Abstract
Context:
The measurement of body temperature is crucial for the initial diagnosis of exertional heat injury and for monitoring purposes during a subsequent treatment strategy. However, little information is available about how different measurements of body temperature respond during and after exertional heat stress.
Objective:
To present the temporal responses of aural canal (Tac), esophageal (Tes), and rectal (Tre) temperatures during 2 different scenarios (S1, S2) involving exertional heat stress and a subsequent recovery period.
Design:
Randomized controlled trial.
Setting:
University research laboratory.
Patients or Other Participants:
Twenty-four healthy volunteers, with 12 (5 men, 7 women) participating in S1 and 12 (7 men, 5 women) participating in S2.
Intervention(s):
The participants exercised in the heat (42°C, 30% relative humidity) until they reached a 39.5°C cut-off criterion, which was determined by Tre in S1 and by Tes in S2. As such, participants attained different levels of hyperthermia (as determined by Tre) at the end of exercise. Participants in S1 were subsequently immersed in cold water (2°C) until Tre reached 37.5°C, and participants in S2 recovered in a temperate environment (30°C, 30% relative humidity) for 60 minutes.
Main Outcome Measure(s):
We measured Tac, Tes, and Tre throughout both scenarios.
Results:
The Tes (S1 = 40.19 ± 0.41°C, S2 = 39.50 ± 0.02°C) was higher at the end of exercise compared with both Tac (S1 = 39.74 ± 0.42°C, S2 = 38.89 ± 0.32°C) and Tre (S1 = 39.41 ± 0.04°C, S2 = 38.74 ± 0.28°C) (for both comparisons in each scenario, P < .001). Conversely, Tes (S1 = 36.26 ± 0.74°C, S2 = 37.36 ± 0.34°C) and Tac (S1 = 36.48 ± 1.07°C, S2 = 36.97 ± 0.38°C) were lower compared with Tre (S1 = 37.54 ± 0.04°C, S2 = 37.78 ± 0.31°C) at the end of both scenarios (for both comparisons in each scenario, P < .001).
Conclusions:
We found that Tac, Tes, and Tre presented different temporal responses during and after both scenarios of exertional heat stress and a subsequent recovery period. Although these results may not have direct practical implications in the field monitoring and treatment of individuals with exertional heat injury, they do quantify the extent to which these body temperature measurements differ in such scenarios.
Keywords: cold-water immersion, core temperature, exercise, hyperthermia
Key Points.
Aural canal, esophageal, and rectal temperatures presented different temporal responses during 2 scenarios of exertional heat stress and a subsequent recovery period.
Rectal temperature increased more slowly than esophageal and aural canal temperatures during exercise-induced hyperthermia.
Rectal temperature decreased more slowly than esophageal and aural canal temperatures after exercise-induced hyperthermia.
Rectal temperature is the only suitable and valid index for the monitoring of body temperature in a field setting; the use of esophageal temperature is not practical in such situations, and aural canal temperature is often influenced by external factors.
Athletic,1 occupational,2 and military3 activities can potentially increase an individual's risk for exertional heat exhaustion and exertional heat stroke. Because individuals with either condition may demonstrate similar physical signs and symptoms, a prompt measurement of rectal temperature (Tre) is critical to establishing an appropriate treatment strategy.1,4 If a collapsed hyperthermic individual has a Tre of less than 40°C and has no signs or symptoms of central nervous system dysfunction, he or she is considered to have exertional heat exhaustion, and immediate treatment should consist of moving the individual to a shaded area.1,4 On the other hand, if the individual has a Tre of greater than 40°C and has signs of central nervous system dysfunction, he or she likely has exertional heat stroke, and cold-water immersion is the recommended treatment.1,5 In both cases, Tre should be monitored until it reaches safe levels.
Many researchers6–14 have evaluated the differences in commonly used measurement sites of body temperature (eg, aural canal, esophagus, rectum). However, most of these investigators6–8 have used passive whole-body cooling or rewarming techniques to manipulate body temperature during surgical procedures. Furthermore, few researchers have examined these different measurements of body temperature during exertional heat stress scenarios, with most studies9–14 conducted to validate the use of Tre as an appropriate indicator of body temperature. To date, a paucity of information exists about the temporal responses of aural canal temperature (Tac), esophageal temperature (Tes), and Tre both during and after exertional heat stress. Therefore, the purpose of our study was to present the temporal responses of Tac, Tes, and Tre during 2 scenarios involving exertional heat stress and a subsequent recovery period. Based on previous studies,6,7 we hypothesized that Tre would increase at a slower rate, compared with both Tes and Tac, during exercise-induced hyperthermia. We also hypothesized that Tre would decrease at a slower rate, compared with Tes and Tac, after exercise-induced hyperthermia.
METHODS
We collected data during 2 separate experiments (scenarios 1 and 2) and compared each index of core temperature within the particular scenario rather than between scenarios. For scenario 1, we reproduced the recommended recovery treatment for individuals with exertional heat stroke and used an aggressive water-immersion strategy.1,5 For scenario 2, we used an inactive seated recovery in a temperate climate, which is, in part, the recommended treatment for individuals with exertional heat exhaustion.1
Participants
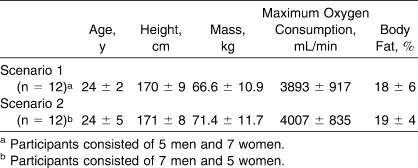
Five men and 7 women volunteered for scenario 1, and 7 men and 5 women volunteered for scenario 2. All participants were physically active, healthy, nonsmoking, and normotensive. We defined physically active as exercising at least 3 times per week at a medium intensity (≥12 on the Borg scale) for at least 20 minutes.15 For both scenarios, body adiposity and maximal oxygen consumption were measured 5 to 7 days before the experiments. Body density was determined by the hydrostatic weighing technique. Calculation of the percentage of body fat was based on the Siri equation.16 Maximal oxygen consumption was measured during a progressive treadmill (Desmo HP; Woodway, Waukesha, WI) running protocol, which consisted of a 2-minute warm-up at 0% grade, followed by 2% increments every 2 minutes until physical exhaustion.17 Running speed was kept constant throughout the protocol at 7 miles per hour for men and at 6 miles per hour for women. Expired gas was analyzed for oxygen (error, ±0.01%) and carbon dioxide concentrations (error, ±0.02%) using electrochemical gas analyzers (AMETEK models S-3A/1 and CD 3A; Applied Electrochemistry, Pittsburgh, PA). The data were used to select the workload for the experimental exercise phase of each scenario. To control for possible hormonal effects, women were tested during the early follicular phase (3–5 days after the onset of menstruation) of their menstrual cycles. Characteristics of the participants from each scenario are presented in the Table.
Participants' Characteristics (Mean ± SD)
The study was approved by the university's Research Ethics Committee, and all participants provided written informed consent.
Instrumentation
We measured Tac using a thermocouple probe (Mon-a-therm model 503-0021; Covidien-Nellcor, Boulder, CO; the same type of thermocouple probe was used for all measurements) that was placed in the aural canal until it rested against the tympanic membrane, after which it was retracted slightly. The probe was held in position and isolated from the external environment with cotton wool. The Tes was measured by placing a pediatric thermocouple probe of approximately 2 mm in diameter through the participant's nostril. The location of the probe tip in the esophagus was estimated to be at the level of the eighth and ninth thoracic vertebrae.18 We measured Tre using a pediatric thermocouple probe inserted to a minimum of 12 cm past the anal sphincter.
Temperatures were collected using a data acquisition module (model 3497A; Agilent Technologies, Inc, Santa Clara, CA) at a sampling rate of 15 seconds throughout scenario 1 and for the recovery period of scenario 2. Data were simultaneously displayed and recorded in spreadsheet format on a personal computer (ThinkCentre M50; IBM Corporation, Armonk, NY) with LabVIEW software (version 7.0; National Instruments Corporation, Austin, TX). For the exercise period of scenario 2, core temperatures were monitored and recorded every 5 minutes using a handheld microprocessor thermometer (model HH21; Omega Engineering, Inc, Stamford, CT).
Experimental Protocol
For each scenario, trials were performed at the same time of day to avoid circadian variation in core temperatures. Participants were instructed to fast for at least 4 hours before experimentation, and water consumption was permitted ad libitum during this time. All participants were instructed to wear shorts and athletic shoes, and female participants were also instructed to wear sports bras. Upon arrival at the laboratory, they were instrumented with the 3 core temperature probes (aural canal, esophageal, and rectal). After instrumentation, participants remained resting in an upright, seated position at ambient temperature conditions. After 15 minutes of seated rest, participants entered an adjoining temperature-controlled chamber maintained at 42°C and 30% relative humidity, with an airflow of approximately 0.2 m/s, and they ran on a treadmill at approximately 70% of their predetermined maximal oxygen consumption.
Scenario 1
Participants exercised until Tre reached 39.5°C. Because Tre values were similar (within the confines of approval by the university Research Ethics Committee) to those values associated with exertional heat stroke, participants were immediately transferred (approximately 2 minutes) postexercise into a circulated water bath (model J-315; Jacuzzi Spas International, Chino, CA) maintained at 2°C. Before entering the water bath, participants were fitted with Neoprene (DuPont Performance Elastomers, Wilmington, DE) mitts and socks to reduce discomfort. Participants were immersed to the clavicles in a semirecumbent position and remained in the water until Tre reached approximately 37.5°C.
Scenario 2
Participants exercised until Tes reached 39.5°C. Because Tre values approached those values typically associated with exertional heat exhaustion (approximately 39°C), participants were immediately transferred (approximately 2 minutes) postexercise to an adjacent chamber, with ambient conditions maintained at 30°C and 30% relative humidity, and began a 60-minute inactive recovery period.
Statistical Analyses
Because total exercise time differed for each participant during exercise for both scenarios and because the period of cold-water immersion differed for each participant in scenario 1, data are presented as a percentage of the total time taken to reach the experimental withdrawal criterion for each period. A 2-way repeated-measures analysis of variance was used to analyze the data for both scenarios with the repeated factors of percentage of total time (0%, 20%, 40%, 60%, 80%, and 100%) and core temperature measurement (aural canal, esophageal, and rectal). The dependent variables were absolute values of Tre, Tes, and Tac. Paired-samples t tests were used to perform pairwise post hoc comparisons. The α level was set at .05 and was adjusted during multiple comparisons to maintain the rate of type 1 error at 5% during Bonferroni post hoc analysis. The data are presented as means ± SDs, unless otherwise indicated. All analyses were performed using SPSS (version 16.0 for Windows; SPSS Inc, Chicago, IL).
RESULTS
Scenario 1
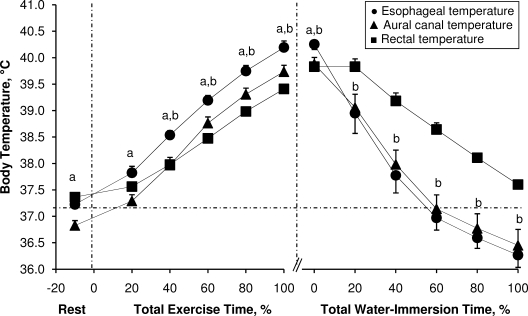
Pre-exercise Tre (t9 = 7.0, P < .001) and Tes (t9 = 6.9, P < .001) were greater than Tac. The average exercise time taken to reach the experimental withdrawal criterion of 39.5°C Tre was 34 ± 8 minutes. During exercise, all 3 indices of core temperature increased as a function of percentage of total exercise time (F10,90 = 317.39, P < .001). However, increases in core temperature during exercise were affected by the measurement index (F2,18 = 22.59, P < .001). This was evidenced by a greater increase in Tes than in Tre at 40% of total exercise time until the end of the exercise period (t11 range, 5.3–8.3, P < .001) (Figure 1). Furthermore, these differences became greater as a function of percentage of total exercise time (F4,40 = 7.74, P < .001) (Figure 2A). Additionally, Tes was greater than Tac throughout the exercise period (t9 range, 4.0–6.9, P < .001) (Figure 1). However, because Tes was higher than Tac at rest (t9 = 6.9, P < .001), the changes from baseline rest in Tes were not different at any time compared with Tac (t9 range, −2.1 to 0.3, P > .05).
Figure 1.
Esophageal, aural canal, and rectal temperatures during exertional heat stress and throughout the subsequent period of cold-water immersion in scenario 1. Values are mean ± standard error for 12 participants. a Indicates different from aural canal temperature (P < .05). b Indicates different from rectal temperature (P < .05). The vertical dashed lines delimit each period. The horizonal dashed line represents the mean of all 3 resting core temperatures.
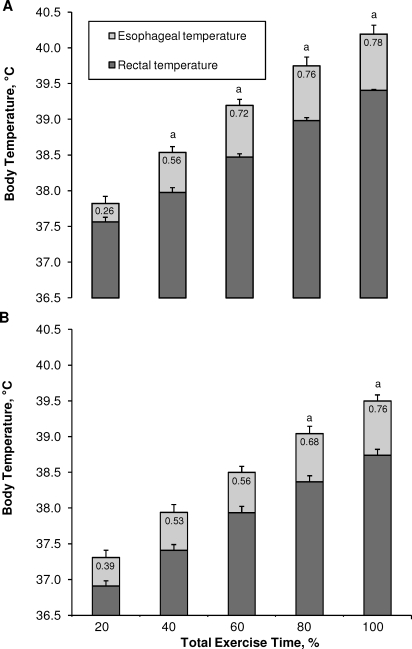
Figure 2.
Differences between esophageal and rectal temperatures as a function of percentage of total exercise time during exertional heat stress as defined by A, an esophageal temperature of 39.5°C or B, a rectal temperature of 39.5°C. Values are mean ± standard error for 12 participants. a Indicates different from 20% of total exercise time (P < .05).
The average time taken to reach the criterion of 37.5°C Tre for water-immersion withdrawal was 14 ± 5 minutes. All 3 indices of core temperature decreased as a function of percentage of total immersion time (F10,50 = 39.81, P < .001). However, we found a difference between core temperature measurements during cold-water immersion (F2,10 = 10.19, P < .001). The Tes was elevated compared with both Tac (t8 = 4.1, P < .001) and Tre (t10 = 3.9, P < .001) at the end of exercise. However, it rapidly decreased during the water immersion such that it was lower than Tre at 20% of the total water-immersion period and remained lower until participants were taken out of the water bath (t10 range, −6.9 to −3.5, P < .001). Similarly, Tac decreased at a greater rate than Tre (t9 range, 2.0–5.3, P < .001); however, we found no differences between Tes and Tac during the immersion period (t7 range, −3.3 to 1.6, P ≥ .05) (Figure 1).
Scenario 2
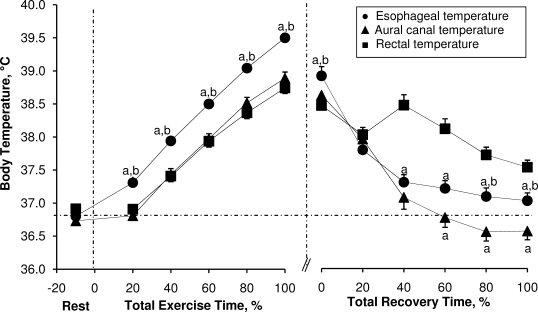
We found no differences between indices of core temperature before exercise (t11 range, −2.4 to 5.6, P ≥ .05). The average time taken to reach the experimental withdrawal criterion of 39.5°C Tes was 29 ± 7 minutes. All 3 indices of core temperature increased as a function of percentage of total exercise time (F5,50 = 278.68, P < .001). However, we found differences between body temperature measurements (F2,20 = 32.75, P < .001). During exercise, Tes was higher than both Tac and Tre at 20% of total exercise time, and this value remained higher until the end of exercise (t11 range, 4.6–7.8, P < .001 for Tac; t11 range, 4.1–9.2, P < .001 for Tre) (Figure 3). Furthermore, the differences between Tes and Tre became greater as a function of percentage of total exercise time (F4,44 = 7.74, P < .001) (Figure 2B).
Figure 3.
Esophageal, aural canal, and rectal temperatures during exertional heat stress and throughout the subsequent inactive recovery in scenario 2. Values are mean ± standard error for 12 participants. a Indicates different from rectal temperature (P < .05). b Indicates different from aural canal temperature (P < .05). The vertical dashed lines delimit each period. The horizonal dashed line represents the mean of all 3 resting core temperatures.
During the 60 minutes of inactive recovery after exertional heat stress, all 3 indices of core temperature decreased as a function of percentage of recovery time (F12,132 = 72.46, P < .001). However, we found differences among core temperature measurements during the recovery period (F2,22 = 22.93, P < .001). Rectal temperature was lower at the end of exercise, compared with Tes (t11 = 3.0, P = .012). After decreasing by approximately 0.5°C during the initial 20% of recovery time, Tre subsequently increased over the next 20% of the total recovery time (approximately 20 minutes), becoming higher than Tes and remaining higher until the end of the recovery period (t11 range, −5.5 to 3.0, P < .001). Additionally, Tre was elevated compared with Tac between 60% and 100% of total recovery time (t11 range, 5.1–11.4, P < .001). Furthermore, Tac decreased at a greater rate than Tes, becoming lower at 80% of total recovery time and remaining lower until the end of the recovery period (t11 range, 2.5–3.6, P < .05) (Figure 3).
DISCUSSION
Although researchers often have documented the differences among measurement sites of body temperature, few investigators have focused on the temporal responses during and after exertional heat stress. As is evident from our data, a single measurement of body temperature does not represent the temperature of the whole body. Therefore, it is important to understand the possible reasons why Tac, Tes, and Tre respond differently during exertional heat stress and the subsequent recovery period. Furthermore, a short discussion about whether these differences should be considered in the treatment of hyperthermic individuals is warranted.
Temporal Response of Core Temperature During and After Exertional Heat Stress
The Tre increased at a slower rate than Tes, resulting in a greater difference between the two as a function of time and, consequently, as body temperature increased. The Tac increased at a rate similar to Tre during both scenarios but was consistently lower than Tes. These results demonstrate that different measurements of body temperature clearly provide different estimates of internal temperature and a different temporal pattern of response. These differences were also evident throughout both recoveries. During cold-water immersion, the slower response time of Tre was evidenced by low points of approximately 36.5°C for Tac and approximately 36.3°C for Tes when participants exited the water bath with a Tre of 37.5°C (Figure 1). However, differences during inactive recovery were mostly due to an increasing Tre (subsequent to an initial drop in temperature) at the beginning (approximately 5–25 minutes) of the recovery period. In fact, although Tes and Tac had decreased by approximately 1.1°C 10 minutes (approximately 16% total recovery time) into recovery relative to their values at the start of the period, Tre actually increased by approximately 0.2°C during this period (Figure 3).
Physical and Physiologic Considerations for Differences in Temporal Responses
To better understand the differences among various measurements of body temperature, one must understand the physical (eg, mass) and physiologic (eg, blood flow) factors that may affect temperature at a given site. Temporal differences between Tre and Tac or Tes can be explained by (1) the physically larger mass of dense tissues associated with the pelvic area that borders the rectal probe and (2) the physiologic differences in regional blood flow during and after exertional heat stress. First, the measurement of temperature of any given body is a function of the change in heat content, its mass, and its composition. Because the rectum is generally assumed to be surrounded by a greater mass and density of tissues compared with the aural canal region and the esophagus, it follows that a greater amount of heat is required to increase the temperature that is measured with a rectal probe. Second, blood flow to the region surrounding the rectal probe (ie, visceral blood flow) is severely reduced during heat stress19 and more severely reduced during combined exercise and heat stress.20 For example, visceral (splanchnic and renal) blood flow is reduced from approximately 2.8 L/min at rest19 to approximately 1 L/min (a decrease of approximately 64%) during passive heat stress19 and to 0.5 L/min (a decrease of approximately 82%) during moderate to heavy exercise in the heat.20 Yet more than 20 L/min of mixed blood (ie, cardiac output) may flow through the region adjacent to the location of the esophageal probe (ie, the left ventricle). The area surrounding the aural canal temperature probe likely receives a relatively large blood flow relative to its mass. Considering that heat exchange within the human body's compartments is mainly achieved by increased blood flow, we were not surprised that Tac and Tes responded much faster than Tre in our study. Note that although Tac values were lower than Tes values in both scenarios, the changes from baseline rest were similar between these indices in scenario 1. In scenario 2, Tac possibly remained lower than Tes because its measurement is influenced by external factors (eg, air movement, sweat).13,21
During both recovery periods, the physical and physiologic differences between measurement sites were also evidenced by the following: (1) Tac and Tes returned to baseline values, but Tre remained elevated after approximately 60% of total immersion time during a subsequent cold-water immersion (scenario 1) (Figure 3), and (2) the “overshoot” in Tre at the beginning of inactive recovery (scenario 2), which was likely due to the restoration of blood flow to the visceral area10 and possibly to postural reorientation (from the standing to the upright, seated position).
Implications
We believe that Tre is the most reliable and valid index of core temperature that can be practically used in emergency situations.1,4 In our study, Tre increased consistently during intense exercise in the heat and decreased consistently and rapidly when the participant was aggressively cooled during cold-water immersion, lending further credence to its validity as a field measure during intense exercise in the heat or during the treatment of exertional heat injury. However, given the apparent disparity in the response profiles of the respective measurement sites, could reliance on a single measurement of body temperature lead to an underestimation or overestimation of the thermal state of the individual? For example, Nybo et al22 reported that jugular venous blood temperature (an index of brain temperature) is approximately 0.3°C to 0.4°C greater than Tes during exercise-induced hyperthermia. Considering that Tes was approximately 0.8°C greater than Tre at the end of exercise in each scenario (Figure 2), brain temperature could be as much as 1°C greater than the temperature measured at the rectum. Therefore, Tre probably does not reflect brain temperature and, therefore, does not reflect the temperature of the central nervous sytem.1
Another important implication for the observed differences in temporal responses among body temperatures lies within the implementation of an appropriate treatment strategy. Current guidelines5 for the immediate care of hyperthermic individuals state that any treatment modality should achieve a Tre cooling rate of at least 0.10°C/min, which is most effectively achieved by immersion in cold water, the temperature of which ranges from 2°C to 10°C. However, Taylor et al23 recently suggested that more temperate water temperatures (14°C and 26°C) offer similar core cooling rates, given the small differences found when examining Tes. Specifically, they23 observed similar Tes cooling rates of 0.88 ± 0.06°C/min at 14°C and 0.71 ± 0.02°C/min at 26°C. They23 suggested that the similarities in esophageal cooling rates were sufficient to question the clinical importance of using cooler water temperatures. In contrast, Proulx et al24 reported Tre cooling rates of 0.15 ± 0.06°C/min during immersion in 14°C water. Therefore, 2 different recovery treatments may be put forth based on core cooling rates obtained by 2 different indices. Taylor et al23 argued that Tes provides a closer approximation of the temperature perfusing the brain and, therefore, is a better indicator of central nervous system temperature, which they believed is the main consideration in the treatment of hyperthermic individuals. On the other hand, Tre offers an extremely valuable window into the stress that vulnerable internal organs face during periods of extreme hyperthermia.1 Therefore, which body temperature provides the best assessment of a hyperthermic individual's thermal status?
Ultimately, Tre is the only suitable and valid index for the monitoring of body temperature in a field setting.1,13,25 In contrast, the use of Tes is not practical in such situations, and Tac is often influenced by external factors, leading to an underestimation of core temperature. We observed differences in regional heat distribution, as highlighted by the different responses of Tac, Tes, and Tre. However, these differences do not change the fact that Tre must be considered the only valid measurement of body temperature for the assessment and monitoring of hyperthermic individuals in a field setting.
One situation in which consideration of these differences is evident is the known risk of overcooling hyperthermic individuals if they remain in cold water until they reach a “normal” resting Tre.26 In fact, investigators1,5,14,26,27 have suggested that a heat-stressed individual does not necessarily have to be cooled until Tre reaches a normal baseline value of 37.5°C because of the delayed response of this value compared with the values of Tes and Tac. Rather, current guidelines5 suggest that hyperthermic individuals should be removed from the water bath at a Tre of approximately 39°C to avoid a potential core temperature after-drop. For example, in scenario 1 of our study, Tre was approximately 38.7°C when Tac and Tes had returned to the mean of all 3 resting temperatures during the period of cold-water immersion (Figure 1).
CONCLUSIONS
We presented the temporal responses of Tac, Tes, and Tre during 2 scenarios of exertional heat stress. Differences among body measurement sites were evident throughout both scenarios, and these are likely due to regional differences in physical (ie, tissue mass) or physiologic (ie, blood flow) characteristics among body sites. However, these differences do not undermine the importance of using Tre as the only valid measurement of body temperature for the field assessment and monitoring of heat-related injuries.
Acknowledgments
This research was supported by grant RGPIN-298159-2004 from the Natural Sciences and Engineering Research Council of Canada and by a research chair award from the University of Ottawa (Dr Kenny).
REFERENCES
- 1.Armstrong L. E., Casa D. J., et al. American College of Sports Medicine. American College of Sports Medicine position stand: exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 2.Bonauto D., Anderson R., Rauser E., Burke B. Occupational heat illness in Washington State, 1995–2005. Am J Ind Med. 2007;50(12):940–950. doi: 10.1002/ajim.20517. [DOI] [PubMed] [Google Scholar]
- 3.Carter R., III, Cheuvront S. N., Williams J. O., et al. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med Sci Sports Exerc. 2005;37(8):1338–1344. doi: 10.1249/01.mss.0000174895.19639.ed. [DOI] [PubMed] [Google Scholar]
- 4.Binkley H. M., Beckett J., Casa D. J., Kleiner D. M., Plummer P. E. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2002;37(3):329–343. [PMC free article] [PubMed] [Google Scholar]
- 5.Casa D. J., McDermott B. P., Lee E. C., Yeargin S. W., Armstrong L. E., Maresh C. M. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev. 2007;35(3):141–149. doi: 10.1097/jes.0b013e3180a02bec. [DOI] [PubMed] [Google Scholar]
- 6.Cooper K. E., Kenyon J. R. A comparison of temperatures measured in the rectum, oesophagus, and on the surface of the aorta during hypothermia in man. Br J Surg. 1957;44(188):616–619. doi: 10.1002/bjs.18004418815. [DOI] [PubMed] [Google Scholar]
- 7.Shiraki K., Konda N., Sagawa S. Esophageal and tympanic temperature responses to core blood temperature changes during hyperthermia. J Appl Physiol. 1986;61(1):98–102. doi: 10.1152/jappl.1986.61.1.98. [DOI] [PubMed] [Google Scholar]
- 8.Robinson J., Charlton J., Seal R., Spady D., Joffres M. R. Oesophageal, rectal, axillary, tympanic and pulmonary artery temperatures during cardiac surgery. Can J Anesth. 1998;45(4):317–323. doi: 10.1007/BF03012021. [DOI] [PubMed] [Google Scholar]
- 9.Cranston W. I., Gerbrandy J., Snell E. S. Oral, rectal and oesophageal temperatures and some factors affecting them in man. J Physiol. 1954;126(2):347–358. doi: 10.1113/jphysiol.1954.sp005214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minard D., Copman L., Dasler A. R. Elevation of body temperature in health. Ann N Y Acad Sci. 1964;121:12–25. doi: 10.1111/j.1749-6632.1964.tb13680.x. [DOI] [PubMed] [Google Scholar]
- 11.Livingstone S. D., Grayson J., Frim J., Allen C. L., Limmer R. E. Effect of cold exposure on various sites of core temperature measurements. J Appl Physiol. 1983;54(4):1025–1031. doi: 10.1152/jappl.1983.54.4.1025. [DOI] [PubMed] [Google Scholar]
- 12.Newsham K. R., Saunders J. E., Nordin E. S. Comparison of rectal and tympanic thermometry during exercise. South Med J. 2002;95(8):804–810. [PubMed] [Google Scholar]
- 13.Casa D. J., Becker S. M., Ganio M. S., et al. Validity of devices that assess body temperature during outdoor exercise in the heat. J Athl Train. 2007;42(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 14.Clements J. M., Casa D. J., Knight J. C., et al. Ice-water immersion and cold-water immersion provide similar cooling rates in runners with exercise-induced hyperthermia. J Athl Train. 2002;37(2):146–150. [PMC free article] [PubMed] [Google Scholar]
- 15.Borg G. A. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 16.Siri W. E. Gross composition of the body. Adv Biol Med Phys. 1956;4:239–280. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Society for Exercise Physiology. Professional Fitness & Lifestyle Consultant Resource Manual. Ottawa, ON, Canada: Canadian Society for Exercise Physiology; 1993. pp. 1–32. [Google Scholar]
- 18.Mekjavic I. B., Rempel M. E. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol. 1990;69(1):376–379. doi: 10.1152/jappl.1990.69.1.376. [DOI] [PubMed] [Google Scholar]
- 19.Rowell L. B. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Sawka M. N., Pandolf K. B. Physical exercise in hot climates: physiology, performance, and biomedical issues. In: Pandolf K. B., Burr R. D., editors. Medical Aspects of Harsh Environments. Vol 1. Washington, DC: Office of the Surgeon General at TMM Publications, Borden Institute, Walter Reed Army Medical Center; 2001. pp. 87–133. http://www.bordeninstitute.army.mil/published_volumes/harshEnv1/Ch3-PhysicalExerciseinHotClimates.pdf. Accessed August 13, 2009. [Google Scholar]
- 21.Roth R. N., Verdile V. P., Grollman L. J., Stone D. A. Agreement between rectal and tympanic membrane temperatures in marathon runners. Ann Emerg Med. 1996;28(4):414–417. doi: 10.1016/s0196-0644(96)70007-0. [DOI] [PubMed] [Google Scholar]
- 22.Nybo L., Secher N. H., Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545(pt 2):697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor N. A., Caldwell J. N., Van den Heuvel A. M., Patterson M. J. To cool, but not too cool: that is the question. Immersion cooling for hyperthermia. Med Sci Sports Exerc. 2008;40(11):1962–1969. doi: 10.1249/MSS.0b013e31817eee9d. [DOI] [PubMed] [Google Scholar]
- 24.Proulx C. I., Ducharme M. B., Kenny G. P. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol. 2003;94(4):1317–1323. doi: 10.1152/japplphysiol.00541.2002. [DOI] [PubMed] [Google Scholar]
- 25.Moran D. S., Mendal L. Core temperature measurement: methods and current insights. Sports Med. 2002;32(14):879–885. doi: 10.2165/00007256-200232140-00001. [DOI] [PubMed] [Google Scholar]
- 26.Proulx C. I., Ducharme M. B., Kenny G. P. Safe cooling limits from exercise-induced hyperthermia. Eur J Appl Physiol. 2006;96(4):434–445. doi: 10.1007/s00421-005-0063-y. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro Y., Seidman D. S. Field and clinical observations of exertional heat stroke patients. Med Sci Sports Exerc. 1990;22(1):6–14. [PubMed] [Google Scholar]