Abstract
Purpose
A critical early step in the establishment of Escherichia coli pyelonephritis is bacterial attachment via the tip protein of P fimbriae. This adhesin, PapG, binds to glycolipid receptors present on vaginal and kidney epithelial surfaces. In this study we investigated the efficacy of vaccination with purified PapDG protein complex in preventing pyelonephritis caused by E. coli.
Materials and Methods
Mature cynomolgus monkeys were intraperitoneally vaccinated with 100 μg purified PapDG protein. Following 3 identical boosters serum antibody titers to PapDG were measured by enzyme-linked immunosorbent assay. Vaccinated and unvaccinated animals were urethrally inoculated with 1 × 108 cfu of E. coli strain DS17, which was isolated from a child with acute pyelonephritis. The infection course was monitored by catheterized urine cultures, and by histological examination of the kidneys, bladder and kidney tissue culture 28 days after infection.
Results
Intraperitoneal administration of purified PapDG vaccine resulted in the development of specific antibody responses in cynomolgus monkeys. In contrast to control monkeys, vaccinated monkeys did not show histological evidence of pyelonephritis after subsequent urethral challenge with pyelonephritogenic E. coli expressing P fimbriae.
Conclusions
Purified PapDG is a tractable vaccine candidate that in our small study demonstrated the ability to elicit adequate serum antibody levels to prevent E. coli mediated pyelonephritis.
Keywords: kidney, pyelonephritis, bacterial vaccines, fimbriae proteins, Macaca fascicularis
The singular importance of bacterial adhesion to uroepithelial cells in the pathogenesis of urinary tract infection was clearly established during the last 25 years.1–4 The binding of P fimbriae of Escherichia coli to receptors in the kidney is essential for the initiation of pyelonephritis.5 P fimbriae adhere to Gal-α-1,4-Gal containing receptors expressed by erythrocytes as well as by cells from the urinary tract of primates, including humans.6,7 Antibodies to P fimbriae prevented adherence of P fimbriated E. coli to cultured uroepithelial cells and a purified bacterial P fimbrial vaccine decreased bacteriuria and protected against pyelonephritis in adult monkeys following urethral bacterial inoculation.8 Infant monkeys of mothers vaccinated with P fimbriae during pregnancy were protected from pyelonephritis when these infants were given a bladder inoculation of E. coli.9
P fimbriae are composite structures, each composed of a rigid fiber made up of the major subunit protein PapA and terminating in a flexible tip fibrillum.10 The periplasmic chaperone PapD ferries pilus subunits via the outer membrane usher PapC to the developing pilus. The adhesin PapG, located at the distal tip of the pilus, is specific for 1 of 3 types of globoside receptors present in the urothelium.11,12 Most human pyelonephritic isolates of E. coli express P fimbriae of class G-II, which bind to globotriaosylceramide (GbO4) receptors in the primate kidney.13 Isogenic papG mutants lacking the tip adhesin were unable to cause pyelonephritis in a monkey model system.5 Therefore, we hypothesized that vaccination of primates against PapGII would provide protection against bacterial colonization of the kidney by uropathogenic E. coli, thereby, preventing pyelonephritis. In the current study PapG conjugated to its chaperone PapD was used in a vaccination strategy in cynomolgus monkeys. After a schedule of intraperitoneal inoculations with PapDG vaccine immunized monkeys showed protective humoral antibodies against P fimbrial components. Vaccinated monkeys did not show histological evidence of pyelonephritis following subsequent urethral challenge with uropathogenic P fimbriated E. coli.
Materials and Methods
Animal care
Adult female cynomolgus monkeys (Macaca fascicularis) were arbitrarily divided into 2 groups, including 5 in the control group and 6 in the experimental group. They were housed individually and given free access to water and food. Experimental procedures were performed with the animals under ketamine anesthesia.
Preparation of vaccines
PapD and PapG were expressed from E. coli strain C600 transformed with plasmids pLS101 (papD) and ptrCGII (papGII). PapDG complexes were purified in an equimolar ratio from periplasmic fractions and purified to greater than 99%, as previously described.14 The PapDG protein complex was dissolved in aluminum phosphate solution (as an adjuvant) adjusted to a pH of 7.5 with sodium acetate.
Experimental infection
The E. coli strain DS17 (O6:K5: H−), which was isolated from a child with acute pyelonephritis, expresses P fimbriae of class II.15 Experimental monkeys were inoculated with 100 μ1 of a suspension containing 1 × 109 E. coli DS17/ml by urethral catheterization. The catheter was slowly inserted until bladder urine flowed and then withdrawn 1 cm to inoculate the urethra.
Immunology and microbiology
Blood was taken monthly for antibody studies during vaccination programs, and weekly for antibody studies and leukocyte counts after bacterial challenge. After vaccination serum antibody titers were determined using enzyme-linked immunosorbent assay (ELISA) with PapDG protein as the coating antigen. Following infection urine samples were obtained weekly by catheter, plated to blood agar and incubated at 37C to monitor for bacteriuria.
Pathological evaluation
At 28 days after infection animals were given an overdose of barbiturates, and the kidneys, ureters, bladder and urethra were collected aseptically. The kidneys were weighed and samples were taken for culture and histological examination. A standard section was taken through the mid portion to contain the cortex, medulla and papilla. Cross sections of the ureter and bladder were taken. Tissues were fixed in buffered formalin before hematoxylin and eosin staining. Slides were evaluated by a veterinary pathologist blinded to monkey vaccination status. Acute pyelonephritis is associated with a marked inflammatory exudate in areas of bacterial growth, tubular damage and death, and microabscess formation. The reparative response includes fibrosis, scarring and mononuclear cell infiltrate, especially in the subcapsular, pelvic and periglomerular regions. These findings are typical following untreated infection in our experimental model and they were considered to indicate chronic pyelonephritis.5,8,9,13,16,17 The kidney cortex and medulla were graded separately and scored as 0 to 4 with 4 being the most severe. Since this infection was urethral, the scores of the left and right kidneys were combined before comparing pathological findings in the control and vaccinated groups.
Statistics
Data were analyzed using Statistica software (Statsoft, Tulsa, Oklahoma). Pathology scores were subjected to a nonparametric 2 × 2 chi-square test. Bacteriuria was evaluated by multiple chi-square log linear scoring and antibody titers were assessed by ANOVA.
Results
Antibody responses to native infection with P fimbriated E. coli
The antibody response to specific bacterial components after pyelonephritis was measured in a cynomolgus monkey model system. After the induction of satisfactory anesthesia control monkeys (vaccinated with phosphate buffered saline) were infected intraurethrally with 100 μ1 of a suspension of 109 E. coli DS17/ml. Antibody titers to PapDG and to whole E. coli DS17 P fimbriae were measured by ELISA at multiple time points. P fimbriae were isolated and purified from E. coli, as previously described.8 There was no detectable IgA response in serum or urine. Serum IgG antibody titers to PapDG in unvaccinated monkeys ranged from 0 to 1:8,000 before infection. Three weeks following infection titers had increased in 4 of 5 monkeys, now ranging from 1:2,000 to 1:256,000 (fig. 1, A). Similarly, serum IgG antibody titers to whole DS17 P fimbriae ranged from 1:2,000 to 1:16,000 before infection and from 1:8,000 to 1:256,000, 3 weeks after infection (fig. 1, B).
Fig. 1.
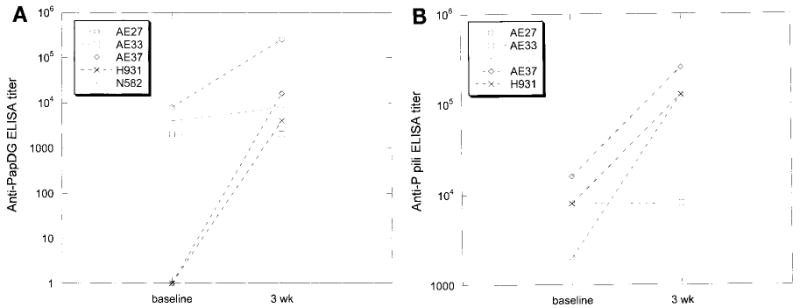
Unimmunized monkeys showed antibodies to PapDG and to whole P fimbriae after native E. coli infection. A, serum antipapDG titers before and 3 weeks following urethral infection with 108 cfu DS17 E. coli on ELISA. B, serum antiP fimbriae titers before and 3 weeks following same infection.
Responses to vaccination
PapG interaction with globo-series glycolipids in the kidney is critical for the initiation of E. coli pyelonephritis and many commensal E. coli strains do not express P fimbriae. Therefore, we hypothesized that PapG adhesin would be immunogenic and offer protection against pyelonephritis. Based on previous vaccination protocols we elected to begin vaccinations with a single intraperitoneal inoculation of 100 μg purified PapDG protein. One month following this injection serum antiPapDG IgG titers ranged from 1:800 to 1:3,200 (fig. 2). After a 6-month hiatus experimental monkeys received additional monthly intraperitoneal injections (100 μg) of PapDG vaccine for 3 months. Following this booster regimen serum antiPapDG IgG titers ranged from 1:2,000 to 1:128,000 (fig. 2). Thus, antiPapDG IgG serum antibody responses to the vaccine were measurable and comparable to those seen after primary infection in unvaccinated animals. These increases in antibody levels were significant at the 5% level on ANOVA.
Fig. 2.
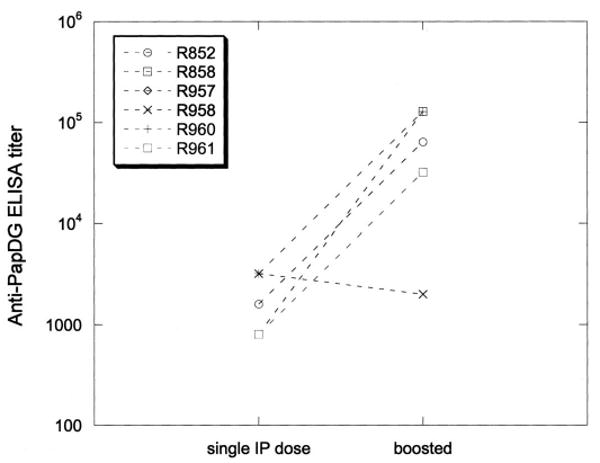
Five of 6 vaccinated monkeys demonstrated increases in antiPapDG titer after 3 monthly booster doses of PapDG vaccine. IP, intraperitoneal.
Efficacy of the PapDG vaccine
Urine samples were obtained aseptically 0, 2, 7, 14, 21 and 28 days after infection. These samples were titered by plating serial dilutions on blood agar plates and enumerating colonies after overnight incubation. There was no significant difference in the proportion of control and immunized monkeys that were bacteriuric at any of the examined time points. In addition, control and vaccinated monkeys demonstrated an increase in peripheral blood leukocyte counts following infection with no significant difference between the groups (data not shown).
Following sacrifice at 28 days after infection the kidneys were cultured and found to be sterile in control and vaccinated animals. A standard section of the mid portion of each kidney was fixed and stained with hematoxylin and eosin. The cortex and medulla were scored separately on the previously described scale of 0 to 4. The table shows the number and proportion of histological sections with a score of 1 or greater for each pathological finding. Compared to vaccinated monkeys a significantly higher proportion of sections from control monkeys demonstrated neutrophilic infiltrate, scarring and interstitial fibrosis. Overall these histological changes suggesting pyelonephritis were observed in 22% to 33% of the sections from control monkeys and they were not observed in any sections from vaccinated monkeys. A mononuclear infiltrate, especially in the pelvic area, was seen in control and vaccinated monkeys but there was a trend toward more extensive mononuclear infiltrate in control monkeys (p not significant).
Histological examination of kidney sections from control and immunized monkeys after urethral E. coli infection.
| Finding | No. Control/Total No. (%) | No. Immunized/Total No. (%) | p Value |
|---|---|---|---|
| Neutrophils: | |||
| Tubular | 4/18 (22) | 0 | 0.007 |
| Interstitial | 4/18 (22) | 0 | 0.007 |
| Mononuclear cells | 15/18 (83) | 11/24 (46) | Not significant |
| Interstitial fibrosis | 4/18 (22) | 0 | 0.007 |
| Scarring | 4/18 (22) | 0 | 0.007 |
| Glomerular involvement | 2/9 (22) | 0 | Not significant |
| Subcapsular involvement | 3/9 (33) | 0 | Not significant |
| Tubular atrophy | 4/18 (22) | 0 | 0.007 |
| Tubular dilatation | 2/18 (11) | 0 | 0.04 |
Discussion
PapG adhesin is assembled on the distal end of P fimbriae and it mediates attachment to specific sugar moieties present in kidney tissue.10 The glycolipids to which PapGII binds are found in humans and primates, arguing that the primate model system used in this study is valid.6,7 In a previous study the pyelonephritic E. coli strain DS17 with a mutation in papG was not able to colonize effectively or cause inflammation in the kidney.5 Previous studies have shown that antibodies to P fimbriae inhibit the binding of E. coli to uroepithelial cells, suggesting a critical role for microbial adherence and in particular for PapG in the pathogenesis of human kidney infections.8 The current finding that a PapDG vaccination regimen offered protection against pyelonephritis is consistent with this important role of PapG mediated adherence during kidney infection.
In the current study monkeys vaccinated by intraperitoneal administration of purified PapDG protein showed high IgG serum titers to PapDG. AntiPapDG titers following vaccination were in the range of those seen in naïve monkeys after E. coli urinary tract infection. Vaccinated monkeys were protected from pyelonephritis, as judged by a review of histological kidney sections.
After infection there were no differences between control and vaccinated animals in peripheral leukocyte counts or bacteriuria. Since bacterial adhesion may occur via several mechanisms, it is not surprising. Indeed, adhesion to uroepithelial cells by hydrophobic interaction, type 1 fimbriae and nonfimbrial adhesins has been shown to occur in vivo and in vitro.13,18 It is the probable explanation of continuing bacteriuria in the vaccinated monkeys in the current study as well as in previous studies of infection with E. coli DS17 papG deletion mutants.5
Identifying attractive vaccine targets involves consideration of many factors. 1) A candidate antigen must be immunogenic. Our vaccination protocol using purified PapDG resulted in robust serum antibody titers against PapDG complex. 2) An optimal vaccine must target a bacterial factor important for virulence and PapG adhesin is firmly established in this regard. 3) By selecting an antigen expressed only by pathogenic organisms one can avoid disturbance of the commensal flora, such as E. coli resident in the primate gut. Our results support the important function of PapG in the pathogenesis of kidney infection and indicate that PapG merits further study as a candidate vaccine for pyelonephritis.
Acknowledgments
Supported by United States Public Health Service Grants DK146818, RR00164, DK64540, DK51406 and F32 A110502-01A1.
References
- 1.Vaisanen V, Elo J, Tallgren LG, Siitonen A, Makela PH, Svanborg-Eden C, et al. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981;2:1366. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- 2.Svanborg Eden C, Hagberg L, Leffler H, Lomberg H. Recent progress in the understanding of the role of bacterial adhesion in the pathogenesis of urinary tract infection. Infection. 1982;10:327. doi: 10.1007/BF01640890. [DOI] [PubMed] [Google Scholar]
- 3.Korhonen TK, Virkola R, Westurlund B, Holthofer H, Parkkinen J. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr Top Microbiol Immunol. 1990;151:115. doi: 10.1007/978-3-642-74703-8_6. [DOI] [PubMed] [Google Scholar]
- 4.Otto G, Sandberg T, Marklund BI, Ulleryd P, Svanborg C. Virulence factors and pap genotype in Escherichia coli isolates from women with acute pyelonephritis, with or without bacteremia. Clin Infect Dis. 1993;17:448. doi: 10.1093/clinids/17.3.448. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, Marklund BI, Ilver D, Haslam D, Kaack MB, Baskin G, et al. The Gal(alpha1–4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc Natl Acad Sci USA. 1994;91:11889. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts JA, Kaack B, Källenius G, Möllby R, Winberg J, Svenson SB. Receptors for pyelonephritogenic Escherichia coli in primates. J Urol. 1984;131:163. doi: 10.1016/s0022-5347(17)50251-7. [DOI] [PubMed] [Google Scholar]
- 7.Kallenius G, Mollby R, Svenson SB, Winberg J, Hultberg H. Identification of a carbohydrate receptor recognized by uropathogenic Escherichia coli. Infection, suppl. 1980;8:S288. doi: 10.1007/BF01639597. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JA, Hardaway K, Kaack B, Fussell EN, Baskin G. Prevention of pyelonephritis by immunization with P-fimbriae. J Urol. 1984;131:602. doi: 10.1016/s0022-5347(17)50513-3. [DOI] [PubMed] [Google Scholar]
- 9.Kaack MB, Roberts JA, Baskin G, Patterson GM. Maternal immunization with P fimbriae for the prevention of neonatal pyelonephritis. Infect Immun. 1988;56:1. doi: 10.1128/iai.56.1.1-6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultgren SJ, Lindberg F, Magnusson G, Kihlberg J, Tennent JM, Normark S. The PapG adhesin of uropathogenic Escherichia coli contains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci USA. 1989;86:4357. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindberg F, Lund B, Johansson L, Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987;328:84. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- 12.Haslam DB, Boren T, Falk P, Ilver D, Chou A, Xu Z, et al. The amino-terminal domain of the P-pilus adhesin determines receptor specificity. Mol Microbiol. 1994;14:399. doi: 10.1111/j.1365-2958.1994.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JA, Kaack MB, Baskin G, Marklund BI, Normark S. Epitopes of the P-fimbrial adhesin of E. coli cause different urinary tract infections. J Urol. 1997;158:1610. [PubMed] [Google Scholar]
- 14.Slonim LN, Pinkner JS, Branden CI, Hultgren SJ. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 1992;11:4747. doi: 10.1002/j.1460-2075.1992.tb05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tullus K, Horlin K, Svenson SB, Kallenius G. Epidemic outbreaks of acute pyelonephritis caused by nosocomial spread of P fimbriated Escherichia coli in children. J Infect Dis. 1984;150:728. doi: 10.1093/infdis/150.5.728. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JA, Kaack MB, Baskin G. Treatment of experimental pyelonephritis in the monkey. J Urol. 1990;143:150. doi: 10.1016/s0022-5347(17)39900-7. [DOI] [PubMed] [Google Scholar]
- 17.Patton JP, Nash DB, Abrutyn E. Urinary tract infection: economic considerations. Med Clin North Am. 1991;75:495. doi: 10.1016/s0025-7125(16)30466-7. [DOI] [PubMed] [Google Scholar]
- 18.Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, et al. Induction and evasion of host defenses by type 1 piliated uropathogenic Escherichia coli. Science. 1998;282:1494. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]


