Abstract
Information about colorectal distension (i.e., colorectal dilation by increased intraluminal pressure) is primarily encoded by stretch-sensitive colorectal afferents in the pelvic nerve (PN). Despite anatomic differences between rectum and distal colon, little is known about the functional roles of colonic vs. rectal afferents in the PN pathway or the quantitative nature of mechanosensory encoding. We utilized an in vitro mouse colorectum-PN preparation to investigate pressure-encoding characteristics of colorectal afferents. The colorectum with PN attached was dissected, opened longitudinally, and pinned flat in a Sylgard-lined chamber. Action potentials of afferent fibers evoked by circumferential stretch (servo-controlled force actuator) were recorded from the PN. Stretch-sensitive fibers were categorized into the following four groups: colonic muscular, colonic muscular/mucosal, rectal muscular, and rectal muscular/mucosal. Seventy-nine stretch-sensitive PN afferents evenly distributed into the above four groups were studied. Rectal muscular afferents had significantly greater stretch-responses than the other three groups. Virtually all rectal afferents (98%) had low thresholds for response and encoded stimulus intensity into the noxious range without obvious saturation. Most colonic afferents (72%) also had low thresholds (<14 mmHg), but a significant proportion (28%) had high thresholds (>18 mmHg) for response. These high-threshold colonic afferents were sensitized to stretch by inflammatory soup; response threshold was significantly reduced (from 23 to 12 mmHg), and response magnitude significantly increased. These results suggest that the encoding of mechanosensory information differs between colonic and rectal stretch-sensitive PN afferents. Rectal afferents have a wide response range to stretch, whereas high-threshold colonic afferents likely contribute to visceral nociception.
Keywords: muscular afferents, muscular/mucosal afferents, single-fiber recording, irritable bowel syndrome
functional gastrointestinal disorders such as irritable bowel syndrome (IBS) are characterized by altered bowel habits, discomfort, pain, and colorectal hypersensitivity (12). Typically, patients have significantly lower response thresholds to provocative visceral stimuli (e.g., distension of hollow organs) and complain of increased sensitivity and discomfort to ingestion of food or beverages (16). Balloon distension of the pelvic colorectum has been widely employed to quantify sensation (principally discomfort and pain) in patients with IBS as a means of assessing colorectal hypersensitivity relative to normal subjects (e.g., Ref. 3). Growing evidence from both clinical and basic science research reveals that peripheral sensory input plays a key role in sustaining visceral pain syndromes (1, 30, 31, 38, 42). Thus a better understanding of colorectal afferent neurons capable of encoding mechanical distending stimuli may inform improved management of IBS symptoms.
Mechanosensory information from the colorectum is conveyed to the central nervous system by two distinct pathways, the lumbar splanchnic nerves (thoracolumbar spinal) (LSN) and pelvic (lumbosacral spinal) nerves (PN). Afferent fibers in both pathways are mechanosensitive although stretch-sensitive receptive endings are fourfold more common in the PN pathway (5). The PN pathway mediates nonnoxious visceral reflexes but is also indispensable for colorectal nociception as documented in studies on humans (15), as well as in rats with only pelvic nerves intact (25).
Recent studies have shown that there are five major functional subtypes of colorectal afferents individually tuned to detect combinations of three different mechanical stimuli including, punctate probing, mucosal stroking, and circumferential stretch (5). Among these three mechanical stimuli, circumferential stretch correlates closely with the distending mechanical stimulus that induces pain in patients with IBS (3). The response characteristics of PN stretch-sensitive afferent fibers have been studied either using in vivo animal models in which colorectal distension was produced by balloon inflation (2, 17, 32, 37) or in vitro models where the colorectum was dissected free, opened longitudinally, and pinned flat in a recording chamber to study receptive endings (5, 19, 34). However, no studies have targeted mechanosensitive rectal afferents to systematically characterize and contrast stretch response functions in the PN between fibers innerving the distal colon vs. rectum. In the present study, we utilized a computer-controlled force actuator and an in vitro mouse colorectum-PN preparation to 1) systematically characterize the subtypes of stretch-sensitive colorectal afferents that innervate the distal colon vs. rectum and 2) investigate the sensitization of high-threshold (HT), stretch-sensitive afferents by a soup of inflammatory mediators. A preliminary report of this work was presented in abstract form (14).
MATERIALS AND METHODS
All experiments were approved by and performed in accordance with the guidelines of the University of Pittsburgh Institutional Animal Care and Use Committee.
In vitro mouse colon-PN preparation.
Male mice (C57BL/6) aged 6–8 wk, 20–30 g (Taconic, Germantown, NJ), were euthanized via CO2 inhalation followed by exsanguination by perforating the right atrium. As described previously (5), the distal colorectum was removed with attached major pelvic ganglion and PN and transferred to ice-cold Krebs solution bubbled with carbogen (95% O2-5% CO2). After further dissection, the distal colon was opened longitudinally along the antimesenteric border and transferred to an acrylic organ bath consisting of two adjacent chambers. The colon was placed in the Sylgard-lined (Sylgard 182 silicone elastomer; Dow Corning, Midland, MI) tissue chamber and pinned flat, mucosal side up. The PN was gently pulled over and laid onto a mirror in the recording chamber, which was filled with paraffin oil (Thermo Fisher Scientific, Waltham, MA). The tissue chamber was superfused with a modified Krebs solution (in mM: 117.9 NaCl, 4.7 KCl, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4-7 H2O, 2.5 CaCl2, 11.1 d-glucose, 2 butyrate, and 20 acetate at a temperature of ∼34°C). In addition, the L-type calcium channel antagonist nifedipine (4 μM; to block spontaneous muscle contractions) and the prostaglandin synthesis inhibitor indomethacin (3 μM; to block synthesis of endogenous prostaglandins) were added to the Krebs solution. Under a dissection microscope, the nerve sheath surrounding the PN was carefully peeled back. Using fine forceps, the nerve trunk was teased into fine bundles of ∼10 μm thick, which were individually placed onto a platinum-iridium recording electrode. A reference electrode of the same material was placed in contact of the Krebs solution in the tissue chamber.
Mechanical characterization of stretch-sensitive afferents.
Colon and rectal PN afferents were mechanically stimulated by homogeneous circumferential stretch. Custom-built claws were firmly attached at 1-mm intervals along the antimesenteric edge of the colorectum. These claws were fixed to a rigid 2.2-cm-long plastic block whose displacement was precisely regulated by a servo-controlled force actuator (series 300B dual mode servo system; Aurora Scientific, Toronto, ON, Canada). With one edge of the colorectum firmly pinned to the Sylgard base of the organ chamber, the colorectum was stretched homogeneously in the circumferential direction by a slow-ramped force (0 to 170 mN at 5 mN/s). This allows correlation of circumferential force (0–170 mN) to intraluminal pressure (0–45 mmHg) by the following equation: pressure = 2·π(force)/(LD) (equation 1), where L is colon length and D colon circumference. The receptive fields of stretch-sensitive afferents were identified by punctate stimulation (1–1.4 g force) with von Frey-like nylon monofilaments. The receptive fields were then tested with mucosal stroking using a calibrated nylon multifilament (10 mg force).
For some afferents, the conduction velocity was measured by electrically stimulating the receptive field with a round-tipped concentric electrode (FHC, Bowdoin, ME; external pole diameter 0.55 mm and internal pole diameter 0.125 mm) to deliver electrical current pulses of 0.5 ms duration and 0.3 Hz frequency. Conduction velocity was computed from the conduction delay and minimum distance between the stimulating cathode at the receptive field and recording site. The minimum distance consisted of two straight lines that intersect at the position the PN dives into the colorectum tissue, representing fibers in the colorectal wall and the PN, respectively.
Chemical stimulation and sensitization of stretch-sensitive afferents.
Some HT stretch-sensitive fibers (threshold >68 mN) were tested for chemical activation and sensitization. Bronze square tubing (height, 10 mm; inner measures, 4 × 4 mm), whose bottom edge was previously lined with petrolatum to avoid damage to the colorectal mucosa, was placed over the receptive ending, and the Krebs solution was removed and 150 μl of an inflammatory soup (IS) (21) applied directly to the receptive ending for 3 min. Subsequently, the IS and the bronze tubing were removed, and responses to the same circumferential stretch (0 to 170 mN) were tested within 4–6 min. The IS was prepared in aliquots of 20 μl by combining bradykinin, serotonin, and histamine dissolved in distilled water with prostaglandin E2 dissolved in dimethylsulfoxide, frozen, and stored at −20°C. On the day of an experiment, an aliquot was diluted to the final concentration (10 μM for all mediators) in freshly oxygenated Krebs solution (21). pH of the IS was adjusted to 6.0 by adding hydrochloric acid. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Histological analysis of mouse distal colon and rectum.
The colorectum from two naive mice were processed for histology and analysis of the thickness of the muscular and mucosal layers of the organ. The distal ∼2 cm of the colorectum was dissected out and, for one of the mice, opened in the longitudinal axis through the mesenteric edge and pinned flat on a silicon platform. The tissue was immediately placed in cold 4% paraformaldehyde and 0.2% picric acid dissolved in 0.16 M phosphate buffer (pH 6.9) and fixed by immersion for 24 h at 4°C. This was followed by incubation in 10–20% sucrose in PBS (pH 7.4) containing 0.01% sodium azide and 0.02% bacitracin (both from Sigma-Aldrich) at 4°C for 48 h. After being embedded in Tissue-Tek optimal cutting temperature compound (Sakura, Torrance, CA) and being frozen, the colorectal tissue was sectioned transversally in a cryostat (CM 3050; Leica, Heidelberg, Germany) at 35 μm. This allowed for the visualization of all layers of the colorectum along its full length. The colorectum from the second mouse was left untouched, immediately placed in 10% formalin for 24 h, embedded in paraffin, and sectioned on a microtome at 10 μm. Following staining with hematoxylin and eosin, the tissue was examined on an Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan) provided with appropriate filters and a Hamamatsu ORCA-ER, C4742-80 digital camera, with the use of Hamamatsu Photonics Wasabi 150 software (Hamamatsu Photonics KK, System Division, Hamamatsu City, Japan).
To analyze the thickness of the tissue, multiple photographs were taken through the length of the colorectum in three sections, each separated by ∼350 μm. Photographs were uploaded to Photoshop software (Adobe Systems, San Jose, CA), where they were stacked, aligned, and merged for subsequent measurements. Pictures thus arranged were opened in ImageJ software (U.S. National Institutes of Health), and the thickness of the muscular and mucosal layers of the colorectum was measured.
Data recording and analysis.
Electrical signals generated by PN afferent endings were filtered (0.3 to 10 kHz) and amplified (×10,000) by a low-noise AC differential amplifier (DAM 80; World Precision Instruments, New Haven, CT). The signal was digitized at 20 kHz using a 1401 interface (Cambridge Electronic Design, Cambridge, UK) and stored on a PC. The signal was also monitored online by an audio monitor (Grass AM8; Astro-med, West Warwick, RI). Action potentials were analyzed offline using the Spike 2 wave mark function and discriminated as single units on the basis of principal component analysis of individual spike waveform. No more than two easily discernable active units in any record were studied to avoid errors in discrimination. The stretch threshold of the fiber was defined as the force that evoked the first action potential in the slow-ramped stretch stimuli (0 to 170 mN at 5 mN/s).
Data are presented throughout as means ± SE. One- and two-way ANOVA or repeated-measures ANOVA were performed as appropriate using SigmaPlot v9.0 (Systat Software, San Jose, CA). Holm-Sidak's post hoc multiple comparison was performed as appropriate when F values for main effects were significant. Differences were considered significant when P < 0.05.
RESULTS
Histology of the distal colon and rectum.
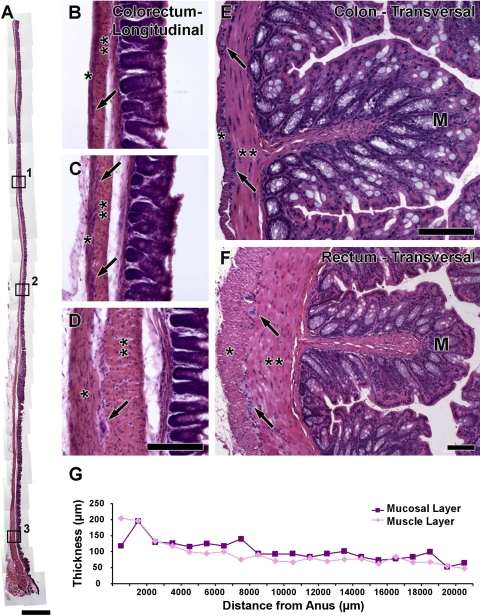
Histological examination of longitudinally (Fig. 1, A–D) or transversally (Fig. 1, E and F) sectioned segments of the colorectum shows a progressive proximal to anus increase in the thickness of the muscle layers of the organ. Figure 1G presents thicknesses of muscle and mucosal layers. Muscle layers are thickest in the distal 1.5 mm of the colorectum (∼200 μm thick) and become less thick moving proximal (∼100 μm thick from 1.5–9.5 mm and ∼50 μm thick from 9.5–20.5 mm). Parallel measurements of the mucosal layer show a similar trend as observed for the muscle layers (Fig. 1G).
Fig. 1.
Brightfield photomicrographs showing the histology of the mouse colorectum in longitudinal (A–D) or transversal (E–F) sections. G: measurements of colorectal muscle and mucosal layer thicknesses. Inspection of the longitudinal section of distal colon and rectum in A clearly shows the progressive decrease in thickness of the tissue layers. Boxes 1, 2, and 3 are shown at high-power magnification in B, C, and D, respectively, to depict the differences between proximal, middle, and distal areas of the colorectum (*longitudinal muscle layer; **circular muscle layer; arrows, myenteric plexus; M, mucosa). A similar difference in muscle layer thickness is shown in transversally cut sections of the colon (E) and rectum (F). Scale bar = 1,000 μm for A and 100 μm for B–F (scale bar in D also applies to B and C).
Categorization of stretch-sensitive colorectal afferents in the PN.
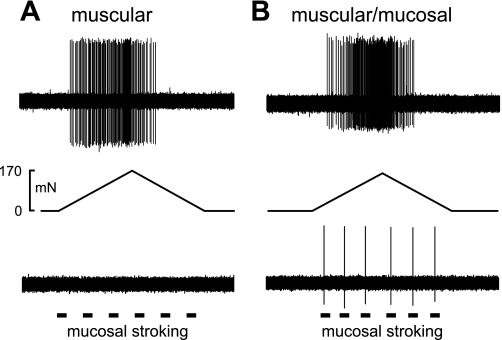
The pressure-encoding features of 79 stretch-sensitive colorectal afferents recorded from 31 male mice were characterized using a computer-controlled, ramped circumferential stretch (0 to 170 mN at 5 mN/s) paradigm. Examples of single afferent fiber recordings from teased PN bundles are displayed in Fig. 2. All fibers studied responded to circumferential stretch; some fibers also responded to mucosal stroking with 10-mg nylon filaments and were categorized as muscular/mucosal afferents. Afferent fibers that responded only to circumferential stretch were categorized as muscular afferents.
Fig. 2.
Representative single-fiber recordings from colorectal stretch-sensitive pelvic nerve afferent fibers. The action potentials were evoked by a ramped circumferential force from 0 to 170 mN at 5 mN/s. A: afferents designated muscular responded only to stretch and not mucosal stroking (10-mg nylon filament) of the receptive field, whereas muscular/mucosal (B) afferents responded to both stretch and mucosal stroking.
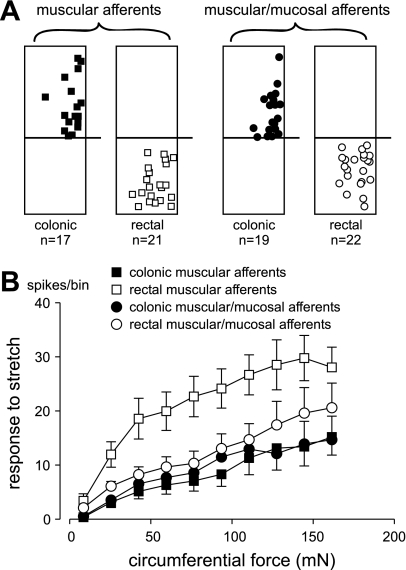
On the basis of the histological evidence presented in Fig. 1, we segregated the colorectum into rectal (the distal 10 mm) and distal colon (the adjacent, proximal colon where muscle thickness is ∼50–100 μm) segments. Figure 3A illustrates the distributions of muscular and muscular/mucosal afferent endings in the colonic and rectal segments of the colorectum. The 79 stretch-sensitive afferents studied were about evenly divided into colonic muscular (21.5%), rectal muscular (26.6%), colonic muscular/mucosal (24%), and rectal muscular/mucosal (27.8%) afferents. Consistent with previous studies (5, 19), the receptive fields of stretch-sensitive afferents were distributed across the circular axis of the colon although it seems that more receptive fields were located along the mesenteric edge of the colorectum (Fig. 3A). In agreement with previous literature (5, 19), there was no marked difference in receptive field distribution between muscular and muscular/mucosal afferents.
Fig. 3.
Topographical distribution (A) and stretch-response functions (B) of colonic muscular, rectal muscular, colonic muscular/mucosal, and rectal muscular/mucosal pelvic nerve afferents. The square and circular symbols in A represent receptive field locations on the colorectum surface, and the horizontal lines designate colonic (top) and rectal (bottom) segments of the mouse colorectum. In B, responses to stretch (action potentials) were evenly binned into 10 bins; data are presented as means ± SE. Responses of rectal muscular afferents significantly differed from all other groups (F = 5.5; P < 0.01).
Responses (action potentials) to the ramped-force stimulus (0 to 170 mN) were evenly binned into 10 bins plotted as stretch-response functions in Fig. 3B. Responses of rectal muscular/mucosal afferents to circumferential stretch were not different from their colonic muscular/mucosal counterparts. However, responses of rectal muscular afferents to circumferential stretch were significantly (P < 0.05) greater than their colonic muscular counterparts as well as from all other afferent fiber groups (F = 5.5; P < 0.01).
In 47 of the 79 stretch-sensitive afferents, conduction velocities were measured using electrical stimulation of receptive fields. Mean conduction velocities did not differ between groups (F = 1.5, P = 0.216): colonic muscular (0.52 ± 0.05 m/s), colonic muscular/mucosal (0.61 ± 0.07 m/s), rectal muscular (0.65 ± 0.02 m/s), and rectal muscular/mucosal (0.62 ± 0.04 m/s). Consistent with previous findings in mouse colorectum (5), the conduction velocities of all fibers studied were below 1 m/s, suggesting that the vast majority of stretch-sensitive PN afferents were unmyelinated slowly conducting C fibers.
Segregation of colonic stretch-sensitive afferents by response threshold.
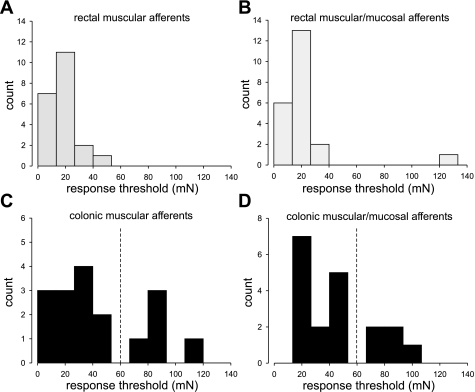
The response threshold of stretch-sensitive afferent fibers was defined as the force that induced the first action potential in the slow-ramped stretch protocol. For simplicity, we linearly correlated the force (mN) to intracolonic pressure (mmHg), assuming constant colon circumference as in equation 1. Response thresholds of all 79 stretch-sensitive afferents were determined and plotted as histograms in Fig. 4. As shown in Fig. 4, A and B, virtually all rectal muscular and muscular/mucosal afferents had thresholds below 60 mN or 16 mmHg (marked as dashed lines in Fig. 4); only one of 43 rectal afferents had a response threshold greater than 60 mN (Fig. 4B). In contrast, the distribution of response thresholds of colonic afferents segregated into HT (>68 mN or 18 mmHg) and low-threshold (LT) (<53 mN or 14 mmHg) subgroups for both colonic muscular (Fig. 4C) and colonic muscular/mucosal (Fig. 4D) afferents. Compared with the LT subgroups, the proportions of HT colonic afferents were relatively small, i.e., 26.3% (5/19) of colonic muscular/mucosal fibers and 29.4% (5/17) of colonic muscular fibers.
Fig. 4.
Response thresholds to stretch of stretch-sensitive afferents are presented in A–D. Response thresholds of rectal muscular and muscular/mucosal afferents were below 42.5 mN (A and B, respectively) with the exception of 1 rectal muscular/mucosal afferent (B). In contrast, response thresholds of colonic afferents segregated into high-threshold (>68 mN) and low-threshold (<53 mN) subgroups as indicated by the dashed vertical line in C and D.
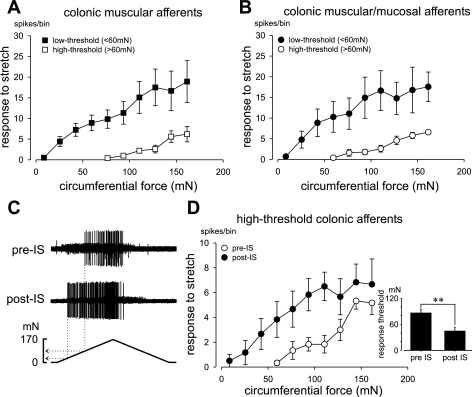
Following segregation by thresholds, colonic muscular and muscular/mucosal fibers were separated into LT and HT subgroups, the stretch-response functions of which differed significantly for both muscular (Fig. 5A; F = 7.5, P < 0.05) and muscular/mucosal fibers (Fig. 5B; F = 7.1, P < 0.05). Sensitization is a defining characteristic of nociceptors. To examine sensitization, an IS (21) was applied directly to the receptive fields of six HT stretch-sensitive colonic afferents (3 muscular and 3 muscular/mucosal). Figure 5C presents an example of sensitization by IS of the response to circumferential stretch; data for all six afferents are summarized in Fig. 5D. Both indices of sensitization, the stretch-response function (i.e., response magnitude; Fig. 5D; F = 8.4; P < 0.05) and stretch-response threshold (Fig. 5D, inset; P < 0.005), were significantly changed after exposure to IS.
Fig. 5.
Stretch-response functions of low- and high-threshold colonic stretch-sensitive afferents are presented in A and B. Responses to stretch (action potentials) were evenly binned into 10 bins; data are presented as means ± SE. An example of sensitization of a high-threshold colonic afferent is shown in C; data are summarized in D. C: response of the same afferent to stretch before (top) and after (bottom) exposure (3 min) of the receptive ending to inflammatory soup (IS). Stretch-response functions of 6 high-threshold colonic afferents (3 muscular, 3 muscular/mucosal) before (pre-IS) and after (post-IS) are presented in D. IS significantly (P < 0.05) increased stretch responses and significantly (P < 0.005) reduced response threshold (inset in D).
DISCUSSION
The present study characterized the response characteristics and sensitization of stretch-sensitive PN afferents innervating the mouse colon and rectum. About one half (52%) of the afferents were also responsive to mucosal stroking (i.e., were muscular/mucosal) in agreement with the literature (5, 18, 19). The receptive ending of each afferent fiber was localized on the colorectal surface using von Frey-like nylon monofilaments and all had delimited areas ∼1 mm2. Consistent with previous studies in mice, we did not find colorectal fibers with multiple receptive fields (5, 18, 19). Significantly, we used a computer-controlled force actuator to apply a homogeneous ramped circumferential stretch to the flattened colorectal tissue, thus allowing calculation of the comparable intracolonic pressure that generates similar local mechanical stress/strain within the intact, cylindrical colorectum. This makes possible comparison of the present findings with other electrophysiological/behavioral studies that have used balloon distension and other strategies to mechanically stimulate the colorectum (5, 18, 20, 32, 37).
To the best of our knowledge, this is the first direct comparison between stretch-sensitive rectal and colonic PN afferents. Whereas the rectal component of the large bowel in humans is anatomically distinguished as the segment distal to the sigmoidal colon (29), the distinction between the rodent rectum and distal colon is relatively subtle (8), as both are contained in the final straight segment of the bowel. Histologically, the human rectum features the merger of flat bands of muscle into a continuous outer longitudinal muscle layer (29) thicker than the smooth muscle layer in the distal colon, and this seems to be preserved in rodent species as well (see Fig. 1). Accordingly, we designated the distal, most muscular 10 mm of colorectum as rectal and the more proximal, adjacent tissue as colonic. When segregated in this manner, we identified four subgroups of stretch-sensitive fibers, including colonic muscular, rectal muscular, colonic muscular/mucosal, and rectal muscular/mucosal afferents. Of the four groups, the accelerating stretch-response function of rectal muscular afferents significantly differed from the other three groups, of which none differed from each other. In related studies, the guinea pig rectum was reported to be densely innervated by a distinct population of LT, slowly adapting mechanoreceptors with specialized intraganglionic laminar endings (IGLEs), representing a group of afferent endings not found more proximally in the colon (22, 41). Rectal IGLEs, which have not been described in the mouse, terminate in the myenteric plexus between circular and longitudinal muscle layers (22); they would likely have been categorized functionally as rectal muscular afferents in the present study.
Virtually all rectal muscular and muscular/mucosal and 72% of colonic muscular and muscular/mucosal afferents had LTs for response, but all encoded stimulus intensity into the noxious range. In distinction from rectal afferents, 28% of colonic stretch-sensitive afferents had HTs (>68 mN or 18 mmHg) for response and also encoded stimulus intensity into the noxious range. This proportion of HT afferents is consistent with what has been reported in other hollow organs (e.g., esophagus, gallbladder, urinary bladder, uterus) and different species (e.g., opossum, ferret, cat, rat, and guinea pig) (see Ref. 33 for details and discussion). In behavioral studies using balloons to distend the colorectum in unanesthetized mice, intracolonic balloon pressures greater than 20 mmHg (20) are considered noxious. The similarity between response thresholds in the present in vitro study (68 mN or 18 mmHg for HT colonic afferents) and the noxious pressure threshold in vivo supports the segregation of colonic afferents into LT and HT subgroups.
HT stretch-sensitive rectal afferents were rare, consistent with a study by Spencer and colleagues (35, 36). They studied mouse rectal nerve afferents (i.e., the proportion of rectal afferents distal to the major pelvic ganglion) using stretch forces up to 8 g and found no HT afferents. Considering the colon and rectum together, the proportion of HT fibers in the present study (14%) is less than the 20–25% typically found in comparable studies that used intracolonic balloon distension in vivo (e.g., Ref. 32). Because balloon distension of the rectal segment is constrained by supporting pelvic tissue and sphincter muscle, in vivo intracolonic balloon distension is likely to impose less deformation in the rectum than colon. We confirmed this by in vivo X-ray microscopy of the mouse distal colorectum during balloon distension (14). We found that colon, but not rectal diameter, increased in a stimulus intensity-dependent manner as balloon pressure was increased from 15 to 60 mmHg. This observation is consistent with the present in vitro results and larger in vivo electrophysiological literature (33); rectal HT afferents are uncommon, and HT afferents reported from in vivo studies using balloon distension likely represent colonic afferents.
A recent ex vivo study in the mouse suggested that the HT proportion of colorectal afferents is much larger than 20–30% (23). When the colon was filled with fluid to stretch/distend the organ, 70% of stretch-responsive colorectal afferents were characterized by low-frequency firing in response. The remaining 30% of afferents were characterized by high-frequency firing and extrapolated response thresholds lower than the larger group of low-frequency afferents. (23). The mean response threshold of those low-frequency afferents was 17.5 cmH2O (equivalent to 12.9 mmHg), a threshold we would characterize as low. An important finding in this study was that most low-frequency afferents also responded to intraluminal infusion of capsaicin, consistent with the finding that 87% of low-frequency afferent somata in the L6 dorsal root ganglion expressed transient receptor potential vanilloid 1 (23). Although there are significant experimental differences and interpretations of findings between this report and the present study, their study nonetheless provides additional support that the colorectal afferent innervation is not homogeneous with respect to either response threshold or chemical phenotype.
In mammals, the afferent innervation of the distal colorectum is contained in two distinct spinal nerve pathways, namely the hypogastric/LSNs and the PNs. It has long been debated which of these two pathways plays the predominant role in conveying nociceptive colorectal mechanosensation to the spinal cord. It has been suggested that serosal afferents in the LSN pathway are most important to visceral nociception because receptive endings located in the serosa have the highest thresholds for response to von Frey probing among the five classes of mechanosensitive colorectal afferents (6, 18). The weight of evidence, however, suggests greater importance of the pelvic pathway in colorectal nociception. In a behavioral study in rats in which colorectal balloon distension was used as a noxious stimulus, partially sympathectomized rats with only pelvic nerves intact retained a passive avoidance behavior (25). Similarly, a recent study showed that cutting the rectal nerves consistently abolished the visceromotor response to colon distension, whereas cutting both the lumbar colonic and hypogastric nerves (with rectal nerves intact) had no effect on the visceromotor response (7). These findings are supported by evidence in the mouse that the PN pathway contains a significantly larger proportion of stretch-sensitive afferents (44% compared with 10% in the LSN) (5), which encodes colorectal distension to drive the visceromotor response, a reliable and widely accepted metric of evoked visceral nociception and hypersensitivity (11, 20, 25, 26). It has been suggested that LSN input assumes a role in colorectal nociception in the presence of inflammation (39).
Significantly, we found that HT colonic afferents were sensitized after brief exposure to an IS, a cocktail containing inflammatory mediators and protons commonly found in inflammatory exudates (10, 21). Acidic IS has been shown to sensitize colonic (19) and bladder (40) afferents to stretch although a significant reduction in response threshold is not always evident (9). Sensitization of mechanosensitive PN colonic afferents is important to colorectal hypersensitivity (e.g., in IBS) because reduced response thresholds of afferent colorectal endings leads to increased afferent input to the central nervous system. In addition, sensitization of normally mechanically insensitive colorectal afferents (i.e., “silent” afferents) contributes to hypersensitivity. We recently reported that ∼24% of PN endings in the mouse colon were mechanically insensitive, about one half of which sensitized after exposure to IS (13).
Accumulating evidence strongly supports the importance of afferent input to the discomfort and pain that characterizes IBS (30, 31). Clinically, assessment of visceral sensitivity in patients with IBS commonly employs barostat distension of the rectum (3, 24). Distension of the descending (left) colon to assess sensations is increasingly uncommon (4, 27), and assessment of sensitivity in both the descending colon and rectum in the same patient is rare (28). The present finding that HT stretch-sensitive afferents are present in the colon and are virtually absent in the rectum suggests that moving the site of distension proximal into the colon might provide a more reliable assessment of visceral hypersensitivity in IBS patients. Another present finding of the greater dynamic range of response of rectal afferents provides an explanation for different sensations reported by patients subjected to rapid as opposed to slow barostat inflation in the rectum.
In summary, we undertook the systematic characterization of stretch-sensitive afferent fibers innervating mouse distal colon and rectum. Virtually all rectal afferents had LTs for response, and muscular rectal afferents gave greater responses to stretch, suggesting greater sensitivity to filling, which would be consistent with a presumed storage and evacuation function. Most colonic afferents were also LT and had similar pressure encoding features as rectal afferents although more than a quarter of colonic afferents had HTs for response and also sensitized after exposure to an IS, which significantly reduced their response threshold and increased response magnitude, suggesting a role in normal nociception and colorectal hypersensitivity in pathophysiological conditions.
GRANTS
This work was supported by National Institutes of Health award NS 19912.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Michael Burcham for assistance with preparation of figures.
REFERENCES
- 1.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil 19: 62–88, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Blumberg H, Haupt P, Janig W, Kohler W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibres from the colon. Pflügers Arch 398: 33–40, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 122: 1771–1777, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bradette M, Delvaux M, Staumont G, Fioramonti J, Bueno L, Frexinos J. Evaluation of colonic sensory thresholds in IBS patients using a barostat. Definition of optimal conditions and comparison with healthy subjects. Dig Dis Sci 39: 449–457, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA, Brierley SM, Jones RC, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, Holtmann G, Liedtke W, Blackshaw LA. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 134: 2059–2069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brookes SJ, Spencer NJ. Identification of the pain pathway activated by rectal distension in mice (Abstract). Proc Aust Soc Neurosci 18: 103, 2008. [Google Scholar]
- 8.Browning KN, Cunningham SM, Duncan L, Timmermans J, Lees GM. Regional differences in the sympathetic innervation of the guinea pig large intestine by neuropeptide Y- and tyrosine hydroxylase-immunoreactive nerves of divergent extrinsic origin. J Comp Neurol 410: 515–530, 1999 [PubMed] [Google Scholar]
- 9.Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol 297: G1250–G1258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bueno L, Fioramonti J. Visceral perception: inflammatory and non-inflammatory mediators. Gut 51, Suppl 1: i19–i23, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christianson JA, Gebhart GF, Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc 2: 2624–2631, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Drossman DA. Rome III: The Functional Gastrointestinal Disorders McLean, VA: Degnon Associates, 2006 [Google Scholar]
- 13.Feng B, Gebhart GF. Silent afferents in the pelvic nerve innervation of the mouse colon (Abstract). Digestive Disease Week 2009 Conference. 2009. [Google Scholar]
- 14.Feng B, Robinson DR, Brumovsky PR, Gebhart GF. In vitro and in vivo characterization of mouse colorectal mechanosensation (Abstract). Biomedical Engineering Society Annual Fall Meeting. 2009 [Google Scholar]
- 15.Gunterberg B, Kewenter J, Petersen I, Stener B. Anorectal function after major resections of the sacrum with bilateral or unilateral sacrifice of sacral nerves. Br J Surg 63: 546–554, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Gupta V, Moshiree B, Verne GN. Treatment of pain symptoms in irritable bowel syndrome patients. Drugs Today (Barc) 40: 829–836, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Habler HJ, Hilbers K, Janig W, Koltzenburg M, Kummel H, Lobenberg-Khosravi N, Michaelis M. Viscero-sympathetic reflex responses to mechanical stimulation of pelvic viscera in the cat. J Auton Nerv Syst 38: 147–158, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Hughes PA, Brierley SM, Martin CM, Brookes SJ, Linden DR, Blackshaw LA. Post-inflammatory colonic afferent sensitization: different subtypes, different pathways, and different time-courses (Abstract). Gut 58: 1317–1318, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamp EH, Jones RC, 3rd, Tillman SR, Gebhart GF. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol 284: G434–G444, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kessler W, Kirchhoff C, Reeh PW, Handwerker HO. Excitation of cutaneous afferent nerve endings in vitro by a combination of inflammatory mediators and conditioning effect of substance P. Exp Brain Res 91: 467–476, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125: 786–794, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Malin SA, Christianson JA, Bielefeldt K, Davis BM. TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci 29: 743–752, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology 109: 40–52, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 450: 153–169, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Ness TJ, Gebhart GF, Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies (Review). Pain 41: 167–234, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain 43: 377–386, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Ng C, Malcolm A, Hansen R, Kellow JE. Distension technique influences the relationship between colonic and rectal hypersensitivity in irritable bowel syndrome. Neurogastroenterol Motil 18: 206–210, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Olsson C, Chen BN, Jones S, Chataway TK, Costa M, Brookes SJ. Comparison of extrinsic efferent innervation of guinea pig distal colon and rectum. J Comp Neurol 496: 787–801, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage 47: 995–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain 7: 529–535, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 71: 2046–2060, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta JN, Gebhart GF. Mechanosensitive afferent fibers in the gastrointestinal and lower urinary tracts. In: Visceral Pain, edited by Gebhart GF. Iowa City, Iowa: IASP, 1995, p. 75–98 [Google Scholar]
- 34.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X(3) receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spencer NJ, Kerrin A, Singer CA, Hennig GW, Gerthoffer WT, McDonnell O. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience 153: 518–534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer NJ, Kerrin A, Zagorodnyuk VP, Hennig GW, Muto M, Brookes SJ, McDonnell O. Identification of functional intramuscular rectal mechanoreceptors in aganglionic rectal smooth muscle from piebald lethal mice. Am J Physiol Gastrointest Liver Physiol 294: G855–G867, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 80: 2632–2644, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain 105: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Tang B, Traub RJ. Differential processing of noxious colonic input by thoracolumbar and lumbosacral dorsal horn neurons in the rat. J Neurophysiol 94: 3788–3794, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Xu L, Gebhart GF, Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol 289: G397–G406, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain 139: 218–224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]