Abstract
Amyloids have traditionally been associated with misfolded protein aggregates and debilitating neurodegenerative diseases. However, a growing number of functional amyloids have now been described that demonstrate that amyloid formation can be an integral part of normal cellular physiology. Functional amyloid production is highly regulated, and the resulting fibers serve a variety of roles for the cells that produce them. A new role for amyloid as storage reservoirs for peptide hormones within mammalian secretory granules has been discovered. More than 30 different peptide hormones have been found to form amyloids in vitro, and both rats and mice have been shown to store hormone amyloid deposits in secretory granules. Thus, the emerging evidence adds to the diverse roles of amyloid and raises intriguing questions for both the peptide hormone and the functional amyloid fields.
The amyloid protein fold is characterized by ordered β-sheet repeats arranged in a fibrous structure in which the β strands orient perpendicular to the fiber axis. The name amyloid derives from the original characterization by Virchow, who observed structures that stained with iodine similar to starch (amylum in Latin) (1). The conserved amyloid structure creates a remarkably stable protein fold that is resistant to heat and chemical treatments that normally dismantle soluble proteins. Amyloids have several distinct biochemical properties, such as causing birefringence of the dye Congo red (CR) and a spectral shift of the dye thioflavin T (ThT) (2, 3). They also exhibit characteristic cross–β sheet x-ray diffraction patterns. Amyloids are thought to assemble through a conserved pathway, with soluble monomers forming ordered oligomeric intermediate structures and finally, fibers. It has been demonstrated that, under optimal conditions, many proteins can aggregate into amyloids, suggesting that amyloid formation is mediated, at least in part, by peptide backbone interactions (4).
Traditionally, amyloids have been associated with protein misfolding, cellular toxicity, and neurodegenerative diseases such as Alzheimer's and Parkinson's (5). However, several functional amyloids have been discovered that contribute to cellular biology without causing measurable cytotoxicity. Unlike disease-associated amyloids, functional amyloids are the product of coordinated and regulated cellular processes that ensure that amyloidogenesis does not result in cell damage and death (6, 7). Functional amyloids were first described in microbes, although they have now been found in many organisms, including humans (8–10) (Table 1). Not only do functional amyloids perform key physiological functions in the cell, they also provide a unique perspective from which to understand protein homeostasis, folding, and misfolding. One of the best-understood functional amyloid assembly systems is curli, which are extracellular amyloids produced by several bacterial species, including Escherichia coli and Salmonella spp. (11). Curli are critical for biofilm formation and are thought to contribute to bacterial pathogenesis (12–14). In curli biogenesis, an amyloidogenic major subunit protein is nucleated into a fiber on the cell surface by a membrane-anchored minor subunit protein that acts as an amyloid-like template for the major subunit (15). Bacteria assemble several other functional amyloids, including the chaplins produced by Streptomyces coelicolor to aid in hyphae formation and spore dispersal (16, 17).
Table 1.
Several eukaryotic functional amyloids have also been described, including functional prion-like proteins produced by yeast and the mammalian protein Pmel17, which assists in melanin production (10, 18, 19). However, our appreciation of the diversity of functional amyloids took a giant leap forward with the discovery by Maji and co-workers (20) that peptide hormones can be stored as amyloids in secretory granules (Fig. 1). Maji et al. identified over 30 human peptide hormones that are stored as amyloids in secretory granules. Found in neuroendocrine and exocrine cells, secretory granules are the home of highly concentrated protein hormones (21). The densely packed cores of secretory granules were previously shown to consist of protein aggregates with distinct protein structure (22). Maji and colleagues now provide evidence that secretory granules store concentrated hormones in an amyloid conformation.
Fig. 1.
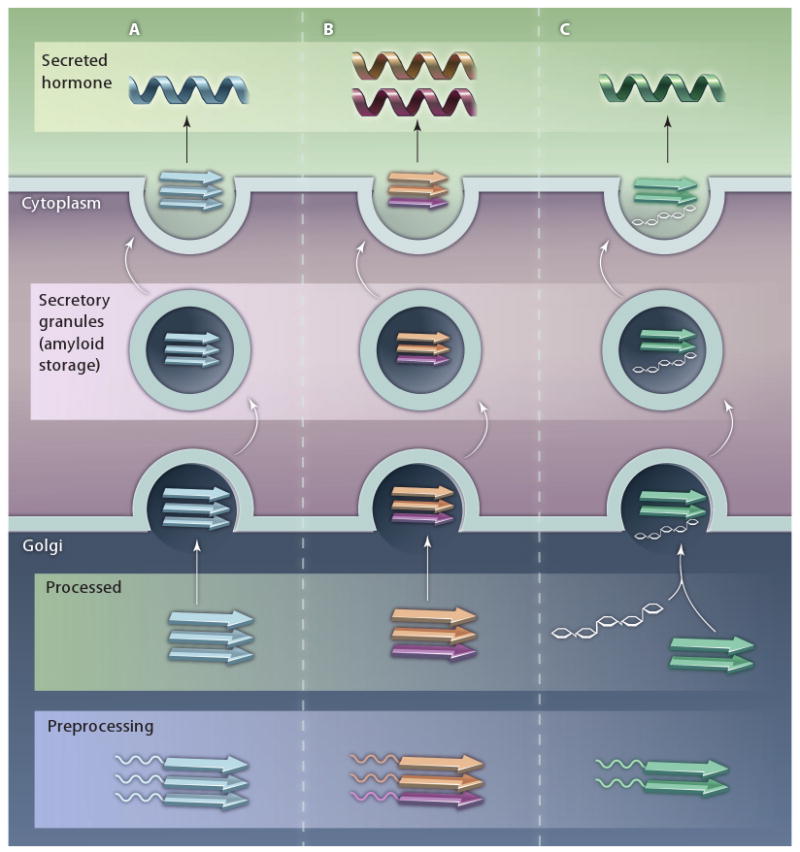
Amyloid storage of peptide hormones. Maji et al. found that 31 out of 42 peptide hormones fold into an amyloid configuration in vitro. From an in vivo perspective, secretory granules purified from AtT20 cells and rat pituitary contained peptide hormones in an amyloid-like structure. Moreover, immunostaining of mouse pituitary with several peptide hormones found that the peptide signal colocalized with the amyloid-specific dye thioflavin-S. Their results suggest a model where peptide hormones are stored in the secretory granules as amyloid fibers. Some peptide hormones form amyloid fibrils spontaneously (A), whereas other peptides form amyloid fibrils when coincubated with another peptide hormone (B) or with GAGs (C). Upon hormone release, the amyloid fibers are broken down by an unknown mechanism to soluble peptides, which are then secreted.
Maji et al. demonstrated the amyloid nature of peptide hormones by using a series of in vitro and in vivo techniques. In vitro amyloid formation was initially detected in only 10 of 42 peptide hormones. However, Maji and co-workers better mimicked in vivo conditions by adding glycosaminoglycans (GAGs) to their in vitro polymerization reactions and found that 31 peptides were then able to form amyloid fibers. GAGs have been previously shown to accelerate amyloid fiber formation, possibly by serving as a template for fiber assembly. A fascinating finding was that two peptides, adrenocorticotropic hormone and β-endorphin, which are processed in the same biosynthetic pathway, were unable to form amyloid on their own. However, these two hormones readily polymerized into amyloid fibrils when incubated together. Additional immunohistochemistry experiments using the AtT20 mouse pituitary cell line demonstrated that adrenocorticotropic hormone and β-endorphin colocalized, implying these hormones are necessary for amyloid fiber formation.
Although the storage of peptide hormones as amyloids seems like an ingenious method for stable packing of potentially aggregating peptides, the densely packed hormones must be able to quickly release active monomers outside the cell. The authors addressed this question by demonstrating that biologically active hormones are released from amyloid fibers. The released monomers contain β-sheet secondary structures but are not as toxic as monomers released from disease-associated amyloids such as β-amyloid (Aβ) (23).
The authors not only characterized the amyloid properties of these peptides in vitro, but also provided direct evidence that secretory granules in vivo contain hormones in the amyloid form. AtT20 cells could make secretory granules that contained hormones in an amyloid state, as measured by an amyloid-specific antibody (24), CR staining, and ThT binding. Also, secretory granules purified from rat pituitary glands and immunohistochemical staining of mouse pituitary tissue showed the presence of hormones in amyloid form. There was almost complete colocalization between thioflavin S, another amyloid-specific dye, and several hormone-specific antibodies, strongly implying that these secretory granules isolated from mammals are composed of hormones stored in an amyloid configuration.
The results presented by Maji et al. add another chapter to the rapidly growing story of functional amyloids. The use of the amyloid configuration for storage of highly concentrated peptide hormones in densely packed aggregates is reasonable, considering the stability that the amyloid structure might afford peptide hormones. However, the presence of large amyloid deposits in human tissues and organs is still somewhat difficult to rationalize, given the extensive neuronal damage caused by amyloids associated with Alzheimer's and Parkinson's diseases. Because proper protein folding and cellular protein homeostasis must be maintained, the cell must have exquisite control of functional amyloid formation. This control is achieved by a number of mechanisms. First, protein hormones must be cleaved from a prohormone or require additional molecules to initiate amyloid packing, allowing the cell greater control of the timing of this process. Second, these amyloids are sequestered in secretory granules and are released only upon stimulation. Third, the results presented by Maji et al. also raise the intriguing possibility that hormone amyloids are slightly less toxic than those associated with disease. Therefore, functional amyloids are not necessarily incongruent with the amyloid hypothesis, which suggests that amyloidogenesis is cytotoxic. Functional amyloids may have simply evolved to be spatially and temporally controlled by cellular machinery so as to minimize the opportunity for interactions with membranes or other cellular components that may lead to toxicity. Understanding the similarities and differences between functional and disease-associated amyloids will undoubtedly provide unique insights into the development of next-generation amyloid therapies.
The findings by Maji et al. are not the only observations that link amyloidogenesis and secretory vesicles. The Aβ peptide precursor can also be cleaved in vesicles within cells (25). Additionally, islet amyloid polypeptide (IAPP) is known to form amyloid fibrils in β-secretory granules, which is the underlying cause of type II diabetes (26). There is even a pharmacological link between secretory granules and amyloidogenesis, as the antimalarial drug chloroquine and a polyphenol derived from tumeric, curcumin, can inhibit secretion of hormones and amyloid formation (27–29). However, the physiological relation between functional and disease-associated amyloidogenesis remains unresolved and ripe for future investigations. Because amyloidogenesis can have cytotoxic consequences if it occurs at the wrong time or place, understanding the fate of newly released soluble peptide hormones will also be important. Elucidating the dynamic structural aspects of the hormone as it is released from the secretory vesicle and moves into the bloodstream will add to our knowledge of hormone protein folding and its relationship to cellular signaling. This work has provided exciting new evidence for how a functional amyloid can be used to store peptide hormones and enhances the understanding of both hormone storage and functional amyloid fields.
References
- 1.Otzen D, Nielsen PH. We find them here, we find them there: Functional bacterial amyloid. Cell Mol Life Sci. 2008;65:910–927. doi: 10.1007/s00018-007-7404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sipe JD, Cohen AS. Review: History of the amyloid fibril. J Struct Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 3.Ghiso J, Frangione B. Amyloidosis and Alzheimer's disease. Adv Drug Deliv Rev. 2002;54:1539–1551. doi: 10.1016/s0169-409x(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 4.Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, Dobson CM. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci USA. 1999;96:3590–3594. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: The molecular basis for Alzheimer's and Parkinson's diseases. Mol Med. 2008;14:451–464. doi: 10.2119/2007-00100.Irvine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer ND, Wang X, McGuffie BA, Chapman MR. Amyloids: Friend or foe? J Alzheimers Dis. 2008;13:407–419. doi: 10.3233/jad-2008-13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid–from bacteria to humans. Trends Biochem Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.True HL, Lindquist SL. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- 9.Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: Involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian Z, Brauner A, Li Y, Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181:602–612. doi: 10.1086/315233. [DOI] [PubMed] [Google Scholar]
- 14.Tukel C, Raffatellu M, Humphries AD, Wilson RP, Andrews-Polymenis HL, Gull T, Figueiredo JF, Wong MH, Michelsen KS, Akcelik M, Adams LG, Baumler AJ. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol Microbiol. 2005;58:289–304. doi: 10.1111/j.1365-2958.2005.04825.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Hammer ND, Chapman MR. The molecular basis of functional bacterial amyloid polymerization and nucleation. J Biol Chem. 2008;283:21530–21539. doi: 10.1074/jbc.M800466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot NJ. Aerial morphogenesis: Enter the chaplins. Curr Biol. 2003;13:R696–R698. doi: 10.1016/j.cub.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 17.Elliot MA, Karoonuthaisiri N, Huang J, Bibb MJ, Cohen SN, Kao CM, Buttner MJ. The chaplins: A family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 2003;17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickner RB, Edskes HK, Shewmaker F, Nakayashiki T. Prions of fungi: Inherited structures and biological roles. Nat Rev Microbiol. 2007;5:611–618. doi: 10.1038/nrmicro1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KPR, Simon R, Schubert D, Eisenberg D, Rivier J, Sawchenko P, Vale W, Riek R. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dannies PS. Protein hormone storage in secretory granules: Mechanisms for concentration and sorting. Endocr Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- 22.Keeler C, Hodsdon ME, Dannies PS. Is there structural specificity in the reversible protein aggregates that are stored in secretory granules? J Mol Neurosci. 2004;22:43–49. doi: 10.1385/JMN:22:1-2:43. [DOI] [PubMed] [Google Scholar]
- 23.Finder VH, Glockshuber R. Amyloid-beta aggregation. Neurodegener Dis. 2007;4:13–27. doi: 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- 24.Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selkoe DJ. Cell biology of protein misfolding: The examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 26.Marzban L, Park K, Verchere CB. Exp Gerontol. 2003;38:347–351. doi: 10.1016/s0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 27.Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci USA. 2001;98:9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore HP, Gumbiner B, Kelly RB. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature. 1983;302:434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 30.Epstein EA, Chapman MR. Polymerizing the fibre between bacteria and host cells: The biogenesis of functional amyloid fibres. Cell Microbiol. 2008;10:1413–1420. doi: 10.1111/j.1462-5822.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Destoumieux-Garzon D, Thomas X, Santamaria M, Goulard C, Barthelemy M, Boscher B, Bessin Y, Molle G, Pons AM, Letellier L, Peduzzi J, Rebuffat S. Microcin E492 antibacterial activity: Evidence for a TonB-dependent inner membrane permeabilization on Escherichia coli. Mol Microbiol. 2003;49:1031–1041. doi: 10.1046/j.1365-2958.2003.03610.x. [DOI] [PubMed] [Google Scholar]
- 32.Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FG, Dijkhuizen L, Wosten HA. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 2003;17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh J, Kim JG, Jeon E, Yoo CH, Moon JS, Rhee S, Hwang I. Amyloidogenesis of type III-dependent harpins from plant pathogenic bacteria. J Biol Chem. 2007;282:13601–13609. doi: 10.1074/jbc.M602576200. [DOI] [PubMed] [Google Scholar]
- 34.Baxa U, Cheng N, Winkler DC, Chiu TK, Davies DR, Sharma D, Inouye H, Kirschner DA, Wickner RB, Steven AC. Filaments of the Ure2p prion protein have a cross-beta core structure. J Struct Biol. 2005;150:170–179. doi: 10.1016/j.jsb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Wosten HA, de Vocht ML. Hydrophobins, the fungal coat unravelled. Biochim Biophys Acta. 2000;1469:79–86. doi: 10.1016/s0304-4157(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 36.Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci USA. 1997;94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]


