Abstract
Objective
The severity, distribution, and pattern of autonomic failure appear to be different in multiple system atrophy (MSA) compared with Parkinson’s disease (PD), but reports have been retrospective reviews and have tended to exclude PD with autonomic failure (PD_AF). We report preliminary results of a prospective ongoing study of MSA and PD, with a large subset of PD_AF (25%) to evaluate autonomic indices that distinguish MSA from PD.
Methods
We used Consensus criteria, detailed autonomic studies (composite autonomic symptom score (COMPASS), composite autonomic severity score (CASS), thermoregulatory sweat test percent anhidrosis (TST%), plasma catecholamines, and functional scales (Unified MSA rating scale (UMSARS) I–IV, Hoehn-Yahr grading) on a prospective, repeated, and ongoing basis.
Results
We report the results of a study based on 52 patients with MSA (61.1±7.8 years; BMI 27.2±4.6; Hoehn-Yahr grade, 3.2±0.9; UMSARS_1 21.5±7.4; UMSARS_2, 22.7±9.0) and 29 patients with PD, including PD_AF (66.0±8.1 years; BMI 26.6±.5.5; Hoehn-Yahr grade, 2.2±0.8; UMSARS_1 10.4±6.1; UMSARS_2, 13.0±5.9). Autonomic indices were highly significantly more abnormal in MSA than PD (P<0.001) for each of: CASS (5.9±1.9 vs. 3.3±2.3), COMPASS (54.4±21.8 vs. 24.7±20.5), TST% (57.4±35.2 vs. 9.9±17.7). These differences were sustained and greater at 1 year follow-up indicating a greater rate of progression of dysautonomia in MSA than PD.
Interpretation
The severity, distribution, and pattern of autonomic deficits at entry will distinguish MSA from PD and MSA from PD_AF. These differences continue and increase with follow-up. Our ongoing conclusion is that autonomic function tests can separate MSA from PD. Autonomic indices support the notion that the primary lesion in PD is ganglionic/postganglionic while MSA is preganglionic.
Introduction
Multiple system atrophy (MSA) is a sporadic multi-system progressive disorder characterized by autonomic failure, with orthostatic hypotension (OH), neurogenic bladder/erectile dysfunction, cerebellar ataxia, corticospinal dysfunction, plus parkinsonism that may be poorly levodopa-responsive.1, 2 A major clinical dilemma for the clinician is whether a patient with parkinsonism has Parkinson’s disease (PD) or MSA, since the prognosis of MSA is much worse. Autonomic involvement is common in Parkinson’s disease but is more variable in severity than MSA.3–5 Mild OH is relatively commonly in PD and occasionally severe OH can occur.6, 7 In the Mayo retrospective studies, we reported that, when quantitative methods were used, the severity and distribution of autonomic deficits appeared to distinguish MSA from PD.4, 5 In contrast to MSA, which is predominantly a preganglionic disorder, autonomic pathology in PD is primarily postganglionic,9 a fact that could be exploited in autonomic function tests. Indeed postganglionic adrenergic neuropathy forms the basis of neuroimaging studies of the postganglionic axons of the heart with [123I]MIBG or other postganglionic adrenergic markers suitable for PET scanning, where uptake is markedly reduced or absent in PD and usually normal in MSA.10–14
The studies above and Consensus criteria1 and its update2 have resulted in significant advances. However, these approaches have been retrospective; lacking is a comprehensive prospective evaluation of autonomic function. As part of an NIH-funded program project on MSA, we undertook a prospective study of MSA and PD patients who underwent full neurological, and autonomic evaluation, using standardized instrument to evaluate symptom (autonomic symptom profile (ASP), motor including autonomic activities of daily living (UMSARS), and deficits (composite autonomic severity score (CASS); percentage anhidrosis on thermoregulatory sweat test (TST%). The composite autonomic symptoms score (COMPASS) provides a score of autonomic symptoms with appropriate weighting.22, 23 We had 2 hypotheses. The first is that the severity, distribution, and pattern of autonomic failure at entry into the study will separate MSA from PD. The second hypothesis is that, for those MSA patients with mild autonomic failure, the increase in autonomic deficit documented at the first return visit, at the end of 1 year, will differentiate MSA from PD and is predictive of the rate of progression of MSA. The relevant indices are changes in CASS, TST%, and COMPASS (COMPASS_change). We defined mild autonomic failure as autonomic deficits of TST% <40% of CASS <4. Presumably, the more severe and rapid progression of autonomic failure reflects the more widespread and intense distribution of neuronal loss and glial cytoplasmic inclusions.
Since autonomic failure is part of the criteria for the diagnosis of MSA,1, 2 we have gone to pains to match the severity of clinical autonomic failure between MSA and PD. We included PD patients with florid autonomic failure, as long as they fulfilled criteria for PD. Specifically, 6/29 PD patient had Parkinson’s disease with autonomic failure (PD_AF), defined as PD patients with symptomatic and severe OH that either preceded or dominated symptomatology. Patients with PD_AF were indistinguishable from MSA in OH and indeed often had more severe OH than MSA.
Methods
Patients
Parkinson’s disease
We utilized criteria modified from Hughes et al.24 that defines clinically definite PD. Patients were required to have the presence of 3 of the 3 cardinal features of PD (resting tremor, bradykinesia, and rigidity). We included PD patients with autonomic failure, defined as PD patients with severe and symptomatic OH that either preceded motor symptoms or comprised a dominant complaint. We excluded parkinsonism due to drugs (including neuroleptics, α-methyldopa, reserpine, metoclopramide), other causes of parkinsonism, dementia, history of stroke, history of brain surgery for Parkinson’s disease, and history of structural brain disease.
Multiple system atrophy
We use the criteria of Gilman et al.1, 2 The diagnosis of probable MSA requires (1) the presence of OH (fall in systolic BP ≥ 30 mm Hg) or urinary incontinence (persistent involuntary partial or total bladder emptying, accompanied by erectile dysfunction (in men) or both; (2) poorly levodopa responsive parkinsonism or cerebellar ataxia.
Tests and Instruments
Patients stopped medications that could interfere with autonomic function for 5 half lives of the drugs prior to autonomic testing.
Thermoregulatory sweat test (TST) is a modification of Guttmann’s25 quinizarin sweat test. Unclothed subjects lie supine and exposed body surface is covered with an indicator powder mixture26, 27 With warming to 38°C, sweating is recognized by indicator color change. The sweat distribution is documented by digital photography. The digital images are processed by a pixel counter to derive an accumulative value for the area of anhidrosis and the percentage of anhidrosis (TST%).
The Unified Multiple System Atrophy Rating Scale (UMSARS) is a validated, disease-specific scale representing the diverse signs and symptoms in MSA.28 It can assess rates of progression and is sensitive to change over time.29 It has an Activities of Daily Living score (UMSARS_1, 12 questions) that evaluates motor including autonomic activities and the Motor Examination score (UMSARS_2, 14 questions). Poorer health is signified by higher scores on the UMSARS scales.
Composite autonomic Symptom Score (COMPASS) is the score derived from the ASP, which is a self-report instrument of 169 questions designed to provide an index of autonomic symptom severity.30 It yields one total score reflecting overall severity of autonomic symptoms and eleven weighted subscale scores that assess severity of symptoms within the following domains: orthostatic intolerance, syncope, sexual failure (males only), bladder dysfunction, diarrhea, constipation, upper gastrointestinal (GI) symptoms, secretomotor dysfunction, sleep dysfunction, vasomotor symptoms, and pupillomotor symptoms. ASP scores correlates with objective indices of autonomic function,30 and we have generated norms for the profile based on a sample of 245 healthy controls who completed the instrument.23 COMPASS_change is a derivative of COMPASS and evaluates the change in symptoms over time on selected domains of symptoms. The focus is on 7 selected domains. We have made the instruments available to selected research centers.31, 32
Composite Autonomic Severity Score (CASS) is the score of autonomic deficits derived of postganglionic sudomotor, adrenergic, and cardiovagal function.33, 34 Results are compared to a normative database of 557 normal subjects.35 A 10-point score (CASS) is generated that corrects for the confounding effects of age and gender.36 It has three subscales: adrenergic (range = 0–4), sudomotor (range = 0–3), and cardiovagal (range = 0–3). Generally, a total CASS score ≤ 3 indicates no or mild autonomic failure, scores from 4–6 indicate moderate autonomic failure, and scores from 7–10 indicate severe autonomic failure.
Statistical analysis
Summary statistics were presented as means and standard deviations (SD). Comparisons of initial evaluation and month twelve measures were based on Wilcoxon signed rank sum/Mann-Whitney U tests. Spearman estimates of correlation were determined. P-values were not adjusted for multiple comparisons.
Results
Comparison of MSA with PD at Initial Evaluation
Patients were compared on age, gender and BMI (Table 1). PD patients were slightly older. Mean age was 61.1±7.8 years in MSA and 66.0±8.1 years in PD (P=0.011). Duration of disease was estimated to be 7.2±2.9 years in MSA and 10.1±5.1 years in PD. Breakdown by type of MSA was as follows: MSA-P = 32, MSA-C = 20.
Table 1.
Summary demographics, autonomic and functional scores at baseline and after one year follow-up.
| Variable | Dx | baseline | year 1 | ||||
|---|---|---|---|---|---|---|---|
| N | mean (SD) | P-Value* | N | mean (SD) | P-Value* | ||
| Gender | MSA PD |
male: 26 female: 26 male: 17 female: 12 |
male: 12 female: 13 male: 11 female: 9 |
||||
| Age | MSA PD |
52 29 |
61.1 (7.8) 66.0 (8.1) |
0.011 | 25 20 |
64.0 (7.7) 68.2 (7.4) |
0.062 |
| BMI | MSA PD |
46 29 |
27.2 (4.6) 26.6 (5.5) |
0.687 | 15 18 |
27.1 (6.4) 27.0 (5.8) |
0.691 |
| CASS | MSA PD |
52 27 |
5.9 (1.9) 3.3 (2.3) |
<0.001 | 14 20 |
5.1 (1.9) 2.9 (2.3) |
0.003 |
| TST% | MSA PD |
45 25 |
57.4 (35.2) 9.9 (17.7) |
<0.001 | 45 26 |
59.0 (34.6) 14.2 (26.2) |
<0.001 |
| UMSARS_1 | MSA PD |
52 29 |
21.5 (7.4) 10.4 (6.1) |
<0.001 | 25 20 |
28.7 (10.7) 11.4 (5.4) |
<0.001 |
| UMSARS_2 | MSA PD |
52 29 |
22.7 (9.0) 13.0 (5.9) |
<0.001 | 14 20 |
24.3 (6.6) 11.5 (6.8) |
<0.001 |
| Hoehn-Yahr | MSA PD |
52 29 |
3.1 (1.0) 2.2 (0.8) |
<0.001 | 22 20 |
3.4 (1.0) 2.1 (0.7) |
<0.001 |
Based on Wilcoxon sign rank sum test
Autonomic Symptoms and Functional Status
COMPASS (Table 2) for MSA was significantly greater (P<0.001) than that of PD. Six of eleven domains were significantly more affected in MSA. Three of the domains related to BP control (syncope, orthostatic intolerance, vasomotor). The other three were domains focused on symptoms of disturbed secretomotor, bladder, and sleep function. These six domains, designated COMPASS_Select, were markedly different between MSA and PD and comprise the domains selected for focus in subsequent analysis. For COMPASS_Select domains, MSA at 47.7±19.7 was significantly higher than PD, at 19.4±16.0 (P<0.001), being 245% that of PD.
Table 2.
Autonomic symptom scores for patients with MSA and PD at baseline.
| Autonomic Symptom Profile Domains | DX | N | Mean | SD | P-Value* |
|---|---|---|---|---|---|
| Orthostatic Intolerance | MSA PD |
47 23 |
22.02 9.89 |
11.83 10.07 |
<0.001 |
| Upper Gastrointestinal Symptoms | MSA PD |
47 24 |
0.89 0.63 |
1.43 1.29 |
0.416 |
| Bladder Dysfunction | MSA PD |
49 25 |
10.73 4.16 |
5.58 3.31 |
<0.001 |
| Syncope | MSA PD |
49 25 |
1.39 0.00 |
2.09 0.00 |
0.001 |
| Vasomotor Symptoms | MSA PD |
48 25 |
3.10 1.34 |
3.04 2.22 |
0.012 |
| Diarrhea | MSA PD |
49 25 |
1.63 1.76 |
3.64 3.07 |
0.621 |
| Constipation | MSA PD |
49 25 |
3.09 2.28 |
2.81 2.57 |
0.179 |
| Pupillomotor Symptoms | MSA PD |
49 25 |
1.62 1.30 |
1.40 0.95 |
0.518 |
| Sleep Dysfunction | MSA PD |
44 23 |
4.33 1.92 |
3.34 2.33 |
0.003 |
| Secretomotor Dysfunction | MSA PD |
44 24 |
5.83 2.94 |
4.28 3.26 |
0.004 |
| Sexual Failure (men only) | MSA PD |
25 15 |
11.80 8.30 |
3.91 7.19 |
0.054 |
| COMPASS (w/o Sexual Failure) | MSA PD |
37 20 |
54.40 24.72 |
21.81 20.44 |
<0.001 |
| COMPASS_Select (6 domains) | MSA PD |
38 21 |
47.66 19.43 |
19.74 15.97 |
<0.001 |
Based on Wilcoxon sign rank sum test.
UMSARS_1 (Table 1), evaluates functional status and was significantly greater (P<0.001) at 21.5±7.4 in MSA than PD (10.4±6.1). UMSARS_2 (Table 1) evaluates neurologic deficits, was also significantly different (P<0.001). The greater impairment in functional status was also reflected in Hoehn-Yahr (P<0.001) and UMSARS_4 (P<0.001) grading in MSA than PD.
Autonomic Function Tests
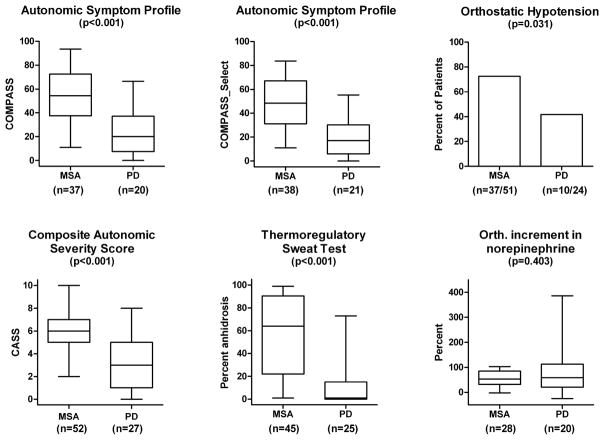
CASS was significantly greater (P<0.001) in MSA (5.9±1.9) than PD (3.3±2.3) (Table 1; Figure 1), indicating more severe and widespread autonomic failure in MSA than PD. The TST% demonstrated more diffuse anhidrosis in MSA than PD (Table 1; Figure 1; P<0.001) with little overlap. Anhidrosis in MSA affected entire regions while that in PD and PD_AF tended to be distal, typical of a length-dependent “neuropathic” pattern. When the QSART component of CASS was separately considered, postganglionic sudomotor involvement was not severe (MSA: N=52, 1.75±1.27; PD: N=28, 0.79±1.17, P=0.001). OH was common in PD and almost invariable in MSA (Figure 1). Supine norepinephrine (NE) and its orthostatic increment was not significantly different between MSA and PD (NS).
Figure 1.
Results at baseline. Autonomic symptoms (COMPASS and COMPASS_Select) based on the Autonomic Symptom Profiles, deficits (composite autonomic scoring scale, thermoregulatory sweat test) are significantly greater in MSA than PD (P<0.001). Orthostatic hypotension is common in both but more so in MSA (P=0.031) while orthostatic increment in plasma norepinephrine was not significantly different.
Figure 2 illustrates typical TST patterns in MSA, PD and PD_AF. MSA (top left) shows regional and progressive anhidrosis not seen in PD or PD_AF. PD patients have a normal pattern or distal anhidrosis (top right) typical of postganglionic length-dependent denervation. PD_AF is associated with either normal TST (bottom left) or a pattern indicative of ganglionic and/or distal denervation (bottom right). Hence while clinical autonomic failure of PD_AF was indistinguishable from MSA, the distribution of anhidrosis was dramatically different.
Figure 2.
Representative thermoregulatory sweat test (TST) findings in Multiple System Atrophy (MSA, left upper panel), Parkinson’s Disease (PD, right upper panel) and PD_AF (lower two panels). The regional (lower extremities) preganglionic sweat loss (TST abnormal, QSART normal) in 2002 with subsequent progression to global anhidrosis in 2005 is nearly pathognomonic of MSA. Distal sudomotor impairment with minimal progression is typical of PD. Note anhidrosis of distal feet and toes in 2004 and progression only to fingers over 4 years (right upper). PD_AF may have a normal TST (left-lower) or may show a more extensive sudomotor deficit (right-lower) that on further testing with QSART reveals a predominantly ganglionic (large truncal segmental sweat loss with reduced QSART) or a postganglionic, length-dependant deficit (feet, fingers). (Sweating in purple shading)
When MSA is compared with the subset of PD_AF patients alone (N=6), PD_AF has a peripheral pattern of anhidrosis and a significantly lower TST% than MSA (MSA 27.1±34.9 vs. PD 10.9±7.4; P=0.01). The other tests (UMSARS_1, COMPASS_Select, CASS) were not significantly different between MSA and PD_AF.
Correlations
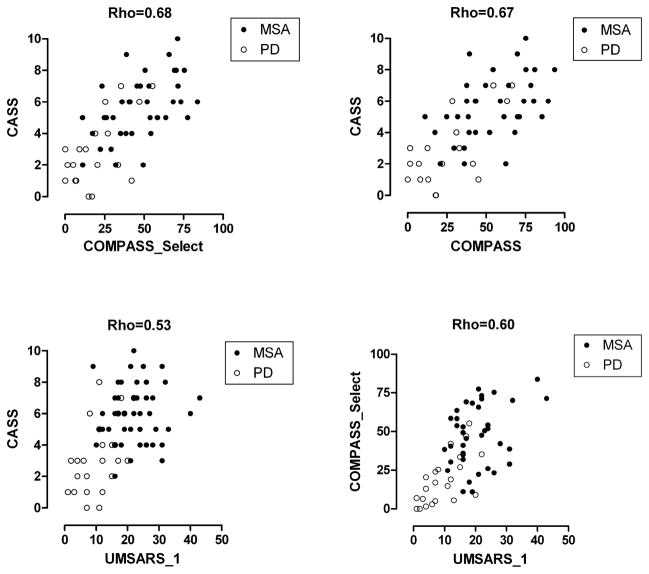
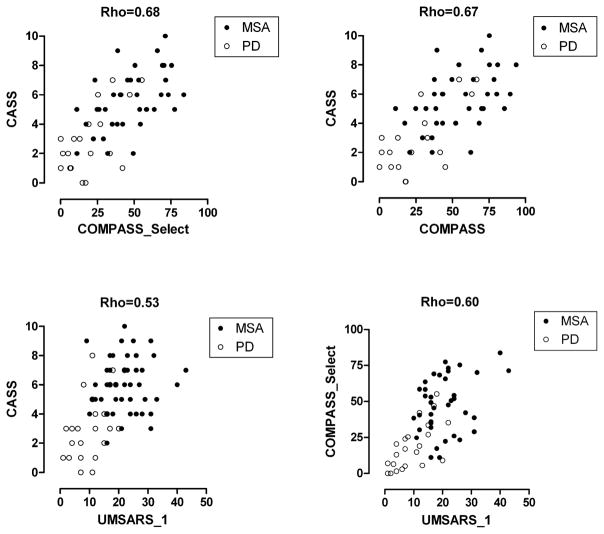
Figure 3 shows the following correlations: Autonomic deficits (CASS) correlate very well with autonomic symptoms (COMPASS) (Rho = 0.67) and with COMPASS_Select (Rho = 0.68). Functional status evaluated by UMSARS_1 correlated well with both COMPASS_Select (Rho=0.60) and CASS (Rho=0.53), suggesting that autonomic failure might be responsible for a significant part of functional deficits (Figure 2).
Figure 3.
Regression of autonomic deficits (CASS) with autonomic symptoms (COMPASS_Select, COMPASS) and functional status (UMSARS) and of autonomic symptoms (COMPASS_Select) with functional status (UMSARS_1).
Progression of Autonomic and Functional Status
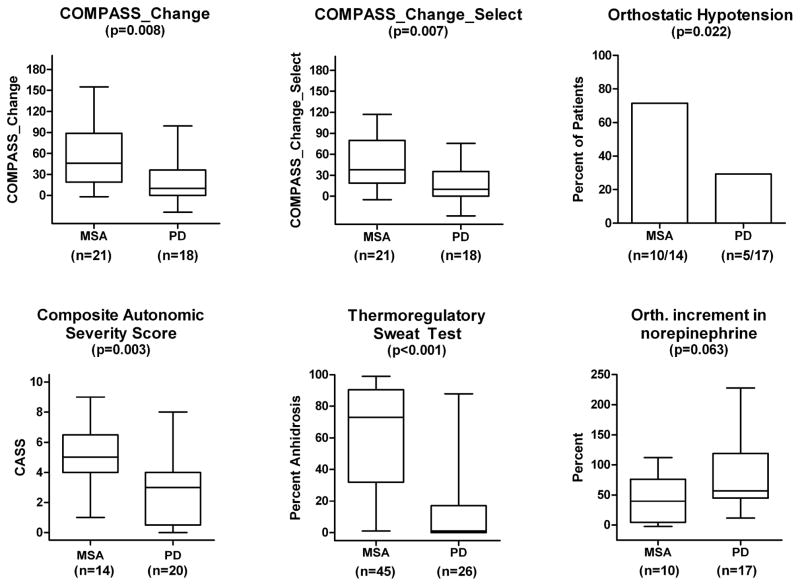
This is based on a preliminary analysis of 25 patients with MSA and 20 patients with PD who had completed follow-up evaluation at 12 months (Tables 1 and 3). The change in autonomic symptoms, (COMPASS_Change) was almost three-fold greater in MSA than PD (Table 3; Figure 4). COMPASS_Change at 56.9±45.9 was significantly greater (P=0.008) in MSA than PD (22.1±32.8). When domains were confined to the five selected (COMPASS_Change_Select), variance was less and the change in autonomic symptoms in MSA at 49.8±37.8 was significantly greater (P=0.007) than in PD (17.7±26.5) (Figure 4).
Table 3.
COMPASS_Change Scores for patients with MSA and PD at 1-year follow-up.
| COMPASS_Change Domains | DX | N | Mean | Std. Dev. | P-Value* |
|---|---|---|---|---|---|
| Orthostatic Intolerance | MSA PD |
21 18 |
29.52 10.50 |
19.14 19.16 |
0.003 |
| Upper Gastrointestinal Symptoms | MSA PD |
21 18 |
1.52 0.22 |
1.99 2.46 |
0.038 |
| Bladder Dysfunction | MSA PD |
21 18 |
8.81 2.50 |
13.68 5.22 |
0.133 |
| Vasomotor Symptoms | MSA PD |
21 18 |
2.14 −0.56 |
2.54 2.36 |
0.001 |
| Diarrhea | MSA PD |
21 18 |
0.95 0.28 |
4.64 1.18 |
0.674 |
| Constipation | MSA PD |
21 18 |
1.43 1.11 |
4.78 2.74 |
0.575 |
| Pupillomotor Symptoms | MSA PD |
21 18 |
2.29 1.67 |
2.78 2.40 |
0.415 |
| Sleep Dysfunction | MSA PD |
21 18 |
2.50 0.28 |
8.25 3.31 |
0.216 |
| Secretomotor Dysfunction | MSA PD |
21 18 |
6.81 5.00 |
8.45 5.99 |
0.760 |
| Sexual Failure (men only) | MSA PD |
11 10 |
1.82 2.00 |
3.37 3.50 |
0.918 |
| COMPASS_Change (max score 200) | MSA PD |
21 18 |
56.93 22.11 |
45.92 32.79 |
<0.001 |
| COMPASS_Change_Select (5 domains) | MSA PD |
21 18 |
49.79 17.72 |
37.84 26.45 |
<0.001 |
Based on Wilcoxon sign rank sum test.
Figure 4.
Results at 1 year of follow up or last available autonomic data.
COMPASS_Change and COMPASS_Change_Select based on a modified Autonomic Symptom Profile, percent of patients with orthostatic hypotension, CASS, TST%, and orthostatic increment in plasma norepinephrine expressed as a percent.
For the same patients, changes in UMSARS_1 and 2 continue to be more than 2 times greater in MSA than PD and progress at a greater rate in MSA (Table 1; Figure 4). Progression is well-exemplified in Figure 2, showing progressive anhidrosis in MSA, not seen in PD.
Discussion
The main findings of this prospective study to date are that functional status (UMSARS_1) and especially certain select autonomic symptom domains (COMPASS_Select) and deficits (CASS; TST%) will distinguish PD from MSA. Additionally, a repeat study in 12 months will further differentiate the two conditions, since MSA is characterized by a much more rapid progression of dysautonomia and functional status. This two-step approach of systematic evaluation at entry and at one year enhances the ability of the clinician to diagnose MSA with greater sensitivity and specificity.
While autonomic failure has long been recognized as being an integral component of MSA, much of the focus has been confined to presence of OH and neurogenic bladder. This study confirms both the observation that OH is almost invariably present in MSA4, 5, 15 but that OH is also relatively common in PD,6, 7 being present in 41.7% (baseline) and 29.4% (1-year). The frequency of OH in PD should not be surprising, since OH is relatively common in PD subjects of this age group37, 38 and there is, additionally, postganglionic adrenergic denervation in PD.9, 37, 38 This study emphasizes the cautionary point that the presence of OH as the sole autonomic manifestation in PD is not a strong red flag for MSA.
The selection of autonomic function tests was done with two goals in mind. We selected tests that we hypothesized, based on preliminary data and autonomic pathophysiology, should discriminate between MSA and PD. Second, we chose tests that were currently available at clinical autonomic laboratories, that are well-validated and reproducible, with a coefficient of variability <20%. MSA differs from PD in the involvement of hypothalamic regions responsible for thermoregulation,39, 40 in addition to involvement of preganglionic neurons,41 so that the distribution of anhidrosis should be more diffuse. The finding in this study that diffuse anhidrosis is usually present in MSA confirms our earlier findings4, 5 that there is more widespread anhidrosis in MSA than PD. The distribution of anhidrosis in PD supports the notion that the lesion is postganglionic in PD irrespective of severity of autonomic failure5, 9 and serves as a reliable diagnostic test to differentiate PD_AF from MSA. The QSART values in MSA are significantly higher than that of PD, indicating that the denervated postganglionic axon undergoes some secondary changes. Of note is that the values are typically <2 indicating that there are areas of anhidrosis on TST that maintain a sweat response to QSART, supporting a preganglionic lesion in MSA. The reason for a lower score in PD reflects the much more restricted or absent area of anhidrosis in PD.
The subset of PD_AF patients where autonomic failure dominates the clinical phenotype is problematic to the clinician since they could closely mimic MSA. When MSA is compared with PD_AF subset alone, COMPASS_Select, UMSARS, and CASS are not significantly different, reflecting the influence of autonomic failure on these scores. On the other hand, the pattern of anhidrosis (peripheral or ganglionic in PD_AF and central-preganglionic in MSA) and %-anhidrosis are significantly different in MSA. Additionally by combining QSART and TST, intact QSART in anhidrotic areas is typical of MSA and confirms a preganglionic site of the lesion.
Plasma norepinephrine measured in both the supine and standing positions has been reported to differentiate a preganglionic from postganglionic disorder.42, 43 Supine norepinephrine is reduced in widespread postganglionic disorder such as PAF and normal in a preganglionic disorder of MSA.42, 43 In contrast, the latter condition is associated with a failure of norepinephrine to approximately double, because of widespread preganglionic failure disorder.42, 43 The poor discriminatory value of norepinephrine or its intracellular metabolite, DHPG, indicates that PD is not characterized by sufficiently widespread postganglionic adrenergic failure to be regularly detectable by this test. This test does not have sufficient sensitivity for routine use to discriminate between MSA and PD.
The autonomic symptom profile provides a comprehensive evaluation of autonomic symptoms, and has been validated.23, 30 The domains that are significantly different between MSA and PD are those on BP control (syncope, orthostatic intolerance, vasomotor) and dysfunctional secretomotor, bladder, and sleep. The high correlation of COMPASS_select with CASS supports the notion that these autonomic symptoms are due to autonomic failure in MSA (and PD). Its high correlation with UMSARS 1 and 2 emphasize that autonomic dysfunction affect activities of daily living and that autonomic dysfunction parallels neurologic deficits. The high discriminatory value of COMPASS_Change over 12 months emphasize that change in autonomic function over time is greater, being almost 3-fold that of PD.
One potential limitation of the study is the reduced number of patients at follow-up compared to the number recruited. We have therefore not been able to test part 2 of our hypothesis, that the rate of change of autonomic failure, in those with initially mild autonomic deficits, will distinguish MSA from PD. The main reasons for inability to return for autonomic evaluation are death and incapacity. We minimized the problem by the use of UMSARS 1 and COMPASS_Change, which allows for an evaluation of autonomic symptoms and activities of daily living by telephone interview. The results at 1 year likely reflect an underestimate of deterioration in MSA since the most common reason for inability to return was incapacity or death. The median time to death from entry in the study, according to Kaplan-Meier estimate of survival was 2.1 years (not shown). Although we use Consensus criteria,1 one limitation is that the diagnosis of MSA is still an assumption. Five patients have died, since starting the study. In all cases, patients who underwent brain autopsy studies have pathologically confirmed MSA, suggesting that the Consensus criteria are relatively robust. Another limitation of the study is the relatively advanced stage of the disease when MSA diagnosis is made. This limitation reflects the strict set of criteria. We plan to explore the predictive value of our evaluative approach in cases of possible MSA within 4 years of onset.
Acknowledgments
This work was supported in part by National Institutes of Health (NS 32352; NS 44233; NS 43364), Mayo CTSA (MO1 RR00585), and Mayo Funds.
References
- 1.Gilman S, Low PA, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 2.Gilman S, Wenning GK, Low PA, et al. Second consensus conference on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aminoff MJ, Wilcox CS. Assessment of autonomic function in patients with a Parkinsonian syndrome. BMJ. 1971;4:80–84. doi: 10.1136/bmj.4.5779.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J, Low P, Fealey R, Sheps S, Jiang NS. Somatic and autonomic function in progressive autonomic failure and multiple system atrophy. Ann Neurol. 1987;22:692–699. doi: 10.1002/ana.410220604. [DOI] [PubMed] [Google Scholar]
- 5.Sandroni P, Ahlskog JE, Fealey RD, Low PA. Autonomic involvement in extrapyramidal and cerebellar disorders. Clin Auton Res. 1991;1:147–155. doi: 10.1007/BF01826212. [DOI] [PubMed] [Google Scholar]
- 6.Senard JM, Rai S, Lapeyre-Mestre M, et al. Prevalence of orthostatic hypotension in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;63:584–589. doi: 10.1136/jnnp.63.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allcock LM, Ullyart K, Kenny RA, Burn DJ. Frequency of orthostatic hypotension in a community based cohort of patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75:1470–1471. doi: 10.1136/jnnp.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson DW, Liu W, Hardy J, et al. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol. 1999;155:1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Braune S, Reinhardt M, Schnitzer R, Riedel A, Lucking CH. Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology. 1999;53:1020–1025. doi: 10.1212/wnl.53.5.1020. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein DS, Holmes C, Li ST, et al. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–347. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 12.Iwasa K, Nakajima K, Yoshikawa H, et al. Decreased myocardial 123I-MIBG uptake in Parkinson’s disease. Acta Neurol Scand. 1998;97:303–306. doi: 10.1111/j.1600-0404.1998.tb05957.x. [DOI] [PubMed] [Google Scholar]
- 13.Satoh A, Serita T, Seto M, et al. Loss of 123I-MIBG uptake by the heart in Parkinson’s disease: assessment of cardiac sympathetic denervation and diagnostic value. J Nucl Med. 1999;40:371–375. [PubMed] [Google Scholar]
- 14.Raffel DM, Koeppe RA, Little R, et al. PET measurement of cardiac and nigrostriatal denervation in Parkinsonian syndromes. J Nucl Med. 2006;47:1769–1777. [PubMed] [Google Scholar]
- 15.Wenning GK, Ben-Shlomo Y, Hughes A, et al. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;68:434–440. doi: 10.1136/jnnp.68.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med. 1994;33:321–325. doi: 10.2169/internalmedicine.33.321. [DOI] [PubMed] [Google Scholar]
- 17.Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117:835–845. doi: 10.1093/brain/117.4.835. [DOI] [PubMed] [Google Scholar]
- 18.Uitti RJ, Ahlskog JE, Maraganore DM, et al. Levodopa therapy and survival in idiopathic Parkinson’s disease: Olmsted County project. Neurology. 1993;43:1918–1926. doi: 10.1212/wnl.43.10.1918. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Marder K, Cote L, Tang M, Mayeux R. Mortality from Parkinson disease. Arch Neurol. 1997;54:260–264. doi: 10.1001/archneur.1997.00550150024011. [DOI] [PubMed] [Google Scholar]
- 20.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. 2008;70:1423–1430. doi: 10.1212/01.wnl.0000310414.85144.ee. [DOI] [PubMed] [Google Scholar]
- 21.Litvan I, Booth V, Wenning GK, et al. Retrospective application of a set of clinical diagnostic criteria for the diagnosis of multiple system atrophy. J Neural Transm. 1998;105:217–227. doi: 10.1007/s007020050050. [DOI] [PubMed] [Google Scholar]
- 22.Suarez GA, Opfer-Gehrking TL, Offord KP, et al. The Autonomic Symptom Profile. A new instrument to assess autonomic symptoms. Neurology. 1999;52:523–528. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 23.Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27:2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 25.Guttmann L. The management of the quinizarin sweat test (QST) Postgrad Med J. 1947;23:353–366. doi: 10.1136/pgmj.23.262.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64:617–628. doi: 10.1016/s0025-6196(12)65338-5. [DOI] [PubMed] [Google Scholar]
- 27.Low PA, Walsh JC, Huang CY, McLeod JG. The sympathetic nervous system in diabetic neuropathy. A clinical and pathological study. Brain. 1975;98:341–356. doi: 10.1093/brain/98.3.341. [DOI] [PubMed] [Google Scholar]
- 28.Wenning GK, Tison F, Seppi K, et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Mov Disord. 2004;19:1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 29.Geser F, Wenning GK, Seppi K, et al. Progression of multiple system atrophy (MSA): a prospective natural history study by the European MSA Study Group (EMSA SG) Mov Disord. 2006;21:179–186. doi: 10.1002/mds.20678. [DOI] [PubMed] [Google Scholar]
- 30.Suarez GA, Opfer-Gehrking TL, Fealey RD, et al. Autonomic symptom profile (ASP): A new questionnaire for assessment of autonomic symptoms. Clin Auton Res. 1995;5:324. [Google Scholar]
- 31.Kollensperger M, Stampfer-Kountchev M, Seppi K, et al. Progression of dysautonomia in multiple system atrophy: a prospective study of self-perceived impairment. Eur J Neurol. 2007;14:66–72. doi: 10.1111/j.1468-1331.2006.01554.x. [DOI] [PubMed] [Google Scholar]
- 32.Schrag A, Selai C, Mathias C, et al. Measuring health-related quality of life in MSA: the MSA-QoL. Mov Disord. 2007;22:2332–2338. doi: 10.1002/mds.21649. [DOI] [PubMed] [Google Scholar]
- 33.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68:748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 34.Low PA. Autonomic nervous system function. J Clin Neurophysiol. 1993;10:14–27. doi: 10.1097/00004691-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Low PA, Denq JC, Opfer-Gehrking TL, et al. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 37.Lipsitz LA. Orthostatic hypotension in the elderly. N Engl J Med. 1989;321:952–957. doi: 10.1056/NEJM198910053211407. [DOI] [PubMed] [Google Scholar]
- 38.Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–2295. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 39.Benarroch EE, Schmeichel AM, Parisi JE. Depletion of mesopontine cholinergic and sparing of raphe neurons in multiple system atrophy. Neurology. 2002;59:944–946. doi: 10.1212/wnl.59.6.944. [DOI] [PubMed] [Google Scholar]
- 40.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Differential involvement of hypothalamic vasopressin neurons in multiple system atriphy. Brain. 2006;129:2688–2696. doi: 10.1093/brain/awl109. [DOI] [PubMed] [Google Scholar]
- 41.Low PA, Thomas JE, Dyck PJ. The splanchnic autonomic outflow in Shy-Drager syndrome and idiopathic orthostatic hypotension. Ann Neurol. 1978;4:511–514. doi: 10.1002/ana.410040606. [DOI] [PubMed] [Google Scholar]
- 42.Polinsky RJ, Kopin IJ, Ebert MH, Weise V. Pharmacologic distinction of different orthostatic hypotension syndromes. Neurology. 1981;31:1–7. doi: 10.1212/wnl.31.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Mathias CJ, Polinsky RJ. Separating the primary autonomic failure syndromes, multiple system atrophy, and pure autonomic failure from Parkinson’s disease. Adv Neurol. 1999;80:353–361. [PubMed] [Google Scholar]