Abstract
The development of alcoholic fatty liver is associated with reduced adipocyte-derived adiponectin levels, decreased hepatic adiponectin receptors, and deranged hepatic adiponectin signaling in animals. Peroxisomal proliferator-activated receptor-γ (PPAR-γ) plays a key role in the regulation of adiponectin in adipose tissue. The aim of the present study was to test the ability of rosiglitazone, a known PPAR-γ agonist, to reverse the inhibitory effects of ethanol on adiponectin expression and its hepatic signaling, and to attenuate alcoholic liver steatosis in mice. Mice were fed modified Lieber-DeCarli ethanol-containing liquid diets for 4 wk or pair-fed control diets. Four groups of mice were given a dose of either 3 or 10 mg·kg body wt−1·day−1 of rosiglitazone with or without ethanol in their diets for the last 2 wk of the feeding study. Coadministration of rosiglitazone and ethanol increased the expression and circulating levels of adiponectin and enhanced the expression of hepatic adiponectin receptors (AdipoRs) in mice. These increases correlated closely with the activation of a hepatic sirtuin 1 (SIRT1)-AMP-activated kinase (AMPK) signaling system. In concordance with stimulated SIRT1-AMPK signaling, rosiglitazone administration enhanced expression of fatty acid oxidation enzymes, normalized lipin 1 expression, and blocked elevated expression of genes encoding lipogenic enzymes which, in turn, led to increased fatty acid oxidation, reduced lipogenesis, and alleviation of steatosis in the livers of ethanol-fed mice. Enhanced hepatic adiponectin-SIRT1-AMPK signaling contributes, at least in part, to the protective action of rosiglitazone against alcoholic fatty liver in mice.
Keywords: lipid metabolism, signal transduction, transcriptional regulators, acetylation
alcoholic fatty liver disease is one of the earliest and most common consequences of chronic and excess alcohol consumption and can lead to more severe forms of liver injury such as steatohepatitis, hepatic fibrosis, and cirrhosis in humans (27, 40). Therefore, the development of effective therapeutic strategies for alcoholic fatty liver is vital. In recent years, several novel mechanisms involved in the development of alcoholic fatty liver have emerged, providing crucial therapeutic leads (27, 40). Chronic ethanol administration in several animal models is associated with impairment of the hepatic sirtuin 1 (SIRT1)-AMP-activated kinase (AMPK) axis, a central signaling system controlling the pathways of lipid metabolism (2, 18, 38, 39).
The activation of SIRT1-AMPK signaling in several metabolic tissues including liver has been found to increase rates of fatty acid oxidation and repress lipogenesis largely by modulating activity of PPAR-γ coactivator-α (PGC-1α)/PPARα or SREBP-1 through deacetylation and phosphorylation, respectively (6, 7, 22, 32, 38). Interestingly, the SIRT1-AMPK axis has emerged as a major signaling system in regulating adiponectin signaling and in the lipid lowering action of adiponectin (27, 40).
Adiponectin is a 30-kDa protein primarily expressed and secreted from adipose tissue, and subsequently circulated in serum (27, 40). Adiponectin is present in the serum as three oligomeric complexes: namely, high (HMW)-, middle-, and low-molecular-weight forms. Accumulating evidence has suggested that adiponectin exerts its biological activities in an oligomer-dependent manner. Two major adiponectin receptors (AdipoR1 and -R2) serve as transducers of adiponectin-mediated signaling, leading to increased fatty acid oxidation and reduced fat accumulation in several organs including liver (27, 40).
Considerable evidence has suggested a pivotal role for adiponectin in the development of alcoholic fatty liver (27, 40). Dysregulation of adiponectin and hepatic AdipoR1/R2 expression can lead to accumulation of hepatic fat in several animal models of alcoholic liver steatosis (8, 9, 11, 31, 33, 35, 37). Administration of recombinant adiponectin has been shown to ameliorate liver steatosis in mouse models of alcoholic- and nonalcoholic fatty liver disease (35). In chronically ethanol-fed animals, induction of adiponectin or activation of AdipoR1/R2 through dietary or pharmacological supplements alleviated liver steatosis and injury (9, 11, 31, 37). Although the precise cellular and molecular mechanisms whereby ethanol dysregulates the expression of adiponectin remain to be established, it has been suggested that peroxisome proliferator-activated receptor-γ (PPAR-γ) may be involved (4, 27, 40).
PPAR-γ is a transcription factor known to be involved in the regulation of lipid metabolism and energy homeostasis and has been implicated in regulating adiponectin gene expression in adipose tissue (4, 40). Activation of PPAR-γ by a group of selective compounds known as thiazolidinediones (TZDs) has been shown to lead to increases in the transcription, translation, and secretion of adiponectin from adipose tissue, resulting in elevated serum levels of the protein, especially the HMW isoform. (4, 19). Pioglitazone, one of the TZDs, has been shown to prevent alcohol-induced liver injury in rats. It is possible that induction of adiponectin by activation of PPAR-γ may contribute to these hepatic protective effects (34).
Several lines of evidence have shown that another TZD, rosiglitazone, improves steatosis and normalizes liver enzyme levels in patients with nonalcoholic fatty liver disease and in some rodent models (4). The molecular mechanisms of such effects appear to involve upregulation of adiponectin in adipose tissue (4, 19). However, the benefits of rosiglitazone for liver injury are controversial. One study has shown that rosiglitazone treatment dramatically increased liver steatosis in ob/ob mice partially through activation of hepatic PPAR-γ (14).
In this study, we examined the effects of rosiglitazone treatment on the development of alcoholic fatty liver in mice and explored the involvement of adiponectin-SIRT1-AMPK signaling in the action of rosiglitazone.
MATERIALS AND METHODS
Animal studies.
The ethanol-feeding model used in this study has been previously described (2, 37). Male C57BL/6J mice (6 to 8 wk old) were purchased from Jackson Laboratory (Bar Harbor, ME). Liquid diets were based on the Lieber-DeCarli formulation and provide 1 kcal/ml (Dyets, Bethlehem, PA) (11). Mice were divided into six dietary groups to test the effects of two doses of rosiglitazone: 1) polyunsaturated fat pair-fed control diet (PUFA, 40% of calories from fat, primarily from corn oil); 2) PUFA diet plus 3 mg·kg body wt−1·day−1 rosiglitazone (R3); 3) PUFA plus 10 mg·kg body wt−1·day−1 rosiglitazone (R10); 4) PUFA diet plus ethanol [E; identical to the control PUFA diet but with ethanol added to account for 29% of total calories and the caloric equivalent of carbohydrate (maltose-dextrin) removed]; 5) PUFA diet plus ethanol and 3 mg·kg body wt−1·day−1 rosiglitazone (E+R3), and 6) PUFA diet plus ethanol and 10 mg·kg body wt−1·day−1 rosiglitazone (E+R10). In the latter two groups, rosiglitazone (Cayman Chemical, Ann Arbor, MI) was added to the diets for the last 2 wk of the study. Ethanol was introduced gradually into the liquid diet. For mice on an ethanol-containing diet, animal cages were placed on heating pads to maintain body temperature, to compensate for potential, ethanol-induced hypothermia. After 4 wk of liquid diet feeding, the animals were euthanized, at which time blood, liver tissue, and adipose tissues were collected. The studies were approved by the Institutional Animal Care Use Committees of University of South Florida.
Pathology and biochemical assays.
Liver sections were stained with hematoxylin and eosin as previously reported (2). Liver triglyceride levels were measured as described previously (2, 37). Liver tissues were deproteinized and the concentrations of lactate and pyruvate were determined by using commercial kits from BioVision (Mountain View, CA). Serum levels of alanine aminotransferase were determined by using a kit from Sigma-Aldrich (St. Louis, MO). Plasma β-hydroxybutyrate (β-OHB) was measured by a β-Hydroxybutyrate LiquiColor procedure (SanBio Laboratory, Boerne, TX). Plasma triglyceride levels were determined with use of the Sigma Diagnostics Triglyceride and Infinity Cholesterol Reagent.
Measurement of serum total or HMW forms of adiponectin levels.
Serum levels of total adiponectin were determined using a commercial ELISA kit from R&D Systems (Minneapolis, MN). Serum levels of HMW forms of adiponectin were measured with a kit from BioVendor (Candler, NC).
Total RNA isolation and qRT-PCR.
Total RNA was prepared from mouse liver or adipose tissue using an RNAeasy Total RNA kit (Qiagen). The real-time quantitative polymerase chain reaction (qRT-PCR) was carried out as described (2). The following primer sets were purchased from SuperArray Bioscience (Frederick, MD): SIRT1 (PPM05054A), glycerol-3-phosphate acyltransferase (GPAT1; PPM33295A), ACCα (PPM05109E), medium-chain acyl-CoA dehydrogenase (MCAD; PPM25604A), carnitine palmitoyltransferase 1a (CPT1a; PPM25930B), PGC-1α (PPM03360E), adiponectin (PPM05260A), AdipoR1 (PPM 35710A), AdipoR2 (PPM 38032E), TNF-α (PPM 03113E), PPAR-γ (PPM 05108B), UCP2 (PPM03034B), and GAPDH (PPM02946E). The relative amount of target mRNA was calculated according to the comparative cycle threshold (Ct) method by normalizing target mRNA Cts to those for GAPDH.
Western blot analysis.
Immunoblot analyses were performed with cell extracts or frozen liver samples separated by electrophoresis in a SDS-polyacrylamide gel and transferred to nitrocellulose filters. SIRT1 and lipin 1 were visualized with antibodies from Santa Cruz. AMPKα, phospho-AMPKα, AMPKβ, phospho-AMPKβ, and phospho-ACC were visualized with antibodies (Cell Signaling Technology, Danvers, MA). Polyclonal rabbit anti-actin β antibody (Sigma) was used to normalize the signal obtained for total liver protein extracts.
PGC-1α and FoxO1 acetylation assays.
PGC-1α protein was immunoprecipitated from nuclear extracts isolated from fresh liver samples with an anti-PGC-1α antibody (Calbiochem, San Diego, CA). PGC-1α and acetylation levels were detected by use of specific antibodies for PGC-1α and acetyl lysine (Cell Signaling). Acetyl and total forkhead transcription factor O 1 (FoxO1) antibodies were from Santa Cruz.
Studies with rat H4IIEC3 hepatoma cells and small interfering RNA transfection.
The small silencing RNAs for SIRT1 (SIRT1 shRNA) and AMPKα (AMPKα shRNA) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The Small Interfering RNA transfection assays using rat H4IIEC3 hepatoma cells were carried out as described (38).
Data analysis.
Data are presented as means ± SE. All data were analyzed by two-way ANOVA followed by Tukey's multiple comparison procedure with P < 0.05 being considered significant.
RESULTS
Rosiglitazone attenuated alcoholic liver steatosis in mice and normalized serum levels of aminotransferases.
Male C57BL/6J mice were fed modified Lieber-DeCarli liquid diet with a high-PUFA diet with ethanol (29% of the total calories) according to a pair-feeding protocol for 4 wk (39). Four groups of mice were given a dose of either 3 mg·kg body wt−1·day−1 (R3) or 10 mg·kg body wt−1·day−1 (R10) of rosiglitazone with or without ethanol in their diets for the last 2 wk of the feeding study. Ethanol intake for 4 wk had no apparent effect on the health status of the mice, and an average 3-g increase in the body weight was observed in all six groups at the end of the feeding period. Ethanol feeding did not cause a significant increase in the liver-to-body weight ratio. Rosiglitazone treatments for the last 2 wk did not significantly affect the average food intake, adiposity, or blood alcohol levels in mice (data not shown).
As shown in Fig. 1A, histopathological analysis revealed that chronic ethanol feeding caused accumulation of fat droplets including microvesicular and macrovesicular steatosis in mouse livers. When the ethanol-fed mice were coadministered rosiglitazone, liver histology showed attenuated accumulation of hepatic lipid droplets. Concordantly, ethanol feeding increased the liver triglyceride levels by approximately threefold compared with pair-fed control mice (Fig. 1B). Treatment of ethanol-fed mice with rosiglitazone significantly reduced the hepatic triglyceride content to nearly the level of controls (Fig. 1B). In addition, plasma alanine aminotransferase and aspartate aminotransferase, two indicators of liver damage, were significantly elevated in the ethanol-fed mice but were reduced to control levels by coadministration of rosiglitazone (Fig. 1, C and D). Collectively, the data demonstrate that rosiglitazone treatment attenuated alcoholic liver steatosis in mice.
Fig. 1.
Rosiglitazone attenuated alcoholic fatty liver in mice. Hematoxylin and eosin stain (original magnification × 20) of liver sections (A), hepatic triglyceride (TG) content (B), plasma aspartate aminotransferase (AST) levels (C), and plasma alanine aminotransferase (ALT) levels (D) of mice fed a polyunsaturated fatty acid (PUFA) control diet (C), a PUFA diet plus 3 mg·kg−1·day−1 rosiglitazone (R3), a PUFA diet plus 10 mg·kg−1·day−1 rosiglitazone (R10), or PUFA plus ethanol diets without rosiglitazone (E) or plus rosiglitazone (E+R3, E+R10). All data are expressed as means ± SD; n = 6–8 animals. Means without a common letter differ, P < 0.05.
Rosiglitazone upregulated adiponectin and normalized HMW form of adiponectin in ethanol-fed mice.
Circulating adiponectin was significantly decreased ∼15% by ethanol feeding compared with the pair-fed control mice (Fig. 2A). Although rosiglitazone treatment alone significantly increased serum adiponectin concentrations compared with controls, rosiglitazone supplementation to ethanol-fed mice also caused a significant increase in circulating adiponectin levels. Ethanol feeding also significantly reduced the amount of the HMW form in circulation (∼14%) (Fig. 2B). The levels of HMW adiponectin were restored to control levels by rosiglitazone supplementation of the ethanol diet (Fig. 2B).
Fig. 2.
Rosiglitazone increased circulating adiponectin and normalized plasma high-molecular-weight (HMW) form of adiponectin in ethanol-fed mice. Circulating adiponectin concentrations (A), plasma HMW levels of adiponectin (B), and relative adipose mRNA levels of adiponectin, TNF-α, peroxisomal proliferator-activated receptor-γ (PPAR-γ), sirtuin 1 (SIRT1), and uncoupling protein-2 (UCP2) (C) of mice fed diets as described in Fig. 1. All data are expressed as means ± SD; n = 6–8 animals. Means without a common letter differ, P < 0.05.
We next examined adiponectin gene expression in mouse visceral adipose tissues. The mRNA expression of adiponectin was significantly suppressed by ethanol feeding and was restored by rosiglitazone treatment (Fig. 2C). The gene expression levels of adiponectin were inversely associated with mRNA levels of TNF-α in adipose tissues in each group (Fig. 2C).
Although adipose PPAR-γ mRNA expression was not affected by ethanol feeding alone, ethanol in combination with rosiglitazone robustly increased mRNA levels of adipose PPAR-γ. This suggests that PPAR-γ might be involved in the upregulation of adiponectin observed in adipocytes when rosiglitazone is supplemented to an ethanol-containing diet (Fig. 2C). Furthermore, the mRNA levels of SIRT1 and uncoupling protein-2 (UCP2), two known regulators of adiponectin gene expression, were each significantly reduced by ethanol feeding and were maintained at control levels by rosiglitazone supplementation of the ethanol diet (Fig. 2C).
Rosiglitazone increased the mRNA expression levels of adiponectin receptors in the livers of ethanol-fed mice.
We examined the effect of rosiglitazone on AdipoR1 and AdipoR2 in the livers of ethanol fed-mice. As shown in Fig. 3A, ethanol feeding selectively reduced mRNA expression of hepatic AdipoR2 in mice. Rosiglitazone supplementation of ethanol diets roughly doubled the mRNA levels of AdipoR1 and AdipoR2 compared with controls whereas rosiglitazone treatment alone resulted in modest increases (Fig. 3A). Moreover, as shown in Fig. 3, B and C, not only did ethanol feeding significantly increase hepatic PPAR-γ mRNA and protein levels, but so did rosiglitazone treatment, with or without ethanol.
Fig. 3.
Rosiglitazone increased the mRNA expression levels of adiponectin receptors in the livers of ethanol-fed mice. Relative hepatic mRNA levels of adiponectin receptors AdipoR1 and AdipoR2 (A), relative hepatic mRNA (B), and nuclear protein (C) levels of PPAR-γ of mice fed diets as described in Fig. 1. All data are expressed as means ± SD; n = 6–8 animals. Means without a common letter differ, P < 0.05.
Rosiglitazone administration stimulated hepatic SIRT1-AMPK signaling system in ethanol-fed mice.
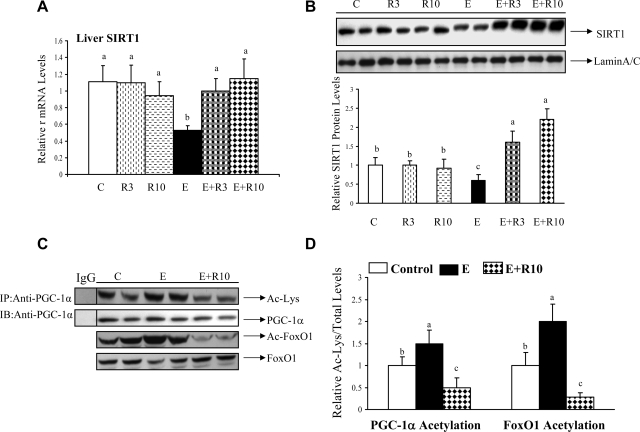
We determined whether the changes in adiponectin concentrations and hepatic AdipoR1/R2 resulted in altered SIRT1-AMPK signaling in the livers of ethanol-fed mice. As shown in Fig. 4, A and B, ethanol feeding significantly reduced the mRNA and protein levels of hepatic SIRT1 compared with control animals. Rosiglitazone treatment alone did not affect SIRT1 expression levels. However, when ethanol-fed mice were given rosiglitazone, the mRNA and protein levels of SIRT1 were increased up to, or higher than, control levels (Fig. 4, A and B).
Fig. 4.
Rosiglitazone administration activated hepatic SIRT1 activity in ethanol-fed mice. Hepatic SIRT1 mRNA (A), SIRT1 protein levels (B), acetylation of PPAR-γ coactivator-α (PGC-1α) or forkhead transcription factor O 1 (FoxO1) (C), and relative PGC-1α or FoxO1 acetylation (D) of mice fed various diets as described in Fig. 1. PGC-1α was immunoprecipitated from liver extracts then immunoblotted with an anti-acetylated lysine (Ac-Lys) antibody to determine the extent of PGC-1α acetylation. The acetylation levels of FoxO1 were determined by use of an anti-acetylated FoxO1 (Ac-FoxO1) antibody. All data are expressed as means ± SD; n = 6–8 animals. Means without a common letter differ, P < 0.05. IP, Immunoprecipitated; IB, immunoblotted.
Furthermore, in the livers of ethanol-fed mice, rosiglitazone abolished ethanol-induced hyperacetylation of PGC-1α and of FoxO1, two known targets of SIRT1, indicating that hepatic SIRT1 enzymatic activity was suppressed by ethanol feeding and restored by rosiglitazone treatment (Fig. 4, C and D).
Concerning the levels and activity of hepatic AMPK, compared with the livers of control mice, livers from ethanol fed mice displayed decreases in both phosphorylated and total protein levels of AMPKα (Fig. 5). In ethanol-fed mice, rosiglitazone supplementation blunted ethanol-mediated inhibition of both phosphorylated and total protein levels of AMPKα as well as phosphorylation of acetyl-CoA carboxylase (ACC), a known downstream target of AMPK (Fig. 5).
Fig. 5.
Rosiglitazone stimulated hepatic AMP-activated kinase (AMPK) activity in ethanol-fed mice. A: Western blots were performed by using anti-phosphorylated-AMPKα (anti-p-AMPKα), anti-AMPKα, and anti-phosphorylated acetyl CoA carboxylase (p-ACC) antibodies from liver extracts of mice fed various diets as described in Fig. 1. β-Actin protein band was used to confirm equal loading and to normalize the data. B: relative levels of p-AMPKα, AMPKα, and p-ACC. Western blots were quantified by a PhosphorImager and MultiAnalyst (Bio-Rad) software analysis. All data are expressed as means ± SD; n = 6–8 animals. Means without a common letter differ, P < 0.05.
We further examined whether levels of hepatic lactate and pyruvate, which represent the ratio of NAD+ and NADH concentrations, were altered by ethanol or rosiglitazone. Although hepatic lactate levels were significantly increased by ethanol feeding, coadministration of rosiglitazone decreased lactate levels by as much as 80% in ethanol-fed mice (Table 1). Hepatic pyruvate levels were unchanged in all of the groups (data not shown).
Table 1.
Effects of rosiglitazone on selected parameters in mice fed ethanol
| Parameters | Control | R3 | R10 | Ethanol | E+R3 | E+R10 |
|---|---|---|---|---|---|---|
| Plasma triglycerides, mg/dl | 78 ± 13b | 59 ± 6b | 69 ± 17b | 139 ± 38a | 64 ± 11b | 74 ± 17b |
| Plasma cholesterol, mg/dl | 84 ± 16 | 86 ± 19 | 78 ± 11 | 70 ± 12 | 77 ± 4 | 69 ± 9 |
| Plasma β-OHB, mg/dl | 3.8 ± 1.6c | 5.8 ± 2.1b | 5.1 ± 1.5b | 6.2 ± 1.7b | 12.4 ± 2.1a | 16.4 ± 3.3a |
| Liver lactate, nmol/mg | 7.3 ± 1.5b | 5.9 ± 1.3c | 4.9 ± 1.2c | 9.8 ± 2.3a | 3.8 ± 1.7d | 2.2 ± 1.3d |
Male C57/BL6J (6-8 wk-old) mice were divided into 6 groups as follows: 1) pair-fed control (Control); 2) control diet supplemented with rosiglitazone (3 mg·kg−1·day−1) (R3); 3) control diet supplemented with rosiglitazone (10 mg·kg−1·day−1) (R10); 4) ethanol-containing diet (29% of total calories); 5) ethanol-containing diet supplemented with rosiglitazone (3 mg·kg−1·day−1) (E+R3); 6) ethanol-containing diet supplemented with rosiglitazone (10 mg·kg−1·day−1) (E+R10). Rosiglitazone was added to the diet for the last 2 wk of the feeding study. The animals were euthanized after 4 wk. Results are expressed as means ± SD of 6-8 mice. β-OHB, β-hydroxybutyrate. Means without a common letter differ, P < 0.05
Rosiglitazone restored PGC-1α and RXRα activity, and enhanced expression of genes involved in fatty acid oxidation in the livers of ethanol-fed mice.
The SIRT1-AMPK axis stimulates hepatic PGC-1α signaling (40). As shown in Fig. 6A, ethanol feeding did significantly suppress mRNA levels of PGC-1α and retinoid X receptor α (RXRα), two known coactivators of PPARα, which were restored to control levels by coadministration of rosiglitazone. However, neither ethanol nor rosiglitazone altered hepatic PPARα gene or protein levels in mice (data not shown).
Fig. 6.
Rosiglitazone induced expression of genes encoding fatty acid oxidation enzymes and blocked the expression of genes encoding lipogenic enzymes in ethanol-fed mice. Relative mRNA levels of PGC-1α, retinoid X receptor α (RXRα), and PPARα signaling-regulated fatty acid oxidation enzymes (A), acetyl-histone H3-Lys9 (Ac-histone H3-Lys 9) and histone H3 levels (B), and relative mRNA levels of lipogenic enzymes (C) in the livers of mice fed various diets as described in Fig. 1. ACCα, acetyl-coenzyme A carboxylase α; MCAD, medium-chain acyl-CoA dehydrogenase; CPTI, carnitine palmitoyltransferase I. All data are expressed as means ± SD; n = 5–8 animals. Means without a common letter differ, P < 0.05.
Accordingly, although mRNAs for mitochondrial MCAD and CPT1a were unchanged in the ethanol-fed group, rosiglitazone supplementation to ethanol-fed mice induced mRNAs of MCAD and CPT1a to levels higher than that in control or ethanol-fed mice (Fig. 6A). These findings agree with a study showing that chronic ethanol feeding impaired DNA binding and transcriptional activity of PPARα without affecting the level of PPARα mRNA or protein in the livers of ethanol-fed mice (12).
Rosiglitazone supplementation to ethanol-fed mice significantly increased the serum β-OHB levels by nearly fourfold compared with controls and over twofold compared with ethanol-fed mice, indicating that rosiglitazone treatment in combination with ethanol feeding was capable of inducing hepatic fatty acid oxidation (Table 1).
Rosiglitazone blunted hyperacetylation of hepatic histone H3 at lysine 9 (Lys9) and attenuated increased expression of genes encoding lipogenic enzymes in ethanol-fed mice.
Ethanol-mediated increase in histone H3-Lys 9 acetylation has been implicated in the transcriptional induction of several lipogenic enzymes (38). Additionally, chromatin immunoprecipitation assays confirmed an increased association of acetylated histone H3-Lys9 with mitochondrial GPAT1 promoters in the livers of ethanol-fed mice compared with the pair-fed control mice (36). As shown in Fig. 6B, the acetylation level of histone H3-Lys9 was enhanced by ethanol feeding compared with pair-fed control mice. In ethanol-fed mice, rosiglitazone treatment completely blocked the ethanol-induced acetylation of histone H3-Lys9 (Fig. 6B). The histone H3 protein levels remained unchanged in all groups (Fig. 6B).
Although ethanol feeding robustly increased the hepatic expression of the mRNAs encoding ACCα and GPAT1 in mice, the rosiglitazone supplementation to ethanol-fed mice restored these gene products to control levels (Fig. 6C). Interestingly, rosiglitazone treatment did not change the levels of the mRNA or active, nuclear form of sterol regulatory element binding protein 1 (SREBP-1) in the livers of ethanol-fed mice (data not shown). This could be due to rapid proteasomal degradation of nuclear SREBP-1 protein.
Rosiglitazone normalized lipin 1 expression levels in the livers and adipose tissues of ethanol-fed mice.
Lipin 1 has recently been identified as a key regulator of lipid metabolism (25). As shown in Fig. 7A, although ethanol administration significantly increased the mRNA expression levels of hepatic lipin 1, rosiglitazone supplementation to ethanol-fed mice normalized hepatic lipin 1 gene expression to control levels. Conversely, lipin 1 mRNA expression levels were markedly reduced in visceral adipose tissue of mice receiving the ethanol diet compared with the pair-fed control mice, whereas coadministration of rosiglitazone and ethanol restored lipin 1 to control levels (Fig. 7A).
Fig. 7.
Rosiglitazone normalized lipin 1 expression levels in the livers and adipose tissues of ethanol-fed mice. A: hepatic or adipose lipin 1 mRNA. B: liver cytosolic lipin 1 protein levels of mice fed various diets as described in Fig. 1. All data are expressed as means ± SD; n = 5–8 animals. Means without a common letter differ, P < 0.05.
Liver cytosolic lipin 1 protein levels were increased in ethanol-fed mice compared with control mice (Fig. 7B). Low (R3)- and high (R10)-dose rosiglitazone supplementation to ethanol-fed mice normalized and reduced (respectively) hepatic lipin 1 protein expression (Fig. 7B). Rosiglitazone treatment alone did not affect the lipin 1 levels (data not shown).
Adiponectin stimulated SIRT1-AMPK signaling in rat hepatoma cells.
In terms of selecting an appropriate model system to study the molecular mechanisms occurring in liver, the rat hepatoma cell line H4IIEC3 expresses sufficient levels of SIRT1 and AMPK gene and protein (38, 39). Also, these cells are able to metabolize ethanol through the activities of alcohol dehydrogenase and aldehyde dehydrogenase (38, 39).
To determine whether the SIRT1-AMPK signaling system is the direct target of adiponectin's action, the effect of adiponectin on SIRT1 protein expression was tested by treating hepatoma H4IIEC3 cells with various concentrations of mouse, full-length, recombinant adiponectin (flAcrp) for 24 h. Treatment with flAcrp significantly increased SIRT1 protein levels with a maximal effect at 0.5 μg/ml (Fig. 8A). The increase of SIRT1 coincided with a significant increase in phosphorylated AMPKα (Fig. 8A).
Fig. 8.
Adiponectin upregulated SIRT1 and stimulated AMPK activity in rat H4IIEC3 cells. A: H4IIEC3 cells were starved in serum-free DMEM overnight, and full-length mouse recombinant adiponectin (flAcrp) was added at the indicated concentrations for 18 h. B: H4IIEC3 cells transfected with AdipoR1 small silencing RNA (shRNA) or AdipoR2 shRNA plasmid were treated with flAcrp (1 μg/ml) for 18 h. C: H4IIEC3 cells transfected with AMPKα shRNA plasmid were treated with flAcrp (1 μg/ml) for 18 h. D: H4IIEC3 cells transfected with SIRT1 shRNA were treated with flAcrp (1 μg/ml) for 18 h. E: H4IIEC3 cells were starved in serum-free DMEM overnight and pretreated with flAcrp (1 μg/ml) followed by addition of ethanol (63 mM) for 18 h. SIRT1 protein levels or phosphorylated-AMPKα (p-AMPKα) were detected by Western blots with anti-SIRT1 and anti-p-AMPKα antibodies, respectively. The data represent at least 3 replications.
Although knocking down either AdipoR1 or AdipoR2 only partially blunted the adiponectin-induced SIRT1 protein increase, knocking down both AdipoR1 and AdipoR2 completely blocked the adiponectin-induced SIRT1 protein increase in H4IIEC3 cells (Fig. 8B). Note that Western blot analysis confirmed the inhibition of both AdipoR1 and AdipoR2 expression by transfection with AdipoR1 shRNA and AdipoR2 shRNA, respectively, in H4IIEC3 cells (data not shown).
We further examined how SIRT1 and AMPKα regulate each other's activities in the presence of adiponectin. Knocking down AMPKα with AMPKα shRNA had negligible effect on adiponectin-induced increases of SIRT1 protein (Fig. 8C). Conversely, knocking down SIRT1 with SIRT1 shRNA significantly diminished adiponectin's ability to increase phosphorylated AMPK (p-AMPK) levels (Fig. 8D). These data suggest that, in cultured hepatic cells, adiponectin may indirectly activate AMPK signaling by upregulating SIRT1. Note that transfection with either AMPK shRNA or SIRT1 shRNA in the absence of flAcrp had no discernable effects on SIRT1 or AMPK expression levels in H4IIEC3 cells (data not shown).
The inhibition of SIRT1 and the phosphorylation of AMPKα by ethanol (63 mM) were largely reversed by the addition of flAcrp (Fig. 8E).
DISCUSSION
In the present study, we have demonstrated that adiponectin signaling plays a role in the ability of rosiglitazone to attenuate alcoholic liver steatosis in mice (Fig. 9). In chronically ethanol-fed mice, circulating total adiponectin, HMW adiponectin and mRNA expression of hepatic AdipoR1/R2 were all significantly elevated by rosiglitazone coadministration. Correlatively, in the livers of ethanol-fed mice, rosiglitazone treatment stimulated SIRT1-AMPK signaling system, activated PPARα signaling, suppressed histone H3-Lys9 hyperacetylation, increased rates of fatty acid oxidation, reduced lipid synthesis, and attenuated liver steatosis. More importantly, our study demonstrates, for the first time to our knowledge, that lipin 1, a vital lipid regulator, exhibits reciprocal patterns of gene expression in livers and adipose tissues of chronically ethanol-fed mice and that rosiglitazone treatment to ethanol fed mice restores lipin 1 expression in both tissues.
Fig. 9.
Proposed role of adiponectin-SIRT1-AMPK signaling in the protective effects of rosiglitazone against alcoholic liver steatosis in mice. Ac-histone H3-Lys9, histone H3 at acetylated lysine 9; FA, free fatty acids; PAP1, phosphatidate phosphatase type-1.
Our present findings show that ethanol feeding caused only a modest decrease in circulating total or HMW adiponectin protein in mice. However, adiponectin transduces its signals through both AdipoR1 and -R2 in liver. The hepatic mRNA expression of AdipoR2 was selectively inhibited by chronic ethanol feeding in mice (2). Therefore, it is likely that the combination of modest decreases in serum total or HMW adiponectin levels and downregulated hepatic AdipoR2 may lead to a substantial decrease in hepatic adiponectin signaling and impaired lipid metabolism in ethanol-fed mice. Conceivably, rosiglitazone treatment reverses abnormalities in hepatic lipid metabolism in ethanol-fed mice synergistically through upregulating both circulating total HMW adiponectin and hepatic adiponectin R1/R2.
PPAR-γ is a major component of the system regulating adiponectin gene expression in adipocytes (4, 27, 40). Our results show that the rosiglitazone-dependent upregulation of adiponectin gene expression in adipose tissues of ethanol-fed mice is partially mediated through upregulation of adipose PPAR-γ. Previous studies, as well as the present one, have consistently shown that chronic ethanol feeding does not alter adipose mRNA expression of PPAR-γ in mice or micropigs (11). However, PPAR-γ transcriptional activity might be inhibited by ethanol (27). Therefore, it is reasonable to speculate that ethanol may inhibit adiponectin gene expression in adipose tissue by somehow suppressing PPAR-γ transcriptional activity, despite the fact that transcription of the PPAR-γ gene itself is not affected. In addition to PPAR-γ, other factors such as TNF-α, SIRT1, and UCP2 may also regulate ethanol- or rosiglitazone-mediated adiponectin expression in adipose tissue (3, 10, 27, 40, 23).
Activation of hepatic PPAR-γ is associated with hepatic lipid accumulation in both alcoholic and nonalcoholic fatty liver (2, 24). Furthermore, rosiglitazone-mediated increases in liver steatosis in ob/ob mice were closely associated with increased hepatic PPAR-γ protein expression (14). Interestingly, our present study showed that treatment with ethanol and/or rosiglitazone significantly increases hepatic PPAR-γ mRNA and protein levels in mice. This finding implies that hepatic PPAR-γ-mediated effects are probably not involved in the protective effect of rosiglitazone against alcoholic fatty liver in mice.
In addition to activation of hepatic adiponectin-SIRT1-AMPK signaling, other non-adiponectin-mediated mechanisms are likely involved in the protective effect of rosiglitazone against alcoholic fatty liver. For instance, pioglitazone has been shown to prevent alcoholic liver steatosis in rats through upregulation of c-Met signaling (34). Also, in cultured muscle cells, where adiponectin is unlikely to be involved, rosiglitazone treatment leads to a marked increase in AMPK activity (13). Moreover, our data show that rosiglitazone reduced hepatic lactate levels in ethanol-fed mice. Lactate downregulates SIRT1 (26). These studies suggest that some of the beneficial effects of rosiglitazone may be mediated by its direct activation of hepatic AMPK and by an indirect upregulation of hepatic SIRT1 via suppression of hepatic lactate levels in ethanol-fed mice. Further studies addressing precise mechanisms of rosiglitazone action would be made possible through use of various animal models such as adipose-specific adiponectin knockout or hepatic-specific adiponectin receptors or SIRT1 null mice. We predict that the protective effects of rosiglitazone against alcoholic liver steatosis would be partially blunted in each of these knockout mouse models.
It is intriguing that ethanol feeding to mice had opposite effects on lipin 1 gene expression in livers and adipose tissues. In adipose tissue, lipin 1 is required for the induction of two important adipogenic genes, PPAR-γ and CAAT enhancer binding protein α (C/EBPα) (21, 25). Both PPAR-γ and C/EBPα are major transcriptional regulators of adiponectin gene expression, and TZDs are able to induce lipin 1 expression in adipocytes (16, 20, 25). Thus it is tempting to hypothesize that lipin 1-PPAR-γ or lipin 1-C/EBPα signaling may be involved in rosiglitazone-mediated upregulation of adiponectin in adipocytes.
In liver, lipin 1 plays two distinct roles in the regulation of lipid metabolism (25). It can act both as an Mg2+-dependent phosphatidate phosphatase type-1 (PAP1) and also as a transcriptional regulator. PAP1 catalyzes the conversion of phosphatidate to diacylglycerol, a direct precursor of triglycerides and phospholipids. Indeed, PAP1 activity was shown to be increased in the livers of human alcoholics and baboons, and activation of PAP1 by ethanol exposure was associated with the development of liver steatosis (5, 28, 30). Therefore, it is likely that the increased hepatic lipin 1 mRNA and protein levels seen with ethanol feeding may largely contribute to enhanced liver PAP1 activity in ethanol-fed mice.
Studies have demonstrated the ability of adiponectin signaling to upregulate SIRT1 in cultured cell systems (29, 40). More importantly, we have shown here that adiponectin-mediated upregulation of SIRT1 requires both AdipoR1 and AdipoR2, further support that SIRT1 is a downstream target of adiponectin signaling. The precise events triggered by adiponectin to alter SIRT1 protein levels remain unknown. Studies have demonstrated that adiponectin reduces NADPH-oxidase-dependent reactive oxygen species (ROS) production induced by stimulators such as LPS or ethanol in Kupffer cells (40). ROS downregulate SIRT1 (1). It is possible that adiponectin-mediated increase in SIRT1 protein levels may be a consequence of such reductions in ROS. However, our results, in macrophages and in hepatoma cells, have shown that adiponectin increases SIRT1 protein levels in the absence of any stimulator of ROS, implying that ROS-independent mechanisms are likely to be involved.
There is accumulating evidence that SIRT1 and AMPK regulate each other's activity (6, 7, 15, 17, 32, 40). Our present findings demonstrate that, in hepatoma cells, knocking down SIRT1 completely blunts the increased p-AMPK levels stimulated by adiponectin, whereas knocking down AMPKα does not negate the adiponectin-mediated increase in SIRT1. These results strongly suggest that the adiponectin-SIRT1 axis lies upstream of AMPK signaling (Fig. 9).
In summary, our present study demonstrates that the protective action of rosiglitazone against alcoholic fatty liver in mice is mediated, at least in part, through stimulating an important hepatic signaling system, the adiponectin-SIRT1-AMPK axis. We suggest that nutritional or pharmacological modulation of adiponectin-SIRT1-AMPK signaling could be therapeutic for treating alcoholic fatty liver disease in patients.
GRANTS
This study was supported by National Institute on Alcoholism and Alcohol Abuse Grants AA-015951 and AA-013623 (to M. You).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol 295: G833–G842, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8: 333–341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouskila M, Pajvani UB, Scherer PE. Adiponectin: a relevant player in PPARgamma-agonist-mediated improvements in hepatic insulin sensitivity? Int J Obes (Lond) 29: S17–S23, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Brindley DN, Cooling J, Burditt SL, Pritchard PH, Pawson S, Sturton RG. The involvement of glucocorticoids in regulating the activity of phosphatidate phosphohydrolase and the synthesis of triacylglycerols in the liver. Effects of feeding rats with glucose, sorbitol, fructose, glycerol and ethanol. Biochem J 180: 195–199, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab 292: E621–E628, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology 49: 1554–1562, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevillotte E, Giralt M, Miroux B, Ricquier D, Villarroya F. Uncoupling protein-2 controls adiponectin gene expression in adipose tissue through the modulation of reactive oxygen species production. Diabetes 56: 1042–1050, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res 31: 1231–1239, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277: 25226–25232, 2002 [DOI] [PubMed] [Google Scholar]
- 14.García-Ruiz I, Rodríguez-Juan C, Díaz-Sanjuán T, Martínez MA, Muñoz-Yagüe T, Solís-Herruzo JA. Effects of rosiglitazone on the liver histology and mitochondrial function in ob/ob mice. Hepatology 46: 414–423, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52: 1655–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Lan F, Cacicedo JM, Ruderman N, Ido Y. modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283: 27628–27635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieber CS, Leo MA, Wang X, Decarli LM. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun 370: 44–48, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPbeta at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol 24: 8671–8680, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan J, Péterfy M, Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem 279: 29558–29564, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9: 327–338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 281: 39915–39924, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, DubÉ GP. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem 224: 29–37, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Reue K, Dwyer JR. Lipin proteins and metabolic homeostasis. J Lipid Res 50: S109–S114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Rogers CQ, Ajmo JM, You M. Adiponectin and alcoholic fatty liver disease. IUBMB Life 60: 790–797, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Savolainen MJ, Baraona E, Pikkarainen P, Lieber CS. Hepatic triacylglycerol synthesizing activity during progression of alcoholic liver injury in the baboon. J Lipid Res 25: 813–820, 1984 [PubMed] [Google Scholar]
- 29.Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-α production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol 296: G1047–G1053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson KJ, Venkatesan S, Martin A, Brindley DN, Peters TJ. Activity and subcellular distribution of phosphatidate phosphohydrolase (EC 3.134) in alcoholic liver disease. Alcohol Alcohol 30: 31–36, 1995 [PubMed] [Google Scholar]
- 31.Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology 47: 867–879, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun 378: 836–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-α production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol 290: G998–G1007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, Inokuchi S, Nishimura T, Suematsu M, Ishii H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology 126: 873–885, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr 138: 497–501, 2008 [DOI] [PubMed] [Google Scholar]
- 37.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008 [DOI] [PubMed] [Google Scholar]
- 39.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 40.You M, Rogers CQ. Adiponectin: a key adipokine in alcoholic fatty liver. Exp Biol Med (Maywood) 234: 2850–2959, 2009. [DOI] [PubMed] [Google Scholar]