Abstract
Context:
Previous researchers have not investigated the thermoregulatory responses to multiple consecutive days of American football in adolescents.
Objective:
To examine the thermoregulatory and hydration responses of high school players during formal preseason football practices.
Design:
Observational study.
Setting:
Players practiced outdoors in late August once per day on days 1 through 5, twice per day on days 6 and 7, and once per day on days 8 through 10. Maximum wet bulb globe temperature averaged 23 ± 4°C.
Patients or Other Participants:
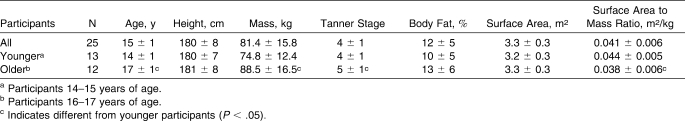
Twenty-five heat-acclimatized adolescent boys (age = 15 ± 1 years, height = 180 ± 8 cm, mass = 81.4 ± 15.8 kg, body fat = 12 ± 5%, Tanner stage = 4 ± 1).
Main Outcome Measure(s):
We observed participants within and across preseason practices of football. Measures included gastrointestinal temperature (TGI), urine osmolality, sweat rate, forearm sweat composition, fluid consumption, testosterone to cortisol ratio, perceptual measures of thirst, perceptual measures of thermal sensation, a modified Environmental Symptoms Questionnaire, and knowledge questionnaires assessing the participants' understanding of heat illnesses and hydration. Results were analyzed for differences across time and were compared between younger (14–15 years, n = 13) and older (16–17 years, n = 12) participants.
Results:
Maximum daily TGI values remained less than 40°C and were correlated with maximum wet bulb globe temperature (r = 0.59, P = .009). Average urine osmolality indicated that participants generally experienced minimal to moderate hypohydration before (881 ± 285 mOsmol/kg) and after (856 ± 259 mOsmol/kg) each practice as a result of replacing approximately two-thirds of their sweat losses during exercise but inadequately rehydrating between practices. Age did not affect most variables; however, sweat rate was lower in younger participants (0.6 ± 0.2 L/h) than in older participants (0.8 ± 0.1 L/h) (F1,18 = 8.774, P = .008).
Conclusions:
Previously heat-acclimatized adolescent boys (TGI < 40°C) can safely complete the initial days of preseason football practice in moderate environmental conditions using well-designed practice guidelines. Adolescent boys replaced most sweat lost during practice but remained mildly hypohydrated throughout data collection, indicating inadequate hydration habits when they were not at practice.
Keywords: fluid, gastrointestinal temperature, hormones, sweat, Tanner stage, heat acclimatization
Key Points.
Heat-acclimatized adolescent boys can safely participate in the initial days of preseason football practice in moderate environmental conditions with well-designed practice guidelines.
Core body temperature and sweat rates in this population appeared to be primarily influenced by environmental conditions.
Although participants hydrated enough during practice sessions to avoid exacerbating their prepractice hypohydration, they hydrated inadequately between practices and, thus, remained mildly hypohydrated throughout the preseason.
Younger players demonstrated lower sweat rates and lower morning testosterone to cortisol ratios than older players, but maximum gastrointestinal temperature did not differ between age groups.
Except for a superficial understanding that adequate hydration benefits exercise performance, participants had little knowledge of hydration physiology and exertional heat illnesses.
American football is the most popular sport played by high school males.1 Exertional heat illnesses are an ever-present danger during the twice-daily preseason practices of many training programs. Multiple successful strategies can decrease the risks associated with intense exercising in the heat while players are wearing full equipment. For example, the National Collegiate Athletic Association (NCAA)2,3 instituted regulations in 2003 that effectively phased variables, such as equipment, duration, and recovery, into preseason college football practices to safely enhance heat acclimatization. Collegiate players demonstrated beneficial physiologic changes during heat acclimatization and remained within “safe” core body temperatures.2,3 Despite this success, governing bodies for high school and junior high school football programs have not uniformly adopted or implemented similar regulations. Written guidelines, such as those instituted by the NCAA, are important, because the demands of football (exercise intensity; environmental conditions; equipment requirements; and, in some cases, body somatotype) may predispose players to developing hyperthermia.4,5 Collegiate players' body temperatures respond to activity and rest periods, as well as environmental conditions.5–7 However, only 1 preliminary study8 has documented core temperature responses in high school football players during preseason practices.
Hypohydration impairs and delays the thermoregulatory benefits characteristic of heat acclimatization and physical fitness.9–11 Investigators have documented hydration status and related variables in professional and collegiate football players, but few researchers have examined high school athletes12 or have compared different age groups within adolescence.13 Examining this population is especially important considering (1) the significant hypohydration noted in collegiate football players3,7,14 and (2) the regularity with which prepubescent and pubescent athletes initiate exercise in a hypohydrated condition.13,15 Therefore, the purpose of our study was to examine thermoregulation and hydration status of heat-acclimatized adolescent high school football players during preseason practices and to determine if any differences existed between younger and older adolescents. We hypothesized that environmental conditions would be strongly correlated with many thermoregulatory and fluid-related variables, maximum gastrointestinal temperature (TGI) would not differ between age groups, sweat rates (SRs) would be lower in younger than in older participants, the testosterone to cortisol ratio (T∶C) before practice began each day (am) would not differ between age groups, and hydration status would not change within practice sessions.
METHODS
Participants
Twenty-five healthy male high school football players volunteered to participate in this study (Table 1). Participants were divided into 2 groups: younger players (aged 14–15 years) and older players (aged 16–17 years). All participants played “first string” for the varsity, junior varsity, or freshman football teams at a private high school in the northeastern United States. Positions were not represented in large enough numbers to explore possible differences in variables among positions. However, both younger and older participants had representation in each player position. Exclusion criteria included obstructive gastrointestinal disease (eg, diverticulitis, inflammatory bowel disease), previous major gastrointestinal surgery, hypersensitive gag reflex, or scheduled magnetic resonance imaging scan. Participants and their parents or guardians attended an orientation meeting during which we described the investigation. Participants and their parents or guardians signed an informed consent document, and the study was approved by the university's institutional review board. Volunteers also completed medical questionnaires and preparticipation physical examinations. These questionnaires included questions about any history of heat illness and medications that might influence thermoregulation. Reviewing physicians (including author J.M.A.) did not identify these issues in any participant. Height, mass, body fat percentage (via skinfold analysis16), recent exercise history, a heat and hydration knowledge questionnaire, and a hydration habits questionnaire were collected before practice initiation.
Table 1.
Participant Characteristics (Mean ± SD)
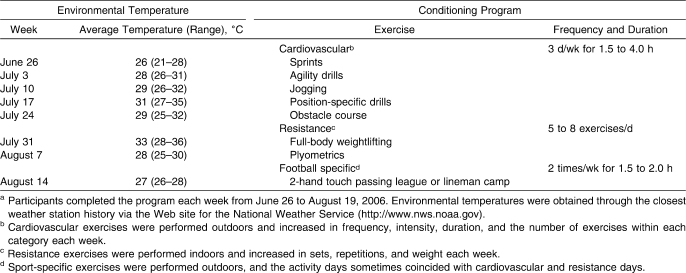
All individuals voluntarily participated in a structured summer conditioning program that was developed and recorded by the assistant football coaches and that occurred during the 8 weeks leading up to preseason practice and data collection (Table 2). During the program, the athletes regularly participated approximately 3 to 5 times per week for approximately 2 to 5 hours per day in warm to hot environmental conditions. The ranges of days and hours represent the minimum encouraged by the coaches and the maximum players experienced during voluntary practices. Individual players' involvement varied; however, coaches recorded and tracked players' completion of the program. Participant compliance with this program promoted heat acclimatization and physical fitness before data collection. As investigators played no role in preseason conditioning, we acknowledge that the assumption of equivalent acclimatization for all participants represents a potential study limitation.
Table 2.
Weekly Environmental Temperature and Summer Conditioning Programa
Practices
Observations occurred over the initial 10 days of preseason practice. Participants completed single practices on days 1, 2, 3, 4, 5, 8, 9, and 10; participants practiced twice daily on days 6 and 7. Data were not collected during practices on days 8 and 9, as these sessions consisted of light “walk-throughs” and minimal physically demanding exercise. Practices averaged 2.8 ± 0.5 hours and consisted of football drills, contact hitting, conditioning, and education. Practices generally began at 4:00 pm on days with 1 practice and at 8:00 am and 4:00 pm on days with 2 practices. Coaches gradually phased in equipment over the first 3 days by starting with helmets only, adding shoulder pads, and then adding thigh pads. Contact or hitting drills did not begin until day 5. Practice occurred only once a day for the first 5 days.
The wet bulb globe temperature (WBGT) (measured with heat stress monitor, model RSS-214; IST Corporation, Horseheads, NY) averaged 23.1°C, 25.5°C, 22.4°C, 22.8°C, 18.6°C, 21.5°C, 21.2°C, 15.3°C, 15.2°C, and 23.3°C for practices occurring on days 1, 2, 3, 4, 5, 6 (morning), 6 (afternoon), 7 (morning), 7 (afternoon), and 10, respectively. Environmental conditions varied over the course of the observational study, allowing days to be grouped and compared. Maximum WBGT and dry bulb temperature for days 1 through 4 and 10 averaged 25.0 ± 1.0°C and 28.5 ± 1.7°C, respectively, and were considered warm. Maximum WBGT and dry bulb temperature for days 5 through 7 averaged 19.2 ± 3.5°C and 21.1 ± 2.8°C, respectively, and were considered cool.17 Warm and cool days were different (t4.647 = 3.732, P = .015).
Protocol
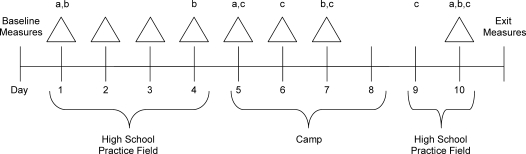
Figure 1 displays a schematic of the 10-day protocol. We collected observational data only, and measures were obtained in a manner that minimized interference with practice and team dynamics. Researchers did not manipulate diet or hydration behaviors before, during, or after practices. Before practice began each day (am), participants were weighed, provided a urine sample, completed a modified Environmental Symptoms Questionnaire (ESQ),18 and rated their perceived thirst and thermal sensations. On days 1, 4, 7, and 10, participants also supplied a saliva sample.
Figure 1.
Schematic of the 10-day preseason practice sessions protocol. Δ Indicates hydration status, sweat rate, fluid consumed, gastrointestinal temperature, and perceptual measures. a Indicates sweat patches. b Indicates hormone responses. c Indicates full contact. For days not noted as full contact, contact was minimal or limited.
Next, participants walked to the practice field (<0.25 miles [0.4 km]), where body mass, TGI via a telemetric temperature sensor, and perceptual sensations were measured. During practice, measured variables included WBGT, TGI (at approximately 30-minute increments), perceptual measures (at approximately 30-minute increments), and ad libitum fluid consumption (continuously via personal bottles). Participants ingested water during all practices except on days 6 and 7, when they consumed carbohydrate-electrolyte beverages (The Gatorade Company, Chicago, IL) with 14 g of carbohydrates, 100 mg of sodium, and 30 mg of potassium per 8-ounce serving. All fluids were provided by the coaches and were determined by team funding. Study participants consumed the same type of fluids as their nonparticipating teammates.
We measured body mass, TGI, and perceptual sensations after participants completed practice but before they left the field. Given the constraints of the field study and practice schedule, every effort was given to weighing the participants before and after practice while they were wearing the same amount of clothing and equipment. After returning to the locker room once practice ended each day (pm), participants repeated prepractice measures.
After practice on day 10, participants self-reported Tanner maturation stage. Physicians provided an explanation of the stages and remained nearby to answer questions. Participants then completed an exit questionnaire about hydration.
Measures
Body Temperature
Investigators monitored TGI with a Food and Drug Administration–approved temperature transmitter (HTI Technologies, Inc, Palmetto, FL). Participants ingested the sensor before breakfast on the morning of day 1. Before each subsequent practice, transmitter checks determined if the sensor remained in the gastrointestinal tract. When necessary, an investigator (S.W.Y.) immediately supplied the player with a new sensor. If a participant ingested a transmitter immediately before practice, final analysis included only physiologically relevant values (37–41°C). The manufacturer had calibrated all sensors to ±0.1°C, and, per manufacturer instructions, the investigator did not handle sensors before dispensing them. Duplicate TGI measures were obtained for most time points. Practice constraints, such as coaches requiring a participant to suddenly participate in a football drill, occasionally prevented duplicate measures.
Hydration Status
For each urine sample provided, we determined urine color (Ucol) via a Ucol chart (range, 1–8),19,20 urine specific gravity (Usg) via refractometer (model A300CL; Spartan, Tokyo, Japan), and urine osmolality (Uosm) via duplicate measures of freezing-point depression (model 3DII; Advanced Instruments, Inc, Needham Heights, MA). Urine osmolality was used to establish and report hydration status. A Uosm value of <700 mOsmol/kg indicated euhydration, a value between 700 and 900 mOsmol/kg indicated mild to moderate hypohydration, and a value >900 mOsmol/kg indicated moderate to severe hypohydration.17,21
Sweat and Fluid Variables
Sweat rate was calculated using the equation: SR = ([prepractice body mass − postpractice body mass] + fluid consumed)/time.9 Body mass measures were obtained from a calibrated scale (model BWB-800A; Tanita Corporation, Tokyo, Japan) with the player wearing minimal clothing; individual players wore similar garments for all body mass measurements. Fluid volume consumed (FC) was determined from personal 1-L bottles. No participant urinated during practice. Percentage of fluid replaced during a practice was calculated as (FC/total sweat loss) × 100.
On days 1, 5, and 10, investigators (M.S.G. and C.M.M.) applied sterile sweat patches (PharmChem Laboratories, Inc, Haltom City, TX) to the players immediately upon their arrival at the practice field and before any warm-up or activity occurred. Investigators cleaned the right forearm with an alcohol preparation pad and distilled water, allowed it to dry, and then applied sweat patches to the posterior midforearm using transparent, water-resistant, bio-occlusive dressings. When saturated, patches were removed with tweezers and immediately placed in sterile tubes. Later, they were centrifuged to obtain the sweat samples, and the samples were analyzed via flame photometry (model IL 943; Instrumentation Laboratory, Lexington, MA) for sodium and potassium.
Salivary Testosterone and Cortisol
On days 1, 4, 7, and 10 participants provided am saliva samples. Per the manufacturer's collection instructions, participants rinsed their mouths with water, waited 10 minutes, and then slowly drooled down a clean straw into the sample container. Morning sampling occurred at the player's home upon awakening and before food consumption, fluid ingestion, or tooth brushing. On the first day, an investigator (S.W.Y.) provided specific written instructions and called participants to encourage compliance. Samples were frozen at −80°C until analysis for free testosterone and cortisol via enzyme-linked immunosorbent assay (Salimetrics, LLC, State College, PA). Samples were thawed only once, and all samples from a given participant were assayed in the same analytical run. Intra-assay coefficients of variation for testosterone and cortisol were 4.9% and 4.3%, respectively. The testosterone to cortisol ratio (T∶C) indicated physiologic stress.22
Perceptual Measures
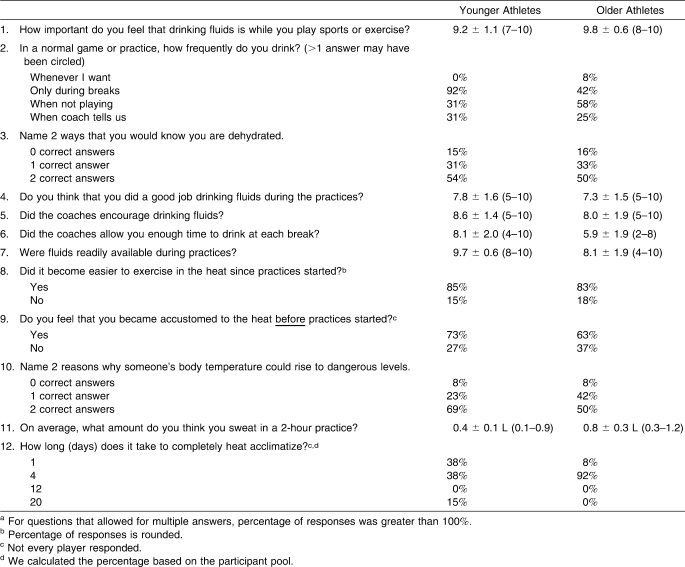
Selected items from an ESQ18 formed a modified and validated questionnaire23 that evaluated signs and symptoms of heat illnesses (eg, headache, dizziness). Participants self-reported thirst and thermal sensations using 9-point24 and 17-point25 visual scales, respectively. Before and after the study, participants also completed questionnaires (multiple-choice, Likert, and open-ended questions) evaluating their knowledge and habits associated with hydration and exercising in the heat (Table 3). Answers equal to or more than 7 on the 10-point Likert scale were considered to be in agreement with the statement provided. Scores equal to or less than 4 were considered to be in disagreement.
Table 3.
Selected Items and Responses From the Hydration Habits and Knowledge Questionnairea
Statistical Analyses
Descriptive data (mean ± SD) were calculated for all variables. Variables measured once daily (maximum TGI, FC, SR, sweat electrolytes, and T∶C) were analyzed with a 2 (younger participants, older participants) by X (days) repeated-measures analysis of variance (ANOVA), where X represented the number of days on which the variable was assessed (eg, for sweat electrolytes, X = 3; for T∶C, X = 4). Variables measured before and after each practice (Uosm, ESQ) were analyzed with a 2 (younger participants, older participants) by 2 (am, pm) by 8 (days) repeated-measures ANOVA. In all cases, a Greenhouse-Geisser correction factor was used for main effects and interactions that violated the assumption of sphericity, and significant findings were further analyzed with post hoc Tukey tests. Two-tailed paired-samples t tests with Bonferroni adjustments for multiple comparisons were used to determine if differences existed between (1) minimal and maximal TGI within each practice and (2) actual and estimated SR. Differences between younger and older participants in fluid replacement were assessed with a 2-tailed unpaired t test. We used Pearson product moment bivariate correlations to determine relationships between variables; Cohen26 described the strength of these relationships. We also used χ2 tests to analyze percentage differences in questionnaire responses. The α level was set at .05. Unless otherwise noted, all data are presented as mean ± SD.
RESULTS
Body Temperature
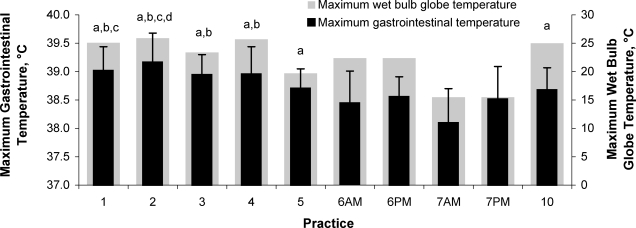
Exercise during football practice increased TGI (minimum = 37.6 ± 0.1°C, maximum = 38.7 ± 0.3°C; t9 = −12.721, P < .001). We found a main effect of time for maximum TGI (F4.322,60.506 = 8.435, P < .001) (Figure 2). No stable, consistent pattern existed across days; however, TGI was lowest on days 6 and 7 and highest on days 1 through 3. We found no interaction between group and day (F4.322,60.506 = 0.808, P = .533). Maximum TGI remained less than 40°C and was similar between younger (38.8 ± 0.6°C) and older (38.7 ± 0.5°C) participants (F1,14 = 0.744, P = .403). Maximum TGI was correlated with maximum WBGT (r = 0.59, P = .009), SR (r = 0.41, P = .044), FC (r = 0.66, P = .004), and pm thirst sensation (r = 0.59, P = .008).
Figure 2.
Maximum gastrointestinal and wet bulb temperature responses across days. a Indicates more than day 7AM. b Indicates more than day 6AM. c Indicates more than day 6PM. d Indicates more than day 7PM. All symbols, P < .05. Abbreviations: AM, before practice began each day; PM, after returning to the locker room once practice ended each day.
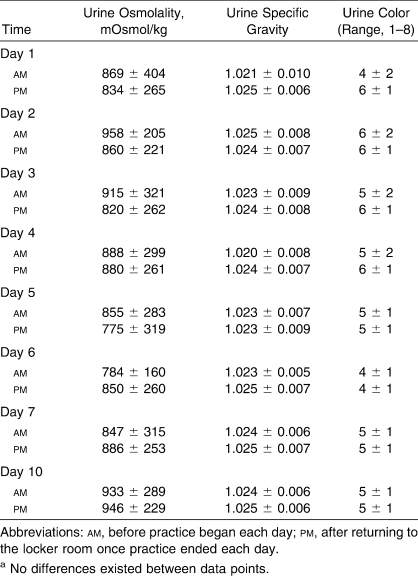
Hydration Status
Table 4 displays hydration measures. Urine osmolality indicated that participants were mildly hypohydrated each day before and after practice. No differences existed in Uosm within days (F1,11 = 2.578, P = .137) or across days (F7,77 = 1.564, P = .159). Age was not a determinant of Uosm (younger participants = 901.1 ± 234.3 mOsmol/kg, older participants = 820.9 ± 328.0 mOsmol/kg; F1,11 = 0.359, P = .561). Urine osmolality was strongly correlated with Usg (r = 0.94, P < .001) and Ucol (r = 0.55, P < .001) but was unrelated to SR, FC, WBGT, or TGI.
Table 4.
Hydration Variables (Mean ± SD)a
The younger (1.3 ± 0.7 L) and older (1.7 ± 0.9 L) participants consumed similar volumes of fluid per practice (F1,17 = 3.962, P = .063). They also consumed similar amounts of fluid per kilogram of body mass (younger participants = 16.8 ± 3.4 mL/kg, older participants = 16.8 ± 4.5 mL/kg). The FC ranged from 1.5 ± 0.6 L to 2.1 ± 0.8 L on warm days (days 1–4 and 10) and from 0.6 ± 0.4 L to 1.7 ± 0.7 L on cool days (days 5–7). The FC was correlated with sweat sodium (r = 0.37, P = .001), maximum WBGT (r = 0.71, P = .002), and TGI (r = 0.67, P = .004).
Sweat Variables
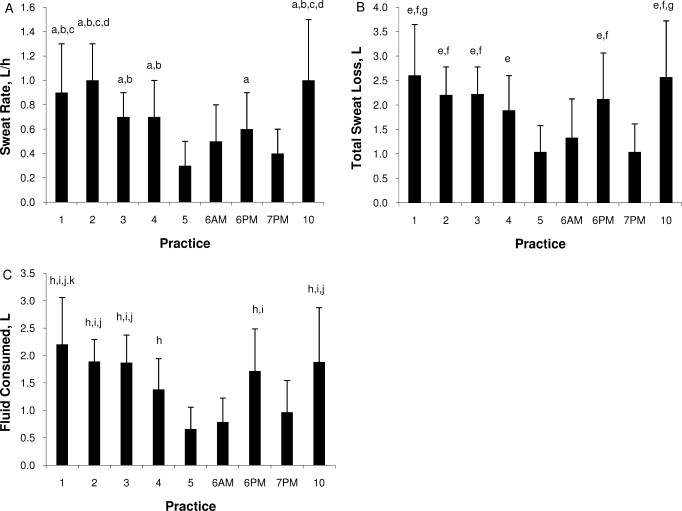
Sweat rate varied across days (F3.855,69.386 = 21.441, P < .001) (Figure 3A). No stable, consistent pattern existed for SR across days; however, SR was lowest on days 5 through 7 and highest on days 1 through 4 and 10. The SR was lower in the younger (0.6 ± 0.4 L/h) than in the older (0.8 ± 0.3 L/h) participants (F1,18 = 8.774, P = .008). Covariate analyses of body mass and of surface area to mass ratio did not reveal effects (P > .05). We found no interaction between age and day for SR (F3.855,69.386 = 0.688, P = .598). The SR was strongly correlated with maximum WBGT (r = 0.61, P = .007). Total sweat loss in a practice ranged from 1.5 ± 0.7 L to 2.9 ± 1.2 L on warm days and from 1.0 ± 0.5 L to 2.1 ± 0.9 L on cool days. We found a main effect of total sweat loss for age group (younger participants = 1.6 L, older participants = 2.3 L; F1,18 = 10.407, P = .005) and for day (F4.820,86.762 = 21.508, P < .001) (Figure 3B). Given the similar FC values, despite apparently different SR values, the percentage of fluid replaced differed between age groups (younger participants = 81%, older participants = 68%; t9 = −6.322, P < .001) (Figure 3C).
Figure 3.
A, Sweat rate across days. a Indicates more than day 5. b Indicates more than day 7PM. c Indicates more than day 6AM and 6PM d Indicates more than days 3 and 4. B, Total sweat loss across days. e Indicates more than days 5 and 7PM. f Indicates more than day 6AM. g Indicates more than day 4. C, Fluid consumed across days. h Indicates more than days 5 and 6AM. i Indicates more than day 7PM. j Indicates more than day 4. k Indicates more than day 6PM. All symbols, P < .05. Abbreviations: AM, before practice began each day; PM, after returning to the locker room once practice ended each day.
Sweat sodium was lower on day 5 than on days 1 and 10 (F1.411,23.987 = 19.721, P < .001). Sweat sodium (younger participants = 27.3 ± 17.2 mEq/L, older participants = 40.4 ± 19.0 mEq/L; F1,17 = 4.308, P = .053) and sweat potassium (younger participants = 6.4 ± 2.3 mEq/L, older participants = 5.1 ± 1.7 mEq/L; F1,17 = 3.556, P = .077) did not differ between age groups. Sweat sodium was strongly correlated with maximum WBGT (r = 0.98, P = .043) and showed a trend toward a relationship with SR (r = 0.98, P = .077).
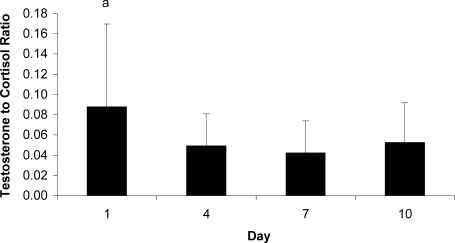
Testosterone to Cortisol Responses
Figure 4 displays T∶C data. Differences existed across days (F1.639,22.947 = 4.103, P = .037), as day 7 values were lower than day 1 values. Younger participants (0.040 ± 0.028) demonstrated lower, but statistically similar, T∶C values compared with older participants (0.080 ± 0.070) (F1,14 = 4.264, P = .058).
Figure 4.
Testosterone to cortisol ratio responses across days. a Indicates more than day 7 (P < .05).
Perceptual Responses
Maximum thermal sensation was correlated with maximum WBGT (r = 0.85, P < .001) and maximum TGI (r = 0.71, P = .002). No association existed between pm thirst sensation and pm Uosm (r = −0.08, P = .483), but thirst sensation was correlated with FC (r = 0.59, P = .008) and maximum WBGT (r = 0.71, P = .002).
Football practices increased the modified ESQ scores from am (11 ± 2) to pm (15 ± 5) (F1,10 = 28.784, P < .001). No stable, consistent statistical pattern existed for ESQ across all days; however, am ESQ on day 6 exceeded am ESQ on day 10, and pm ESQ scores were elevated on days 1 and 2 (F7,70 = 18.137, P < .001). Age affected am and pm ESQ scores (younger participants = 13 ± 6, older participants = 18 ± 7; F1,10 = 8.088, P = .017).
Heat and Hydration Knowledge and Habits
Table 3 presents selected responses from the questionnaires. No differences existed between the younger and older adolescents for any question (P > .05). Responses not provided in Table 3 included the following: (1) most participants (92%) understood the importance of drinking before exercise, during exercise, and postexercise; (2) most participants (96%) reported drinking only during structured breaks; (3) few participants (23%) described obstacles to hydration (eg, insufficient time or fluid availability); (4) most participants (84%) indicated that coaches provided a good environment for hydration; and (5) all participants (100%) (t24 = 10.738, P < .001) underestimated their sweat losses.
Although only 64% of participants could list at least 1 exertional heat illness, all participants indicated that appropriate hydration best prevented these conditions. Fifty percent of participants understood the concept of heat acclimatization; 85% incorrectly believed acclimatization occurred in less than 4 days. Most participants (68%) believed summer conditioning successfully acclimatized them before preseason, providing great benefits.
DISCUSSION
The primary findings of our study indicate that heat-acclimatized adolescent boys can safely (TGI < 40°C) complete the initial days of preseason football practice in moderate (cool to warm) environmental conditions. As expected, environmental conditions were strongly correlated with many thermoregulatory and fluid-related variables. Despite self-perception of consistently good hydration habits, participants replaced most sweat loss during practice but remained mildly hypohydrated throughout the preseason, indicating inadequate rehydration habits outside of practice.
Body Temperature Responses
Throughout preseason, participants' average maximum TGI remained less than 40°C, which is the typical threshold core temperature that medical experts associate with increased risk of exertional heat stroke.17 In preliminary studies, investigators2,8 observing collegiate and high school preseason football practices have reported similar findings; however, we are the first to report this observation in adolescent boys. We attribute the core body temperatures of the participants to (1) practice guidelines using the gradual addition of exercise duration and football equipment, (2) generally moderate environmental conditions, and (3) participants' previously developed heat acclimatization. These logistical, environmental, and physiologic factors combined to minimize heat gain and maximize heat loss during practice, keeping participants safe throughout practice.27–30 Although undocumented, appropriate exercise intensity and exercise to rest ratios likely also contributed to the participants' safe core temperatures. The greatest average TGI occurred on days 1 and 2, likely as a result of the challenging environmental conditions (as supported by the strong correlation between TGI and WBGT) and the intense exercise characteristic of the first days of preseason football practice.
Consistent with the literature, maximum TGI did not differ between age groups, as we hypothesized. Researchers31–34 have attributed these similarities between age groups to heat acclimatization and efficient sweating mechanisms. The participant characteristics in our study supported this conclusion, as the first-string status of all participants indicates that they completed similar exercise bouts (ie, intensity and duration) and experienced similar thermoregulatory demands. In previous studies, investigators have observed different age groups within laboratory settings. To our knowledge, we are the first to compare age groups in an organized sport setting. Our findings indicated that even with the different stresses of a field environment (eg, solar radiation, spontaneous changes in exercise intensity), boys aged 14 to 17 years still thermoregulate efficiently.
Hydration Status
Similar to previous research in which collegiate football players3,7 and adolescent athletes8,12,13,15 were examined, urinary hydration measures indicated that individual participants experienced mild to severe hypohydration each day. Urine osmolality has been found35 to be a valid and reliable measurement of hydration status. In studies of youth football and soccer players (aged 9–18 years), investigators13,15 (also R.M.L. et al, unpublished data, 2009) have indicated that most athletes initiate exercise in a hypohydrated condition and maintain their pre-exercise hydration state throughout exercise. As hypothesized, we found similar results; hydration status did not change within practice sessions, indicating that participants hydrated during practice at least enough to avoid exacerbating their prepractice hypohydration. It is surprising that the participants maintained status throughout practice despite the distracting factor of natural competition. We attribute this unique finding of “successful” hydration (65%–80% of sweat loss replaced) to appropriate practice rest intervals, fluid availability, and coaches' external motivation and education. Of these, fluid availability might be paramount, as researchers15 examining hydration of adolescent football players without individual bottles or team coolers have shown that 56% of players developed serious hypohydration.
As has been the case with other investigations,13,15 the magnitude of hypohydration did not change across days. Participants' similar hypohydration states before and after practice throughout preseason indicated inadequate between-practices rehydration. Lack of adequate rehydration between practices is relevant not only in a camp setting, as investigated by previous researchers,13,15 but also in high school sports settings in which players go home each night. These findings, coupled with those of similar research,36,37 indicated that coaches and athletes must emphasize drinking between exercise bouts, as well as during exercise, especially considering that fluid intake after practice should exceed sweat loss (by about 25%) to ensure complete rehydration 4 to 6 hours after the exercise bout.35,38
Sweat Variables
The SR for this population matched previous data.12,36,39 However, we are the first to demonstrate that the SRs in this population appeared to be primarily influenced by environmental conditions, as demonstrated by (1) the strong correlation between SR and maximum WBGT and (2) the greater SR noted on days 1, 2, 3, 4, and 10 (the warmest days of preseason), compared with other days. Although SR generally increases during preseason football, participants' complete heat acclimatization during summer conditioning likely maximized SR before preseason practices. Additionally, WBGT may have stressed the participants differently each day of preseason. Undocumented exercise intensity also likely played a role. Because of the constraints of a field study, participants were not weighed nude. Our SR results likely were underestimated.
As we expected, SRs were lower in younger participants than in older participants. Physical maturation increases total body SR and the amount of sweat excreted per gland, but these developments might not affect evaporative cooling, as aging bears no influence on the percentage of skin covered with sweat.40 Our results support this conclusion, as the older participants' greater SR did not lower TGI compared with younger participants. Additionally, older participants' FC was similar to that of younger participants, despite higher SRs (similar to previous research13,36,41), indicating less effective fluid replacement in older participants. Yet differences in SRs between age groups may only be due to body mass and surface area to mass ratio changes that accompany maturation. However, when trying to isolate the effect of maturation, covariate analyses of body mass and of surface area to mass ratio did not demonstrate effects because of the small participant numbers in the younger and older groups.
As was the case with SR, environmental conditions also seemed to influence sweat sodium, as indicated by the correlation between sweat sodium and maximum WBGT and the differences in sweat sodium between warm (days 1 and 10) and cool (day 5) practices. This may be explained by the trend toward a relationship between sweat sodium and SR. As sweating increased, more sodium was collected in the patch. Some researchers42,43 have suggested that sweat sodium concentrations increase with maturation, but they examined a greater age range (prepubescent to adolescence or young adulthood); our results were unique because they indicated that sweat sodium changes little during the late phases of adolescence. The lack of differences between older and younger participants' sweat potassium noted in our study may indicate a similar new conclusion during late phases of adolescence. The difference in potassium from children to adults42 may occur at an earlier stage.
Testosterone to Cortisol Responses
The T∶C ratio estimates physiologic stress based on the ratio of anabolic (testosterone) to catabolic (cortisol) hormones, respectively; a low T∶C ratio indicates increased stress, whereas a high T∶C ratio indicates recovery.22 We proposed that comparing am values across days would quantify the stress experienced throughout preseason. We attribute the lower am T∶C values on day 7 to the cumulative stresses added throughout the week and to the preceding day's double practice session. The importance of environmental conditions, another potential concern, must be viewed cautiously given (1) previous research44,45 suggesting a lack of environmental effect on T∶C response to short-duration exercise and (2) the lack of correlations between T∶C and WBGT or T∶C and TGI. Although not a significant finding and not as we had expected, younger participants demonstrated lower am T∶C than did older participants, whose more advanced pubertal development would lead to greater circulating testosterone.46 These are novel findings, as T∶C values have not been reported for adolescent football players.
Perceptual Responses
Similar to previous research examining adolescents47 and collegiate3 football players, thermal sensation scores were strongly correlated with maximum WBGT and TGI. Thirst sensation was not correlated with hydration status, which also corroborated the findings of previous research.13 Although competition and other distractions may hinder the accuracy of thirst measures, participants' mild hypohydration best explains the lack of relationship, as body mass loss usually needs to exceed 1% to 2% to stimulate thirst.48
Heat and Hydration Knowledge and Habits
Questionnaires completed on day 1 indicated that participants understood the importance of hydration in terms of exercise performance. Supporting these qualitative findings, participants generally avoided severe hypohydration and self-reported good hydration habits. Alternately, researchers of unpublished work from our laboratory have noted a poor correlation between hydration knowledge and actual hydration status in adolescent and child football players. We are unaware of any other research on this topic. Participants felt coaches provided appropriate breaks, accessibility to fluids, and encouragement to drink, combating primary obstacles to good hydration.
All participants expressed reliance on the presence and severity of symptoms (eg, thirst, headache, nausea) to indicate hypohydration. Fifty-two percent of participants also listed objective measures, including Ucol and SR. Participants underestimated their sweat losses and overestimated their ability to rehydrate during practice, which was a combination predisposing them to insufficient drinking after practice and the chronic mild hypohydration noted throughout the study. Participants also displayed a lack of understanding about heat acclimatization and the preventive measures for exertional heat stroke. In total, these original findings indicated that high school football players possess little knowledge of hydration physiology and exertional heat illnesses, other than a superficial understanding that adequate hydration benefits exercise performance.
CONCLUSIONS
Heat-acclimatized adolescent football players can safely complete appropriately controlled preseason football practices in moderate environmental conditions. Environmental conditions strongly influenced many physiologic variables and, together with exercise intensity (duration, intensity, work to rest ratio, etc), likely represented the 2 most important characteristics guiding safe participation in adolescent football. Participants adequately replaced their sweat losses with fluid intake during practice with encouragement from outside influences. However, participants poorly rehydrated between exercise bouts, leading to a chronic mild to moderate hypohydration. Participants' misconceptions about their sweat losses and rehydration techniques might partially explain this condition. The findings from this study are novel, as the variables have never been observed in this population in sport.
Several practical applications arose from the study. Previous heat acclimatization occurring over the summer and immediately before preseason and appropriate preseason practice guidelines (eg, limiting the number of practices for the first 5 days, gradually increasing equipment worn, ensuring suitable exercise to rest ratios) appear to promote safe participation in adolescent football. Without disregarding other predisposing factors (primarily exercise intensity), certified athletic trainers might focus on environmental conditions when working with heat-acclimatized athletes. As athletes tend to underestimate fluid losses and overestimate their rehydration, coaches and medical professionals must provide good hydration protocols (hydration education; appropriate breaks; and, if possible, individual drinking bottles) to minimize the deleterious effects of hypohydration.
Acknowledgments
This study was funded in part by a doctoral research grant from the National Athletic Trainers' Association Research & Education Foundation (Dallas, TX) and in part by The Gatorade Company (Chicago, IL).
REFERENCES
- 1.Metzl J. D. Sports-specific concerns in the young athlete: football. Pediatr Emerg Care. 1999;15(5):363–367. doi: 10.1097/00006565-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Stofan J. R., Zachwieja J. J., Horswill C. A., Murray R., Eichner E. R., Anderson S. A. Core temperature responses during two-a-day practices in NCAA Division-1 college football [abstract] Med Sci Sports Exerc. 2004;36(5):S48. [Google Scholar]
- 3.Yeargin S. W., Casa D. J., Armstrong L. E., et al. Heat acclimatization and hydration status of American football players during initial summer workouts. J Strength Cond Res. 2006;20(3):463–470. doi: 10.1519/20596.1. [DOI] [PubMed] [Google Scholar]
- 4.McCullough E. A., Kenney W. L. Thermal insulation and evaporative resistance of football uniforms. Med Sci Sports Exerc. 2003;35(5):832–837. doi: 10.1249/01.MSS.0000064998.48130.22. [DOI] [PubMed] [Google Scholar]
- 5.Godek S. F., Bartolozzi A. R., Burkholder R., Sugarman E., Dorshimer G. Core temperature and percentage of dehydration in professional football linemen and backs during preseason practices. J Athl Train. 2006;41(1):8–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Fowkes Godek S., Godek J. J., Bartolozzi A. R. Thermal responses in football and cross-country athletes during their respective practices in a hot environment. J Athl Train. 2004;39(3):235–240. [PMC free article] [PubMed] [Google Scholar]
- 7.Godek S. F., Godek J. J., Bartolozzi A. R. Hydration status in college football players during consecutive days of twice-a-day preseason practices. Am J Sports Med. 2005;33(6):843–851. doi: 10.1177/0363546504270999. [DOI] [PubMed] [Google Scholar]
- 8.Bergeron M. F., McKeag D. B., Casa D. J., et al. Youth football: heat stress and injury risk. Med Sci Sports Exerc. 2005;37(8):1421–1430. doi: 10.1249/01.mss.0000174891.46893.82. [DOI] [PubMed] [Google Scholar]
- 9.Casa D. J., Armstrong L. E., Hillman S. K., et al. National Athletic Trainers' Association position statement: fluid replacement for athletes. J Athl Train. 2000;35(2):212–224. [PMC free article] [PubMed] [Google Scholar]
- 10.Convertino V. A., Armstrong L. E., Coyle E. F., et al. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 1996;28(1):i–vii. doi: 10.1097/00005768-199610000-00045. [DOI] [PubMed] [Google Scholar]
- 11.Casa D. J., Clarkson P. M., Roberts W. O. American College of Sports Medicine roundtable on hydration and physical activity: consensus statements. Curr Sports Med Rep. 2005;4(3):115–127. doi: 10.1097/01.csmr.0000306194.67241.76. [DOI] [PubMed] [Google Scholar]
- 12.Stover E. A., Zachwieja J., Stofan J., Murray R., Horswill C. A. Consistently high urine specific gravity in adolescent American football players and the impact of an acute drinking strategy. Int J Sports Med. 2006;27(4):330–335. doi: 10.1055/s-2005-865667. [DOI] [PubMed] [Google Scholar]
- 13.McDermott B. P., Casa D. J., Yeargin S. W., Ganio M. S., Lopez R. M., Mooradian E. A. Hydration status, sweat rates, and rehydration education of youth football campers. J Sport Rehabil. 2009;18(4):535–552. doi: 10.1123/jsr.18.4.535. [DOI] [PubMed] [Google Scholar]
- 14.Murphy R. J., Ashe W. F. Prevention of heat illness in football players. JAMA. 1965;194(6):650–654. [PubMed] [Google Scholar]
- 15.Decher N. R., Casa D. J., Yeargin S. W., et al. Hydration status, knowledge and behavior in youths at summer sports camps. Int J Sport Physiol Perform. 2008;3(3):262–278. doi: 10.1123/ijspp.3.3.262. [DOI] [PubMed] [Google Scholar]
- 16.Jackson A. S., Pollock M. L., Gettman L. R. Intertester reliability of selected skinfold and circumference measurements and percent fat estimates. Res Q. 1978;49(4):546–551. [PubMed] [Google Scholar]
- 17.Armstrong L. E., Casa D. J., et al. American College of Sports Medicine. American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 18.Sampson J. B., Kobrick J. L. The Environmental Symptoms Questionnaire: revisions and new field data. Aviat Space Environ Med. 1980;51(9 pt 1):872–877. [PubMed] [Google Scholar]
- 19.Armstrong L. E., Maresh C. M., Castellani J. W., et al. Urinary indices of hydration status. Int J Sport Nutr. 1994;4(3):265–279. doi: 10.1123/ijsn.4.3.265. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong L. E., Soto J. A., Hacker F. T., Jr, Casa D. J., Kavouras S. A., Maresh C. M. Urinary indices during dehydration, exercise, and rehydration. Int J Sport Nutr. 1998;8(4):345–355. doi: 10.1123/ijsn.8.4.345. [DOI] [PubMed] [Google Scholar]
- 21.Cheuvront S. N., Sawka M. N. Hydration assessment of athletes. Gatorade Sports Sci Exch. 2005;18(2):1–6. [Google Scholar]
- 22.Kraemer W. J., Rogol A. D., editors. The Encyclopedia of Sports Medicine. Malden, MA: Blackwell Publishing; 2005. The Endocrine System in Sports and Exercise; vol 11. [Google Scholar]
- 23.Yamamoto L. M., Casa D. J., Stearns R. L., et al. Validation of a modified Environmental Symptoms Questionnaire for exercise in the heat [abstract] Med Sci Sports Exerc. 2008;40(5):S190. [Google Scholar]
- 24.Engell D. B., Maller O., Sawka M. N., Francesconi R. N., Drolet L., Young A. J. Thirst and fluid intake following graded hypohydration levels in humans. Physiol Behav. 1987;40(2):229–236. doi: 10.1016/0031-9384(87)90212-5. [DOI] [PubMed] [Google Scholar]
- 25.Toner M. M., Drolet L. L., Pandolf K. B. Perceptual and physiological responses during exercise in cool and cold water. Percept Mot Skills. 1986;62(1):211–220. doi: 10.2466/pms.1986.62.1.211. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Philadelphia, PA: Lawrence Erlbaum; 1988. [Google Scholar]
- 27.Armstrong L. E. Performing in Extreme Environments. Champaign, IL: Human Kinetics; 2000. [Google Scholar]
- 28.Armstrong L. E., Casa D. J. Predisposing factors for exertional heat illnesses. In: Armstrong L. E., editor. Exertional Heat Illnesses. Champaign, IL: Human Kinetics; 2003. p. 151. [Google Scholar]
- 29.Armstrong L. E., Maresh C. M. The induction and decay of heat acclimatisation in trained athletes. Sports Med. 1991;12(5):302–312. doi: 10.2165/00007256-199112050-00003. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen B. Heat acclimation: mechanisms of adaptation to exercise in the heat. Int J Sports Med. 1998;19(suppl 2):S154–S156. doi: 10.1055/s-2007-971984. [DOI] [PubMed] [Google Scholar]
- 31.Falk B., Bar-Or O., MacDougall J. D. Thermoregulatory responses of pre-, mid-, and late-pubertal boys to exercise in dry heat. Med Sci Sports Exerc. 1992;24(6):688–694. [PubMed] [Google Scholar]
- 32.Davies C. T. Thermal responses to exercise in children. Ergonomics. 1981;24(1):55–61. doi: 10.1080/00140138108924830. [DOI] [PubMed] [Google Scholar]
- 33.Shibasaki M., Inoue Y., Kondo N., Iwata A. Thermoregulatory responses of prepubertal boys and young men during moderate exercise. Eur J Appl Physiol Occup Physiol. 1997;75(3):212–218. doi: 10.1007/s004210050150. [DOI] [PubMed] [Google Scholar]
- 34.Drinkwater B. L., Horvath S. M. Heat tolerance and aging. Med Sci Sports. 1979;11(1):49–55. [PubMed] [Google Scholar]
- 35.Shirreffs S. M. Markers of hydration status. J Sports Med Phys Fitness. 2000;40(1):80–84. [PubMed] [Google Scholar]
- 36.Iuliano S., Naughton G., Collier G., Carlson J. Examination of the self-selected fluid intake practices by junior athletes during a simulated duathlon event. Int J Sport Nutr. 1998;8(1):10–23. doi: 10.1123/ijsn.8.1.10. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell I. D., Cleary M. A., Line S. M. Voluntary, chronic dehydration in adolescent American football players [abstract] J Athl Train. 2004;39(suppl 2):S56–S57. [Google Scholar]
- 38.Shirreffs S. M., Taylor A. J., Leiper J. B., Maughan R. J. Post-exercise rehydration in man: effects of volume consumed and drink sodium content. Med Sci Sports Exerc. 1996;28(10):1260–1271. doi: 10.1097/00005768-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Horswill C. A., Passe D. H., Stofan J. R., Horn M. K., Murray R. Adequacy of fluid ingestion in adolescents and adults during exercise. Pediatr Exerc Sci. 2005;17(1):41–50. [Google Scholar]
- 40.Falk B., Bar-Or O., Calvert R., MacDougall J. D. Sweat gland response to exercise in the heat among pre-, mid-, and late-pubertal boys. Med Sci Sports Exerc. 1992;24(3):313–319. [PubMed] [Google Scholar]
- 41.Falk B., Bar-Or O., MacDougall J. D., McGillis L., Calvert R., Meyer F. Sweat lactate in exercising children and adolescents of varying physical maturity. J Appl Physiol. 1991;71(5):1735–1740. doi: 10.1152/jappl.1991.71.5.1735. [DOI] [PubMed] [Google Scholar]
- 42.Meyer F., Bar-Or O., MacDougall D., Heigenhauser G. J. Sweat electrolyte loss during exercise in the heat: effects of gender and maturation. Med Sci Sports Exerc. 1992;24(7):776–781. [PubMed] [Google Scholar]
- 43.Araki T., Toda Y., Matsushita K., Tsujino A. Age differences in sweating during muscular exercise. Jpn J Phys Fitness Sports Med. 1979;28(3):239–248. [Google Scholar]
- 44.Hoffman J. R., Falk B., Radom-Isaac S., et al. The effect of environmental temperature on testosterone and cortisol responses to high intensity, intermittent exercise in humans. Eur J Appl Physiol Occup Physiol. 1997;75(1):83–87. doi: 10.1007/s004210050130. [DOI] [PubMed] [Google Scholar]
- 45.Kenefick R. W., Maresh C. M., Armstrong L. E., et al. Plasma testosterone and cortisol responses to training-intensity exercise in mild and hot environments. Int J Sports Med. 1998;19(3):177–181. doi: 10.1055/s-2007-971900. [DOI] [PubMed] [Google Scholar]
- 46.Elmlinger M. W., Kuhnel W., Wormstall H., Doller P. C. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab. 2005;51(11–12):625–632. [PubMed] [Google Scholar]
- 47.Anderson G. S., Mekjavic I. B. Thermoregulatory responses of circum-pubertal children. Eur J Appl Physiol Occup Physiol. 1996;74(5):404–410. doi: 10.1007/BF02337720. [DOI] [PubMed] [Google Scholar]
- 48.Greenleaf J. E. Problem: thirst, drinking behavior, and involuntary dehydration. Med Sci Sports Exerc. 1992;24(6):645–656. [PubMed] [Google Scholar]