Abstract
The cytoplasmic tail of the amyloid precursor protein (APP) contains two putatively cytotoxic peptides, Jcasp and C31, derived by caspase cleavage of APP. Jcasp is a fragment starting from the ε-secretase site to position 664, while C31 is a fragment from position 665 to the C-terminus. Our studies now showed that compared to C31, Jcasp appeared to play a minor role in cytotoxicity. In particular, inhibition of Jcasp generation by treatment of γ-secretase inhibitor did not lead to any attenuation of C31 induced toxicity. Secondly, because C31 toxicity is largely absent in cells lacking endogenous APP, we determined, using a split β-galactosidase complementary assay to monitor protein-protein interactions, the presence of APP associated complexes. Our results demonstrated that both APP homomeric and C31/APP heteromeric complexes were correlated with cell death, indicating that C31 complexes with APP to recruit the interacting partners that initiate the signals related to cellular toxicity.
Keywords: Amyloid precursor protein, Asp-664 cleavage, C31, cytotoxicity, Jcasp, caspase
Alzheimer’s disease (AD) is the most common age-associated neurodegenerative disease and is characterized by the progressive accumulation of amyloid β-protein (Aβ) in brain, a process that is considered to play an important and potentially causal role in the pathogenesis of AD [1]. Although deposition of amyloid in senile plaques is a hallmark of AD, it is synapse loss and neuronal death that likely represent the basis of cognitive impairment in AD [2]. At present, the causes of these changes are not known, but it has been hypothesized that the presence of both extra- and intracellular Aβ may play an important role in neuronal loss and synaptic alterations [1]. Nevertheless, how Aβ induces these changes in the brain is unclear. Recently, others and we have proposed that cleavage of amyloid precursor protein (APP) at the aspartate residue at position 664 (APP 695 numbering) mediated by caspases or a caspase-like protease is another mechanism of cell toxicity in AD. Specifically, we have proposed that in this pathway, release of the C-terminal 31 amino acid peptide, termed C31, following cleavage at Asp664 activates various cell death pathways [3]. Interestingly, Aβ also facilitates this cleavage pathway and we have hypothesized that Aβ enhances the release of C31 from APP by promoting the dimerization of APP at the cell surface [4]. Apparent In vivo support for this pathway was demonstrated by the finding of a relatively normal phenotype in transgenic mice with age-associated amyloid pathology that overexpress an APP transgene encoding the D664A mutation to prevent cleavage [5–6]. Therefore, the cytoplasmic domain of APP through release of C31 may represent another pathological pathway relevant to synapse loss and neuronal death in AD.
In addition to C31, the cytoplasmic domain contains at least two other death-inducing domains. It has been shown that the APP cytoplasmic region or the APP intracellular domain (AICD) following γ-secretase mediated cleavage at the ε-cleavage site (termed C50 hereon) can be pro-apoptosis [7–8]. Further, cleavage of C50 by caspases at position 664 releases not only C31 from the C-terminus but also a small peptide from the N-terminus, called Jcasp (from positions 649 to 664). Transduction of Jcasp into primary cultured neurons by fusion to a cell permeable peptide resulted in apoptosis that is dependent on the tyrosine residue at position 653 [9]. Subsequently, it was reported that Jcasp binds to SET protein and this interaction contributes to Jcasp induced neuronal death [10]. Importantly, the D664A mutation that prevented caspase mediated cleavage would theoretically abrogate the generation of both Jcasp and C31 [11–12], the former requiring an additional cleavage by γ-secretase to release the N-terminus. Consequently, in the studies with D664A mutation, it is not possible to conclusively implicate a role of C31 in cytotoxicity because Jcasp generation is also prevented at the same time. Therefore, one of the goals of this study was to examine the toxicity of both Jcasp and C31. Indeed, using comparable methods, we found that C31 rather than Jcasp is the major cytotoxic peptide in vitro.
An additional intriguing aspect of C31 mediated toxicity is the dependence on APP. That is, we noticed that C31 has an APP-dependent component such that in the absence of endogenous APP, expression of C31 does not induce any detectable cytotoxicity. The reason for this curious APP dependency is unclear. However, because we have proposed that dimerization of APP is one pathway that leads to the cleavage of APP at D664 and putatively the release of C31, we hypothesized that C31 toxicity might be initiated by the binding of C31 to the APP cytoplasmic domain. In this way, C31:APP heterodimers and APP:APP homodimers would represent the seminal event that activates the cell death signal, and the generation of C31 is a way by which this putative signaling pathway may be amplified. In this study, we provided direct evidence to support this hypothesis by correlating cell death to the formation of C31:APP heterodimers.
MATERIALS AND METHODS
Expression constructs
cDNAs encoding APP695, APP-C99 and APP695 deleted of the last 31 amino acid residues (APPΔC31) have been described previously [3]. Two constructs deleted of the Jcasp domains were engineered from full-length APP by PCR: APPΔJ1 is a 13 amino acid deletion (QYTSIHHGVVEVD) construct while APPΔJ2 is a 9 amino acid deletion (QYTSIHHGV) that preserves the VEVD caspase cleavage motif of the Jcasp fragment. From the latter construct, an additional C31 deletion construct was made (APPΔJ2ΔC31). EGFP was appended to the C-terminus of C31, C50, and Jcasp to stabilize these short polypeptides by subcloning into pEGFP-N3 (Clontech, Mountain View, CA). Caspase cleavage mutants were engineered in full length APP and C50 by substituting alanine for aspartate at amino acid 664 (D664A using APP695 numbering) by site-directed mutagenesis (Stratagene, La Jolla, CA). Jcasp-Y653D, in which Tyr-653 of APP695 in Jcasp-EGFP was mutated to Asp, was engineered by similar method.
Cell Culture, transfection and western blot
Mouse N2A neuroblastoma and rat B103 neuroblastoma cells were cultured in DMEM with 10% fetal bovine serum. Cells were transfected at 70–80% cell confluency using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations. Western blot analyses were performed as previously described [3]. To inhibit γ-secretase activity, the transfected cells were exposed to 10µM DAPT (N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester) in dimethylsulphoxide six hours after transfection
Detection of dimerization event
For quantification of APP dimerization, the β-galactosidase (β-gal) complementation assay was used. For this assay, various APP constructs were fused to either of the two β-gal deletion mutants (Δα or Δω) that were kindly provided by Dr. Helen Blau, Stanford University [13]. In this system, the proximity of both Δα and Δω mutants will reconstitute β-gal activity, but neither β-gal mutant alone will exhibit such activity. Therefore, this is an assay for protein-protein interaction, specifically, interaction of the non-β-gal portion of the chimeric proteins when they are both co-expressed in the same cell. Measurement of β-gal activity was carried out from cell lysates following transient transfection with a commercial colorimetric ELISA kit (Roche, Indianapolis, IN).
Antibodies
Polyclonal antibodies include: CT15, recognizing the C-terminal 15 amino acids of APP [4] and APP-α664 recognizing the neoepitope at position 664 after cleavage [14]. Monoclonal antibodies include anti-GFP (Stressgen, Victoria, Canada) and 6E10 detecting 1–16 sequence of human Aβ (Senetek, Maryland Heights, MO).
Cell death studies
Cells were transiently co-transfected with a GFP-expressing plasmid together with the respective APP-derived constructs (at 1:1 to 1:2 molar ratios of GFP:APP). Assessment of cell death was carried out as described [4] using either either ethidium homodimer (Molecular probes, Eugene, OR) or by Hoechst 33342 staining. Positively stained cells were scored as a percentage of total GFP-positive cells 48 hours after transfection. Four or more independent experiments were carried out and the results are expressed as averages of all experiments ±standard error. One way ANOVA with post-hoc Tukey was used for statistical analysis.
RESULTS AND DISCUSSION
C31 toxicity is dependent on APP
Previously, we observed that over-expression of APP was able to augment C31 toxicity [15]. We further noticed that in the absence of endogenous APP, C31 cytotoxicity was abolished, leading to the conclusion that there is an APP-dependent component of C31-induced toxicity. To determine whether this APP dependency extended to the β-secretase cleaved APP C-terminal fragment (C99), we transfected a C31 expression construct, alone or in combination with C99, into B103 rat neuroblastoma cells that do not express APP or APP-like proteins [16]. Consistent with our previous results with full length APP, the expression of C99 alone demonstrated moderate toxicity, and this was markedly amplified when C99 was co-expressed with C31 (Supp. Fig. 1). However, as shown before, C31 by itself was unable to induce any cytotoxicity.
C31 toxicity requires heterodimerization with C99 and intact an Asp-664 site
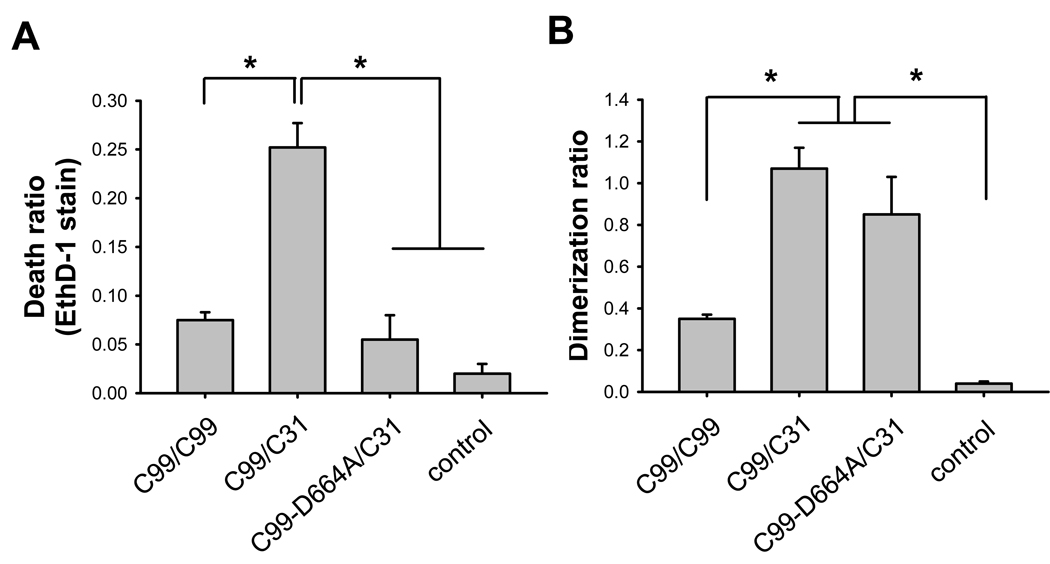
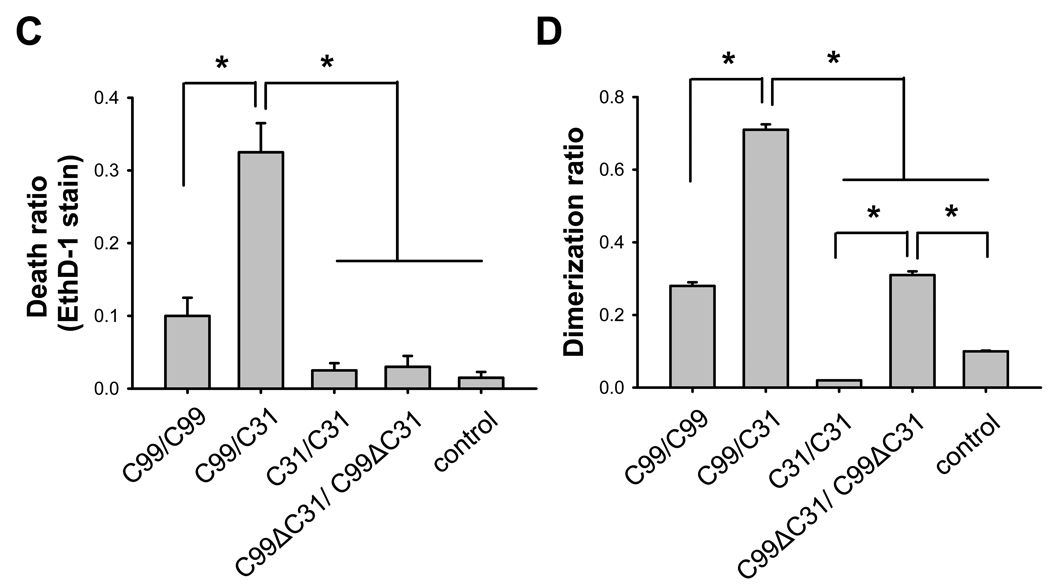
Having established the synergistic cytotoxic effects of C99 and C31 in transfected cells, we next turned to the question of whether dimerization can be detected and quantified in various C99-derived constructs with C31. We hypothesize that there is a direct interaction between APP (or C99) and C31 and that this interaction is a necessary step in C31-mediated toxicity. To test this hypothesis, we appended the β-gal complementing deletion mutants (Δα or Δω) to the C-termini of these APP constructs (Supp. Fig. 2). In this system, when two molecules containing the respective complementary β-gal deletion mutants interact with each other, then β-gal activity is reconstituted and represents a quantitative measure of the interaction of the non-β-gal portions of the chimeric proteins. Consequently, N2A cells were transfected with the respective paired constructs and 24 hours later, the cells were assayed in parallel for cell death and dimerization to determine the relationship between these two measures. Comparable levels of protein were expressed from all the constructs (data not shown). As expected, expression of differentially tagged C99 constructs led to increased β-gal activity over the control condition, representing homo-dimerization of C99; and further, transfection of these two C99 constructs was correlated with cell death (Fig. 1A–D). Interestingly, the highest β-gal activity was seen when C99 and C31 were co-expressed, and again this correlated with the highest level of cell death. This result suggested a direct interaction between C31 and C99. Interestingly, there was no apparent C31:C31 interaction, providing a possible explanation as to why C31 itself is not toxic in the absence of C99 or APP: because there is no propensity for C31 to homo-multimerize. Lastly, although as expected there was no cell death in the C99ΔC31 (C99 deleted of the last 31 amino acids) transfected cells, there was significant β-gal activity. This observation was not surprising, as other regions in APP have been shown to mediate APP dimerization and one such motif is the GXXXG sequence in the transmembrane domain that is present in C99ΔC31 [17]. Importantly, however, this result also suggested that dimerization itself is insufficient to initiate cell death in the absence of C31.
Fig. 1.
C31-induced toxicity in B103 cells is correlated with C31/C99 dimer formation. B103 cells were transiently transfected with control vector or APP-β-gal chimeric constructs based on C99, C31, C99-D664A, or C99-ΔC31. 24 hours after transfection, cells were assayed in parallel samples for cell death and β-gal activity. (A, C) Cell death was determined by ethidium homodimer (EthD-1, Live Dead Assay) staining. (B, D) Dimerization was determined by β-gal activity. Results showed that toxicity was limited to C99 or C99/C31 co-expression but not when C99-D664A or C99ΔC31 was expressed. However, elevated β-gal activity was seen with all C99 and C99-derived constructs even in the absence of cell death (*p < 0.001 one way ANOVA, post-hoc Tukey).
To relate the above findings to the cleavage of APP at the Asp-664 site, the experiments were repeated with a C99-D664A construct, in which mutation of the Asp to Ala residue prevented caspase mediated cleavage of C99. Interestingly, there was significant β-gal activity when the cells were co-transfected with C99-D664A and C31, indicating substantial heterodimerization to a level comparable to C99:C31 (Fig. 1B). However, cell death was comparable to control levels (Fig. 1A). This finding again suggested that Asp-664 is important for cytotoxicity, presumably through cleavage or protein-protein interaction, but not for dimerization.
Both C31 and Jcasp show cytotoxicity
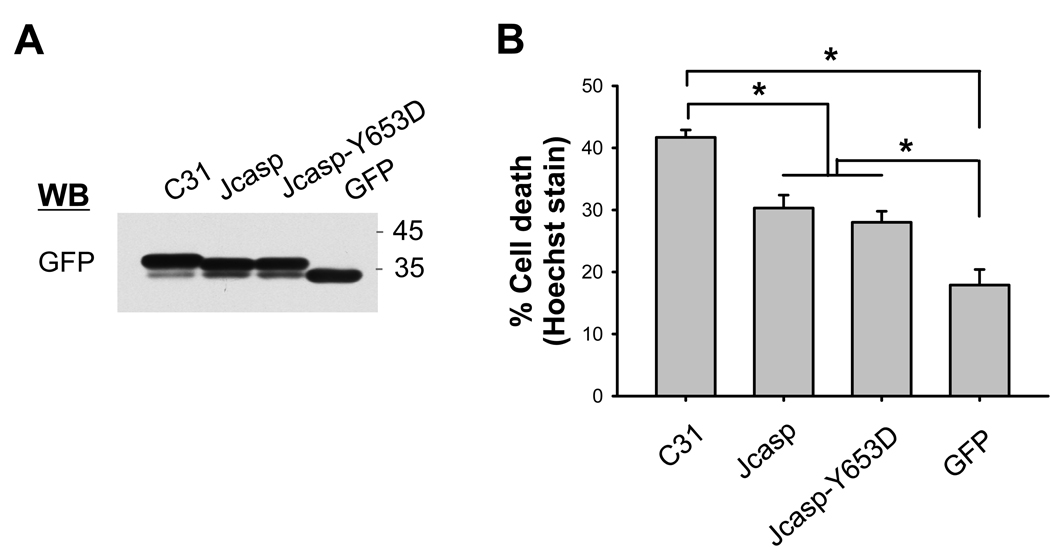
The cleavage at position 664 results in the release of not only C31, but also the short ~20 N-terminal amino acid polypeptide (termed Jcasp) following cleavage at the ε-secretase site. This latter cleavage mediated by γ-secretase releases the intact AICD if there is no cleavage at position 664. Thus, the D664A mutation abrogates the formation of both C31 and Jcasp. To examine the possible differential toxicity of these polypeptides, chimeric Jcasp and C31 proteins, fused to EGFP to stabilize the rapid turnover of these short peptides, were expressed in N2A cells. Following transfection into N2A cells, cytotoxicity was assessed by Hoechst or ethidium homodimer staining. In this in vitro culture system, although comparable levels of protein expression were seen (Fig. 2A), C31-GFP showed the highest level of toxicity in both assays (Fig. 2B and Suppl. Fig. 3A). Jcasp and Jcasp- Y653D mutation showed similar and significant levels of toxicity, but intermediate between C31 and GFP control transfection. Previously, Jcasp- Y653D was reported to show substantially reduced toxicity [9], but this was not evident in our study.
Fig. 2.
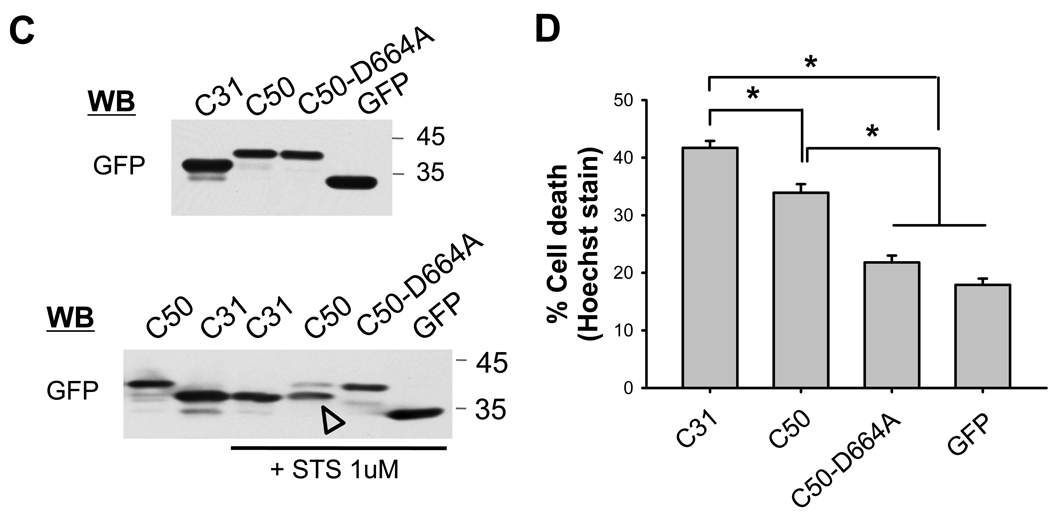
(A, B) Cytotoxicity following Jcasp and C31 expression. GFP-stabilized Jcasp or C31 fragments were expressed in N2A cells and assessed for cell death by Hoechst staining. (A) Western blot of transfected cell lysates using GFP antibody. (B) C31 showed the highest level of cell death, with Jcasp and Jcasp-Y653D peptides showing significant but lower levels of toxicity (*p ≤ 0.001). Cytotoxicity of APP C-terminal fragment was due primarily to C31. (C, D) N2A cells were transiently transfected with GFP-stabilized C31, C50, and C50-D664A mutant. (C) Western blots of transfected cell lysates using GFP antibody. After treatment with staurosporine (STS), C50 peptide, but not the C50-D664A mutant, was cleaved into a shorter fragment (arrowhead) that co-migrated with C31 (lower panel). The Hoechst staining (D) showed cytotoxicity following C31 and C50 expression. However, toxicity was lost in C50-D664A transfected cells (*p < 0.001).
To validate the importance of Asp-664 cleavage in cytotoxicity, we examined the APP AICD fragment, or C50, and compared this to a similar C50 construct with the aspartate mutation at position 664 (Fig. 2C). Similar to the Jcasp results above, C50 peptide was also cytotoxic but less than C31 expression. Further, C50-D664A showed no toxicity, again confirming the importance of the D664 residue (Fig. 2D and Supp. Fig. 3B). To confirm the ability of C50 to undergo further cleavage, the transfected cells were treated with staurosporine (1 µM) to induce cell death. As predicted, western blotting with a GFP antibody showed the expected cleavage of C50 but not C50-D664A construct (Fig. 2C). Taken together, these studies showed that while all the APP C-terminal cytoplasmic peptides can induce cytotoxicity when expressed in N2A cells, C31 showed the highest level of toxicity, with C50 and Jcasp showing similar, but lesser, degree of toxicity. These results also showed that the toxicity that has been ascribed to AICD is likely to arise from its ability to be proteolytically cleaved to produce C31 and Jcasp, not to full-length AICD itself [4,6].
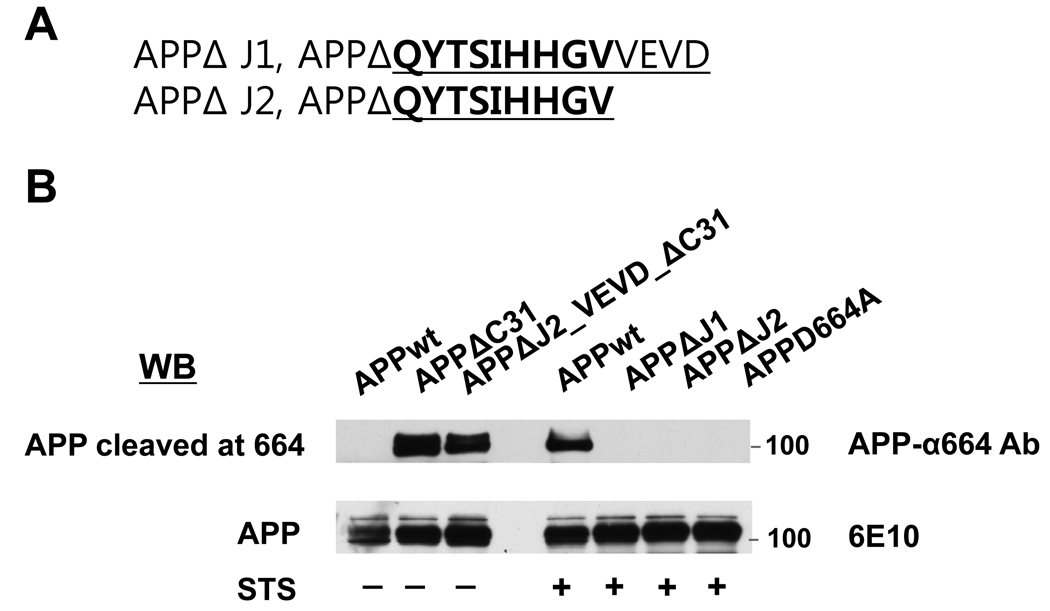
Deletion of Jcasp from APP695 reduced APP-dependent C31 cytotoxicity
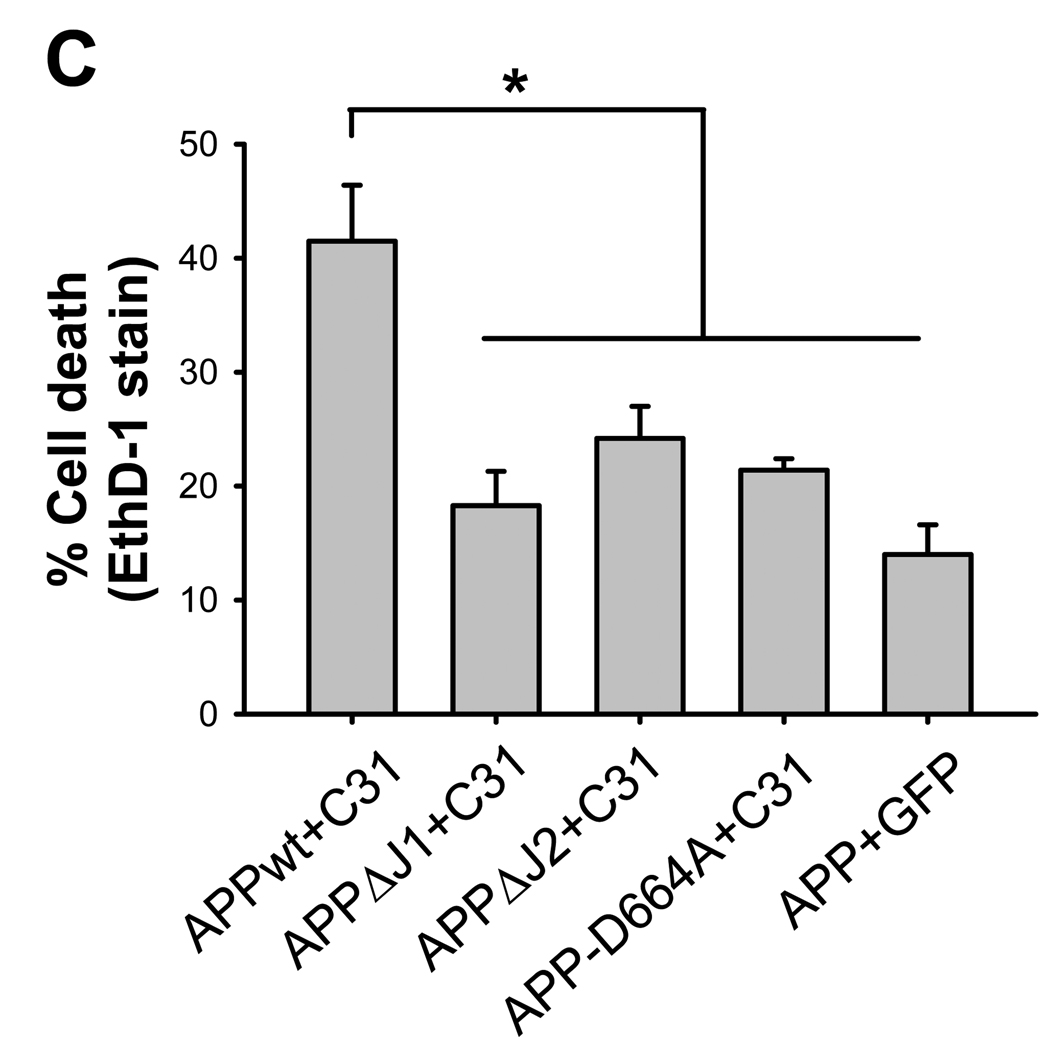
To address the potential toxicity of Jcasp further, we took advantage of the observation that APP can augments C31 toxicity [4,15]. In this experiment, we asked whether APP that is deleted of the Jcasp domain enhances C31 toxicity similar to full-length APP. We engineered two different Jcasp deleted APP constructs, APPΔJ1 and APPΔJ2, the latter representing a smaller deletion to preserve the VEVD caspase cleavage recognition site (Fig. 3A and B). From the latter construct, C31 was additionally deleted in a second construct, APPΔJ2ΔC31, designed as a positive control for antibody reactivity. When these various APP constructs were co-transfected with C31, we observed that APP without the Jcasp domain showed markedly reduced toxicity (Fig. 3C and Supp. Fig. 4). In fact, APPΔJ1 transfected with C31 showed essentially no toxicity, comparable to either APP-D664A or control, while APPΔJ2 together with C31 showed marginal toxicity only. At first glance, these results suggested that the Jcasp can contribute substantially to C31 toxicity. However, because deletion of the entire Jcasp domain would prevent caspase cleavage because the VEVD motif is lost, it essentially behaves like the D664A mutation and is not particularly informative. However, the smaller Jcasp deletion construct, APPΔJ2, contains the DEVD domain and should retain caspase cleavage. To assess this possibility, the transfected cells were treated with staurosporine and cleavage was assessed with the α664 antibody specific to cleaved APP. APPΔC31 was also expressed as a control for the antibody because this APP construct is deleted of the C-terminal 31 amino acids and thus resembles caspase-cleaved APP. As expected, APPΔC31 but not full length APP was immunoreactive to the α664 antibody (Fig. 3B). APPΔJ2ΔC31 was immunoreactive, perhaps slightly less than APPΔC31. However, following staurosporine treatment, we could not detect any cleavage in APPΔJ2, suggesting that even though the VEVD motif was present, caspase mediated cleavage of this construct was substantially less than wild type APP. Thus, the inability of APPΔJ1 or APPΔJ2 to increase C31 toxicity might be secondary to a lack of cleavage, rather than because these molecules did not release Jcasp.
Fig. 3.
Toxicity of APP-C31 co-transfection was lost in APP deleted of Jcasp domain. (A) APPΔJ1 is a construct with a 13 amino acid deletion consisting of the entire Jcasp region while APPΔJ2 was a 9 amino acid deletion construct preserving the VEVD caspase cleavage motif. (B) Western blot using an end-specific antibody, APP-α664 (upper most). APPΔC31 and APP695ΔJ2_VEVD_ΔC31 transfected cells, but not wild type APP (APPwt) were immunoreactive to APP-α664 antibody. Following staurosporine treatment (STS), Asp-664 cleavage was detected in APP695wt transfected cells, but not in APP695ΔJ1, APP695ΔJ2 or APPD664A transfected cells. Expression of all constructs was comparable as determined by immunoblotting with 6E10 antibody. Cell death assay by ethidium homodimer staining (C) showed significantly increased cytotoxicity only for C31 cotransfected with APPwt. Neither Jcasp deleted constructs was able to augment C31 induced cytotoxicity (*p ≤ 0.001).
Inhibition of γ- or ε-cleavage did not alter APP-dependent C31 cytotoxicity
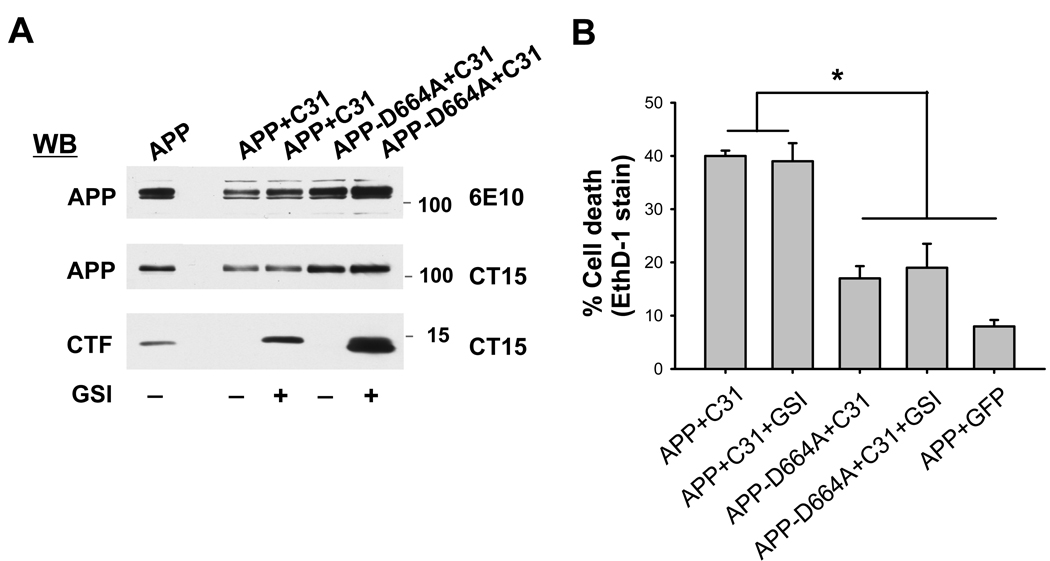
Because the previous experiment could not reliably ascertain the role of the Jcasp domain in C31-induced cell death, we asked whether prevention of Jcasp formation by γ-secretase inhibition had any effect on C31 toxicity. This is because Jcasp is released after γ-secretase (at the ε-site) and caspase cleavages. Therefore, treatment with γ-secretase inhibitor would effectively prevent the generation of Jcasp from APP but not C31. Accordingly, N2A cells were co-transfected with APP and C31 and then treated with or without the γ-secretase inhibitor (GSI) DAPT. As expected, treatment with DAPT led to a marked accumulation of APP C-terminal fragments at ~12–15 kDa in both the wild type APP and D664A mutant (Fig. 4A). However, treatment with DAPT had no effect on C31-induced toxicity as APP and C31 co-transfected cells demonstrated marked cell death regardless of GSI treatment (Fig 4B and Supp. Fig. 5). As expected, APPD664A showed no additional toxicity with C31 and this was unchanged in the presence of DAPT. Because Jcasp formation is blocked by treatment with DAPT, this result indicated that release of Jcasp is likely not an important component of cell death secondary to cleavage of endogenous APP by caspases.
Fig. 4.
The γ-secretase inhibitor DAPT did not affect cell death induced by C31 expression. (A) Western blot showing no substantive change in APP processing as seen by immunoblotting with 6E10 and CT 15 antibodies. The levels of C-terminal fragment markedly increased following exposure to DAPT. (B) The ethidium homodimer staining showed substantial cell death after co-expression of C31 and APP that were unchanged after DAPT treatment. Expression of APP-D664A in place of wild type APP resulted in an absence of cytotoxicity that was not affected by DAPT treatment. (*p < 0.001).
In summary, our results support a model whereby C31 is the principal toxic intermediate that is generated following cleavage of APP at position 664. In particular, our findings indicated that APP/C31 complex formation is upstream of APP cleavage by caspases and that this step is necessary but not sufficient to result in cytotoxicity. Upon generation, C31 must complex with APP or C99 to initiate or propagate the cytotoxic signal. Indeed, whether C31 itself is necessary for cell death beyond forming the putative signaling complex with APP is unknown. Thus, the molecular details of C31 toxicity remain to be clearly defined.
Supplementary Material
Acknowledgement
This work was supported by the KRF Grant funded by the Korean Government (MOEHRD) KRF-2006-611-E00004 (SAP) and NIH grant AG 05131 (DEB and EHK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 3.Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Ye X, Salvesen GS, Koo EH, Bredesen DE. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat. Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- 4.Shaked GM, Kummer MP, Lu DC, Galvan V, Bredesen DE, Koo EH. Aβ induces cell death by direct interaction with its cognate extracellular domain on APP (APP 597–624) FASEB J. 2006;20:1254–1256. doi: 10.1096/fj.05-5032fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galvan V, Gorostiza OF, Banwait S, Ataie M, Logvinova AV, Sitaraman S, Carlson E, Sagi SA, Chevallier N, Jin K, Greenberg DA, Bredesen DE. Reversal of Alzhemier’s-like pathology and behavior in human APP transgenic mice by mutation of Asp664. Proc. Natl. Acad. Sci. USA. 2006;103:7130–7135. doi: 10.1073/pnas.0509695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saganich MJ, Schroeder BE, Galvan V, Bredesen DE, Koo EH, Heinemann SF. Deficits in synaptic transmission and learning in amyloid precursor protein (APP) transgenic mice require C-terminal cleavage of APP. J. Neurosci. 2006;26:13428–13436. doi: 10.1523/JNEUROSCI.4180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinoshita A, Whelan CM, Berezovska O, Hyman BT. The gamma secretase-generated carboxyl-terminal domain of the amyloid precursor protein induces apoptosis via Tip60 in H4 cells. J. Biol. Chem. 2002;277:28530–28536. doi: 10.1074/jbc.M203372200. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Kim EM, Lee JP, Park CH, Kim S, Seo JH, Chang KA, Yu E, Jeong SJ, Chong YH, Suh YH. C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3 beta expression. FASEB J. 2003;17:1951–1953. doi: 10.1096/fj.03-0106fje. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand E, Brouillet E, Caille I, Bouillot C, Cole GM, Prochiantz A, Allinquant B. A short cytoplasmic domain of the amyloid precursor protein induces apoptosis in vitro and in vivo. Mol. Cell Neurosci. 2001;18:503–511. doi: 10.1006/mcne.2001.1030. [DOI] [PubMed] [Google Scholar]
- 10.Madeira A, Pommet JM, Prochiantz A, Allinquant B. SET protein (TAF1β, I2PP2A) is involved in neuronal apoptosis induced by an amyloid precursor protein cytoplasmic subdomain. FASEB J. 2005;19:1905–1907. doi: 10.1096/fj.05-3839fje. [DOI] [PubMed] [Google Scholar]
- 11.Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeq LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 12.Galvan V, Chen S, Lu D, Logvinova A, Goldsmith P, Koo EH, Bredesen DE. Caspase cleavage of members of the amyloid precursor family of proteins. J. Neurochem. 2002;82:283–294. doi: 10.1046/j.1471-4159.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- 13.Blakely BT, Rossi FM, Tillotson B, Palmer M, Estelles A, Blau HM. Epidermal growth factor receptor dimerization monitored in live cells. Nat. Biotechnol. 2000;18:218–222. doi: 10.1038/72686. [DOI] [PubMed] [Google Scholar]
- 14.Soriano S, Lu DC, Chandra S, Pietrzik CU, Koo EH. The amyloidogenic pathway of amyloid precursor protein (APP) is independent of its cleavage by caspases. J. Biol. Chem. 2001;276:29045–29050. doi: 10.1074/jbc.M102456200. [DOI] [PubMed] [Google Scholar]
- 15.Lu DC, Soriano S, Bredesen DE, Koo EH. Caspase cleavage of the amyloid precursor protein modulates amyloid beta-protein toxicity. J. Neurochem. 2003;87:733–741. doi: 10.1046/j.1471-4159.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya H, Roch JM, Jin LW, Saitoh T. Secreted from of amyloid beta/A4 protein precursor (APP) binds to two distinct APP binding sites on rat B103 neuron-like cells through two different domains, but only one site is involved in neuritotropic activity. J. Neurochem. 1994;63:495–500. doi: 10.1046/j.1471-4159.1994.63020495.x. [DOI] [PubMed] [Google Scholar]
- 17.Munter LM, Voigt P, Harmeier A, Kaden D, Gottschalk KE, Weise C, Pipkorn R, Schaefer M, Langosch D, Multhaup G. GxxxG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Abeta42. EMBO J. 2007;26:1702–1712. doi: 10.1038/sj.emboj.7601616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.