infant Behavior& Development. 2001;24:27–39. [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, Bendell D. Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development. 2004;27(2):216–229. [Google Scholar]
- Field T, Pickens J, Fox NA, Gonzalez J, Nawrocki T. Facial expression and EEG responses to happy and sad faces/voices by 3-month-old infants of depressed mothers. British Journal of Developmental Psychology. 1998;16:485–494. [Google Scholar]
- Field T, Fox NA, Pickens J, Nawrocki T. Relative right frontal EEG activity in 3- to 6-month-old infants of "depressed" mothers. Developmental Psychology. 1995;31:358–363. [Google Scholar]
- Field T, Pickens J, Prodromidis M, Malphurs J, Fox N, Bendell D, Yando R, Schanberg S, Kuhn C. Targeting adolescent mothers with depressive symptoms for early intervention. Adolescence. 2000;35:381–414. [PubMed] [Google Scholar]
- Field T, Reite M. Children's responses to separation from mother during the birth of another child. Child Development. 1984;55:1308–1316. [PubMed] [Google Scholar]
- Fox NA. If it's not left, it's right. Electroencephalograph asymmetry and the development of emotion. American Psychol. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. In: Fox NA, editor. The development of emotion regulation: Behavioral and biological considerations. Vol. 2 3. Monographs of the Society for Research in Child Development, 59; 1994. pp. 152–166. Serial No.240. [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia. 1985;24:417–422. doi: 10.1016/0028-3932(86)90028-x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10-month-old infants. Developmental Psychology. 1988;24:230–236. [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: a glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. [PubMed] [Google Scholar]
- Hagne I. Development of the EEG in normal infants during the first year of life. A longitudinal study. Acta Paediatrica Scandinavica Suppl. 1972;232:1–53. [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Hernandez-Reif M, Field T, Diego M, Ruddock M. Greater arousal and less attentiveness to face/voice stimuli by neonates of depressed mothers on the Brazelton Neonatal Behavioral Assessment Scale. Infant Behavior and Development. 2006;29:594–598. doi: 10.1016/j.infbeh.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- Jones NA, Field T. Brain electrical activity and biochemical levels in infants of depressed mothers. SPR Abstracts. 1998:S44. [Google Scholar]
- Jones NA, Field T, Davalos M, Pickens J. EEG stability in infants/children of depressed mothers. Child Psychiatry and Human Development. 1997a;28:59–70. doi: 10.1023/a:1025197101496. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Lundy B, Davalos M. EEG activation in 1-month-old infants of depressed mothers. Developmental Psychopathology. 1997b;9:491–505. doi: 10.1017/s0954579497001260. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Davalos M, Malphurs J, Carraway K, Schanberg S, Kuhn C. Infants of intrusive and withdrawn mothers. Infant Behavior & Development. 1997c;20:175–186. [Google Scholar]
- Jones NA, Field T, Fox NA, Davalos M, Lundy B, Hart S. Newborns of mothers with depressive symptoms are physiologically less developed. Infant Behavior and Development. 1998;21:537–541. [Google Scholar]
- Jones NA, Field T, Fox NA, Davalos M, Gomez J. EEG during different emotions in 10-month-old infants of depressed mothers. Journal of Reproductive and Infant Psychology. 2001;19:295–312. [Google Scholar]
- Jones NA, Fox NA. Electroencephalogram asymmetry during emotionally evocative films and its relation to positive and negative affectivity. Brain Cognition. 1992;20:280–299. doi: 10.1016/0278-2626(92)90021-d. [DOI] [PubMed] [Google Scholar]
- Lindsley DB. Electroencephalography. Oxford, England: Ronald Press; 1944. [Google Scholar]
- Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, Almqvist F. Longitudinal study of maternal depressive symptoms and child well-being. J Am Acad Child Adolescent Psychiatry. 2001;40:1367–1374. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Yamauchi N, Watanabe A, Komatsushiro M, Takagi T. Maturation patterns of EEG basic waves of healthy infants under twelve-months of age. Tohoku Journal of Experimental Medicine. 1970;102:91–98. doi: 10.1620/tjem.102.91. [DOI] [PubMed] [Google Scholar]
- Monk C, Fifer W, Myers M, Sloan R. Newborn infants exposed to maternal psychiatric illness during pregnancy have diminished HR responses to downward tilting; Paper presented at the International Society on Infant Studies; Toronto. 2002. [Google Scholar]
- Patel V, DeSouza N, Rodrigues M. Postnatal depression and infant growth and development in low income countries: a cohort study from Goa, India. Archives of Disease in Childhood. 2003;88:34–37. doi: 10.1136/adc.88.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology. 1992;62:676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency. Psychophysiology. 1992;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Field T. Maternal Depression and Infant Disturbance. San Francisco: Josey-Bass; 1986. [Google Scholar]
- Warner V, Weissman MM, Mufson L, Wickramaratne PJ. Grandparents, parents, and grandchildren at high risk for depression: a three-generation study. Journal of the American Academy of Child Adolescent Psychiatry. 1999;38:289–296. doi: 10.1097/00004583-199903000-00016. [DOI] [PubMed] [Google Scholar]
- Wilcox H, Field T, Prodromidis M, Scafidi F. Correlations between the BDI and CES-D in a sample of adolescent mothers. Adolescence. 1998;33:565–574. [PubMed] [Google Scholar]