Abstract
Vasoactive intestinal peptide (VIP) immunoreactive neurons are important secretomotor neurons in the submucous plexus. They are the only submucosal neurons to receive inhibitory inputs and exhibit both noradrenergic and nonadrenergic inhibitory synaptic potentials (IPSPs). The former are mediated by α2-adrenoceptors, but the receptors mediating the latter have not been identified. We used standard intracellular recording, RT-PCR, and confocal microscopy to test whether 5-HT1A, SST1, and/or SST2 receptors mediate nonadrenergic IPSPs in VIP submucosal neurons in guinea pig ileum in vitro. The specific 5-HT1A receptor antagonist WAY 100135 (1 μM) reduced the amplitude of IPSPs, an effect that persisted in the presence of the α2-adrenoceptor antagonist idazoxan (2 μM), suggesting that 5-HT might mediate a component of the IPSPs. Confocal microscopy revealed that there were many 5-HT-immunoreactive varicosities in close contact with VIP neurons. The specific SSTR2 antagonist CYN 154806 (100 nM) and a specific SSTR1 antagonist SRA 880 (3 μM) each reduced the amplitude of nonadrenergic IPSPs and hyperpolarizations evoked by somatostatin. In contrast with the other antagonists, CYN 154806 also reduced the durations of nonadrenergic IPSPs. Effects of WAY 100135 and CYN 154806 were additive. RT-PCR revealed gene transcripts for 5-HT1A, SST1, and SST2 receptors in stripped submucous plexus preparations consistent with the pharmacological data. Although the involvement of other neurotransmitters or receptors cannot be excluded, we conclude that 5-HT1A, SST1, and SST2 receptors mediate nonadrenergic IPSPs in the noncholinergic (VIP) secretomotor neurons. This study thus provides the tools to identify functions of enteric neural pathways that inhibit secretomotor reflexes.
Keywords: somatostatin, serotonin, secretomotor neurons, inhibitory interneurons, nonadrenergic inhibitory synaptic potentials
the enteric nervous system contains a network of neurons that regulates gut water and electrolyte secretion. The key secretomotor neurons in this network are immunoreactive for vasoactive intestinal peptide (VIP) and are located within the submucosal plexus (3). These VIP neurons receive inputs from several sources and display at least six different types of inhibitory and excitatory postsynaptic potentials (IPSPs and EPSPs, respectively) (23). They are the only neurons in the submucous plexus to receive inhibitory inputs (2, 12).
It has been known for over 20 years that there are at least two classes of IPSPs in submucosal neurons (24, 25). One comes from sympathetic nerve terminals and is well established to be mediated by norepinephrine (NE) acting on α2-adrenoceptors (1, 43). The other persists after sympathetic denervation of the intestine and is insensitive to blockade of α-adrenoceptors and to guanethidine, a blocker of transmitter release from sympathetic nerve terminals (1). These nonadrenergic IPSPs, however, disappear when sympathetic denervation is combined with removal of the overlying myenteric plexus, suggesting that they arise from myenteric neurons (1, 36).
Somatostatin (Som) is accepted as the most likely candidate for the neurotransmitter mediating nonadrenergic IPSPs. Local application of Som produces a hyperpolarization that mimics IPSPs (37). Som is found within nerve terminals in submucosal ganglia (35), and Som desensitization depressed IPSPs in sympathetically denervated submucosal neurons (46). However, desensitization studies are subject to the uncertainties of potential cross-desensitization with unidentified alternative receptors, and the Som receptors expressed by submucosal neurons in the guinea pig have not been identified. One aim of this present study was to identify the Som receptor (SSTR) subtypes expressed by guinea pig submucosal neurons and to determine whether these mediate nonadrenergic IPSPs in these neurons. We focused on SST1 and SST2A receptors, because mRNA for the former (45) and immunoreactivity for the latter (49) are found in the rat submucous plexus and specific antagonists for each of these receptor subtypes are now available.
More recently, we reported that the 5-HT1A receptor antagonist NAN-190 depresses the noradrenergic component of the IPSPs in VIP neurons via an action on α2-adrenoceptors (14). In the course of that study, we noted that NAN-190 also appeared to depress nonadrenergic IPSPs, although whether 5-HT1A receptors are involved was difficult to confirm because of lack of specificity of the drug. Accordingly, another aim of this study was to examine the effects of a highly specific antagonist for 5-HT1A receptors, WAY 100125 (13), on the nonadrenergic IPSPs, testing the novel hypothesis that these responses are mediated by serotonin.
MATERIALS AND METHODS
Electrophysiology: Intracellular Recording
Tissue preparation.
Guinea pigs of either sex weighing 200–400 g were killed by stunning followed by severing of carotid arteries and spinal cord, a method approved by the University of Melbourne Animal Experimentation Ethics Committee. The abdominal cavity was opened and a 3–5 cm segment of ileum, 15–30 cm oral to the ileocecal junction, was removed. The tissue was immediately placed in physiological saline (composition in mM: NaCl 118, NaHCO3 25, d-glucose 11, KCl 4.8, CaCl2 2.5, MgSO4 1.2, NaH2PO4 1.0), bubbled with 95% O2-5% CO2. The intestinal segment was cut open along the mesenteric border and pinned flat with the mucosa side up on a dissecting dish lined with silicone elastomer (Sylgard, compound 184; Dow Corning, Midland, MI). The mucosa was stripped off with fine forceps, and a 1.5 × 1.5 cm piece of submucous plexus was removed from the underlying circular muscle layers and pinned flat with the serosa side up in an organ bath and viewed under an upright microscope (Olympus BX51WI, Olympus Australia, Victoria, Australia).
In another series of experiments, segments of ileum were placed in physiological saline containing nicardipine (1.25 μM) and hyoscine (1 μM). The tissue was opened and the uppermost mucosa, submucosa, and circular muscle were removed to reveal the myenteric plexus attached to longitudinal muscle.
The preparations were continually superfused with physiological saline bubbled with 95% O2-5% CO2, maintained at 33–34°C, and allowed to equilibrate for at least 1 h before beginning electrophysiological experiments.
Electrophysiology.
Electrical recordings were made from submucosal neurons by conventional intracellular recording methods (38). Submucosal ganglia were viewed at ×200 magnification under an Olympus LMPlanF1 20×/0.40 ∞/o microscope (long-distance objective lens). Neurons were impaled with glass microelectrodes (resistance 100–200 MΩ, 1 M KCl). A monopolar stimulating electrode (50 μm, noninsulated tungsten wire) was positioned on an internodal strand leading into the chosen ganglion to apply focal stimulation to nerve fibers, evoking synaptic potentials in the impaled cells. Trains of 3 (12 V, 30 Hz, 100 ms) or 15-pulses were applied to internodal strands. These stimulus parameters evoke IPSPs, and sometimes slow EPSPs (2, 14). Where indicated, a single pulse stimulus (12 V) was also applied to evoke the one pulse, or purinergic slow EPSPs in these neurons (15, 38, 50). Unless otherwise indicated, experiments were only undertaken on neurons that displayed IPSPs in response to stimulus trains. Only VIP immunoreactive submucosal neurons display IPSPs (2, 12).
S neurons in myenteric ganglia were impaled by conventional methods (22). Hyperpolarizing current was applied to keep the membrane potential at −85 mV to enlarge the amplitudes of fast EPSPs evoked by single pulses (1–2 nA) applied to an interganglionic fiber tract.
Drugs used.
The receptor agonists dl-arterenol · HCl (NE) and Som (somatostatin-14, both from Sigma-Aldrich, Castle Hill, New South Wales, Australia) were prepared as stock solutions dissolved in physiological saline kept at −20°C. About 8%, 100 mM acetic acid was added to the Som solution to facilitate dissolving of the peptide. 8-Hydroxy-2-(di-n-propylamino)tertraline (8-OH-DPAT, Sigma-Aldrich) was dissolved in distilled water and kept at −20°C.
Receptor antagonists idazoxan hydrochloride and hyoscine hydrobromide (both from Sigma-Aldrich) were prepared as stock solutions in distilled water kept at 4°C. Nicardipine (Sigma-Aldrich) and (S)-WAY 100135 dihydrochloride (both from Tocris Bioscience, East Brisbane, Queensland, Australia) were prepared as stock solutions in distilled water kept at −20°C. CYN 154806 (Tocris Bioscience) was dissolved in water and 10%, 0.1 M acetic acid kept at −20°C. PKF225-880-AA-1 (SRA 880, Novartis Pharmaceuticals, North Ryde, New South Wales, Australia) was prepared as stock solution in 100% ethanol and kept at −20°C.
All antagonists were prepared as 1,000-fold concentration stock solutions and dissolved to their final concentration in physiological saline before addition to the organ bath.
Antagonists and 8-OH-DPAT (where indicated) were added to the superfusing solution. Agonists were applied directly to cell bodies via pressure ejection (15 psi, 50–150 ms) via a micropipette (tip diameter ∼10 μM) positioned just above the preparation near the targeted cell in the submucosal ganglion.
Data measurements and statistics.
The data recorded were analyzed with the computer software Axoscope 9.2.0.12 (Axon Instruments, Union City, CA). The parameters analyzed for fast EPSPs, IPSPs, slow EPSPs, and agonist pressure ejection-evoked responses were amplitude (mV) and, where applicable, duration (ms). The membrane potential of an impaled neuron immediately before a stimulus was taken as the baseline. The duration was measured as the time between a point immediately after the stimulus artifact, which corresponds to the baseline, and a point when the synaptic potential returned to baseline. Each stimulus was repeated two to three times. The average of each preparation was obtained for each parameter of each stimulus regime in control, drug and, where possible, wash conditions. The mean values obtained for the preparations were used for statistical comparison.
All results are presented as means ± SE. A two-tailed Student's paired t-test, one-tailed paired t-test, or, where possible, repeated-measures ANOVA were conducted. P values less than 0.05 were considered to be significant.
Identification of 5-HT Contacts on VIP Neurons
Impaled neurons displaying IPSPs were filled with biocytin during recordings. After the experiment, the submucous preparation was fixed for 80 min in 4% formaldehyde (freshly prepared from paraformaldehyde in 0.1 M phosphate buffer, pH 7.4) at room temperature, a technique established (4) for immunohistochemical identification of 5-HT (4). The preparation was given three washes with phosphate-buffered saline (PBS), followed by a 25-min incubation submerged in 10% universal blocking agent CAS block (Invitrogen Australia, Mount Waverley, Victoria, Australia) to suppress nonspecific background staining and 1% Triton X-100 (ProSciTech, Thuringowa, Queensland, Australia). The tissue was then incubated in rabbit antiserum against 5-HT (1:2,000, Immunostar, Hudson, WI) at 4°C for 48 h. After three washes with PBS, the preparation was incubated for ∼2 1/2 h with secondary antibodies (Alexa donkey anti-rabbit 594 1:100; Strepavidin Alexa 488 1:100, both from Molecular Probes, Invitrogen Australia). The submucous preparation was given another three washes with PBS and then mounted on a slide with Dakocytomation fluorescent mounting medium (Carpinteria, CA).
The impaled neurons and 5-HT immunoreactive varicosities were identified and viewed under a confocal microscope (Zeiss Pascal LSM 510). A Z-series of the preparation was obtained by use of a ×100 objective lens and a step distance of ∼0.4 μm to ensure that adjacent planes overlapped. The number of varicosities apposed to filled neurons was counted as described previously (34, 41, 42). The number of 5-HT varicosities on the identified VIP-immunoreactive cell was counted by merging the images by use of a Zeiss LSM Image Browser (version 4,2,0,121, Carl Zeiss MicroImaging 1997–2006). The outline of the cell body and visible processes was traced onto transparent sheets for each focal plane directly by overlaying the sheets on the computer monitor. Varicosities that contacted the cell body or a process with no intervening pixel (0.2 μm), viewed with a ×100 objective, were defined as appositions. Outlines of these varicosities were traced on the transparent sheets in a different color. The transparent sheets of all the planes in the z-series were then overlaid on each other and every unique apposition was counted (34, 41, 42).
Identification of 5-HT1A, SSTR1, and SSTR2 Messenger RNA
A 3- to 4-cm piece of ileum was removed from the abdomen of each animal and placed into 4°C PBS. Six stripped submucosal preparations and six myenteric plexus preparations with attached longitudinal muscle were obtained by microdissection (details above) on ice with sterile forceps and spring scissors.
Total RNA was extracted from frozen tissues by using TRI Reagent (Ambion; Applied Biosystems, Scoresby, Victoria, Australia) according to the manufacturer's instructions. The RNA was then treated with 2 U DNase I to remove genomic DNA contamination. RNA concentrations were determined by using a Bio-Rad Smart Spec 3000 with A260:A280 absorbance ratios of >1.9. First-strand cDNA synthesis used 1 μg total RNA in a 20-μl reaction, with random hexamers (50 ng/μl) and 200 U SuperScript III (Invitrogen). We also included a reverse transcriptase (RT) negative control in which the RT enzyme was replaced with water.
RT-PCR assessed the presence or absence of gene transcripts for 5-HT1A, SSTR2, and SSTR1 in the submucous plexus or myenteric plexus. Oligonucleotide primers (Geneworks, Hindmarsh, South Australia, Australia) were designed from the rat 5-HT1A (NCBI 6981053), SSTR2 (NCBI 11225271), and SSTR1 (NCBI 6981583) genes (Table 1). Each 25-μl reaction mix consisted of 5 μl, 5× GoTaq Green Master Mix (Promega, Annandale, New South Wales, Australia), 1.5 μl MgCl2, 1 μl 10 mM dNTP mix, 1 μl forward and 1 μl reverse oligonucleotide primer (20 μM), 0.1 μl Taq enzyme and 1 μl sample cDNA or nuclease free H2O as a negative control. The PCR reaction conditions were as follows: 1 cycle (85°C, 2 min) and then 40 cycles (94°C, 1 min; specific annealing temperatures for each primer, 1 min; 72°C, 1 min) with a final extension at 72°C for 10 min. To assess the quality of the cDNA template from every sample, PCR reactions were run using oligonucleotide primers specific for the housekeeping gene ribosomal 18S. Each PCR product (15 μl) was separated by gel electrophoresis on 2% 0.5× Tris-borate-EDTA buffer agarose gels with ethidium bromide (Invitrogen). The DNA marker HyperLadder IV (Bioline, Alexandra, New South Wales, Australia) was used to estimate PCR product size.
Table 1.
Receptor-specific primers used for RT-PCR
| Primer | Sequence | Size, bp | Annealing Temperature, °C |
|---|---|---|---|
| Rat 5 HT1A | 199 (Ref. 31) | 52 | |
| Forward | 5′-GATGTGTTCAGTTTTGGCCAGG-3′ | ||
| Reverse | 5′-GGAGCGCTCCAAGGCGATGGCA-3′ | ||
| Rat 5-HT1A | 433 (NCBI: 6981053) | 50 | |
| Forward | 5′-TACTAGCATCTCCGACGTGAC-3′ | ||
| Reverse | 5′-GATGGAGATGAGAAAGCCAAT-3′ | ||
| GP 5-HT1A | 339 (NCBI: 226432451) | 55 | |
| Forward | 5′-GTGATCACCTCTCTGCTGCTA-3′ | ||
| Reverse | 5′-CGTCCTCTTGTTCACGTAGTC-3′ | ||
| Rat SSTR1 | 374 (NCBI: 6981583) | 55 | |
| Forward | 5′-TGTGTTACGTGCTCATCATTG-3′ | ||
| Reverse | 5′-CACACTGTAGGCACGACTCTT-3′ | ||
| GP SSTR1 | 288 (NCBI: 213391418) | 58 | |
| Forward | 5′-ACTCTCATGGTGATGATGGTG-3′ | ||
| Reverse | 5′-CACACTGTAGGCACGACTCTT-3′ | ||
| Rat SSTR2 | 351 (NCBI: 11225271) | 57 | |
| Forward | 5′-TCATCAAGGTGAAGTCCTCTG-3′ | ||
| Reverse | 5′-GGGATTTGTCCTGCTTACTGT-3′ | ||
| GP SSTR2 | 270 (NCBI: 213391416) | 53 | |
| Forward | 5′-CCTCCAAGAGGAAGAAATCTG-3′ | ||
| Reverse | 5′-CACTCAATTTGACCAAGAACG-3′ |
GP, guinea pig.
The remaining 10 μl of each PCR product was purified with Sure-Clean Plus (Bioline), and the resulting DNA was sequenced by the Applied Genetic Diagnostics laboratory in the Department of Pathology (The University of Melbourne, Parkville, Victoria, Australia). Guinea pig oligonucleotide primers (Geneworks) were designed from the sequenced guinea pig 5-HT1A (NCBI 226432451), SSTR2 (NCBI 213391416), and SSTR1 (NCBI 213391418) genes (refer to Table 1), and PCR was performed again on the same cDNA as described above.
RESULTS
Electrophysiological experiments were conducted on 156 submucous plexus preparations and 5 myenteric plexus preparations, with a total of 254 submucosal and 6 myenteric neurons impaled. Pharmacological experiments were conducted on 188 of the impaled submucosal neurons and all of the myenteric neurons.
IPSPs and Hyperpolarization to NE
Focal electrical stimulation of internodal strands in the submucosal plexus with a train of 3 (Fig. 1A) or 15 pulses (Fig. 1B) produced an IPSP in 239 submucosal neurons often followed by a slow EPSP (Fig. 1A, B). Neurons that display IPSPs have previously been shown to be the VIP-immunoreactive neurons (1, 2, 12, 28, 38).
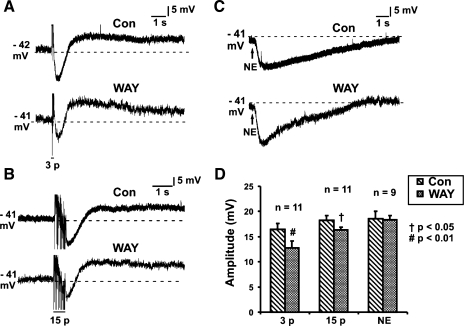
Fig. 1.
Effects of WAY 100135 (1 μM) on inhibitory postsynaptic potentials (IPSPs). Examples of 3-pulse (3p; 12 V, 30 Hz; A) and 15-pulse (15p; 30 Hz; B) evoked IPSPs and norepinephrine (NE; 1 mM; C) evoked hyperpolarizations in control (Con) and in the presence of WAY 100135 (WAY). D: quantified data for 3-pulse (n = 11) and 15-pulse (n = 11) IPSPs and NE hyperpolarization (n = 9). Amplitudes of all IPSPs were significantly reduced by WAY 100135 (2-tailed paired t-test, †P < 0.05, #P < 0.01). WAY 100135 had no effect on NE evoked hyperpolarizations.
NE (1 mM in pipette) was applied to 90 submucosal neurons; all responded with a long hyperpolarization (Fig. 1C) associated with a decrease in input resistance. The mean amplitude of the hyperpolarization was 18.2 ± 0.5 mV (n = 90), and the mean duration was 23.8 ± 1.6 s (n = 71). Focal stimulation of internodal strands evoked IPSPs in 89 of these 90 neurons.
Addition of idazoxan (α2-adrenoceptor antagonist, 2 μM) to the superfusing physiological saline depressed the IPSPs (3-pulse, n = 31; 15-pulse, n = 36) as reported previously (43) and revealed a nonadrenergic component. Slow EPSPs (3-pulse: control 5.9 ± 0.7 mV, idazoxan 6.9 ± 0.9 mV, n = 15, P = 0.2; 15-pulse: control 6.3 ± 0.9 mV, idazoxan 7.0 ± 0.8 mV, n = 16, P = 0.4) were not affected by idazoxan. There was no time-dependent rundown of the idazoxan-insensitive IPSPs (3-pulse: 1st set 6.4 ± 1.4 mV, 2nd set 6.5 ± 1.6 mV, 3rd set 6.5 ± 1.6 mV n = 5; 15-pulse: 1st set 11.9 ± 0.6 mV, 2nd set 11.6 ± 0.9 mV, 3rd set 12.6 ± 1.0 n = 5) observed over a test period of 45 min (3 sets of 3-stimulus trains separated by ∼15 min each). Idazoxan also reversibly abolished the hyperpolarization evoked by NE (n = 5) (14, 43).
NE rapidly desensitized the α2-adrenoceptors. Thus, for these experiments, the micropipette was positioned at a distance at which the membrane potential of the neuron was not affected and immediately returned to this point after pressure ejection of the agonist to prevent receptor desensitization.
Effects of Blocking and Activating 5-HT1A Receptors
The specific 5-HT1A receptor antagonist WAY 100135 (1 μM) did not affect the amplitude of slow EPSPs in submucosal neurons (3-pulse slow EPSPs: control 4.8 ± 0.7 mV, WAY 100135 6.2 ± 1.1 mV, n = 6, P = 0.3; 15-pulse slow EPSPs: control 5.5 ± 1.2 mV, WAY 100135 7.6 ± 1.1 mV, n = 6, P = 0.1) but reduced the amplitude of IPSPs (3-pulse IPSPs: control 16.5 ± 1.1 mV, WAY 100135 12.8 ± 1.3 mV, n = 11, P = 0.002, Fig. 1, A and D; 15-pulse IPSPs: control 18.3 ± 0.9 mV, WAY 100135 16.3 ± 0.6 mV, n = 11, P = 0.01, Fig. 1, B and D). WAY 100135 did not affect the amplitude of the hyperpolarization evoked by local application of NE (1 mM in pipette) (n = 9, P = 0.9, Fig. 1, C and D).
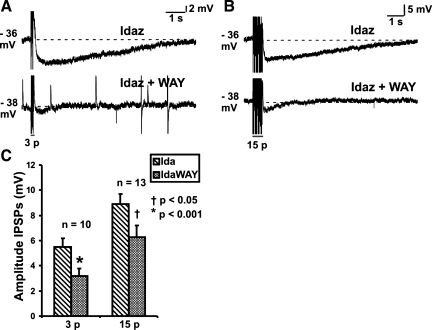
In the presence of idazoxan (2 μM), which unmasked nonadrenergic IPSPs, WAY 100135 (1 μM) also reduced the amplitudes of the IPSPs (3-pulse IPSPs: idazoxan 5.5 ± 0.7 mV, idazoxan and WAY 100135 3.2 ± 0.6 mV, n = 10, P = 0.0007, Fig. 2, A and C; 15-pulse IPSPs: idazoxan 8.9 ± 0.8 mV, idazoxan and WAY 100135 6.3 ± 0.9 mV, n = 13, P = 0.01, Fig. 2, B and C). Although the illustrated traces (Fig. 2, A and B) appear to show a reduction in the duration of the nonadrenergic IPSPs in the presence of WAY 100135, this was not a routinely observed effect and no significant difference in durations was observed for the full sample.
Fig. 2.
Effects of WAY 100135 (1 μM) on nonadrenergic IPSPs. Examples of 3-pulse (12 V, 30 Hz; A) and 15-pulse (30 Hz; B) evoked IPSPs in idazoxan (Idaz or Ida; 2 μM), and in idazoxan together with WAY 100135 (IdaWAY). C: quantified data for 3-pulse (n = 10) and 15-pulse (n = 13) evoked IPSPs. Amplitudes of all nonadrenergic IPSPs were significantly reduced by WAY 100135 (2-tailed paired t-test; †P < 0.05, *P < 0.001).
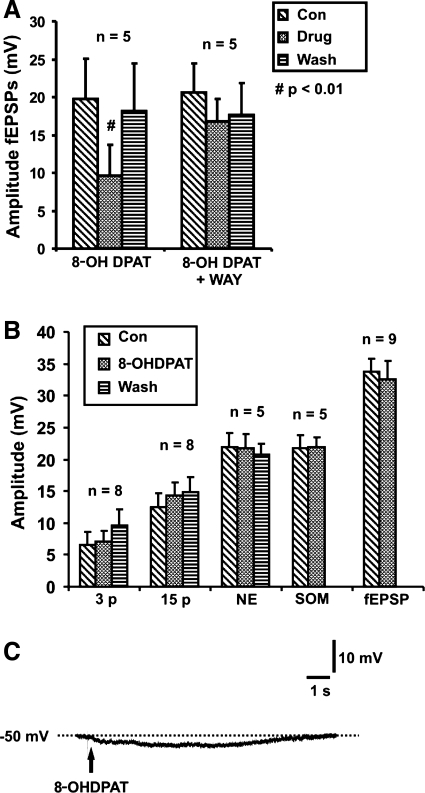
To test whether the concentration of WAY 100135 was appropriate, its effects on the inhibition of fast EPSPs in myenteric neurons by the 5-HT1A agonist 8-OH-DPAT were tested (20). 8-OH-DPAT (300 nM) reversibly reduced the amplitude of fast EPSPs evoked in myenteric neurons by focal electrical stimulation (control 19.7 ± 5.4 mV, 8-OH-DPAT 9.6 ± 4.1 mV, wash 18.1 ± 6.4 mV, n = 5, P = 0.008, Fig. 3A). 8-OH-DPAT produced no significant change to the amplitude of fast EPSPs in the presence of WAY 100135 (1 μM) (n = 5, P = 0.1, Fig. 3A).
Fig. 3.
Effects of 8-hydroxy-2-(di-n-propylamino)tertraline (8-OH-DPAT; 300 nM) and WAY 100135 (1 μM). A: quantified data for the amplitudes of fast excitatory postsynaptic potentials (fEPSPs) in myenteric neurons in control, 8-OH-DPAT (n = 5) or 8-OH-DPAT together with WAY 100135 (n = 5) and after washout. 8-OH-DPAT reversibly reduced the amplitude of fast EPSPs (repeated-measures ANOVA, #P < 0.01). This reduction is blocked by WAY 100135. B: quantified data from submucosal neurons for amplitudes of 3-pulse (n = 8) and 15-pulse (n = 8) evoked IPSPs and NE hyperpolarizations (n = 5) in control, in the presence of 8-OH-DPAT and after washout, as well as somatostatin (Som) hyperpolarizations (n = 5) and fEPSPs (n = 9), in control and in the presence of 8-OH-DPAT. No significant changes were observed. C: example of a hyperpolarization evoked by pressure ejection of 8-OH-DPAT (10 μM) onto a VIP submucosal neuron.
In contrast with its actions in the myenteric plexus, 8-OH-DPAT did not affect the amplitude of fast EPSPs, IPSPs, or agonist-evoked hyperpolarizations in submucosal neurons (Fig. 3B).
Pressure ejection of 8-OH-DPAT locally onto submucosal neurons either had no effect or produced a small hyperpolarization (4.0 ± 0.4 mV, n = 9, Fig. 3C) in some submucous neurons that displayed IPSPs. Three different concentrations of 8-OH-DPAT were tested. At 3 μM, the two neurons tested responded with small hyperpolarizations of 3 and 5 mV. Nine neurons were tested with 10 μM of the agonist; two did not respond, and seven exhibited hyperpolarizations ranging from 2–5 mV. The five neurons tested with 8-OH-DPAT (30 μM) did not hyperpolarize.
5-HT Immunoreactive Varicosities Were Apposed to VIP Submucosal Neurons
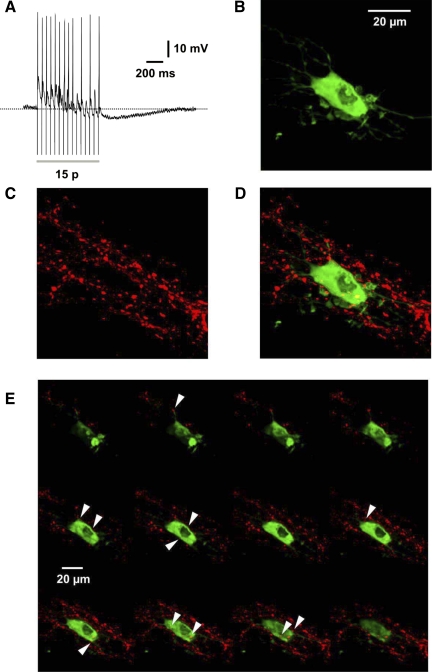
To determine whether submucosal VIP neurons receive anatomical inputs from the terminals of 5-HT neurons, as has been reported for cholinergic secretomotor neurons (41), closely apposed 5-HT varicosities were counted around four biocytin-filled VIP neurons in one submucous plexus preparation. This method was chosen because VIP immunoreactivity does not clearly delineate the cell bodies of the submucosal neurons (2, 6) and so simple double-labeling immunohistochemistry could not be used (41, 42). The biocytin-filled neurons were as assumed to be VIP immunoreactive from their ability to display IPSPs upon electrical stimulation (Fig. 4A) (1, 2, 12, 28, 38). An average of 22 varicosities (ranging from 5 to 36 appositions) immunoreactive for 5-HT was found in close apposition with the cell bodies and processes of each of these neurons (Fig. 4).
Fig. 4.
Example confocal micrographs of 5-HT immunoreactive varicosities apposing a VIP-IR submucosal neuron. A: recording of a 15-pulse IPSP in a submucosal neuron that was filled with biocytin (B, green fluorescence). C: 5-HT immunoreactivity (red fluorescence). D: green and red immunofluorescence superimposed showing 5-HT-immunoreactive varicosities closely apposed to the soma and individual processes of the biocytin-filled neuron. This image is comprised of multiple focal planes. E: Z-series of the submucosal neuron and surrounding 5-HT terminals (apposed varicosities identified by the absence of any pixel between the terminal and the biocytin staining are indicated by white arrowheads; note several of these contacts are at some distance from the cell body; scale bar represents 20 μm).
Som Hyperpolarized Submucosal Neurons With IPSPs
Som (100 μM) was applied to 47 submucosal neurons; 35 responded with a long hyperpolarization (Fig. 5C) associated with a decrease in input resistance. The mean amplitude of the hyperpolarization was 16.3 ± 0.8 mV (n = 35), and the mean duration was 95.4 ± 48.4 s (n = 12). Thirty-two of these 35 neurons also displayed IPSPs upon electrical nerve stimulation. Interestingly, 7 of the 47 neurons were depolarized (21.0 ± 3.5 mV, 6.4 ± 1.1 s, n = 7) by Som, also associated with a decrease in input resistance: three neurons responded solely with a depolarization (28.0 ± 1.2 mV, 7.5 ± 0.9 s, n = 3); and four neurons showed a hyperpolarization (10.1 ± 1.9 mV, 53.1 ± 24.8 s, n = 4) after the depolarization (23.3 ± 12.3 mV, 5.6 ± 2.0 s, n = 4). Six of these seven neurons displayed IPSPs. Five of the 47 neurons did not respond to Som and also did not display IPSPs with nerve stimulation. Vehicle controls (n = 4) were conducted, and local pressure ejection of 8% acetic acid had no effect on submucosal neurons. Only hyperpolarizations evoked by Som were studied further.
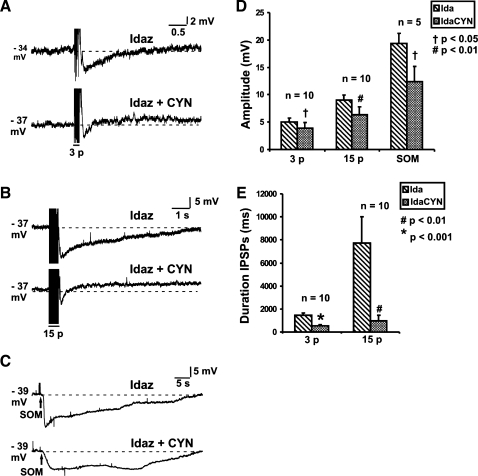
Fig. 5.
Effects of CYN 154806 (CYN; 100 nM) on nonadrenergic IPSPs. Examples of 3-pulse (A; 12 V 30 Hz) and 15-pulse (B; 30 Hz) evoked IPSPs and Som (C; 100 μM) evoked hyperpolarizations in idazoxan (2 μM), and in idazoxan together with CYN 154806 (IdaCYN). D: quantified data for the amplitudes of 3-pulse (n = 10) and 15-pulse (n = 10) evoked IPSPs and Som (100 μM) evoked hyperpolarizations (n = 5). Amplitudes of all nonadrenergic IPSPs and Som hyperpolarizations were significantly reduced by CYN 154806. E: quantified data for the durations of 3-pulse (n = 10) and 15-pulse (n = 10) evoked IPSPs. CYN 154806 produced a greater reduction on the durations of all the nonadrenergic IPSPs compared with their amplitude (1-tailed paired t-test, †P < 0.05, #P < 0.01, *P < 0.001).
Idazoxan (2 μM) did not affect the hyperpolarization evoked by Som (control 21.1 ± 1.2 mV, idazoxan 21.1 ± 1.6 mV, n = 5, P = 0.98). Som was found to desensitize its relevant Som receptor. Therefore, the application protocol used for NE was also used for Som. Som-evoked hyperpolarizations were confirmed to be consistent over a 45-min test period (n = 5; 3 sets of at least two repeated pressure ejections of Som, at 15-min intervals).
SST2 Receptors
Idazoxan (2 μM) was added to the superfusate throughout these experiments to reveal the nonadrenergic IPSP component. CYN 154806 (selective SST2 receptor antagonist, 100 nM) reduced the amplitude of the nonadrenergic IPSPs (3-pulse IPSPs: idazoxan 5.0 ± 0.7 mV, idazoxan and CYN 154806 3.9 ± 1.0 mV, n = 10, P = 0.02, Fig. 5, A and D; 15-pulse IPSPs: idazoxan 9.0 ± 0.9 mV, idazoxan and CYN 154806 6.4 ± 1.4 mV, n = 10, P = 0.01, Fig. 5, B and D) and the hyperpolarization evoked by Som (idazoxan 19.4 ± 1.8 mV, idazoxan and CYN 154806 12.4 ± 2.8 mV, n = 5, P = 0.02, Fig. 5, C and D).
CYN 154806 produced a consistent and marked shortening of the duration of nonadrenergic IPSPs (3-pulse IPSP duration: idazoxan 1.4 ± 0.2 s, idazoxan and CYN 154806 0.5 ± 0.1 s, n = 10, P = 0.0008, Fig. 5, A and E; 15-pulse IPSP duration: idazoxan 7.7 ± 2.3 s, idazoxan and CYN 154806 1.0 ± 0.5 s, n = 10, P = 0.003, Fig. 5, B and E), with a 87% reduction in duration of 15-pulse nonadrenergic IPSPs compared with controls, unlike SRA 880 (see below SST1 Receptors).
Effects of WAY 100135 and CYN 154806 on Nonadrenergic IPSPs Were Additive
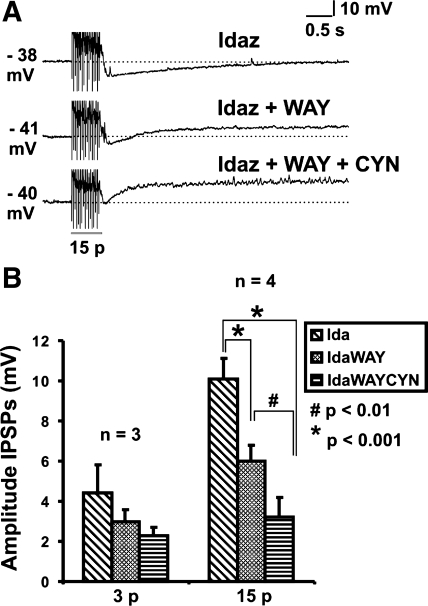
To determine whether the effects of blocking SSTR2 and 5-HT1A receptors were additive, the antagonists were added in sequence. The effects of CYN 154806 were observed to diminish when it was kept in the superfusing solution for more than 15 min. Accordingly, WAY 100135 was added to the bath first. In general, nonadrenergic IPSPs evoked by three-pulse stimulation were too small to analyze as addition of WAY 100135 or CYN 154806 individually in the presence of idazoxan often almost abolished three-pulse IPSPs (idazoxan 4.4 ± 1.4 mV, idazoxan and WAY 100135 3.0 ± 0.6 mV, idazoxan and WAY 100135 and CYN 154806 2.3 ± 0.4 mV, n = 3, P = 0.1, Fig. 6B). As a result, only the 15-pulse responses were large enough to provide a sufficient sample to be analyzed, and it was found that the antagonist effects were indeed additive. Four neurons were tested with idazoxan, WAY 100135, and CYN 154806; the 15-pulse-evoked IPSPs tested in all four neurons were additive. The mean amplitudes of the 15-pulse-evoked IPSPs were idazoxan 10.1 ± 1.0 mV, idazoxan and WAY 100135 6.0 ± 0.8 mV, idazoxan and WAY 100135 and CYN 154806 3.2 ± 1.0 mV, n = 4 idazoxan against WAY, P < 0.001; idazoxan and WAY against CYN, P < 0.01; idazoxan against WAY and CYN, P < 0.001; Fig. 6, A and B.
Fig. 6.
Effects of idazoxan (2 μM) and WAY 100135 (1 μM) together with CYN 154806 (100 nM) on nonadrenergic IPSPs. Examples of 15-pulse (A; 12 V, 30 Hz) evoked IPSPs in idazoxan, idazoxan with WAY 100135, idazoxan, and WAY 100135 together with CYN 154806 (IdaWAYCYN). B: quantified data for the amplitudes of 3-pulse (n = 3) and 15-pulse (n = 4) evoked IPSPs. Amplitudes of the 15-pulse evoked nonadrenergic IPSPs were reduced more in the presence of both WAY 100135 and CYN 154806 than with either antagonist alone (repeated-measures ANOVA, #P < 0.01, *P < 0.001).
SST1 Receptors
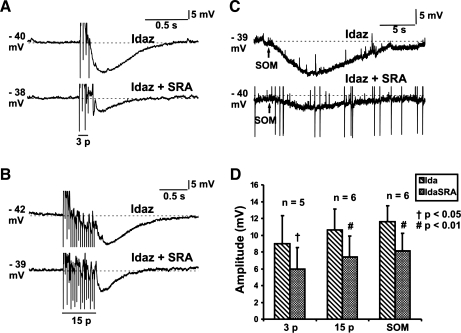
Idazoxan was again used to reveal nonadrenergic IPSPs. Only data obtained from neurons that displayed IPSPs and were hyperpolarized by Som were used for statistical analysis. SRA 880 (selective SST1 receptor antagonist, 3 μM) reduced the amplitudes of nonadrenergic IPSPs (3-pulse IPSPs: idazoxan 9.0 ± 3.3 mV, idazoxan and SRA 880 6.0 ± 2.5 mV, n = 5, P = 0.04, Fig. 7, A and D; 15-pulse IPSPs: idazoxan 10.6 ± 2.5 mV, idazoxan and SRA 880 7.4 ± 2.5 mV, n = 6, P = 0.006, Fig. 7, B and D) and the hyperpolarizations evoked by Som (idazoxan 11.6 ± 1.9 mV, idazoxan and SRA 880 8.1 ± 2.1 mV, n = 6, P = 0.003, Fig. 7, C and D).
Fig. 7.
Effects of SRA 880 (SRA; 3 μM) on nonadrenergic IPSPs. Examples of 3-pulse (12 V, 30 Hz; A) and 15-pulse (30 Hz; B) evoked IPSPs and Som (100 μM; C) evoked hyperpolarizations in idazoxan (2 μM), and in idazoxan together with SRA 880. D: quantified data for the amplitudes of 3-pulse (n = 5) and 15-pulse (n = 6) evoked IPSPs and Som hyperpolarizations (n = 6). Amplitudes of all nonadrenergic IPSPs and Som hyperpolarizations were significantly reduced by SRA 880 (1-tailed paired t-test, †P < 0.05, #P < 0.01).
Unlike the blockade of SST2 receptors with CYN 154806, blocking SST1 receptors with SRA 880 had no effect on duration of the nonadrenergic IPSPs (3-pulse IPSPs: idazoxan 0.9 ± 0.2 ms, idazoxan and SRA 880 0.6 ± 0.1 ms, n = 5, P = 0.06; 15-pulse IPSPs: idazoxan 5.1 ± 2.6 mV, idazoxan and SRA 880 2.2 ± 0.8 ms, n = 6, P = 0.09). The mean duration of IPSPs in SRA 880 decreased; this effect was not consistent between the cells tested, with one or two showing an increased duration, which accounts for the failure of the reduction to achieve statistical significance with a paired t-test. The mean reduction in duration with SRA 880 (57% reduction for 15-pulse nonadrenergic IPSPs) was substantially less than that seen with CYN 154806.
Expression of 5-HT1A, SST1, SST2 Receptor Gene Transcripts in the Guinea Pig Submucous Plexus and Myenteric Plexus
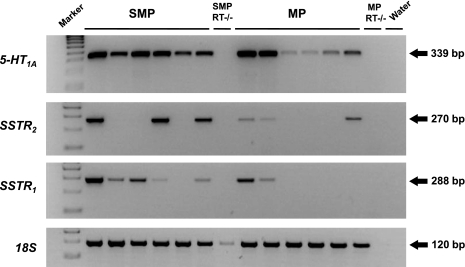
RT-PCR demonstrated the presence of 5-HT1A receptor mRNA in the guinea pig myenteric plexus and submucous plexus (Fig. 8). Sequence analysis confirmed that this PCR product corresponded to a 143-amino acid fragment of the 5-HT1A receptor, with 100 and 98% homology to the rat and human 5-HT1A receptors, respectively. Qualitatively, the intensity of the 5-HT1A PCR bands was generally higher in the submucous plexus compared with the myenteric plexus, indicating higher expression in the submucous plexus. That the PCR product was amplified from cDNA and not genomic DNA was confirmed by the absence of a PCR product in the RT negative controls (Fig. 8). We also identified gene transcripts for both the SST1 and SST2 receptor. SST1 was expressed in five of six submucous plexus samples and two of six myenteric plexus samples, whereas SST2 was expressed in three of six submucous plexus and myenteric plexus samples (Fig. 8). The variation in gene expression between samples was not due to quality of the cDNA template because 18S was equally expressed across all samples in the linear range of amplification (Fig. 8). The SST1 PCR product encoded a 124-amino acid fragment, with 99% homology to both rat and human SST1 receptors, whereas the SST2 PCR product encoded a 116-amino acid fragment, with 89 and 91% homology to the rat and human SST2 receptors, respectively. The absence of PCR products in the RT negative controls demonstrated amplification from cDNA (Fig. 8).
Fig. 8.
RT-PCR analysis of guinea pig 5-HT1A, SST2, and SST1 receptor gene expression in stripped submucous plexus (SMP) and the myenteric plexus (MP) with its attached longitudinal muscle. The ribosomal 18S shows the equivalent quality of cDNA between samples. Negative control, water; RT−/−, no reverse transcriptase in cDNA synthesis reaction. Marker = 100 bp DNA ladder.
DISCUSSION
Several studies have shown that enteric inhibitory interneurons with cell bodies in the myenteric plexus project to the submucosal plexus where they produce nonadrenergic IPSPs in noncholinergic secretomotor neurons (1, 24, 36). This present study shows that 5-HT1A, SST1, and SST2 receptors each play a role in mediating these nonadrenergic IPSPs, thus indicating that 5-HT and Som are transmitters employed by these inhibitory interneurons. Although the findings on SST receptors confirm and sharpen the focus of previous data suggesting that Som mediates IPSPs in these neurons (37, 46), identification of a role for 5-HT1A receptors is entirely unexpected.
Role of 5-HT1A Receptors
A major finding of this study is that the specific 5-HT1A antagonist WAY 100135 depresses IPSPs evoked in VIP neurons in the presence, or absence, of the α2-adenoceptor antagonist idazoxan. Consistent with the latter, WAY 100135 has no effect on hyperpolarizations evoked in these neurons by local application of NE, which mediates IPSPs arising from the sympathetic innervation of the submucosa (1, 43). The present study employed a more specific antagonist, WAY 100135, which enabled us to obtain clear effects, differing from our previous finding that another 5-HT1A antagonist, NAN-190, blocks α2-adrenoceptors on these neurons (14). The effect of WAY 100135 suggests that neurally released 5-HT mediates a component of the nonadrenergic IPSPs in the VIP neurons.
A necessary precondition for 5-HT to mediate transmission of any kind to VIP neurons is that 5-HT containing nerve terminals form synaptic contacts with them. There are numerous contacts between 5-HT terminals and neuropeptide Y (NPY) immunoreactive submucosal neurons, which are cholinergic secretomotor neurons (41). However, until now, no data about contacts between 5-HT terminals and VIP neurons was available. We found that VIP neurons have an average of more than 20 close contacts from 5-HT terminals. We used confocal, rather than electron, microscopy so the actual numbers of synapses formed on VIP neurons is unknown, but previous correlations of confocal and electron microscopy indicate that many close contacts correspond to morphological synapses (33, 34, 44, 54). Several studies suggest that 5-HT plays no role in excitatory transmission to VIP neurons, despite the presence of excitatory 5-HT receptors on these neurons (14, 38–40, 51), so any role for 5-HT at synapses on VIP neurons is probably inhibitory.
The idea that 5-HT acts postsynaptically to inhibit the VIP neurons is consistent with the effects of locally applied 8-OH-DPAT, a specific agonist at 5-HT1A receptors. This agonist produced a consistent, if small, hyperpolarization of VIP neurons when the concentration in the application pipette was 10 μM or less. By contrast, a higher concentration in the pipette (30 μM) had no effect on any neuron to which it was applied, a difference that was statistically significant (P < 0.05, χ2 test, 1 df). This suggests that the higher concentration desensitized the 5-HT1A receptors, which may account for the small hyperpolarizations we observed and earlier failures to detect direct responses to 8-OH-DPAT in submucosal VIP neurons (21, 51). Bath application of 300 nM 8-OH-DPAT depressed synaptic transmission in the myenteric plexus but did not affect fast EPSPs or IPSPs in the submucosal neurons. It also had no effect on hyperpolarizations evoked by local application of either NE or Som, suggesting that activation of 5-HT1A receptors does not enhance the postsynaptic effects of these two transmitters.
Our results show that mRNA for the 5-HT1A receptor is expressed in the submucous plexus. This is consistent with a previous study using both in situ hybridization and immunohistochemistry that showed expression of 5-HT1A receptors in a majority of submucosal neurons (32). However, one important difference is that the earlier study concluded that VIP neurons, identified indirectly by their ability to take up and retrogradely transport the B-chain of cholera toxin from the intestinal lumen, do not express 5-HT1A receptors (32). This is incompatible with our proposal that 5-HT acts postsynaptically to mediate IPSPs in VIP neurons. However, both the figures and the text in the earlier paper indicate that well over 50% of submucosal neurons express 5-HT1A mRNA (32) and VIP neurons make up ∼45% of all submucosal neurons in the guinea pig ileum (3), which suggests that many VIP neurons do express the 5-HT1A receptor. Furthermore, the same study indicates that 5-HT-immunoreactive varicosities tend to be apposed to rings of 5-HT1A immunoreactivity, with the latter being more frequent than the former (32). Since VIP neurons have many apposed 5-HT varicosities, this suggests that 5-HT1A receptors are expressed by these neurons. Unfortunately, the antisera available to localize VIP and 5-HT1A receptors by immunohistochemistry are both raised in rabbits, making it difficult to demonstrate this colocalization directly. Indeed, we have yet to identify antisera against 5-HT1A receptors or VIP suitable for this purpose.
An alternative explanation for the effects of WAY100135 and 8-OH-DPAT is that they act presynaptically on either sympathetic or inhibitory interneuron nerve terminals. However, for this to be a valid explanation, 5-HT1A receptors would have to facilitate release of Som from these terminals. This is unlikely because activation of presynaptic 5-HT1A receptors has been found to inhibit transmitter release wherever they have been studied (27).
Role of Som
There are five different Som receptor subtypes (SST1-5), and they all belong to the G protein-coupled receptor superfamily (45). Both SST1 and SST2 (mRNA) are higher than the other receptor subtypes in the upper gastrointestinal tract of the rat. SST1 receptor mRNA has the highest level and have been localized in myenteric and submucosal neurons (45). SST2A receptor has been colocalized with VIP-immunoreactive submucosal neurons in the rat intestine (49). The data here strongly support the idea that Som and, in particular, the SST1 and SST2 receptor subtypes play major roles in inhibitory transmission to submucosal VIP neurons. Blocking each of the two receptor subtypes substantially depresses both nonadrenergic IPSPs and hyperpolarizations evoked by local application of Som itself. Furthermore, RT-PCR shows that mRNA for both classes of Som receptor are expressed within isolated submucosa from guinea pig ileum, indicating that the effects of the antagonists are indeed on these receptors. Finally, it is well established that nerve terminals containing Som can be found within submucosal ganglia with all sympathetic terminals being immunoreactive for this peptide (7, 8, 30) and many terminals coming from myenteric interneurons (8, 35). Thus Som may contribute to both the intrinsic IPSPs identified in lesion studies (1, 36) and the sympathetically mediated IPSPs that are predominantly mediated by NE (43).
The NPY-immunoreactive cholinergic secretomotor neurons represent a third potential source of Som that might mediate the nonadrenergic IPSPs. These neurons contain Som, in addition to choline acetyltransferase and several other peptides (19), have been shown immunohistochemically to express 5-HT1A receptors (32), and receive abundant contacts from 5-HT interneurons (41). Thus, if 5-HT excites these neurons via 5-HT1A receptors, they may act as intermediates that would account for the anomalies in the data relating to our hypothesis that 5-HT acts to directly hyperpolarize VIP neurons via 5-HT1A receptors. However, several lines of evidence indicate that this is a highly unlikely possibility. First, 5-HT1A receptors are negative coupled to adenylyl cyclase and hence are likely to inhibit rather than excite neurons on which they are found (27). Indeed, we have found no instance of direct neuronal excitation via 5-HT1A receptors. Second, injection of markers into submucosal neurons subsequently identified as immunoreactive for NPY has not revealed any possible synaptic connections from these neurons to other submucosal ganglia (2, 12, 16), and direct tracing of the axons of these neurons identified immunohistochemically has also not revealed such contacts (18). Finally, when extrinsic denervation is combined with removal of the overlying myenteric plexus, all IPSPs, whether adrenergic or nonadrenergic, disappear (1), indicating that the latter do not come from neurons in the submucosal plexus.
The possibility of two different sources for Som-mediated IPSPs suggests that the two receptor subtypes might be pathway specific. IPSPs mediated by SST2 receptors have longer durations than those mediated by SST1 or 5-HT1A receptors, because blocking SST2 receptors markedly reduced the duration of the nonadrenergic IPSPs. Previous results suggest that the intrinsic IPSPs have much longer time courses than the sympathetically mediated IPSPs (1). Perhaps SST2 receptors are associated with the intrinsic inhibitory pathway, whereas SST1 receptors are associated with sympathetic input to VIP neurons. These intriguing possibilities remain to be tested, as do possible roles for SST4 receptors that have been implicated in inflammatory responses in the submucosa of the mouse intestine (9, 10).
Functions of the Intrinsic Inhibitory Interneurons?
Although it has been clear that there are intrinsic inhibitory interneurons with their cell bodies in the myenteric plexus for over two decades (1, 36), no physiological function for them has been identified. This is partly due to the lack of specific antagonists for the intrinsic IPSPs and to the rarity of the relevant neurons in the myenteric plexus. Both 5-HT interneurons and Som interneurons have been identified, but in each case they make up only a very small proportion of the myenteric neurons (35). Furthermore, both neuron types are immunoreactive for choline acetyltransferase and so may act as excitatory interneurons, even on the submucosal VIP neurons (18, 48). They each project anally along the myenteric plexus and then down to the submucosal plexus, where they run for a short distance anally before making synapses (17, 35, 48). There has been only one study of neurons with projections like these, and this focused on fast EPSPs (39); thus there is little direct evidence as to function.
There is also little useful evidence from pharmacological studies of neurally mediated mucosal water and electrolyte secretion. Som clearly inhibits secretion (5, 11, 26, 29, 47), but this may be due in part to direct effects of Som on the mucosa (10, 29, 52, 53). Effects on secretion of Som mediated by submucosal neurons have been reported (5, 47), but there have been no studies using receptor-specific antagonists and there is no evidence as to what physiological stimuli activate the intrinsic inhibitory pathways. Similar to 5-HT1A, the variation in SST1 and SST2 mRNA may reflect the proportion of neurons in each sample that express these receptors.
In Conclusion
The present results provide a strong indication that 5-HT1A receptors play an important role in a hitherto unidentified enteric inhibitory pathway regulating mucosal secretion. They also show that Som mediates a major component of the intrinsic inhibitory activity in the same neurons. Although the exact site of the 5-HT1A receptors remains to be determined, interactions of these receptors with the other inhibitory transmitters regulating secretomotor activity are likely to be important for both the physiological and pathophysiological regulation of intestinal secretion.
GRANTS
The study was supported by a grant from the National Health and Medical Research Council Australia (NHMRC Grant No. 40053) and an Australian Postgraduate Award (J. P. P. Foong).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol 398: 371–390, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein JC, Costa M, Furness JB. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol 381: 465–482, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornstein JC, Furness JB. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst 25: 1–13, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Chen JJ, Li ZS, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci 21: 6348–6361, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke HJ, Wang YZ, Wray D, O'Dorisio MS, Woltering EA, Coy DH, Murphy WA, Christofi FL, Gosh P, O'Dorisio TM. A multi-tyrosinated sst1/2 receptor preferring somatostatin agonist inhibits reflex and immune-mediated secretion in the guinea pig colon. Regul Pept 114: 51–60, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Costa M, Furness JB. The origins, pathways and terminations of neurons with VIP-like immunoreactivity in the guinea-pig small intestine. Neuroscience 8: 665–676, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Costa M, Furness JB. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience 13: 911–919, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Costa M, Furness JB, Smith IJL, Davies B, Oliver J. An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience 5: 841–852, 1980 [DOI] [PubMed] [Google Scholar]
- 9.Den Bosch JV, Adriaensen D, Van Nassauw L, Timmermans JP. The role(s) of somatostatin, structurally related peptides and somatostatin receptors in the gastrointestinal tract: a review. Regul Peptides 156: 1–8, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Den Bosch JVO, Van Nassauw L, Lantermann K, Van Marck E, Timmermans JP. Effect of intestinal inflammation on the cell-specific expression of somatostatin receptor subtypes in the murine ileum. Neurogastroenterol Motil 19: 596–606, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Emery PTJ, Higgs NB, Warhurst AC, Carlson GL, Warhurst G. Anti-secretory properties of non-peptide somatostatin receptor agonists in isolated rat colon: luminal activity and possible interaction with p-glycoprotein. Br J Pharmacol 135: 1443–1448, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans RJ, Jiang MM, Surprenant A. Morphological properties and projections of electrophysiologically characterized neurons in the guinea-pig submucosal plexus. Neuroscience 59: 1093–1110, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA, Haskins JT, Jones D, Mansell HL, Reilly Y. WAY100135-a novel, selective antagonist at presynaptic and postsynaptic 5-HT(1a) receptors. Eur J Pharmacol 237: 283–291, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Foong JP, Bornstein JC. 5-HT antagonists NAN-190 and SB 269970 block alpha2-adrenoceptors in the guinea pig. Neuroreport 20: 325–330, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Foong JPP, Bornstein JC. mGluR1 receptors contribute to non-purinergic slow excitatory transmission to submucosal VIP neurons of guinea-pig ileum. Front Ent Neurosci 1: doi:10.3389/neuro.21.001.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furness JB, Alex G, Clark MJ, Lal VV. Morphologies and projections of defined classes of neurons in the submucosa of the guinea-pig small intestine. Anat Rec A Discov Mol Cell Evol Biol 272: 475–483, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Furness JB, Costa M. Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience 7: 341–349, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Furness JB, Costa M, Gibbins IL, Llewellyn-Smith IJ, Oliver JR. Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res 241: 155–163, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Furness JB, Costa M, Keast JR. Choline acetyltransferase- and peptide immunoreactivity of submucous neurons in the small intestine of the guinea-pig. Cell Tissue Res 237: 329–336, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Galligan JJ, North RA. Opioid, 5-HT1A and alpha-2 receptors localized to subsets of guinea-pig myenteric neurons. J Autonom Nerv System 32: 1–11, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Galligan JJ, Surprenant A, Tonini M, North RA. Differential localization of 5-HT1 receptors on myenteric and submucosal neurons. Am J Physiol Gastrointest Liver Physiol 255: G603–G611, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Gwynne RG, Bornstein JC. Electrical stimulation of the mucosa evokes slow EPSPs mediated by NK1 tachykinin receptors and by P2Y1 purinoceptors in different myenteric neurons. Am J Physiol Gastrointest Liver Physiol 297: G179–G186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwynne RM, Bornstein JC. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol 5: 1–17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirst GD, McKirdy HC. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol 249: 369–385, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirst GD, Silinsky EM. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol 251: 817–832, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogenauer C, Aichbichler B, Santa Ana C, Porter J, Fordtran J. Effect of octreotide on fluid absorption and secretion by the normal human jejunum and ileum in vivo. Aliment Pharmacol Therapeut 16: 769–777, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550: 493–504, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurst RD, Ballantyne GH, Modlin IM. Octreotide inhibition of serotonin-induced ileal chloride secretion. J Surg Res 59: 631–635, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Keast JR, Furness JB, Costa M. Origins of peptide and norepinephrine nerves in the mucosa of the guinea pig small intestine. Gastroenterology 86: 637–644, 1984 [PubMed] [Google Scholar]
- 31.Kim SW, Paick JS. Peripheral effects of serotonin on the contractile responses of rat seminal vesicles and vasa deferentia. J Androl 25: 893–899, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kirchgessner AL, Liu MT, Raymond JR, Gershon MD. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J Comp Neurol 364: 439–455, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn-Smith IJ, Costa M, Furness JB. Light and electron-microscopic immunocytochemistry of the same nerves from whole mount preparations. J Histochem Cytochem 33: 857–866, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Mann PT, Southwell BR, Young HM, Furness JB. Appositions made by axons of descending interneurons in the guinea-pig small intestine, investigated by confocal microscopy. J Chem Neuroanat 12: 151–164, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Meedeniya AC, Brookes SJ, Hennig GW, Costa M. The projections of 5-hydroxytryptamine-accumulating neurones in the myenteric plexus of the small intestine of the guinea-pig. Cell Tissue Res 291: 375–384, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Mihara S, Nishi S, North RA, Surprenant A. A non-adrenergic, non-cholinergic slow inhibitory post-synaptic potential in neurones of the guinea-pig submucous plexus. J Physiol 390: 357–365, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mihara S, North RA, Surprenant A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurones. J Physiol 390: 335–355, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monro RL, Bertrand PP, Bornstein JC. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J Physiol 556: 571–584, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore BA, Vanner S. Organization of intrinsic cholinergic neurons projecting within submucosal plexus of guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 275: G490–G497, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Moore BA, Vanner S. Properties of synaptic inputs from myenteric neurons innervating submucosal S neurons in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 278: G273–G280, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Neal KB, Bornstein JC. Mapping 5-HT inputs to enteric neurons of the guinea-pig small intestine. Neuroscience 145: 556–567, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Neal KB, Bornstein JC. Targets of myenteric interneurons in the guinea-pig small intestine. Neurogastroenterol Motil 20: 566–575, 2008 [DOI] [PubMed] [Google Scholar]
- 43.North RA, Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol 358: 17–33, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pompolo S, Furness JB. Sources of inputs to longitudinal muscle motor-neurons and ascending interneurons in the guinea-pig small-intestine. Cell Tissue Res 280: 549–560, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Schafer J, Meyerhof W. sst1 mRNA is the prominent somatostatin receptor mRNA in the rat gastrointestinal tract: reverse transcription polymerase chain reaction and in situ-hybridization study. Neuropeptides 33: 457–463, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Shen KZ, Surprenant A. Somatostatin-mediated inhibitory postsynaptic potential in sympathetically denervated guinea-pig submucosal neurones. J Physiol 470: 619–635, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjoqvist A. Difference between the antisecretory mechanisms of opioids and the somatostatin analog octreotide in cholera toxin-induced small intestinal secretion in the rat. Regul Pept 40: 339–349, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Steele PA, Brookes SJH, Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small-intestine. Neuroscience 45: 227–239, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Sternini C, Wong H, Wu SV, de Giorgio R, Yang M, Reeve J, Jr, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol 386: 396–408, 1997 [PubMed] [Google Scholar]
- 50.Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol 351: 343–361, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surprenant A, Crist J. Electrophysiological characterization of functionally distinct 5-hydroxytryptamine receptors on guinea-pig submucous plexus. Neuroscience 24: 283–295, 1988 [DOI] [PubMed] [Google Scholar]
- 52.Warhurst G, Barbezat GO, Higgs NB, Reyldesmars F, Lewin MJM, Coy DH, Ross I, Grigor MR. Expression of somatostatin receptor genes and their role in inhibiting Cl− secretion in HT-29cl.19a colonocytes. Am J Physiol Gastrointest Liver Physiol 269: G729–G736, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Warhurst G, Higgs NB, Fakhoury H, Warhurst AC, Garde J, Coy DH. Somatostatin receptor subtype 2 mediates somatostatin inhibition of ion secretion in rat distal colon. Gastroenterology 111: 325–333, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Young HM, Furness JB. Ultrastructural examination of the targets of serotonin-immunoreactive descending interneurons in the guinea-pig small-intestine. J Comparative Neurol 356: 101–114, 1995. [DOI] [PubMed] [Google Scholar]