Abstract
Early life stress is a predisposing factor for the development of chronic intestinal disorders in adult life. Here, we show that stress associated with early weaning in pigs leads to impaired mucosal barrier function. Early weaning (15- to 21-day weaning age) resulted in sustained impairment in intestinal barrier function, as indicated by reductions in jejunal transepithelial electrical resistance and elevations in mucosal-to-serosal flux of paracellular probes [3H]mannitol and [14C]inulin measured at 5 and 9 wk of age, compared with that shown in late-weaned pigs (23- to 28-day weaning age). Elevated baseline short-circuit current was observed in jejunum from early-weaned pigs and was shown to be mediated via enhanced Cl− secretion. Jejunal barrier dysfunction in early-weaned pigs coincided with increased lamina propria immune cell density particularly mucosal mast cells. The mast cell stabilizer drug sodium cromoglycolate ameliorated barrier dysfunction and hypersecretion in early-weaned pigs, demonstrating an important role of mast cells. Furthermore, activation of mast cells ex vivo with c48/80 and corticotrophin-releasing factor (CRF) in pig jejunum mounted in Ussing chambers induced barrier dysfunction and elevations in short-circuit current that were inhibited with mast cell protease inhibitors. Experiments in which selective CRF receptor antagonists were administered to early-weaned pigs revealed that CRF receptor 1 (CRFr1) activation mediates barrier dysfunction and hypersecretion, whereas CRFr2 activation may be responsible for novel protective properties in the porcine intestine in response to early life stress.
Keywords: weaning age, mast cells, corticotropin releasing factor
the single layer of epithelial cells lining the gastrointestinal (GI) tract forms a selective barrier to the harsh environment of the intestinal lumen. Disturbances in the intestinal barrier, characterized by increased intestinal permeability, result in the translocation of luminal bacteria, toxins, and antigens into subepithelial tissues, inciting mucosal and systemic inflammatory responses that are central to many GI disorders (15). Psychological stress is an important cause of intestinal barrier injury and is linked to the onset and severity of clinical GI disorders, including irritable bowel syndrome (IBS), inflammatory bowel diseases, and infectious diarrhea (1, 12, 22, 25). The mechanisms by which stress causes breakdown in intestinal barrier function are not fully understood but thought to be mediated by the release of central and peripheral stress mediators, including corticotrophin-releasing factor (CRF) and adrenal glucocorticoids (40, 46, 60). Subsequent activation of CRF and glucocorticoid receptors (GR) has been shown to trigger disturbances in intestinal physiology, including increased mucosal permeability (10, 55, 62), secretion (61, 62), visceral hypersensitivity (30, 51), and motility, altogether contributing to clinical disease.
Mast cells (MCs) are hematopoietic-derived immune cells that migrate to peripheral tissues to mature and regulate a variety of effector functions. MCs are strategically positioned at epithelial barriers, signifying their important role in mucosal surveillance; MCs coordinate both innate and adaptive immune responses. Although they are mostly well known for their role in allergy and inflammatory disorders, MCs are becoming increasingly recognized as an important cell type mediating stress-related intestinal disorders (7, 19, 30, 44, 57). MCs are abundant in preformed (tryptases, histamine, etc.) and synthesized bioactive mediators (prostanoids and leukotrienes), which, when released, have a profound influence on intestinal function, including increased intestinal permeability, secretion, and visceral hypersensitivity (58). MCs express receptors that can respond to a variety of external stimuli, including bacterial toxins, complement, IgE, and peptides. Of the peptide receptors, MCs express at least two G-protein-coupled CRF receptors, CRFr1 and CRFr2 (16, 49), which may provide the link between stress and intestinal disease.
Although stress can cause intestinal barrier disturbances in normal individuals, the developmental period during which stress occurs can dictate the duration and severity of intestinal barrier dysfunction. For example, stress occurring during the neonatal period can have long-lasting effects on GI barrier health. Neonatal maternal separation models in rodents have shown that psychological stress during the neonatal period can induce permanent defects in intestinal barrier properties (28, 59). This is supported by epidemiological studies that show that psychological stress occurring early in life is associated with the development of chronic, persistent GI disorders in adult life (13, 14, 52). The pathophysiology of early life stress-induced intestinal dysfunction is poorly understood.
In our previous work (48, 49), our group demonstrated that early life stress in the pig, as a result of early weaning, induces increased intestinal permeability, net electrogenic ion transport, and mucosal inflammation measured within 24 h after weaning. Furthermore, we identified an important role of intestinal CRF receptor activation pathways and mucosal MCs in this acute response. Subsequent experiments in this model demonstrated that weaning age (weaning at 18 vs. 28 days of age) can significantly affect both central and intestinal stress responses in the pig (49). In the present study, our aim is to determine whether early weaning stress in piglets induces long-term changes in intestinal barrier function and intestinal mucosal health and whether minor alterations in weaning age can influence this response.
METHODS
Animals and weaning protocol.
The North Carolina State University Institutional Animal Care and Use Committee approved all studies. Yorkshire cross-bred piglets of either sex were housed in standard farrowing crates with sows of similar parity and subjected to routine management practices. Piglets were assigned to one of five weaning age groups: weaning at 15, 18, 21, 23, or 28 days of age. At respective weaning ages, piglets were removed from the sow and housed in nursery pens for the remainder of the study. Weaned pigs were offered ad libitum access to water and a commercial nursery diet (Renaissance Nutrition, Roaring Spring, PA). At 35 days of age, all of the pigs were sedated with a combination of xylazine (1.5 mg im) and ketamine (11 mg/kg im) followed by euthanasia with an intravenous overdose of pentobarbital via a catheterized ear vein. Initial sedation was used to minimize stress before we obtained intestine for subsequent intestinal studies. Segments of midjejunum were harvested immediately after euthanasia and prepared for Ussing chamber studies. To confirm weaning age-induced long-term effects on mucosal barrier function, we conducted Ussing chamber experiments with samples from early-weaned (15 days of age) and late-weaned (23 days of age) pigs at 9 wk of age.
Ussing chamber experiments.
Segments of midjejunum were harvested from the pig, and the mucosa was stripped from the seromuscular layer in oxygenated (95% O2-5% CO2) Ringer solution (in mmol/l: 154 Na+, 6.3 K+, 137 Cl−, 0.3 H2PO4, 1.2 Ca2+, 0.7 Mg2+, 24 HCO3−; pH 7.4). Tissues were then mounted in 1.13-cm2 aperture Ussing chambers, as described in a previous study (4). Tissues were bathed on the serosal and mucosal sides with 10 ml of Ringer solution. The serosal bathing solution contained 10 mM glucose, which was osmotically balanced on the mucosal side with 10 mM mannitol. Bathing solutions were oxygenated (95% O2-5% CO2) and circulated in water-jacketed reservoirs maintained at 37°C. The spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes, and the PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Tissues were maintained in the short-circuited state, except for brief intervals to record the open-circuit PD. Transepithelial electrical resistance (TER; Ω·cm2) was calculated from the spontaneous PD and short-circuit current (Isc), as previously described (5). After a 30-min equilibration period on Ussing chambers, TER was recorded at 15-min intervals over a 1-h period and then averaged to derive the basal TER values for a given animal. For experiments assessing the role of Cl− transport in alterations in jejunal Isc, mucosal and serosal normal Ringer bathing solutions were replaced with a Cl−-free Ringer solution after a 30-min equilibration period. In additional experiments, BaCl2 (5 mM) was added to the mucosal bathing solutions to assess the role of apical K+ channels in the Isc response.
Mucosal-to-serosal fluxes of 3H-labeled mannitol and [14C]inulin.
Mucosal-to-serosal fluxes of [3H]mannitol and [14C]inulin were performed at the same time as TER was measured. After a 15-min period on Ussing chambers, 0.2 μCi/ml of [3H]mannitol (180 kDa) and [14C]inulin (5,000 kDa) (Sigma, St. Louis, MO) were added to the mucosal side of Ussing chamber-mounted tissues. The isotope was allowed to equilibrate for 15 min after which standards were taken from the mucosal side of each chamber (t = 30 min) and a 60-min flux period was established by taking 0.5-ml samples from the serosal compartment at the beginning and end of the 60-min flux period. The presence of 3H and 14C was established by measuring β-emission in a liquid-scintillation counter (model 1219 Rack Beta, LKB Wallac, Perkin Elmer Life and Analytical Sciences, Boston, MA). Unidirectional [3H]mannitol and [14C]inulin mucosal-to-serosal fluxes were evaluated by determining mannitol and inulin specific activity added to the mucosal bathing solution and by calculating the net appearance of 3H and 14C over time in the serosal bathing solution on a chamber unit area basis.
MC stabilizer drug experiments.
Pigs (35 days) that were previously early weaned at 15 days of age were given intraperitoneal injections of saline vehicle or the MC stabilizer drug cromolyn (20 mg/kg) 24 h before assessment of intestinal mucosal barrier function on Ussing chambers. Pigs were redosed with saline and cromolyn treatments at 16 and 8 h before jejunal tissues were mounted on Ussing chambers for measurement of Isc, TER, and [3H]mannitol fluxes according to protocols described above.
Ex vivo MC activation experiments.
Porcine jejunal mucosa samples from 35-day-old pigs that were previously weaned at 15 days of age were mounted on Ussing chambers as described above. The MC degranulator drug c48/80 (5 μg/ml; Sigma) or CRF (1 μM) was added to the serosal chamber of Ussing chambers. TER, Isc, and [3H]mannitol flux were measured over a 180-min period on chambers. To investigate the role of MC activation and protease activity in response to CRF, cromolyn (10−4 M) or complete serine and cysteine protease inhibitor cocktail tablets (Roche Applied Science, Indianapolis, IN) were dissolved in water according to manufacturer's recommendations and added to the serosal chamber 30 min before CRF addition.
CRF receptor antagonist experiments.
The 35-day-old pigs that were previously weaned at 15 days of age were administered via intraperitoneal injection saline vehicle, the CRFr1/r2 antagonist astressin B (30 μg/kg), or the selective CRFr2 antagonist astressin 2B (30 μg/kg). CRF receptor antagonists were obtained from J. E. F. Rivier (The Salk Institute, La Jolla, CA). Injections for each pig (n = 6 pigs/treatment) were performed at 24, 16, and 8 h before intestinal tissue collection and determination of Isc, TER, and [3H]mannitol flux on Ussing chambers.
MC counts.
Frozen sections of jejunum (5 μm thick) were prepared and then fixed for 1 h in Carnoy's fixative (60% ethanol-30% chloroform-10% glacial acetic acid). Sections were then stained for 45 min at room temperature with 0.5% toluidine blue in 0.5 N HCl in PBS. Sections were then viewed at ×20 objective, and MCs were counted using a grid = 0.043 mm2. MC counts were conducted on five different fields per slide and 6 slides per treatment group (cell counts were expressed as number of MCs/mm2). All cell counts were performed by a blinded reviewer.
Western blotting.
Jejunal mucosal scrapings from pigs were snap frozen and stored at −70°C before SDS-PAGE analysis. Tissue aliquots were thawed at 4°C and added to 3-ml chilled lysis buffer, including protease inhibitors at 4°C, as previously described (48). This mixture was homogenized on ice and then centrifuged at 4°C, and the supernatant was saved. Protein analysis of extract aliquots was performed (DC protein assay; Bio-Rad, Hercules, CA). Tissue extracts (amounts equalized by protein concentration) were mixed with an equal volume of 2× SDS-PAGE sample buffer and boiled for 4 min. Lysates were loaded on a 10% SDS-polyacrylamide gel, and electrophoresis was carried out according to standard protocols. Proteins were transferred to a nitrocellulose membrane (Hybond ECL; Amersham Life Science, Birmingham, UK) by using an electroblotting minitransfer apparatus. Membranes were blocked at room temperature for 60 min in Tris-buffered saline plus 0.05% Tween 20 and 5% dry powdered milk. Membranes were washed and incubated with primary antibody (goat CRF-r1, CRF-r2) and glucocorticoid receptor (GR) polyclonal antibodies (Santa Cruz, Santa Cruz, CA) or mouse MC tryptase monoclonal antibody (Chemicon International, Temecula, CA). A β-actin antibody (Abcam, Cambridge, MA) was used as a control for protein loading. After additional washing was performed, membranes were incubated with horseradish peroxidase-conjugated secondary antibody and developed for visualization of protein by the addition of enhanced chemiluminescence reagent (Amersham, Piscataway, NJ) as previously described (48). After primary immunoblot analysis, membranes were stripped and reprobed with a β-actin antibody (Abcam) to ensure equal protein loading within lanes. Quantitative results were obtained by scanning the resulting images and analyzing them densitometrically using SigmaScan Pro 5 software (Point Richmond, CA).
CRF and cortisol ELISA.
Blood samples were obtained from pigs via venipuncture before euthanasia. Pigs were sedated before blood collection to minimize the stress of sampling procedures. All samples were taken at the same time of day to minimize the effects of diurnal rhythms. Serum was separated by centrifugation (20 min, 10,000 g), and the serum was stored at −80°C until analysis. Serum levels of CRF and cortisol were determined using commercial ELISA kits (CRF from Phoenix Pharmaceuticals, Belmont, CA; cortisol from R&D Systems, Minneapolis, MN). For CRF ELISA analyses on intestinal mucosa, intestinal samples were prepared according to manufacturer's instructions.
Histopathology.
Jejunal tissues were harvested from pigs and fixed in 10% neutral buffered formalin until processing. Paraffin-embedded intestinal samples were sectioned (5 μm) and stained with hematoxylin and eosin for histological analysis. Measurements for crypt depth, villous height, and villous width were taken by utilizing the measurement caliper option in the Infinity Analyze Software (Lumenera, Ottawa, Ontario, Canada). Lamina propria cell counts were performed at ×20 using a 0.043-mm2 grid (lamina propria cell counts were expressed as number of lamina propria cells/mm2).
Statistical analyses.
Data are reported as means ± SE based on the experimental number (n). Data were analyzed by using a standard one-way ANOVA (Sigmastat, Jandel Scientific, San Rafael, CA). A post hoc Tukey test was used to determine differences between treatments following ANOVA. For time course experiments on Ussing chambers, repeated-measures ANOVA was employed.
RESULTS
Influence of weaning age on postweaning intestinal barrier function.
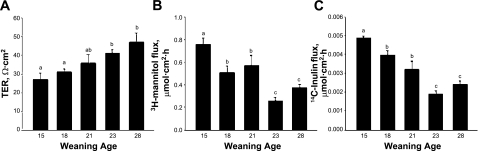
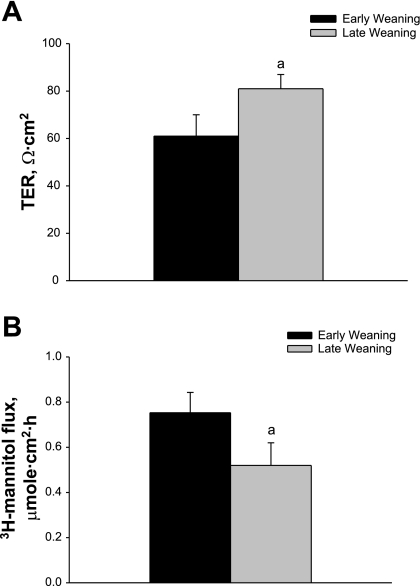
In previous studies (48, 49), we demonstrated that early weaning occurring at 18 days of age in the piglet induces acute disturbances in intestinal barrier measured 24 h after weaning. Delaying weaning to 28 days of age was protective against weaning-induced alteration in barrier function (48, 49). In the present study, we examined whether incremental alterations in weaning age could alter long-term intestinal barrier function in pigs. To determine this, piglets were weaned at 15, 18, 21, 23, or 28 days of age, and jejunal barrier function was assessed on Ussing chambers in all pigs at 35 days of age. Results from this study show that, as weaning age was incrementally increased, improvements in jejunal barrier function were observed, as indicated by higher baseline TER and lower [3H]mannitol and [14C]inulin permeability (Fig. 1). Pigs weaned at 15 days of age exhibited the lowest TER and highest [3H]mannitol and [14C]inulin permeability compared with all other weaning age treatments. Pigs weaned at 18 or 21 days of age had similar barrier function measurements that were improved (P < 0.05) compared with 15-day-old weaned pigs; however, these results were lower (P < 0.05) than those for pigs weaned ≥23 days of age. Pigs weaned at 28 days of age exhibited TER, [3H]mannitol, and [14C]inulin flux rates similar to pigs weaned at 23 days of age, suggesting that increasing weaning age beyond 23 days of age in the pig did not result in measureable improvements in mucosal barrier function. Similar findings were observed in porcine colonic tissues, suggesting that other regions of the GI tract are similarly affected (data not shown). To confirm that observed alterations in mucosal barrier function were indeed sustained, we conducted experiments with early-weaned (15 day weaning age) and late-weaned (28 day weaning age) pigs at 9 wk of age. These experiments revealed findings (reduced TER and elevations in [3H]mannitol permeability) similar to those observed in 35-day-old pigs (Fig. 2).
Fig. 1.
Effects of weaning age on postweaning barrier function in porcine jejunum. Pigs were subjected to different weaning ages (at 15, 18, 21, 23, and 28 days old), and jejunal tissues were harvested from 35-day-old pigs for measurement of transepithelial electrical resistance (TER) (A), mucosal-to-serosal [3H]mannitol flux (B), and mucosal-to-serosal [14C]inulin flux (C). Values are means ± SE (n = 6 pigs per weaning age treatment). a,b,cSignificantly different (P < 0.05) by ANOVA.
Fig. 2.
Effects of weaning age on postweaning short-circuit current (Isc) in porcine jejunum. Pigs were subjected to different weaning ages (15, 18, 21, 23, and 28 days old), and jejunal tissues were harvested from 35-day-old pigs for measurement of Isc. Values are means ± SE (n = 6 pigs per weaning age treatment). a,b,cSignificantly different (P < 0.05) by ANOVA.
Influence of weaning age on jejunal Isc.
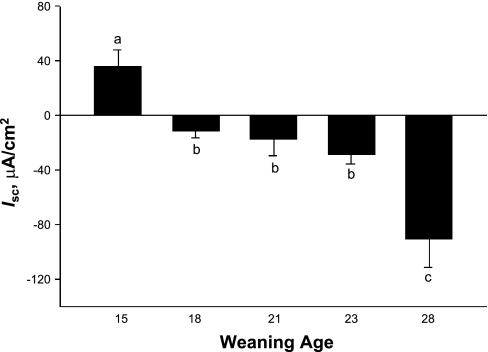
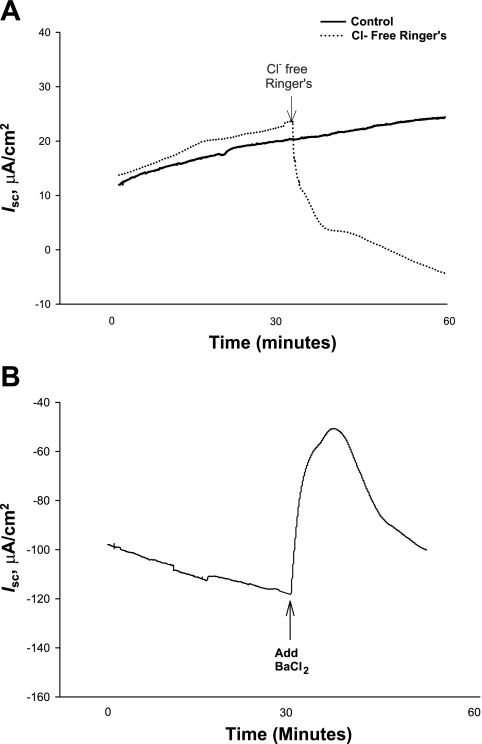
Weaning age influenced long-term net electrogenic ion transport in terms of Isc across the porcine jejunum. Pigs weaned at 15 days of age displayed the highest (P < 0.05) baseline Isc compared with all other weaning age groups (Fig. 3). As weaning age increased from 15 to 28 days of age, the baseline jejunal Isc values became negative, with pigs weaned at 28 days of age exhibiting the largest negative Isc values. To determine the nature of elevated baseline Isc observed in jejunum from early-weaned piglets, we conducted studies with Cl−-free Ringer. Replacement of Cl−-free Ringer caused reductions in Isc in jejunum from early-weaned piglets (P < 0.01), indicating that Cl− secretion is contributing to heightened baseline secretory activity (Fig. 4A). Studies were also conducted in jejunum from late-weaned pigs (at 28 days) to investigate the nature of the large, negative baseline Isc that we observed. Given that negative Isc is likely a result of cation secretion rather that electrogenic anion absorption in the jejunum (26), we focused our studies on luminal K+ secretion, which has been demonstrated to occur in the rat jejunum (18). Mucosal application of the K+ channel blocker BaCl2 (5 mM) led to a positive deflection in jejunal Isc (Fig. 4B), thus indicating that K+ secretion is mediating a significant portion of the negative Isc observed in jejunum from late-weaned pigs.
Fig. 3.
Effects of weaning age on long-term mucosal barrier function in porcine jejunum. Pigs were weaned either at 15 days of age (early weaning) or 28 days of age (late weaning), and jejunal tissues were harvested from 9-wk-old pigs for measurement of TER (A) and mucosal-to-serosal [3H]mannitol flux (B). aSignificantly different (P < 0.05) by ANOVA.
Fig. 4.
Mechanisms of altered Isc as influenced by weaning age. A: jejunum from 35-day-old pigs that were early weaned (at 15 days of age) was mounted on Ussing chambers. After a 30-min equilibration period, normal Ringer solution was replaced with Cl−-free Ringer solution, and resultant changes in Isc were monitored. Data represent averages from 5 pigs and were analyzed using repeated-measures ANOVA. Treatments differed by P < 0.05; pooled SE value = 4.3. B: jejunum from late-weaned pigs (at 28 days of age) was treated with the K+ channel blocker BaCl2 on the mucosal surface, after which changes in Isc were monitored. Data represent averages from 5 pigs and were analyzed using repeated-measures ANOVA. BaCl2 treatment induced a positive deflection in Isc (P < 0.05).
Histological analyses of jejunal mucosa.
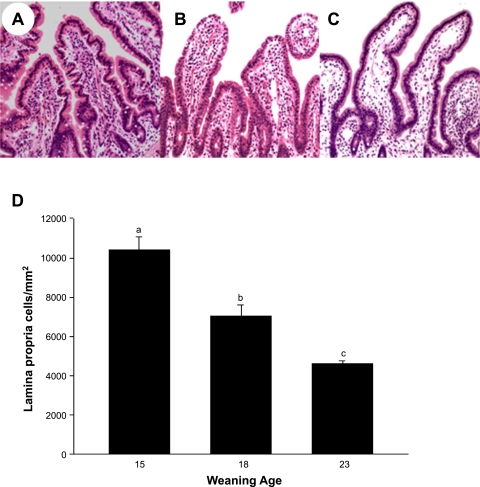
We conducted morphological analyses (villus height and width and crypt depth) of jejunal tissues to determine whether changes in intestinal mucosal physiology were attributable to physical changes in the intestinal mucosa. Weaning age had no influence on long-term morphological measurements of the jejunal mucosa, suggesting that changes observed with regard to intestinal permeability and Isc were functional rather than physical changes in mucosal architecture (data not shown). Although there were no morphological changes, there were marked differences in the numbers of cellular infiltrates within the lamina propria between weaning age groups. Jejunum from pigs weaned at 15 days of age had the greatest density of lamina propria cell infiltrates (Fig. 5) compared with samples from other weaning ages, suggesting increased mucosal inflammation and antigen stimulation, presumably due to impaired mucosal barrier function. The cellular infiltrates were predominantly lymphocytes and macrophages with occasional neutrophils. As weaning age increased, lamina propria cell density was reduced.
Fig. 5.
Histological analysis of porcine jejunum as influenced by weaning age. Results show representative jejunal sections from 35-day-old pigs previously subjected to different weaning ages. A: weaning at 15 days old. B: weaning at 18 days old. C: weaning at 23 days old. D: lamina propria cell counts. Notice the increased cellularity of the lamina propria in jejunum from early-weaned pig compared with jejunum from pigs weaned at older ages. Values are means ± SE (n = 6 pigs per weaning age treatment). a,b,cSignificantly different (P < 0.05) by ANOVA.
Influence of weaning age on intestinal mucosal MC activity.
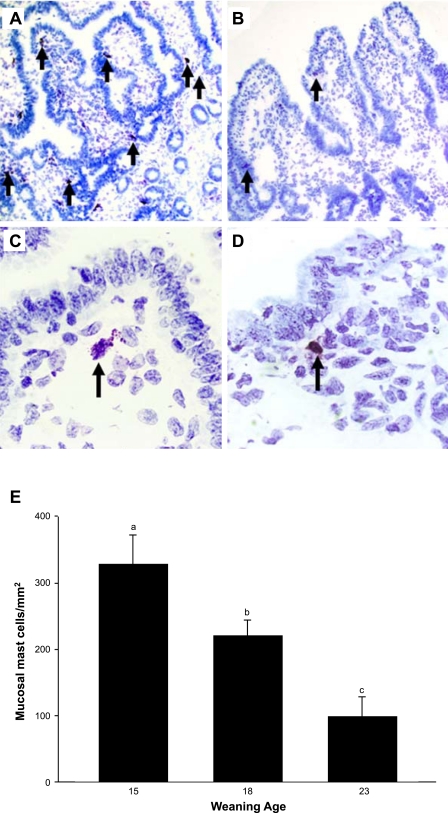
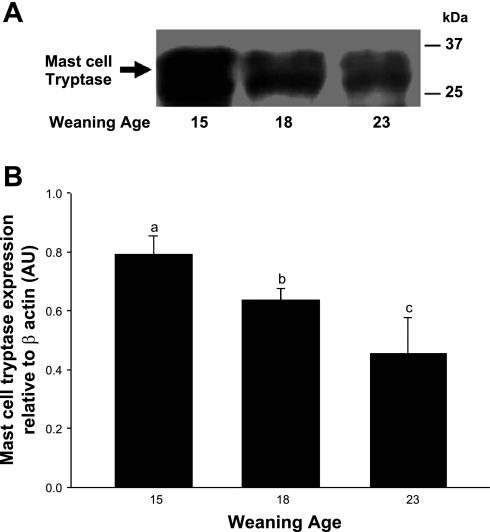
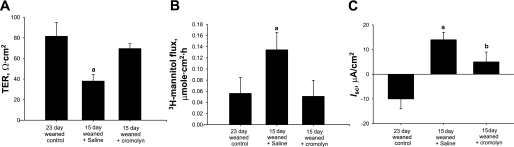
In our previous study (49), we showed that early weaning at 18 days of age in the piglet resulted in intestinal MC hyperplasia and activation. Furthermore, inhibition of MC activation by administration of cromolyn before early weaning prevented intestinal barrier dysfunction, demonstrating the central role of MCs in weaning-induced intestinal mucosal dysfunction. In the present study, we assessed whether weaning age influenced long-term mucosal MC activity by conducting studies in 35-day-old pigs. Jejunal mucosa from early-weaned piglets had greater numbers of mucosal MCs present within the lamina propria than the later-weaned pigs (P < 0.01; Fig. 6). Jejunum from early-weaned pigs also exhibited greater numbers of MCs that were actively releasing their intracellular contents than shown in late-weaned pigs (Fig. 6C), indicative of ongoing MC activation. Intestinal mucosal protein levels of the most abundant MC mediator, MC tryptase, was greatest in jejunal mucosa of pigs weaned at 15 days of age and levels decreased as weaning age increased (Fig. 7). The role of MC function in early weaning-induced jejunal barrier dysfunction was confirmed in in vivo experiments, as administration of cromolyn to early-weaned pigs ameliorated mucosal barrier dysfunction and hypersecretion (Fig. 8).
Fig. 6.
Effect of weaning age on mast cell (MC) numbers and activation. Porcine jejunum from early-weaned (A and C) and late-weaned (B and D) pigs were fixed in Carnoy's fixative, sectioned, and stained with toluidine blue to visualize MCs. Arrows indicate toluidine blue-positive MCs. Increased numbers of degranulated MCs were observed in tissues from early-weaned pigs (C; ×100 magnification) compared with jejunum from late-weaned pigs (D). E: MC counts were performed in sections from 4 pigs and are expressed as the number of toluidine blue-positive cells/mm2 tissue. a,b,cSignificantly different (P < 0.05) by ANOVA.
Fig. 7.
MC tryptase expression in porcine jejunum. Porcine jejuna mucosa from pigs weaned at different ages was harvested from pigs at 35 days of age, and Western blot analysis was performed to assess MC tryptase expression levels. Jejunal MC tryptase expression decreased as weaning age increased as determined by SDS-PAGE (A) and densitometric analysis relative to β-actin (B). AU, arbitrary units. a,b,cSignificantly different (P < 0.05) by ANOVA.
Fig. 8.
Effect of in vivo MC inhibition on intestinal barrier function and Isc in early-weaned porcine jejunum. Values represent means ± SE; n = 6 animals. Pigs (35 days of age) were injected with cromolyn (20 mg/kg ip) or saline vehicle (control) 24, 16, and 8 h before mounting jejunum on Ussing chambers. Compared with jejunum from late-weaned control (23 day weaning), jejunum from early-weaned (15 day weaning) pigs treated with saline vehicle had lower TER and greater mucosal-to-serosal [3H]mannitol flux and Isc. Cromolyn treatment restored TER, [3H]mannitol flux, and Isc to late-weaned control levels. aP < 0.05 (ANOVA).
Effect of weaning age on stress signaling pathways.
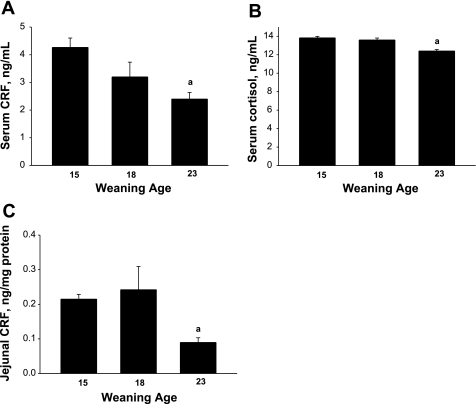
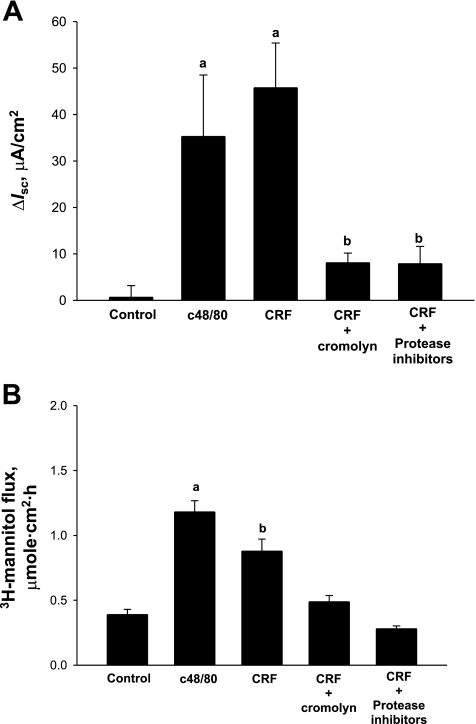
To determine whether weaning age influenced baseline activity of the hypothalamic-pituitary-adrenal (HPA) axis, we measured serum CRF and cortisol in early-weaned and late-weaned 35-day-old pigs. Serum CRF and cortisol levels were greater in early-weaned pigs than in late-weaned pigs (P < 0.05), indicating alterations in HPA axis activity (Fig. 9). Given that CRF can be produced locally in the gut (50) and independent of the HPA axis, we analyzed jejunal mucosa for CRF protein. CRF levels were lower in jejunum from late-weaned pigs than in jejunum from early-weaned pigs, indicating enhanced production of local CRF in intestinal tissues of early-weaned pigs (Fig. 9C). To investigate the function of local CRF on disturbances in intestinal mucosal function, we treated porcine jejunum mounted on Ussing chambers with CRF (1 μM) and measured changes in TER, [3H]mannitol flux, and Isc. CRF triggered elevations in Isc and [3H]mannitol flux (Fig. 10). However, no alterations were noted with TER (data not shown). Activation of MCs with c48/80 (5 μg/ml) elicited similar responses to CRF. To assess whether CRF-induced changes in mucosal function were due to MC activation, we pretreated jejunal tissues with the MC stabilizer agent cromolyn. This approach inhibited CRF-induced elevations in Isc and [3H]mannitol flux. Furthermore, CRF-induced changes were also blocked with protease inhibitor treatments. Functional alterations in MC activation by CRF were confirmed with MC histochemistry, demonstrating enhanced MC activation and granule release in CRF-treated tissues (data not shown). Overall, these data demonstrate that early weaning enhances production of CRF in the jejunum, which contributes to mucosal dysfunction via MC protease-dependent mechanisms.
Fig. 9.
Effect of weaning age on hypothalamic-pituitary-adrenal axis activity. Serum samples from 35-day-old pigs that were weaned at 15, 18, and 23 days of age were analyzed for corticotrophin-releasing factor (CRF) and cortisol levels. Early-weaned pigs (at 15 and 18 days) exhibited higher baseline serum CRF (A) and cortisol (B) than late-weaned pigs. Jejunal CRF was measured using ELISA methods (C), which demonstrated that early-weaned pigs (at 15 and 18 days) exhibited higher CRF levels than late-weaned pigs. Values are means (n = 6 animals/treatment) ± SE. aP < 0.05 (ANOVA).
Fig. 10.
Effects of CRF on Isc (A) and barrier function (B) in porcine jejunum mounted on Ussing chambers. Early-weaned porcine jejunum was mounted on Ussing chambers and treated with CRF (1 μM) or the MC activator c48/80 (5 μg/ml) on the serosal surface of tissues. Cromolyn (10−4 M) and protease inhibitors (Roche Complete Mini tablets) were added 30 min before CRF. Values represent means (n = 6 animals/treatment) ± SE. a,bSignificantly different (P < 0.05) by ANOVA.
CRF receptor signaling in early weaning-induced mucosal barrier dysfunction.
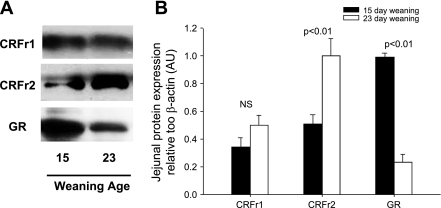
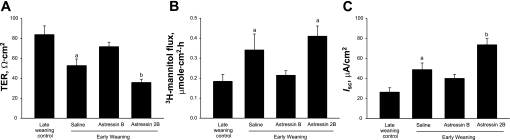
CRF mediates its effects through binding to G-protein-coupled CRF receptors that are expressed on multiple intestinal cell types, including enteric neurons, lamina propria immune cells, and epithelial cells (36, 50, 64). In our previous study (48), we demonstrated that peripheral CRF receptor activation mediates intestinal mucosal disturbances induced by early weaning. In the present study, we aimed to determine whether CRF receptor activation was mediating sustained mucosal barrier impairment in porcine jejunum and to investigate which CRF receptor subtype was involved. We first investigated expression of CRF receptor subtypes (CRFr1 and CRFr2) in jejunum from early- and late-weaned pigs (Fig. 11). Jejunal CRFr1 protein expression was not influenced by weaning age; however, CRFr2 expression was greater (P < 0.001) in jejunum from late-weaned pigs. We also investigated the expression of the glucocorticoid receptor and found that GR expression was greater in jejunal mucosa from early-weaned pig than in jejunum from late-weaned pig. To elucidate the role of CRF receptor subtypes in early weaning-induced mucosal dysfunction, we administered selective CRFr antagonist compounds [astressin B (inhibits both CRFr1 and CRFr2) and astressin 2B (selectively inhibits CRFr2)] to 35-day-old pigs that were previously weaned at 15 days of age. Pharmacological blockade of both CRFr1 and CRFr2 ameliorated disturbances in barrier function (increased jejunal TER and reduced [3H]mannitol flux and Isc) (Fig. 12). However, selective inhibition of CRFr2 with astressin 2B enhanced mucosal barrier dysfunction (reduced TER and elevated [3H]mannitol flux and Isc). Overall, these data suggest that, in jejunum from the early-weaned pig, CRFr1 is mediating barrier disruption and hypersecretion, whereas CRFr2 acts in an opposing manner.
Fig. 11.
CRF receptor subtype and glucocorticoid receptor (GR) expression in jejunum from early- and late-weaned pigs. A: Western analysis of CRFr1 (50–55 kDa), CRFr2 (50–55 kDa), and GR (95 kDa) in porcine jejuna mucosa. B: densitometric analysis revealed enhanced expression of CRFr2 in jejunum from late-weaned pigs, whereas GR expression was elevated but decreased as weaning age increased. Values, expressed relative to β-actin control (n = 6 animals/treatment), are means ± SE.
Fig. 12.
Effect of in vivo CRF receptor antagonists on intestinal barrier function and Isc in early-weaned porcine jejunum. Values represent means ± SE; n = 8 animals. Pigs (35 days of age) were injected intraperitoneally with saline vehicle, astressin B (CRFr1/r2 antagonist, 30 μg/kg), or astressin 2B (CRFr2 antagonist, 30 μg/kg) 24, 16, and 8 h before jejunum was mounted on Ussing chambers. Compared with jejunum from late-weaned controls (23 day weaning), jejunum from early-weaned pigs (15 day weaning) treated with saline vehicle had lower TER (A) and greater mucosal-to-serosal [3H]mannitol flux (B) and Isc (C). Astressin B treatment restored TER, [3H]mannitol flux, and Isc to late-weaned control levels. Astressin 2B treatment enhanced barrier dysfunction, as indicated by reductions in TER and elevations in Isc compared with tissues from late-weaned controls. a,bSignificantly different (P < 0.05) by ANOVA.
DISCUSSION
The intestinal epithelial barrier is the first line of defense against a hostile environment within the intestinal lumen. Disruption in the intestinal barrier leads to translocation of luminal antigens across the epithelium and into lamina propria tissues, triggering mucosal inflammation and sepsis (3, 6, 15, 21, 23, 35, 45). Here, we show in a porcine model that early weaning can markedly impair the postweaning development of mucosal barrier function, an effect mediated by activation of CRF signaling pathways and chronic activation of intestinal MCs.
The stress response involves coordinated regulation of central and peripheral signaling pathways that are critical to maintain homeostasis and survival. However, excessive or chronic stress can overwhelm the ability of the individual to mount an adequate stress response, leading to disturbances in homeostasis and subsequently disease. In the present study, we demonstrate that early weaning stress in pigs induces chronic activation of the HPA stress axis, as indicated by higher serum levels of CRF and adrenal cortisol measured 20 days postweaning. This response is similar to that shown in rodent models of neonatal maternal separation, in which chronic elevations in serum corticosterone levels were triggered (2, 27). The precise mechanisms for sustained elevations of serum CRF and cortisol in early-weaned pigs are presently unclear. It is well known that adrenal corticosteroid secretion is regulated by negative feedback systems where circulating cortisol binds to glucocorticoid receptors in the hippocampus and downregulates CRF, ACTH, and adrenal corticosteroid release. However, this negative feedback system has been shown to be impaired in adult animals that were previously subjected to maternal separation stress in the neonatal period (63), which may explain our findings in the early-weaned pig. It remains unclear as to the exact role of HPA activation in intestinal barrier dysfunction observed in the present study. Intestinal permeability and hypersecretion induced by restraint stress in rats were shown to be independent of the HPA axis activation (55). However, other studies have shown that adrenal corticosteroids released in response to stress can cause disruption in intestinal barrier function, suggesting that HPA axis-derived glucocorticoids may be involved (46). In the present study, we observed increased expression of GC receptors in the jejunum of early-weaned pigs; however, the exact role of this signaling pathway in this model remains to be elucidated.
The present study demonstrated that intestinal MCs play a central role in early weaning-induced mucosal barrier dysfunction in the porcine jejunum. MCs play an important role in the innate responses to epithelial disturbances by releasing mediators that cause increases in intestinal secretion and motility to flush out invading pathogens and recruit inflammatory cells to the area to control the infection at the site of the breached epithelial barrier (47, 53). However, excessive or chronic MC activation is detrimental and is implicated in several prevalent human GI disorders, including food allergies, C. difficile enteritis, IBS, and inflammatory bowel diseases (7, 20). MCs express receptors for CRF and therefore have been shown to be activated by CRF and psychological and physical stressors (55, 57). In the present study, we showed that CRF is upregulated in the jejunum from pigs that were previously subjected to early weaning. Furthermore, CRF applied to isolated mucosa on Ussing chambers mimicked early weaning-induced mucosal barrier dysfunction and hypersecretion via MC-dependent pathways. This in line with rodent studies reporting that CRF-induced intestinal permeability can be prevented by MC stabilizer agents and MC-deficient rats do not exhibit stress-induced intestinal barrier dysfunction (55, 62). Although the CRF-MC association clearly exists, the signaling between CRF and MCs has not been studied in detail. MCs express CRF receptors, indicating the possibility for direct activation of MCs by CRF (16). Our group has previously shown that CRF receptors are colocalized with mucosal MCs in the porcine intestine (49), and in the present study we show that CRF activates intestinal mucosal MCs initiating breakdown in barrier function. Similar findings have been observed in colonic biopsies from humans (65). Overall, data from this study demonstrate that early weaning stress in pigs triggers sustained elevations in mucosal CRF that are capable of inducing MC activation and mucosal barrier dysfunction. The exact source of CRF remains to be fully elucidated, although CRF was shown to be expressed in multiple cell types within the intestine, including enteric neurons, epithelial cells, and immune cells (50).
The mechanism by which MCs trigger chronic barrier dysfunction in response to early weaning is still unclear. Here, we show that MC tryptase was increased in jejunum from early-weaned pigs and that CRF-induced intestinal barrier dysfunction in porcine jejunum ex vivo was MC protease dependent. In agreement with these findings, MC tryptase was shown to induce increased epithelial permeability in the T84 colonic epithelial cell line, mediated by activation of protease-activated receptor 2 on colonic epithelial cells, triggering tight junction protein breakdown and increased T84 cell paracellular permeability (32). Tryptase has also been shown to have a protective role in the intestine, especially in bacterial and parasitic infections (17). Tryptase can upregulate adhesion molecules, increase vascular permeability, recruit eosinophils, and increase epithelial and fibroblast proliferation (66). It should also be noted that other MC mediators such TNF-α initiate changes in epithelial permeability (37). In fact, studies have shown TNF-α to trigger disruption of tight junction proteins in a model of restraint stress (43). Our findings suggest a key role of MC proteases in intestinal barrier dysfunction triggered by CRF. However, the contributing roles of other MC mediators released in response to stress require further investigation.
In the present study, we showed differential roles of CRF receptor subtypes in intestinal function as a result of early life stress in the pig. Based on CRF receptor antagonist experiments, we show that CRFr1 is likely mediating impaired barrier dysfunction and hypersecretion in early-weaned pigs, whereas CRFr2 may play a protective role as inhibition of CRFr2 enhanced mucosal barrier dysfunction and hypersecretion. Furthermore, enhanced barrier function in late-weaned pigs coincided with enhanced intestinal expression of CRFr2. In contrast to our findings, CRFr2 or combinations of CRFr1 and CRFr2 activation were shown to be responsible for intestinal barrier dysfunction in response to stress in rodents (62). Recently, Wallon et al. (65) showed that CRF induced hyperpermeability in human colonic biopsies that were inhibited with both CRFr1- and CRFr2-selective antagonists.
Weaning age had a pronounced effect on postweaning development of electrogenic ion transport in the jejunum. Jejunum from early-weaned pigs exhibited a net positive Isc contributed by electrogenic Cl− secretion. As weaning age increased, there was a decline in baseline Isc, which was shown to be attributed to apical K+ secretion.
In the present study, we confirmed that enhanced Isc in jejunum from early-weaned pig was due to MC activation and CRFr1 activation. Furthermore, in ex vivo experiments, we showed that CRF-induced Isc was attenuated with MC protease inhibitors, indicating a prominent role of proteases in mucosal secretion. The exact protease regulating mucosal secretion is presently unclear. Several studies have indicated that tryptase can induce electrogenic ion transport in colon and airway epithelium via activation of protease-activated 2 receptors (33, 34, 39). Other factors such as enhanced intestinal inflammation due to impaired mucosal barrier function and activation of neurogenic secretory pathways are also likely contributing or enhancing intestinal secretory tone in the jejunum from early-weaned pig. As mentioned above, electrogenic K+ secretion, through barium-sensitive channels, contributes to the negative jejunal Isc observed in late-weaned pigs. The significance of this phenomenon is presently unclear. Luminal K+ secretion is a homeostatic mechanism to rid the body of excess K+. Luminal K+ secretion is also involved in other homeostatic processes, including regulatory cell volume decrease, Na+ transport, wound healing, and apoptosis (29, 31). Therefore, the enhanced K+ may represent the enhanced control of homeostatic mechanisms in the jejunum for older weaned pigs. It should be noted that, in the present study, we observed differences in baseline Isc and permeability across the different experiments. For example, in our initial experiments, baseline Isc measurements ranged from largely negative values in late-weaned pigs, which we determined to be due to electrogenic K+ secretion. However, in some experiments, baseline Isc values were positive (Fig. 12). Differences in baseline permeability measurements also differed across experiments. The discrepancies in these data could result from several different experimental factors. First, in the CRFr antagonist experiments (Fig. 12), pigs were handled more frequently to administer intraperitoneal injections, which could have increased colonic Isc with the additional and likely stressful procedures. Other factors that could have contributed to the data variation in the present study include inherent differences between different litters, environmental temperature changes, as studies were conducted in different seasons, and management of animals within the swine barn. Nonetheless, within each experiment, treatment results were consistent with regard to early vs. late weaning, with early-weaned pigs exhibiting high baseline Isc compared with late-weaned pigs.
In previous experiments (49), we showed that early weaning stress triggers MC activation responsible for mucosal barrier disturbances. In the present study, we observed increased MC numbers and activation in jejunal mucosa from early-weaned pigs at 20 days postweaning, suggesting chronic MC activation in these tissues. The signaling events leading to MC hyperplasia are not fully understood. In infection models, MC recruitment was shown to be T-cell dependant as T-cell-deficient mice or athymic nude mice do not exhibit MC hyperplasia in Nippostrogylus brasiliensis (41, 42) or Trichinella spiralis infections (54). Antibodies to IL-3 and IL-4 also suppressed intestinal mastocytosis in parasite-infected mice, demonstrating a role of these cytokine in MC recruitment (38). Stress has also been shown to increase MCs numbers in the intestinal mucosa (9, 11, 56). Patients with stress-related GI disorders such as IBS often exhibit MC hyperplasia and increased MC activation that correlate with symptom severity (7, 8, 24). The mechanism of MC hyperplasia in these disorders is not well understood but may be related to CRF levels. Teitelbaum et al. (62) showed that administration of CRF to rats induced colonic MC hyperplasia, indicating that CRF may be involved in regulating MC numbers to intestinal tissues.
Early life stress is increasingly recognized as an important risk factor in the development and onset of chronic intestinal disease (13, 67). Here, we show that early weaning in the pig triggers long-term defects in intestinal barrier function and electrogenic ion transport mediated through CRFr-MC interactions. In addition, we have identified divergent roles for CRFr subtypes in the postweaning development of mucosal barrier function in pigs subjected to early weaning stress.
GRANTS
This work was supported by the United States Department of Agriculture National Research Initiative Grant 0701366 and National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK-34987, K08 DK-084313 (A. J. Moeser), and PO1 DK-26741 (J. E. F. Rivier).
ACKNOWLEDGMENTS
J. E. F. Rivier is the Dr. Frederik Paulsen Chair in Neurosciences Professor and Founder of Sentia Medical Sciences.
REFERENCES
- 1.Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology 106: 945–950, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience 154: 1218–1226, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Ammori BJ, Leeder PC, King RF, Barclay GR, Martin IG, Larvin M, McMahon MJ. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg 3: 252–262, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Argenzio RA, Henrikson CK, Liacos JA. Restitution of barrier and transport function of porcine colon after acute mucosal injury. Am J Physiol Gastrointest Liver Physiol 255: G62–G71, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Argenzio RA, Liacos JA. Endogenous prostanoids control ion transport across neonatal porcine ileum in vitro. Am J Vet Res 51: 747–751, 1990 [PubMed] [Google Scholar]
- 6.Barbara G. Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am J Gastroenterol 101: 1295–1298, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126: 693–702, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 132: 26–37, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology 127: 524–534, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol 580: 347–356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut 57: 582–590, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 43: 256–261, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard EB, Keefer L, Lackner JM, Galovski TE, Krasner S, Sykes MA. The role of childhood abuse in Axis I and Axis II psychiatric disorders and medical disorders of unknown origin among irritable bowel syndrome patients. J Psychosom Res 56: 431–436, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Blanchard EB, Keefer L, Payne A, Turner SM, Galovski TE. Early abuse, psychiatric diagnoses and irritable bowel syndrome. Behav Res Ther 40: 289–298, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Cao J, Cetrulo CL, Theoharides TC. Corticotropin-releasing hormone induces vascular endothelial growth factor release from human mast cells via the cAMP/protein kinase A/p38 mitogen-activated protein kinase pathway. Mol Pharmacol 69: 998–1006, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev 217: 141–154, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cermak R, Evelgunne A, Lawnitzak C, Scharrer E. K+ secretion in rat distal jejunum. J Membr Biol 171: 235–243, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Crowe SE, Perdue MH. Functional abnormalities in the intestine associated with mucosal mast cell activation. Reg Immunol 4: 113–117, 1992 [PubMed] [Google Scholar]
- 20.Crowe SE, Perdue MH. Gastrointestinal food hypersensitivity: basic mechanisms of pathophysiology. Gastroenterology 103: 1075–1095, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Deitch EA, Rutan R, Waymack JP. Trauma, shock, and gut translocation. New Horiz 4: 289–299, 1996. [PubMed] [Google Scholar]
- 22.Delvaux MM. Stress and visceral perception. Can J Gastroenterol 13, Suppl A: 32A–36A, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med 158: 444–451, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Farhadi A, Fields JZ, Keshavarzian A. Mucosal mast cells are pivotal elements in inflammatory bowel disease that connect the dots: stress, intestinal hyperpermeability and inflammation. World J Gastroenterol 13: 3027–3030, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farhadi A, Keshavarzian A, Van de Kar LD, Jakate S, Domm A, Zhang L, Shaikh M, Banan A, Fields JZ. Heightened responses to stressors in patients with inflammatory bowel disease. Am J Gastroenterol 100: 1796–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931–943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut 56: 1522–1528, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol 293: G198–G203, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Grishin A, Ford H, Wang J, Li H, Salvador-Recatala V, Levitan ES, Zaks-Makhina E. Attenuation of apoptosis in enterocytes by blockade of potassium channels. Am J Physiol Gastrointest Liver Physiol 289: G815–G821, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Gue M, Del Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 9: 271–279, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88: 1119–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, Coelho AM, Singh P, Grady EF, Perdue M, Bunnett NW. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem 280: 31936–31948, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Kunzelmann K, Schreiber R, Konig J, Mall M. Ion transport induced by proteinase-activated receptors (PAR2) in colon and airways. Cell Biochem Biophys 36: 209–214, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Kunzelmann K, Sun J, Markovich D, Konig J, Murle B, Mall M, Schreiber R. Control of ion transport in mammalian airways by protease activated receptors type 2 (PAR-2). FASEB J 19: 969–970, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc Sport Sci Rev 32: 185–190, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang GD, Xia Y, Wood JD. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J Comp Neurol 481: 284–298, 2005 [DOI] [PubMed] [Google Scholar]
- 37.MA TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol 286: G367–G376, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Madden KB, Urban JF, Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol 147: 1387–1391, 1991 [PubMed] [Google Scholar]
- 39.Mall M, Gonska T, Thomas J, Hirtz S, Schreiber R, Kunzelmann K. Activation of ion secretion via proteinase-activated receptor-2 in human colon. Am J Physiol Gastrointest Liver Physiol 282: G200–G210, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Martinez V, Rivier J, Wang L, Tache Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther 280: 754–760, 1997 [PubMed] [Google Scholar]
- 41.Mayrhofer G. The nature of the thymus dependency of mucosal mast cells. II. The effect of thymectomy and of depleting recirculating lymphocytes on the response to Nippostrongylus brasilliensis. Cell Immunol 47: 312–322, 1979 [DOI] [PubMed] [Google Scholar]
- 42.Mayrhofer G, Fisher R. Mast cells in severely T-cell depleted rats and the response to infestation with Nippostrongylus brasiliensis. Immunology 37: 145–155, 1979 [PMC free article] [PubMed] [Google Scholar]
- 43.Mazzon E, Cuzzocrea S. Role of TNF-α in ileum tight junction alteration in mouse model of restraint stress. Am J Physiol Gastrointest Liver Physiol 294: G1268–G1280, 2008 [DOI] [PubMed] [Google Scholar]
- 44.McKay DM, Perdue MH. Establishing epithelial-immune cell co-cultures. Effects on epithelial ion transport and permeability. Methods Mol Biol 188: 359–371, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Meddings J. Barrier dysfunction and Crohn's disease. Ann NY Acad Sci 915: 333–338, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology 119: 1019–1028, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 77: 1033–1079, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol 292: G173–G181, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol 293: G413–G421, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides 21: 1799–1809, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Nozu T, Kudaira M. Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J Gastroenterol 41: 740–744, 2006 [DOI] [PubMed] [Google Scholar]
- 52.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65: 263–267, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann NY Acad Sci 1143: 83–104, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature 264: 258–260, 1976 [DOI] [PubMed] [Google Scholar]
- 55.Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol Gastrointest Liver Physiol 277: G391–G399, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut 48: 630–636, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santos J, Yates D, Guilarte M, Vicario M, Alonso C, Perdue MH. Stress neuropeptides evoke epithelial responses via mast cell activation in the rat colon. Psychoneuroendocrinology 33: 1248–1256, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Siddiqui AA, Miner PB., Jr The role of mast cells in common gastrointestinal diseases. Curr Allergy Asthma Rep 4: 47–54, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Soderholm JD, Yates DA, Gareau MG, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol 283: G1257–G1263, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Tache Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol 13, Suppl A: 18A–25A, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol 141: 1321–1330, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teitelbaum AA, Gareau MG, Jury J, Yang PC, Perdue MH. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol 295: G452–G459, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 23: 663–700, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Von Mentzer B, Murata Y, Ahlstedt I, Lindstrom E, Martinez V. Functional CRF receptors in BON cells stimulate serotonin release. Biochem Pharmacol 73: 805–813, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Soderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 57: 50–58, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Walls AF, He S, Teran LM, Buckley MG, Jung KS, Holgate ST, Shute JK, Cairns JA. Granulocyte recruitment by human mast cell tryptase. Int Arch Allergy Immunol 107: 372–373, 1995 [DOI] [PubMed] [Google Scholar]
- 67.Whitehead WE. Psychosocial aspects of functional gastrointestinal disorders. Gastroenterol Clin North Am 25: 21–34, 1996. [DOI] [PubMed] [Google Scholar]