Abstract
The aim of the present study was to determine the association between prenatal stress and immune function in human adults. Peripheral blood mononuclear cells (PBMCs) from 34 healthy young women whose mothers experienced major negative life events during their pregnancy (Prenatal Stress, PS group, mean age 25, SD ± 4.34 years), and from a female comparison group (n = 28, CG, mean age 24 ± 3.40 years), were stimulated with phytohemagglutinin (PHA), and subsequent cytokine production was measured. A bias for T-helper 2 (Th2) cytokine production due to an overproduction of IL-4 relative to IFN-γ after PHA stimulation was observed in PS subjects. In addition, IL-6 and IL-10 were also significantly elevated. To the best of our knowledge, this study is the first to suggest a direct association between prenatal stress exposure and alterations in immune parameters in adult women.
Keywords: prenatal stress, psychosocial, cytokines, Th1, Th2
Introduction
Epidemiological studies across the world have reported associations between birth phenotype such as low birth weight or small body size at birth and subsequent risk of disease in adult life, including hypertension, coronary heart disease, type 2 diabetes mellitus, depression and other cognitive and affective disorders (Barker, 1998; Cannon, Jones, & Murray, 2002; Gluckman & Hanson, 2004a; Thompson, Syddall, Rodin, Osmond, & Barker, 2001). There is emerging evidence that immune-related disorders such as asthma, allergies and autoimmune conditions like autoimmune thyroiditis also are related to size and weight at birth (Braback & Hedberg, 1998; Fergusson, Crane, Beasley, & Horwood, 1997; Kajantie, Phillips, Osmond, Barker, Forsen, & Eriksson, 2006; Phillips, Osmond, Baird, Huckle, & Rees-Smith, 2002; Shaheen, Sterne, Montgomery, & Azima, 1999). It is unlikely that birth phenotypes, per se, play a causal role in increasing risk of adult disease. Instead they constitute more likely a crude reflection of developmental processes in intrauterine life that also may influence the structure and function of physiological systems that underlie health and disease risk in later life (Gluckman & Hanson, 2004b; Morley, Owens, Blair, & Dwyer, 2002). The link from prenatal environment to adult health/disease might not necessarily be mediated via adverse birth outcomes. Thus, measures of prenatal conditions may be more sensitive predictors than birth size and weight at birth.
Two major evolutionary forces that act upon and shape the development of living organisms are those related to availability and utilization of energy substrates (nutrition) and those involved in adaptation to challenges or threats to homeostasis (stress). Several studies of the effects of adverse early environment have focused on the role of pre- and perinatal nutrition. We and others have proposed that prenatal stress exposure represents yet another adverse environment that may contribute to weight and size at birth and the physiology of the developing organism (Wadhwa, 2005). Maternal stress during pregnancy has been shown in humans to predict low birth weight (Paarlberg, Vingerhoets, Passchier, Dekker, Heinen, & van Geijn, 1999; Paarlberg, Vingerhoets, Passchier, Dekker, & Van Geijn, 1995; Wadhwa, Sandman, & Garite, 2001) and preterm delivery (Hedegaard, Henriksen, Sabroe, & Secher, 1993; Paarlberg et al., 1995; Wadhwa et al., 2001), and other studies have linked these birth outcomes with asthma, allergies (for review see Remes & Pekkanen, 2005) and autoimmune disorders (Kajantie et al., 2006; Phillips et al., 2002).
It is well known that psychosocial stress has an impact on the immune system (for a recent review see Segerstrom & Miller, 2004). Almost all immune cells have receptors for one or more hormones that are associated with the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) axis (Glaser & Kiecolt-Glaser, 2005). Chronic stress was first believed to generally suppress the immune system. New findings support the hypothesis that chronic stress specifically alters the pattern of cytokines that are secreted in response to an antigen. Under conditions of chronic stress there is a shift from T-helper 1 (Th1) cytokines to Th2 cytokines, mediated by glucocorticoids, which suppress cellular immunity and activate humoral immunity (Elenkov, 2004; Glaser & Kiecolt-Glaser, 2005).
There is evidence from animal studies in different species that prenatal stress has an impact on the immune system of the offspring later in life. For example, offspring of rats stressed during pregnancy showed reduced lymphocyte proliferation and cytolytic responses (Kay, Tarcic, Poltyrev, & Weinstock, 1998; Klein & Rager, 1995), as well as a decrease in the total peripheral leukocyte count and alterations in the differential count by decreasing lymphocytes and increasing neutrophil and eosinophil counts, and a significant reduction in the percentage of peripheral CD8+ cells (Llorente, Brito, Machado, & Gonzalez, 2002). Several studies conducted by Coe and colleagues (summarized in Coe & Lubach, 2005) revealed effects of hormone-treatment and psychological stress during pregnancy on rhesus monkeys. Monkeys born from these pregnancies showed lower production of cytokines after lipopolysaccharide (LPS) stimulation, altered proliferative response of mononuclear cells in response to self- and non-self antigens, and a tendency for a reduction in circulating CD4+ cells compared to monkeys from undisturbed pregnancies. Maternal stress during pregnancy also affects placental transfer of antibodies from the mother to the neonate, and this effect may depend on the sex of the fetus (Coe & Crispen, 2000). The offspring of prenatally-stressed mice were shown to have an increased vulnerability toward airway hyperresponsiveness and inflammation, accompanied by a Th2 biased immune response after Ag challenge (Pincus-Knackstedt, Joachim, Blois, Douglas, Orsal, Klapp, Wahn, Hamelmann, & Arck, 2006). Additionally, a study in an outbred rat model found significant differences between offspring of prenatally stressed rats relative to offspring of control rats, including an increased pro-inflammatory immunologic stance in the prenatally stressed adult animals, whereas young prenatally stressed animals showed an increase in the B lymphocyte compartment and dramatically elevated IL-5 mRNA levels (Vanbesien-Mailliot, Wolowczuk, Mairesse, Viltart, Delacre, Khalife, Chartier-Harlin, & Maccari, 2007).
To the best of our knowledge there are no studies in humans that have examined the effects of maternal psychosocial stress during pregnancy on immune function of their adult offspring. Thus, the three major objectives of the present study were to investigate 1) whether there is an association between measures of prenatal stress, operationalized as major negative life events that occurred during pregnancy, and measures of immune function in adult female offspring, 2) whether this association is mediated by or independent of birth phenotype like birth weight and length of gestation, and 3) the possible role of postnatal factors on the association between prenatal stress and adult immune function, e.g., poor maternal care, presence of traumatic events during childhood, and subjects’ present depression and neuroticism scores. There are three elements for effective immune function, in both the innate and adaptive arms of the immune system: production, activation and effector function. Soluble immune mediators, cytokines, regulate these processes. As an indicator of production we examined the numbers of circulating lymphocytes and lymphocyte subsets. The capacity for activation and intrinsic bias to the immune response was assessed by in vitro phytohemagglutinin (PHA) induced cytokine production. Functional assays to look at effectiveness of activated immune cells are more difficult to conduct and were not run in this preliminary study.
Methods
Subjects
The study sample included a total of 62 subjects. Thirty-four young women (mean age 25, SD ± 4.34 years), whose mothers experienced a high level of psychosocial stress (negative life events during pregnancy) constituted the prenatal stress group (PS). An age-matched sample of 28 women (mean age 24 ± 3.40 years) constituted the comparison group (CG). Subjects were recruited through an announcement in local newspapers and via emails that were sent to students and staff of the University of Trier, Germany. Before entering the study, the absence of acute or chronic physical health problems was ascertained by self-report and confirmed by a medical examination, and subjects were asked about their medical history. Women with a history of psychiatric disorders and chronic illnesses were excluded from the study. All subjects were non-smokers, reported to be medication free and did not have vaccinations two weeks prior to the entry to the study. A copy of the maternal prenatal medical record (which is handed to the mother by the obstetrician during her first prenatal visit) was obtained from each participant. From this record, information about maternal parity, maternal age at birth, length of gestation and subjects’ weight, height and head circumference at birth were extracted. Written informed consent was obtained from all subjects. The investigation described in this manuscript was conducted in accordance with the guidelines described in the declaration of Helsinki and the study protocol was approved by the ethics committee of the German Psychological Society, DGPs.
Conceptualization and assessment of prenatal psychosocial stress exposure
We adopted a conservative strategy for the conceptualization of prenatal stress in the present study. We defined a high level of prenatal psychosocial stress exposure as the presence of major negative life events that occurred to the mother while she was pregnant (see Table 1 for list and frequency of events). Psychosocial stress is a multi-component construct that includes the occurrence of negative life events, appraisal of the stress (e.g., degree of predictability and control), and psychological symptoms such as anxiety and negative affect. Because retrospective assessment of stress appraisals and symptoms is known to be unreliable, we focused on only the presence or absence of negative life events during the index pregnancy. Moreover, we selected those events that are considered as highly stressful across individuals (Table. 1).
Table 1.
List of events during pregnancy included in the study. Each woman is listed with one life event. One woman that reported marital infidelity of her husband followed by a divorce during her pregnancy is only listed once under the category “relationship conflicts”. Three other women that lost a family member suffering of a disease were each listed once under “death of someone close” and not under “severe illness of someone close”
| Event | N (34) | % | |
|---|---|---|---|
| Relationship conflicts | -divorce -break up -paternity denial -marital infidelity |
15 | 44 |
| Death of someone close | -partner -parent -other child |
6 | 19 |
| Severe illness of someone close | -cancer -heart attack -stroke |
5 | 14 |
| Severe financial problems | -loss of house by flooding -sudden unemployment of husband -foreclosure |
3 | 8 |
| Car accident | 2 | 5 | |
| Unmarried, father not accepted by family | 1 | 3 | |
| Becoming political refugee | 2 | 5 |
In all subjects we conducted semi-structured interviews based on a questionnaire about exposure to major negative life events during the prenatal period that subjects were instructed to review with their mothers prior to the interview. In most of the cases (70%) we were able to verify this information by communicating directly with the mothers by phone, e-mail or letters. The subjects that were recruited to constitute the comparison group were asked to review the same questionnaire with their mothers to ascertain that their mothers had not experienced any negative life events during pregnancy.
Questionnaires
Since it is possible that prenatal stress exposure is associated with adverse postnatal experiences such as poor maternal care and presence of other stressors during childhood we assessed several measures to control for these potential confounding factors.
In order to obtain an important aspect of the family environment during the postnatal period we administered the maternal care scale of the Parental Bonding Inventory (PBI; German version by Lutz et al., 1995, originally developed by Parker et al., 1979). The PBI measures the self reported perception of being parented to the age of 16 years. Studies assessing re-test reliability of the PBI suggest that the parental evaluation is a rather stable measure, which is not affected by confounding variables like dysthymia, neuroticism, depressive episode or gender (Lizardi & Klein, 2005; Parker, 1990; Plantes, Prusoff, Brennan, & Parker, 1988; Wilhelm, Niven, Parker, & Hadzi-Pavlovic, 2005). Good validity of the PBI can be concluded e.g., from high agreement between sibling ratings (Parker, 1990). Furthermore, subjects’ and subjects’ mothers’ socio-economic status (SES) was assessed by educational level.
A translated version of the Childhood Traumatic Events Survey (Pennebaker & Susman, 1988) was administered. The instrument screens adverse experience during childhood in six questions. In addition, subjects completed a German version of the Centre for Epidemiological Studies Depression Scale (CES-D, Hautzinger & Bailer, 1993), and the NEO Five Factor Inventory (Borkenau & Ostendorf, 1993).
Immune Assays
All subjects reported to the laboratory between 1400 and 1500 h. A blood sample for each subject was collected in heparinized tubes. Samples were stored at room temperature for no longer than 1 h. PBMCs (peripheral blood mononuclear cells) were isolated using differential density centrifugation with Ficoll (Pharmacia, Freiburg, Germany). In each sample, cell numbers were obtained using a cell counter (AcT Diff, Coulter, Krefeld, Germany), and cell numbers were adjusted to 1 × 106 cells/ml by adding according amounts of cell culture medium (RPMI 1640, Rosewell Park Memorial Institute, Biochrome Berlin, Germany). Cell suspensions were stimulated by 5 μg/ml phytohaemagglutinin (PHA, Remel, Santa Fe, USA), a potent T cell stimulant, in 24-well microtiter plates and incubated at 37°C in 5% CO2 humidified atmosphere. After 24 h the samples were centrifuged, culture supernatant was harvested, and stored at - 80°C until processed for cytokine assays.
Multiplex cytokine analysis kits were obtained from Linco Research Inc. (St. Charles, MO, USA). Millipore multiscreen 96 well filter plates (Bedford, MA, USA) were used for all multiplex cytokine kits. Assays were run in triplicate according to the manufacturers’ protocol. Data was collected using the Luminex-100 system Version 1.7 (Luminex, Austin, TX, USA). Data analysis was performed using the MasterPlex QT 1.0 system (MiraiBio, Alameda, CA, USA). A five-parameter regression formula was used to calculate the sample concentrations from the standard curves. Intra- and interassay precision varied between 5.4 and 14.1%. This technology has been shown to be a valid alternative method to ELISA for the evaluation of cytokines (Dupont, Wang, Wadhwa, Culhane, & Nelson, 2005).
An additional peripheral blood sample was collected at the same time point in EDTA coated tubes to determine lymphocyte subpopulations by flow cytometry. Total numbers of leukocytes were determined in each sample, using a cell counter (Coulter, AcT Diff, Krefeld, Germany). After that, 5 × 106 leukocytes (approximately 100 μl whole blood) were incubated with the following sets of two monoclonal murine antibodies and isotype control antibodies (Simultest TS IMK-Lymphocyte, Becton Dickinson, Heidelberg, Germany) that had been conjugated with flourescein isothiocyanate (FITC) and phycoerythrin (PE) for 15 minutes: CD45 FITC / CD14 PE, CD3 FITC / CD19 PE, CD3 FITC / CD4 PE, CD3 FITC / CD8 PE, CD3 FITC / CD16 PE + CD56, IgG1 FITC and IgG2A PE (control). In the next step, FACS lysing solution was added (Becton Dickinson, Heidelberg, Germany), the sample was washed 3 times in cell wash (Becton Dickinson, Heidelberg, Germany) and fixed in formaldehyde solution (Merck, Darmstadt, Germany). The samples were run on a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany) and raw data was processed using SimulSET v. 3. (Becton Dickinson, Heidelberg, Germany). The percentage of the following lymphocyte subpopulations was determined: CD3-/CD19+ (B cells), CD3+/CD4+ (T-helper cells), CD3+CD8+ (T-cytotoxic cells), CD3-/CD16, 56+ (NK cells).
Statistical Analysis
General Linear Model (GLM) analyses were performed to assess differences between the two groups in birth weight, length of gestation, SES, depression, degree of neuroticism and maternal care, cytokine levels after PHA stimulation, and differences in lymphocyte subpopulations as well as CD4:CD8 ratio. Chi-square analyses were conducted to assess differences between the two groups in the frequency of the events assessed by the Childhood Traumatic Events Survey. The interrelationship between birth weight and length of gestation and cytokine levels was assessed applying Pearson correlations. Greenhouse-Geisser corrections were applied where appropriate and only adjusted results are reported. All results are presented as the mean ± standard error of the mean (SEM). All analyses were performed using SPSS Inc. statistical software package (Version 13.0).
Results
Birth Weight, Length of Gestation and Mode of Delivery
There was no difference between the two groups in birth weight (PS: 3280 g ± 83; CG: 3258 g ± 74, F(1;55) = .03, p = .86), length of gestation (PS: 39.33 wk ± .30; CG: 39.39 wk ± .37, F(1;53) = .01, p = .91) or birth weight adjusted for length of gestation as expressed by growth percentiles at birth according to the 2000 US Natality dataset (PS: 37.8 ± 1.9; CG: 40.8 ± 2.6, F(1;50) = .36, p = .55). Birth weight and length of gestation of all subjects were within the normal range. There were no C-section deliveries in the PS group, while two subjects in the CG were delivered by C-section. Birth weight and length of gestation of these two subjects were within the normal range. There was no association between birth weight or length of gestation and cytokine levels. The two groups did not differ in maternal parity (PS: 1.1, ± .21, CG: 1.0 ± .18, F(1;49) = .14, p= .71) and maternal age at birth (PS: 28.23 ± .90, CG: 30.13 ± 1.1, F(1;49) = 1.8, p = .18).
Lymphocyte Subpopulations
There were no differences in any of the lymphocyte subpopulations assessed (all p>.30, see table 2), and there was no difference in the CD4:CD8 ratio between the two groups.
Table 2.
Concentrations of white blood cells (WBC) and lymphocyte subsets in prenatal stress (PS) and comparison group (CG) subjects
| WBC | Lymph | CD3+ | CD4+ | CD8+ | CD19+ | CD16+/CD56+ | |
|---|---|---|---|---|---|---|---|
| Group | |||||||
| PS | 7.54 ±. 25 | 1.44 ± .07 | 1.03 ± .06 | .46 ± .03 | .28 ± .02 | .16 ± .01 | .18 ± .01 |
| CG | 7.26 ± .31 | 1.53 ± .10 | 1.11 ± .04 | .50 ± .04 | .32 ± .03 | .15 ± .02 | .20 ± .02 |
WBC = white blood cells (mio/ml); Lymph = lymphocytes, CD3+ = T cells (mio/ml); CD4+ = T-helper cells (mio/ml); CD8+ = T-cytotoxic cells (mio/ml); CD19+ = B cells (mio/ml); CD16+/CD56+ = NK cells (mio/ml). Values are total numbers of cells ± SEM. None of the group comparisons reached statistical significance (p>.30).
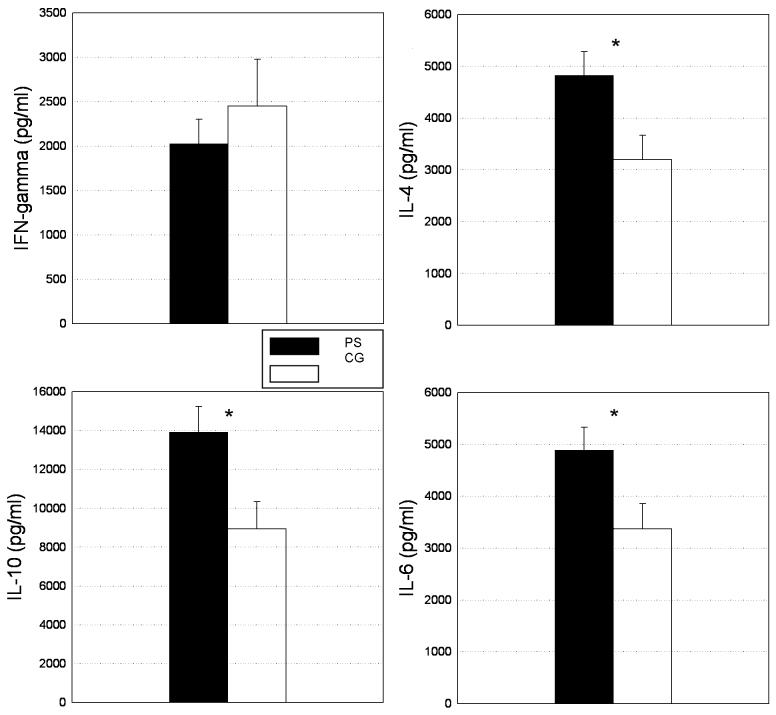
Cytokine Production
Differences in cytokine production after PHA stimulation between the two groups are summarized in Figure 1. PS subjects showed significantly higher levels of IL-4 (4825.15 ± 452.06 vs. 2635.91 ± 468.73 pg/ml, F(1; 60) = 6.19, p< .05), IL-10 (13919.79 ± 1317.19 vs. 8938.11 ± 1400.13 pg/ml, F(1;60) = 6.67, p < .05) and IL-6 (4886.94 ± 439.73 vs. 3377.23 ± 480.23 pg/ml, F(1;60) = 5.37, p < .05). Levels of IFN-γ were lower in PS subjects but this difference was not statistically significant (2024.76 ± 277.44 vs. 2447.74 ± 529.77 pg/ml, F(1;60) = .55, p = .46). However, IFN-γ/IL-4 ratio was significantly lower in the PS group (.56 ± .14 vs. 1.2 ± .30, F(1;61) = 5.88, p < .05, see Figure 2).
Figure 1.
Levels of IFN-γ, IL-4, IL-10 and IL-6 after PHA stimulation in prenatal stress (PS) and comparison group (CG) subjects. Significance is indicated by an asterisk (*, p < .05)
Figure 2.
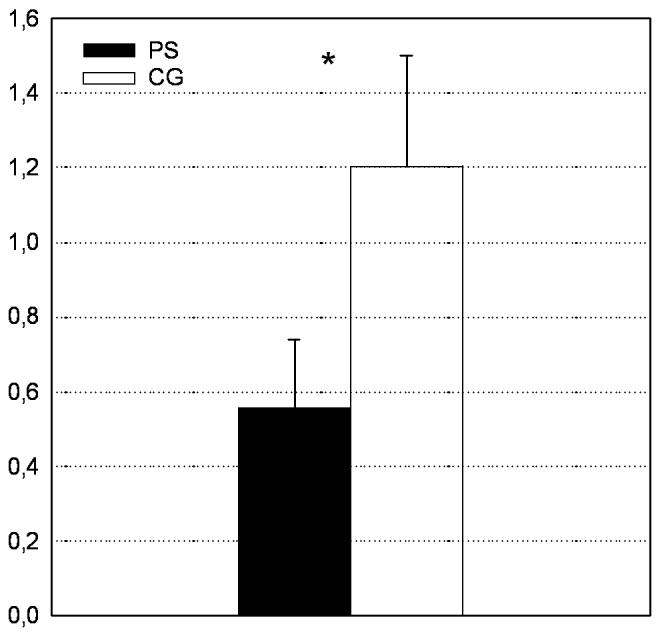
IFN-γ/IL-4 ratio after PHA stimulation in prenatal stress (PS) and comparison group (CG) subjects. (*, p < .05)
Questionnaires
Subjects and subjects‘ mothers did not differ in SES, assessed by educational level, and there were no differences in maternal care scores between the two groups (F(1;49) = 1.74, p = .19). The two groups neither differed in the frequency of the events assessed by the Childhood Traumatic Events Survey (p > .25 for all events), nor in their depression score (F(1;61) = .12, p = .73) or their degree of neuroticism (F(1;59) = 1.29, p = .24).
Discussion
Our results suggest an association between maternal psychosocial stress during pregnancy and changes in cytokine production in response to antigen stimulation in the adult (female) offspring in humans. Young women that were exposed to prenatal stress showed a bias for Th2 cytokine production due to an overproduction of IL-4 relative to IFN-γ, as well as higher IL-10 and IL-6 levels after PHA stimulation. There were no differences in the lymphocyte subpopulations, as well as the CD4:CD8 ratio assessed by FACS analysis; the higher concentration of Th2 cytokines seemed therefore not to be due to generally higher concentrations of T-cells in PS subjects. The observed changes seemed to be independent of birth weight and length of gestation, and postnatal factors like maternal care and traumatic events during childhood, as well as subjects’ present depression and neuroticism scores.
The prenatally stressed women in the present study showed a bias for similar cytokine production (shift towards Th2) to that of chronically stressed individuals. The findings of a decreased IL-4: IFN-γ ratio along with the increased production of both IL-10 and IL-6 are consistent with this enhanced Th2 bias. Although IL-10 is more pleiotropic in humans than in rodents and is not an exclusive Th2 associated cytokine (Mosmann & Sad, 1996), there is a substantial body of evidence to support its increase and association with a more pronounced Th2 immune bias in the setting of chronic stress. Caregivers of persons with dementia exhibited higher percentage of IL-10(+) lymphocytes (Glaser, MacCallum, Laskowski, Malarkey, Sheridan, & Kiecolt-Glaser, 2001), and Marshall et al. (1998) observed a decrease in IFN-γ accompanied by an increase in IL-10 during exam stress that resulted in a decreased IFN-γ/IL-10 ratio. The PS subjects in our study also showed higher levels of IL-6. IL-6 supports B lymphocyte maturation and survival. Thus, this finding is consistent with the T- helper type 2 responses that support antibody responses and suppress T cytotoxic responses. The shift from Th1 to Th2 may be related to changes in catecholamine levels which are elevated as a result of psychological stress. Elenkov and Chrousos (1999) reported that an increase in IL-10 production was associated with an increase in catecholamines, and these changes resulted in a shift from the Th1 to the Th2 direction. Possible implications for HPA axis activation in inducing a shift from Th1 to Th2 cytokine responses have also been demonstrated (Agarwal & Marshall, 1998). Th1 and Th2 responses are mutually inhibitory. Thus, the stress-induced Th2 shift might have profound effects on the susceptibility of the organism to specific infections, and /or might influence the course of an infection, the defense against which is primarily through cellular immune mechanisms (Elenkov & Chrousos, 1999). Furthermore, a Th2 predominant cytokine pattern, associated with systemic lupus erythematosus (SLE) and atopies such as asthma, eczema, hay fever, urticaria and food allergy (Elenkov & Chrousos, 1999), might predispose the prenatally stressed individuals to develop atopic and autoimmune disease in later life.
Wadhwa and others (e.g., Huizink, Mulder, & Buitelaar, 2004; Wadhwa, 1998) have discussed possible mechanisms of how stress is transduced from pregnant mother to fetus, such as: a) transplacental transport of maternal stress hormones to the fetus b) maternal stress induced release of placental hormones that enter the fetal circulation c) and maternal stress induced effects on placental physiology including blood flow and changes in fetal metabolism impacting on oxygen and glucose usage. According to Coe et al. (2002) these changes can cause deleterious effects on organ development, especially for hormone-sensitive glands like the thymus, a critical immune organ, which is extremely sensitive to the stress-responsive adrenal corticosteroids during fetal development (Sawyer, Hendrickx, Osburn, Terrell, & Anderson, 1977). Activation of the HPA axis has profound effects on the inflammatory immune response because virtually all components of the immune response are influenced by cortisol, including alterations of leukocyte traffic and function, decrease in production of cytokines and other mediators of inflammation, and inhibition of the latter’s effect on target tissue. Exposure to glucocorticoids in utero due to maternal psychosocial stress during pregnancy may thus program immune function of the developing organism resulting in life-long alterations. In rodents and non-human primates the impact of prenatal stress on the immune system of the offspring later in life could already be confirmed (see e.g., Coe & Lubach, 2005; Kay et al., 1998; Klein & Rager, 1995). Our findings are in line with a recent study done in mice showing that prenatally stressed adult offspring exhibit a Th2 biased immune response after Ag challenge (Pincus-Knackstedt et al., 2006). The consequences of maternal stress during pregnancy on the immune system of the offspring seem to depend, in part, on the both the species and the age of the offspring investigated. Vanbesien et al. (2007) report prominent increases in IFN-γ expression and the CD8+ T cell compartment in 6 month old adult offspring, whereas none of the PS-induced cellular perturbations reported in adult rats could be evidenced in younger (7 week old) rats. But, interestingly, the younger rats showed a marked increase in IL-5 mRNA expression in basal condition, and an increase in mRNA levels for IL-10 (1.54 fold) and IL-6 (6.96 fold) in stimulated splenocytes from adult offspring of prenatally stressed rats relative to controls, supporting an element of increased Th2 bias.
At birth, the immune system of the neonate is primed towards a Th2 dominance. Within the first two years of life the immune system is activated, probably via childhood infections, leading to a naturally occurring shift from Th2 to Th1 immunity. It has been suggested that maternal stress during pregnancy may delay this crucial part of postpartum immune adaptation (Knackstedt, Hamelmann, & Arck, 2005). Atopy predisposed children show a delay in the shift towards a Th1-predominance. An increased production of Th2 cytokines such as IL-4, has been described in those newborns that later developed atopic diseases (Prescott, Macaubas, Smallacombe, Holt, Sly, & Holt, 1999). Th2 primed T-cells producing high levels of IL-4 accompanied by high serum levels of IgE and increased numbers of eosinophils immunologically predispose these children to the onset of atopic disease. Accordingly, Coe et al. (2002) showed that offspring of rhesus monkeys that have been stressed during their pregnancies showed lower levels of Th1 cytokines, especially TNF-α, in response to Lipopolysaccharide (LPS).
There are some limitations to our study. First and foremost, prenatal stress exposure was assessed retrospectively. Although retrospective assessments of psychosocial factors such as stress are prone to biases such as “after-the-fact” reporting, biases produced by personality/mood, and memory biases, we believe it is unlikely that any of these biases operated in our study sample for the following reasons: Subjects with adverse health outcomes are more prone to retrospectively reporting higher levels of prior adverse exposures (i.e., after-the-fact retrospective reporting bias), however, it is unlikely that this bias was present in the current study because all subjects received the same information before entering the study; the subjects were not informed about the hypothesized direction of the effects; subjects (as well as the experimenters) were blind to and had no a priori knowledge about the results of the study outcome; and none of the subjects had any underlying disease. In terms of reporting bias produced by personality/mood state, the subjects in the two groups did not differ in either neuroticism or depression scores (i.e., the major personality/mood constructs that underlie self-report bias). Last, retrospective assessments also are prone to memory biases, but it also is unlikely that this potential bias affected one group of subjects more than the other. If at all, memory bias and under-reporting stress in the comparison group could only bias the results in the direction of possibly dilute the observed effects.
Retrospective life event assessments certainly are less biased than retrospective assessments of other components of stress such as perceived severity of stress appraisals and symptoms. Thus instead of relying on a subjective distress measure of mothers’ stress appraisal, we focused on only the presence of negative life events during the index pregnancy, and we selected those events that are considered as highly stressful across individuals. While it is known that stressful life events are more likely to occur in women of lower social class, there were no differences in SES in our two study groups. Finally, it is possible that prenatal stress exposure is associated with adverse postnatal experiences such as poor maternal care and presence of other stressors during childhood. We, however, assessed these constructs in our sample and found no differences across the two study groups in perceived maternal care after birth, in the frequency of early traumatic events assessed by the Childhood Traumatic Events Survey, in depression and neuroticism levels.
Unfortunately only very few men responded to our study announcements and thus were not include in the present analysis since we did not have the power to look at potential sex effects on the association between prenatal stress and immune function. We speculate that women were more willing to take part in the present study because they are in general more interested in issues and research questions concerning pregnancy. Because sex-specific prenatal programming effects may occur for HPA axis related parameters (Fameli, Kitraki, & Stylianopoulou, 1994; Koehl, Darnaudery, Dulluc, Van Reeth, Le Moal, & Maccari, 1999; McCormick, Smythe, Sharma, & Meaney, 1995; Szuran, Pliska, Pokorny, & Welzl, 2000; Weinstock, Matlina, Maor, Rosen, & McEwen, 1992), our findings cannot be assumed to generalized to men.
In conclusion, the present findings provide support for an impact of prenatal stress exposure on immune function in humans. Additional future studies including both sexes are necessary to clarify how these findings may generalize to men. After having revealed preliminary differences in cytokine production, it also may be interesting to assess functional immune assays. Together with the findings of the present study this may lead to a better understanding of the pathogenesis of stress-related immune disorders, which may have their origin very early in life.
Acknowledgements
Supported, in part, by the German Research Foundation grant WU 324/ 3-(1-3) to SW and US PHS (NIH) grants HD-047609, HD-041696 and HD-33506 to PDW.
References
- Agarwal SK, Marshall GD., Jr. Glucocorticoid-induced type 1/type 2 cytokine alterations in humans: a model for stress-related immune dysfunction. J Interferon Cytokine Res. 1998;18(12):1059–1068. doi: 10.1089/jir.1998.18.1059. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers, babies and health in later life. Livingstone; Churchill: 1998. [Google Scholar]
- Borkenau P, Ostendorf F. NEO-Fünf-Faktoren Inventar (NEO-FFI) nach Costa und McCrae. Handanweisung. Hogrefe; Göttingen: 1993. [Google Scholar]
- Braback L, Hedberg A. Perinatal risk factors for atopic disease in conscripts. Clin Exp Allergy. 1998;28(8):936–942. doi: 10.1046/j.1365-2222.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Coe CL, Crispen HR. Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol. 2000;19(6):554–559. [PubMed] [Google Scholar]
- Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87(2):675–681. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Prenatal origins of individual variation in behavior and immunity. Neurosci Biobehav Rev. 2005;29(1):39–49. doi: 10.1016/j.neubiorev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dupont NC, Wang K, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of luminex multiplex cytokine analysis kits with ELISA: determinations of a panel of nine cytokines in clinical sample culture supernatants. J Reprod Immunol. 2005;66(2):175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999;10(9):359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Fameli M, Kitraki E, Stylianopoulou F. Effects of hyperactivity of the maternal hypothalamic-pituitary-adrenal (HPA) axis during pregnancy on the development of the HPA axis and brain monoamines of the offspring. Int J Dev Neurosci. 1994;12(7):651–659. doi: 10.1016/0736-5748(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Crane J, Beasley R, Horwood LJ. Perinatal factors and atopic disease in childhood. Clin Exp Allergy. 1997;27(12):1394–1401. doi: 10.1046/j.1365-2222.1997.1430947.x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, MacCallum RC, Laskowski BF, Malarkey WB, Sheridan JF, Kiecolt-Glaser JK. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci. 2001;56(8):M477–482. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004a;15(4):183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004b;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M. Manual. Beltz Test GmbH; Göttingen: 1993. Allgemeine Depressions Skala. [Google Scholar]
- Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. Bmj. 1993;307(6898):234–239. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull. 2004;130(1):115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI, Osmond C, Barker DJ, Forsen T, Eriksson JG. Spontaneous hypothyroidism in adult women is predicted by small body size at birth and during childhood. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-1093. [DOI] [PubMed] [Google Scholar]
- Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav. 1998;63(3):397–402. doi: 10.1016/s0031-9384(97)00456-3. [DOI] [PubMed] [Google Scholar]
- Klein SL, Rager DR. Prenatal stress alters immune function in the offspring of rats. Dev Psychobiol. 1995;28(6):321–336. doi: 10.1002/dev.420280603. [DOI] [PubMed] [Google Scholar]
- Knackstedt MK, Hamelmann E, Arck PC. Mothers in stress: consequences for the offspring. Am J Reprod Immunol. 2005;54(2):63–69. doi: 10.1111/j.1600-0897.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudery M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40(3):302–315. [PubMed] [Google Scholar]
- Lizardi H, Klein DN. Long-term stability of parental representations in depressed outpatients utilizing the Parental Bonding Instrument. J Nerv Ment Dis. 2005;193(3):183–188. doi: 10.1097/01.nmd.0000154838.16100.36. [DOI] [PubMed] [Google Scholar]
- Llorente E, Brito ML, Machado P, Gonzalez MC. Effect of prenatal stress on the hormonal response to acute and chronic stress and on immune parameters in the offspring. J Physiol Biochem. 2002;58(3):143–149. doi: 10.1007/BF03179851. [DOI] [PubMed] [Google Scholar]
- Lutz R, Heyn C, Kommer D. Fragebogen zur elterlichen Bindung - FEB. In: Lutz R, Mark N, editors. Wie gesund sind Kranke? Zur seelischen Gesundheit Kranker. Verlag für angewandte Psychologie; Göttingen: 1995. [Google Scholar]
- Marshall GD, Jr., Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun. 1998;12(4):297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84(1):55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Morley R, Owens J, Blair E, Dwyer T. Is birthweight a good marker for gestational exposures that increase the risk of adult disease? Paediatr Perinat Epidemiol. 2002;16(3):194–199. doi: 10.1046/j.1365-3016.2002.00428.x. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Heinen AG, van Geijn HP. Psychosocial predictors of low birthweight: a prospective study. Br J Obstet Gynaecol. 1999;106(8):834–841. doi: 10.1111/j.1471-0528.1999.tb08406.x. [DOI] [PubMed] [Google Scholar]
- Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Van Geijn HP. Psychosocial factors and pregnancy outcome: a review with emphasis on methodological issues. J Psychosom Res. 1995;39(5):563–595. doi: 10.1016/0022-3999(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Parker G. The Parental Bonding Instrument. A decade of research. Soc Psychiatry Psychiatr Epidemiol. 1990;25(6):281–282. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A Parental Bonding Instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26(3):327–332. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Osmond C, Baird J, Huckle A, Rees-Smith B. Is birthweight associated with thyroid autoimmunity? A study in twins. Thyroid. 2002;12(5):377–380. doi: 10.1089/105072502760043440. [DOI] [PubMed] [Google Scholar]
- Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, et al. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J Immunol. 2006;177(12):8484–8492. doi: 10.4049/jimmunol.177.12.8484. [DOI] [PubMed] [Google Scholar]
- Plantes MM, Prusoff BA, Brennan J, Parker G. Parental representations of depressed outpatients from a U.S.A. sample. J Affect Disord. 1988;15(2):149–155. doi: 10.1016/0165-0327(88)90083-3. [DOI] [PubMed] [Google Scholar]
- Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353(9148):196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- Remes ST, Pekkanen J. Perinatal chracteristics and asthma and allergies in offspring. In: Hodgson D, Coe CL, editors. Perinatal Programming. Taylor & Francis; London and New York: 2005. pp. 155–168. [Google Scholar]
- Sawyer R, Hendrickx A, Osburn B, Terrell T, Anderson J. Abnormal morphology of the fetal monkey (Macaca mulatta) thymus exposed to a corticosteroid. J Med Primatol. 1977;6(3):145–150. doi: 10.1159/000459736. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54(5):396–402. doi: 10.1136/thx.54.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71(3-4):353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–455. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- Vanbesien-Mailliot CC, Wolowczuk I, Mairesse J, Viltart O, Delacre M, Khalife J, et al. Prenatal stress has pro-inflammatory consequences on the immune system in adult rats. Psychoneuroendocrinology. 2007;32(2):114–124. doi: 10.1016/j.psyneuen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Prenatal Stress and Life-Span Development. In: Friedman HS, editor. Encyclopedia of Mental Health. Vol. 3. Academic Press; San Diego: 1998. pp. 265–280. [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595(2):195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Niven H, Parker G, Hadzi-Pavlovic D. The stability of the Parental Bonding Instrument over a 20-year period. Psychol Med. 2005;35(3):387–393. doi: 10.1017/s0033291704003538. [DOI] [PubMed] [Google Scholar]