Abstract
Studies have suggested the reversibility of liver fibrosis, but the mechanisms of fibrosis reversal are poorly understood. We investigated the possible functional link between apoptosis, macrophages, and matrix turnover in rat liver during reversal of fibrosis secondary to bile duct ligation (BDL). Biliary fibrosis was induced by BDL for 4 wk. After Roux-en-Y (RY)-bilio-jejunal-anastomosis, resolution of fibrosis was monitored for up to 12 wk by hepatic collagen content, matrix metalloproteinase (MMP) expression and activities, and fibrosis-related gene expression. MMP expression and activities were studied in macrophages after engulfment of apoptotic cholangiocytes in vitro. Hepatic collagen decreased to near normal at 12 wk after RY-anastomosis. During reversal, profibrogenic mRNA declined, whereas expression of several profibrolytic MMPs increased. Fibrotic septa showed fragmentation at week 4 and disappeared at week 12. Peak histological remodeling at week 4 was characterized by massive apoptosis of cytokeratin 19+ cholangiocytes, >90% in colocalization with CD68+ macrophages, and a 2- to 7.5-fold increase in matrix-degrading activities. In vitro, phagocytosis of apoptotic cholangiocytes induced matrix-degrading activities and MMP-3, -8, and -9 in rat peritoneal macrophages. We concluded that reconstruction of bile flow after BDL leads to an orchestrated fibrolytic program that results in near complete reversal of advanced fibrosis. The peak of connective tissue remodeling and fibrolytic activity is associated with massive apoptosis of cholangiocytes and their phagocytic clearance by macrophages in vivo. Macrophages upregulate MMPs and become fibrolytic effector cells upon apoptotic cholangiocyte engulfment in vitro, suggesting that phagocytosis-associated MMP induction in macrophages significantly contributes to biliary fibrosis reversal.
Keywords: animal model, bile duct, collagen, collagenase, cirrhosis, extracellular matrix, gelatinase, hepatic stellate cell, Kupffer cell, integrin αvβ6, liver fibrosis resolution, matrix metalloproteinase, phagocyte, PDGF, repair, transforming growth factor-β, tissue inhibitor of matrix metalloproteinases, zymography, urokinase plasminogen activator receptor-1
liver fibrosis has long been considered static and irreversible. However, case reports and small cohort studies suggest potential reversibility of liver fibrosis once the pathological trigger is eliminated. Moreover, larger therapeutic studies in patients with chronic hepatitis B or C that were successfully treated with antivirals such as lamivudine and interferon-α, respectively, suggest reversal even in patients with cirrhosis (17, 43). However, these studies have limitations because of biopsy sampling error or small numbers of patients, and the statement that advanced fibrosis (cirrhosis) may reverse has been criticized as premature (11). Despite the controversies, remarkable progress has been made in the last decade in our understanding of the dynamic nature of liver fibrosis (16, 21, 43), which results from an imbalance of extracellular matrix (ECM) production (fibrogenesis) and its degradation (fibrolysis).
Reversibility of liver fibrosis in small rodents is well established (21) although it appears to depend significantly on the degree of preestablished fibrosis and on the particular model. Thus we and others (35) have shown that, despite normalization of profibrogenic gene expression and significant improvement of hepatic architecture and function, collagen content remains unchanged up to 8 wk after toxin withdrawal in thioacetamide (TAA)-induced fibrosis. This contrasts with significant reversal reported in the CCl4 model (22) though reversibility is limited in advanced fibrosis/cirrhosis, possibly attributable to matrix crosslinking (24). In earlier studies, reversibility of secondary biliary fibrosis after restoration of bile flow ranged from complete reversal (1) to improvement in liver architecture and function without significant reduction in collagen content (2). These earlier reports did not study potential mechanisms, but a more recent investigation proposed that apoptosis of activated hepatic stellate cells/myofibroblasts (HSC/MF) drives biliary fibrosis reversal (23), similarly to the mechanistically distinct CCl4 recovery model (22). Although substantial experimental evidence exists for the involvement of HSC/MF apoptosis in liver fibrosis reversal, it remains unclear how their disappearance would mechanistically contribute to the dissolution of fibrous septa, especially because HSC themselves can upregulate matrix-degrading machinery (35). Furthermore, several other reports have suggested that apoptosis plays a key role in fibrosis progression. Thus Fas-deficient (apoptosis-resistant) mice developed less fibrosis attributable to bile duct ligation (BDL) (5), and pharmacological inhibition of apoptosis attenuated BDL-induced fibrosis (3). Taken together, our present state of knowledge cannot easily reconcile these findings, and further studies are needed to develop a clear model and design a safe strategy to target apoptosis in liver fibrosis.
Recently, Duffield et al. (12) in an elegant study used selective depletion of macrophages to show that they participate in both progression and resolution of fibrosis. Thus macrophages promoted progression during induction of murine CCL4-induced liver fibrosis but facilitated fibrosis reversal once CCL4 was discontinued (12). In vivo, the ultimate fate of apoptotic cells is engulfment by neighboring cells, primarily by macrophages, often referred to as “professional phagocytes.” Macrophage-mediated phagocytosis is a multistep process regulated by a complex system of highly redundant receptors and bridging molecules (25). Importantly, macrophages have a striking potential to degrade extracellular matrices through expression of a variety of matrix metalloproteinases (MMPs), including interstitial collagenase MMP-13 (14), gelatinase MMP-9 (47), and elastase MMP-12 (45). The striking similarities between the dual role of macrophages and apoptosis in fibrosis progression or reversal prompted us to study the link between apoptosis, macrophage activation, and fibrosis reversal.
We therefore used the model of secondary biliary fibrosis and its reversal after bilio-jejunal anastomosis to characterize the temporal pattern of apoptotic cell death and its link to macrophage activation in relation to fibrogenic and fibrolytic gene expression and levels of MMP activities. We show that reversal is associated with increased apoptosis of fibrogenic cholangiocytes, the active recruitment of macrophages to clear these apoptotic cholangiocytes via phagocytosis, and activation of a fibrolytic cascade that peaks at 4 wk of reversal.
MATERIALS AND METHODS
Animal Experiments
Male Sprague-Dawley rats (BRL, Füllinsdorf, Switzerland) were housed on a 12-h:12-h dark/light cycle and fed a standard rat chow and tap water ad libitum. Animal experiments had been approved by the Animal Ethics Board of the State of Berne and Government of Lower Franconia. Thirty-six animals underwent BDL for 4 wk and Roux-en-Y (RY)-anastomosis as described previously (27).
TUNEL/CK19 and CD68 Double Immunohistochemistry
Formalin-fixed liver sections were deparaffinized and pretreated with proteinase K followed by horseradish peroxidase TdT-mediated dUTP nick end labeling (TUNEL) using the In Situ Cell Death Detection Kit (Roche, Indianapolis, IN). For double labeling, TUNEL-stained sections were boiled in a water bath with 10 mM citric acid, pH 6.0, for 20 min, blocked with normal horse serum, and incubated with anti-cytokeratin 19 (CK19) (1:20; Dako, Carpinteria, CA) or anti-CD68 antibody (1:50; AbD Serotec, Raleigh, NC) for 60 min, followed by horse anti-mouse IgG that was preadsorbed on rat immunoglobulin (Vector Laboratories, Burlingame, CA) 1:200 and soluable immunocomplexes of alkaline phosphatase and mouse monoclonal antialkaline phosphatase-complex (Dako) 1:25, 30 min each. Staining was developed with Fast Red (Sigma, St. Louis, MO). TUNEL-positive and TUNEL/CK19 double positive cells were counted in at least 10 random high-power fields (HPF)/animal at ×200 magnification independently by two observers (G. Millonig and Y. Popov). TUNEL/CD68 colocalization was scored positive if the CD68 signal was in immediate proximity to a TUNEL-positive nucleus. Cell numbers were expressed as mean positive cells/10 HPF ± SE. The immunohistochemical results were confirmed by immunofluorescence double staining for TUNEL/CD68 and TUNEL/pan-CK (1:100, Dako) and the appropriate fluorophore-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) in formalin-fixed, paraffin-embedded tissue sections after proteinase K retrieval.
Hepatic Collagen Determination
Hepatic collagen content was determined as relative hydroxyproline (μg/g liver) in 300–400-mg liver samples from two different lobes after hydrolysis in 6 N HCl for 16 h at 110°C as described (35). Total hydroxyproline (mg/whole liver) was calculated on the basis of individual liver weights and the corresponding relative hydroxyproline content (35, 36).
Quantitative Real-time RT-PCR
A sample (300–400 mg) of liver tissue from two lobes was homogenized, and total RNA was extracted using RNAPure (peqLab, Erlangen, Germany), and 1 μg of total RNA was reverse transcribed as described previously (35, 37). Relative transcript levels were quantified by real-time RT-PCR on a LightCycler 1.5 instrument (Roche, Mannheim, Germany) using the TaqMan and SYBR Green methodology as described previously (35, 36, 37). TaqMan probes (dual-labeled with 5′-FAM and 3′-TAMRA) and primers (Supplemental Table S1; supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website) were designed using the Primer Express software (Perkin Elmer, Wellesley, MA), synthesized at MWG Biotech AG (Ebersberg, Germany), and validated as described (35, 36, 37). The housekeeping gene β-2 microglobulin (β2MG) was amplified in parallel reactions for normalization.
Western blotting for MMP-9.
Western blotting for MMP-9 was performed using a standard technique as described before (35, 36, 37). Briefly, 10 μg of tissue or cell lysate or 25 μl of conditioned medium were boiled for 10 min under reducing conditions and extracts separated on a 10% SDS-polyacrylamide gel, blotted onto nitrocellulose, and stained with 0.5% Ponceau S to assure equal protein loading and transfer. Membranes were blocked with 5% powdered milk and incubated overnight at 4°C with rabbit anti-rat MMP-9 (1:1,000, AB 19016; Chemicon, Temecula, CA), followed by the appropriate alkaline phosphatase-conjugated secondary antibody for 2 h (Promega, Madison, WI). Immunodetected proteins were visualized utilizing nitroblue tetrazolium and bromo-chloro-indolyl-phosphate. Standardization was achieved by loading equal amounts of protein (tissue and cell lysates) or using equal cell numbers (cell lysates and conditioned medium).
Cell Isolation and Culture
Cell lines.
Mouse macrophage cell lines RAW 264.7 (ATCC no. TIB-71) and J774A.1 (ATCC no. TIB-67), human hepatoma cells Huh-7, the rat HSC line CFSC-2G (18) (kindly provided by Dr. M. Rojkind, Washington, DC), and the mouse cholangiocyte cell line 603B (19) were cultured under standard conditions as previously described (32, 33, 35) at 95% air-5% CO2 in a humidified atmosphere.
Rat peritoneal macrophages.
Rat peritoneal macrophages (pMΦ) were isolated using a standard protocol (13). Briefly, adult male Sprague-Dawley rat (250 g) were euthanized in a CO2 chamber and injected intraperitoneally through a 20-gauge needle with 50 ml of cold Ca2+, Mg2+-free PBS, followed by aspiration of macrophage-containing medium using the same syringe and centrifugation for 10 min at 400 g. All procedures were done at 4°C and under sterile conditions. Macrophage yields were 1.4 × 107 to 2.3 × 107 cells/rat at a purity of 85% or higher as determined by phagocytosis of 3 μm latex beads as previously described (35).
Apoptosis was induced in vitro in 603B cholangiocytes by a single UV irradiation (200 mJ, Bio-Rad GS Gene Linker UV-chamber; Bio-Rad, Hercules, CA). Apoptotic cells were collected after 3 h, centrifuged at 13,000 g for 5 min, and resuspended in serum-free (1% penicillin/streptomycin; 4 × 106 cells/ml) before each experiment. Apoptotic cell death was confirmed as previously established (9), assessing cellular criteria (cell rounding, cytoplasmatic membrane blebbing), nuclear morphology (condensed and fragmented nuclei by DAPI staining), and annexin V positivity by fluorescence-activated cell sorting. It was routinely 80% or higher. For apoptotic cell engulfment experiments, 1× 104 to 4 × 104 apoptotic 603B cholangiocytes were added to unstimulated macrophages in serum-free medium (freshly isolated rat pMΦ or RAW cells, 3 × 104 cells/well) for 1–16 h.
Determination of ECM-degrading Activities
Determination of net interstitial collagenase and gelatinase activities.
Determination of net interstitial collagenase and gelatinase activities in liver homogenates was performed with a 96-well plate fluorescent assay on the basis of degradation of self-quenched fluorogenic substrates DQ-collagen type I and DQ-gelatin, respectively (Molecular Probes, Eugene, OR) as described (37). Human recombinant activated MMP-1 and -2 were used for calibration. Extraction procedures were performed in the presence of EDTA-free complete protease inhibitor (Roche) to prevent ex vivo MMP activation (37).
Substrate gel zymography.
Substrate gel zymography was performed as outlined (37). Briefly, liver extracts (see above) or cell lysates at 20 μg protein/lane were separated on 10% polyacrylamide gels copolymerized with 1 mg/ml gelatin, incubated in activity buffer for 24 h, stained with 0.25% Coomassie Blue, and destained in acetic acid/methanol/H2O (10:1:89). Proteolytic bands corresponding to MMP-2 and MMP-9 were identified and quantified as described (37).
Substrate in situ zymography.
Substrate in situ zymography was performed as detailed (37). Briefly, liver sections were dried and overlaid with 0.1 mg/ml DQ-gelatin in MMP activity buffer supplemented with 0.5% low-melt agarose, covered with coverslips, gelled at 4°C for 1 h, and incubated at room temperature for 2–16 h. Images were documented on a Nikon E800 photodocumentation microscope.
Live cell matrix-degrading activities.
Live cell matrix-degrading activities were determined as follows. Freshly isolated rat peritoneal macrophages or cell lines representing other liver cell types were plated in 96-well culture plates at constant density (3 × 104 cells/well) in phenol red and serum-free DMEM (1% penicillin/streptomycin). Gelatinase and collagenase activities were measured as an absolute increase in fluorescence after addition of self-quenched fluorogenic substrates DQ-gelatin or DQ-collagen (0.02 mg/ml), respectively, as previously described (37). These substrates were added to cells for 1–16 h, either alone or immediately before addition of the apoptotic cholangiocytes prepared as described above.
Statistical Analyses
Data are expressed as means ± SE, and statistical analyses were performed using Microsoft excel and GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). Multiple comparisons were performed by one-way ANOVA. Two planned comparisons were performed to each of the control groups, healthy nonfibrotic rats (sham) and rats at peak of fibrosis (BDL) using the Dunnett's posttest. Differences among selected experimental groups were compared using the Tukey posttest. P values >0.05 were considered significant.
RESULTS
Advanced Secondary Biliary Fibrosis Reverses After Bilio-digestive Anastomosis
At week 4 after BDL (peak of fibrosis), serum markers of cholestasis and inflammation were significantly increased (Supplemental Table S2). After RY-anastomosis these parameters rapidly decreased to near normal on day 3 and remained at this level throughout the following 12 wk (Supplemental Table S2).
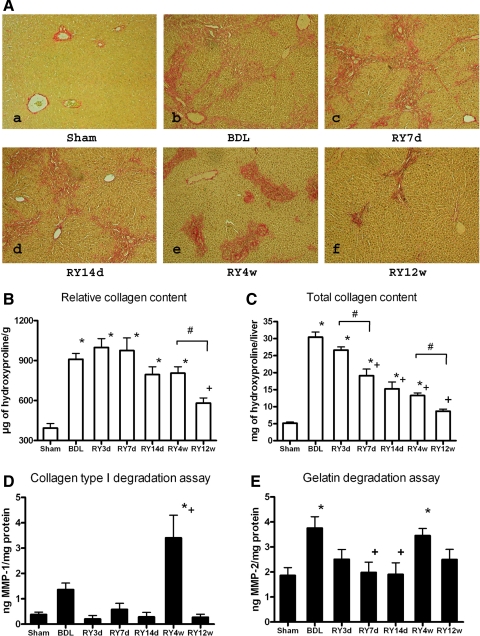
At peak fibrosis connective tissue staining demonstrated severe portal and bridging fibrosis (Fig. 1A). No significant changes in fibrosis were observed until week 4, when striking remodeling of the fibrous tissue was apparent, including decrease in periportal fibrosis, fragmentation of the septa, and condensation of the portal connective tissue (Fig. 1A). At 12 wk, septa had disappeared and portal tracts were only slightly enlarged (Fig. 1F). Because liver volume decreased dramatically over the course of reversal (Supplemental Table S2), connective tissue staining only allowed an assessment of architectural changes rather than of quantitative fibrosis reversal. Therefore, we measured both relative (per g of liver) and total (per whole liver) collagen content via hepatic hydroxyproline determination. Relative hepatic hydroxyproline increased 2.3-fold at peak fibrosis compared with sham-operated controls, reaching a significant reduction at 12 wk of reversal (Fig. 1B). Total hepatic collagen increased sixfold at peak fibrosis and decreased steadily and significantly from 7 days to 12 wk after RY-anastomosis (Fig. 1C). To establish during which period fibrolysis was most active, we compared the collagen content at each time-point to the preceding one. Significant changes in total liver collagen occurred between 3 and 7 days and 4 and 12 wk of reversal (Fig. 1, B and C).
Fig. 1.
Histological features of fibrosis reversal, changes in hepatic collagen content, and collagenase activities after bilio-jejunal anastomosis. A: connective tissue staining (Sirius Red, representative images) of liver sections of rats after 4 wk of bile duct ligation (BDL) (a) and 7 and 14 days and 4 and 12 wk after Roux-en-Y (RY)-anastomosis (b, RY7d; c, RY14d; d, RY4w and e, RY12w, respectively). Original magnification: ×200. B and C: relative (μg/g of liver) and total (per liver) hepatic collagen content as determined by hydroxyproline in livers of rats after 4 wk of BDL (n = 6) and 3, 7, and 14 days and 4 and 12 wk after RY-anastomosis (n = 4 for RY3d and n = 6 for all other time points). D and E: interstitial collagenase and gelatinase activities in liver homogenates as determined by degradation of DQ-collagen type I and DQ-gelatin, respectively. Sham-operated rats served as normal and BDL as fibrotic controls (n = 6). MMP, matrix metalloproteinase. *P < 0.05 compared with the sham-operated group; +P < 0.05 compared with the peak of fibrosis group (BDL). #P < 0.05 compared with the preceding time-point (ANOVA, Tukey posttest).
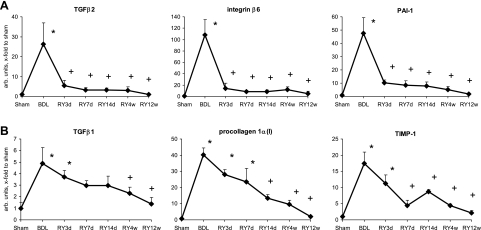
Fibrosis Reversal is Associated With Rapid Downregulation of Profibrogenic Genes Followed by Increase in Collagen-degrading Activities
During fibrosis resolution, profibrogenic gene expression was rapidly downregulated, following two distinct patterns, either declining immediately [transforming growth factor (TGF)-β2, integrin-β6, plasminogen activator inhibitor (PAI)-1, procollagen-α2(IV), connective tissue growth factor (CTGF)] (Fig. 2A; Supplemental Fig. S1), or gradually [TGF-β1, procollagen-α1(I), tissue inhibitor of MMP (TIMP)-1, TIMP-2, PDGF receptor (PDGFR)-β] after RY-anastomosis (Fig. 2B; Supplemental Fig. S1). Attenuated profibrogenic gene expression alone is not sufficient to reverse established fibrosis even when the causative agent is removed (35), requiring also active proteolytic degradation of excess ECM by the coordinated action of collagenases and gelatinases. Therefore, we quantified interstitial collagenase, which is rate limiting for the degradation of triple-helical collagens type I and III, and gelatinase activities in liver homogenates. Collagenase activity peaked 7.5-fold over normal and 2.5-fold over peak fibrosis (BDL) levels at week 4 of reversal (Fig. 1D), when histological remodeling was most pronounced, i.e., when dissolution of septa occurred (Fig. 1A). Gelatinase activity was upregulated twofold at peak fibrosis and increased again during fibrolysis (Fig. 1E). Of note, advanced fibrosis induced by TAA, which does not reverse (35), is characterized by persistence of septa that are only sparsely populated by cells. In sharp contrast, regression of BDL fibrosis was characterized by a relative increase in septal cell densities throughout all stages of reversal, suggesting that scar tissue needs repopulation by cells for resolution (Supplemental Figs. S2 and S3).
Fig. 2.
Temporal patterns of profibrogenic gene expression during fibrosis reversal. Hepatic transforming growth factor (TGF)-β1, procollagen-α1(I), tissue inhibitor of MMP (TIMP)-1, TGF-β2, integrin-β6, and plasminogen activator inhibitor (PAI)-1 transcript levels as quantified by quantitative RT-PCR. Note the rapid decline of transcripts that are primarily expressed by activated cholangiocytes (A) and the slow decline of transcripts that are characteristic of activated hepatic stellate cells/myofibroblasts (HSC/MF) (B) after RY-anastomosis. Data are expressed as means ± SE and in arbitrary units relative to β-2 microglobulin (β2MG) mRNA. *P < 0.05 compared with the sham-operated group; +P < 0.05 compared with the peak fibrosis group (BDL).
Disappearance of Activated Cholangiocytes Via Apoptosis is a Hallmark of Biliary Fibrosis Reversal
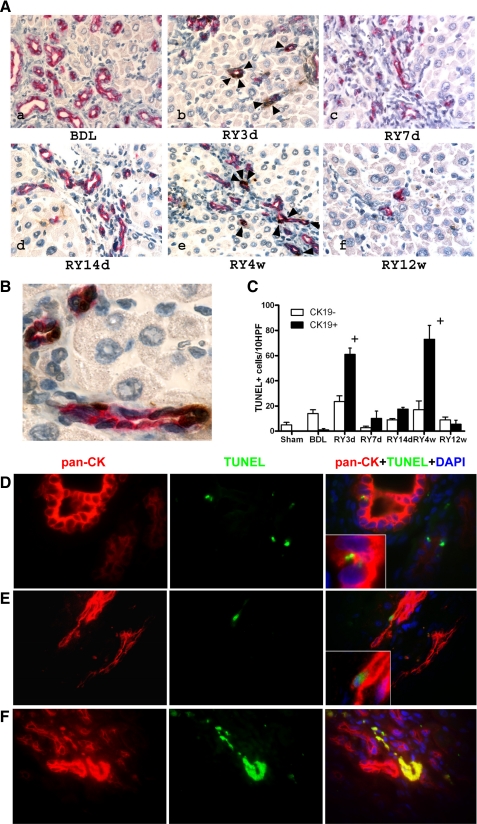
In parallel with progressive decrease in liver mass after restoration of bile flow, fibrosis reversal was accompanied by a massive loss of bile ducts, which had actively proliferated at peak of fibrosis and occupied roughly half the liver volume, with only a few ducts observed at 12 wk after anastomosis (185 ± 33.5 compared with 23 ± 5.26 CK19+ cells/HPF in BDL at 4 wk and RY at 12 wk groups, respectively). To assess the role of cholangiocyte apoptosis in the disappearance of the bile ductular structures, we performed double labeling for apoptotic cells (TUNEL) and the cholangiocyte marker CK19. At peak fibrosis, there was a threefold increase in overall apoptosis compared with sham-operated controls, but >90% of apoptotic cells were CK19 negative (15 out of 16 TUNEL+ cells per 10 HPF) (Fig. 3, A and B). In contrast, apoptotic cholangiocytes (CK19+/TUNEL+ cells) represented the majority (>90%) of apoptotic cells immediately after biliary decompression, with two prominent peaks of increased cholangiocyte apoptosis (60- to 70-fold vs. peak fibrosis, respectively) at day 3 and week 4 of reversal (Fig. 3, A and B). In addition, double immunofluorescence with the alternative cholangiocyte marker pan-CK (TUNEL/pan-CK) confirmed that bile ductular structures that expanded during BDL disappeared during the recovery phase through cholangiocyte apoptosis (Fig. 3, C, D, and E). Interestingly, both peaks of cholangiocyte apoptosis were followed by a significant decrease in total hepatic collagen content (Fig. 1C).
Fig. 3.
Cholangiocyte apoptosis is a prominent feature of biliary fibrosis reversal. A: double staining for bile duct epithelial (cytokeratin 19, CK19) and apoptotic cells (TdT-mediated dUTP nick end labeling, TUNEL) demonstrates a sharp increase in double-positive cells 3 days (b) and 4 wk (e) after RY-anastomosis (arrows), whereas there are no apoptotic cholangiocytes at peak fibrosis (a). Original magnification: ×200. B: high-power magnification (×600) of a representative section 4 wk after RY-anastomosis when maximal apoptosis occurs, demonstrating that almost all CK19+ cholangiocytes (red staining) forming bile ducts are TUNEL positive (brown nuclear staining), whereas other portal cells and hepatocytes are TUNEL negative (light blue nuclear counterstain). C: quantification of apoptosis: CK19-negative/TUNEL-positive (open bars) and CK19/TUNEL-double-positive cells (shaded bars) were counted in 3–5 animals per group in at least 10 random fields at a magnification of ×200. Numbers are expressed as positive cells/10 high-power fields (HPF), means ± SE. D–F: detection of apoptotic cholangiocytes by immunofluorescent double-labeling for TUNEL and the alternative cholangiocyte marker pan-cytokeratin (pan-CK), confirming the results obtained using TUNEL/CK-19 double immunohistochemistry. D: no detectable cholangiocyte apoptosis is found at the peak of fibrosis (BDL4w). Small TUNEL-positive nuclei are rare and belong to intraepithelial (inflammatory) cells in proliferating bile ducts (inset). E: early apoptotic cholangiocyte at the peak of resolution (RY4w) identified by the large TUNEL+ nucleus, pan-CK+ cytoplasm, typical morphology and its location within a bile duct (inset). F: late apoptotic cholangiocytes within a vanishing bile duct. TUNEL+, p-CK+ material demonstrates colocalization in the merged image (yellow). Original magnification, ×100.
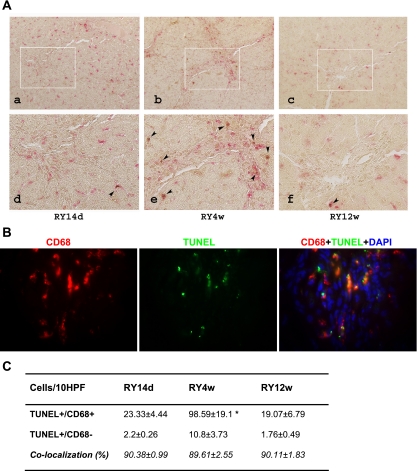
Fibrolytic Matrix Remodeling is Associated With Clearance of Apoptotic Cholangiocytes by Infiltrating Macrophages
In vivo, apoptotic cells are recognized and efficiently cleared via phagocytosis. The high cholangiocyte apoptosis rate observed during biliary fibrosis resolution is expected to trigger their phagocytosis and removal, preferentially by macrophages. Therefore, we performed double labeling for apoptotic cholangiocytes (TUNEL) and the macrophage marker CD68 at 14 days, 4 wk (maximal fibrolysis and apoptosis), and 12 wk of reversal. In all animals, ∼90% of all TUNEL+ cells colocalized with macrophages despite different apoptosis rates between the groups (Fig. 4, A, B, and C). Accordingly, the severalfold elevated apoptosis at 4 wk of reversal resulted in a corresponding increase in infiltrating macrophage numbers within the portal tracts (Supplemental Fig. S4). During this active macrophage recruitment into the resolving scar tissue, no change in the numbers and distribution of lobular (resident) macrophages/Kupffer cells was observed (Supplemental Fig. S4).
Fig. 4.
Scar-associated macrophages increase at maximal fibrolytic remodeling and colocalize with apoptotic cholangiocytes. A: double immunohistochemistry staining for the macrophage marker CD68 (red, cytoplasmic) and apoptosis (TUNEL: brown, nuclear) on liver sections from rats 14 days (a and d), 4 wk (b and e, maximal fibrolysis), and 12 wk (c and f) after RY-anastomosis; representative portal areas (top, original magnification, ×200; bottom, high-power magnification, ×400). Apoptotic cells in colocalization with macrophages are highlighted by arrows. A marked increase of colocalized cells is observed at week 4 of reversal. B: double immunofluorescence staining for the macrophage marker CD68 and apoptosis (TUNEL) on liver sections from rats 4 wk after RY-anastomosis, i.e., at maximal fibrolysis; representative portal area (original magnification, ×100). C: quantification of TUNEL-positive apoptotic cells colocalized with CD68-positive macrophages as assessed immunohistochemically and counted at a magnification of ×200 (see materials and methods). Data are expressed as the number of cells per 10 HPF; means ± SE. *P < 0.05 compared with the RY14d group.
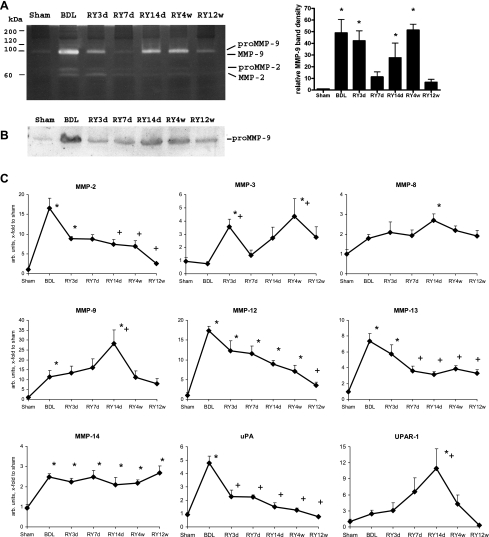
Mapping of MMP Expression and Activities Reveals a Distinct Subset of MMPs Associated With Fibrosis Reversal
Both net interstitial collagenase and gelatinase activities were elevated at maximal scar remodeling (Fig. 1, D and E), indicating that induction and/or activation of the respective MMPs occurred along with diminished expression of their inhibitors, especially TIMP-1 (Fig. 2B). In situ zymography revealed that gelatinase activity was spatially associated with the connective tissue in portal tracts and septa at any time point (Supplemental Fig. S5). To assess the contribution of particular MMPs, we performed gelatin zymography, which showed that, although both gelatinases (MMP-2 and -9) were upregulated at the peak of fibrosis, they demonstrated divergent patterns of regulation during reversal. MMP-9 was highly upregulated starting at 14 days and peaking at 4 wk of reversal, representing most of gelatinase activity, whereas MMP-2 declined during resolution and was barely detectable at day 7 of reversal (Fig. 5A). Similar results were obtained with immunodetection of MMP-9 protein in liver homogenates by Western blotting (Fig. 5B). MMP activity requires proteolytic activation, which can be achieved by several proteases including plasmin and MMPs themselves (38). We therefore determined transcript levels of MMP-2, -3, -8, -9, -12, -13, -14, and of molecules involved in the plasmin activation cascade, i.e., urokinase plasminogen activator (uPA) and uPA receptor (UPAR)-1, to test whether their expression pattern was consistent with the observed peak of MMP activity and fibrolytic remodeling. MMP-3, -8, -9, -12, and -14 were most prominently upregulated at those time points that preceded or coincided with peak fibrolytic activity (Fig. 5, A–C; Fig. 1, D and E). Whereas MMP-12 and -14 transcripts were elevated throughout reversal, MMP-13 transcripts decreased at day 7 and remained slightly elevated above sham-operated controls (Fig. 5C). MMP-8 and -9 mRNA peaked at day 14 (Fig. 5C), preceding maximal gelatinase and interstitial collagenase activities, respectively (Fig. 1, D and E). The mRNA expression of the MMP activator UPAR-1, but not of uPA, was almost superimposable on that of MMP-9 mRNA during reversal (Fig. 5C), suggesting that UPAR-1 might act as an MMP-9 proactivator, whereas PAI-1 mRNA fell quickly after RY-anastomosis (Fig. 2A). Interestingly, MMP-3 transcripts (Fig. 5C) were elevated at both early (3 days) and late (4 wk) peaks of cholangiocyte apoptosis (Fig. 3C). MMP-2 transcript levels continuously declined during reversal, in agreement with zymography and protein and activity data (Fig. 5, A–C; Supplemental Fig. S5).
Fig. 5.
Activation of collagen-degrading enzymes in the liver during fibrosis reversal. A: gelatin gel zymography of representative liver homogenates demonstrates that MMP-9 is the most prominent gelatinase, reaching highest levels at the peak of fibrolysis (week 4 of reversal), whereas MMP-2 declines. Densitometry of the MMP-9 band was performed from zymographies of all livers (n = 4–6 per bar) and is expressed as arbitrary units relative to the sham-operated controls (means ± SE). B: corresponding changes in MMP-9 protein as detected by Western blotting in representative liver homogenates. C: expression of MMPs and components of the plasmin proteolytic system during fibrosis reversal. Transcript levels of MMPs, urokinase plasminogen activator (uPA) and uPA receptor (UPAR)-1 were quantified by quantitative RT-PCR. Data are expressed as means ± SE and in arbitrary units relative to β2MG mRNA. *P < 0.05 compared with the sham-operated group; +P < 0.05 compared with the peak fibrosis group (BDL).
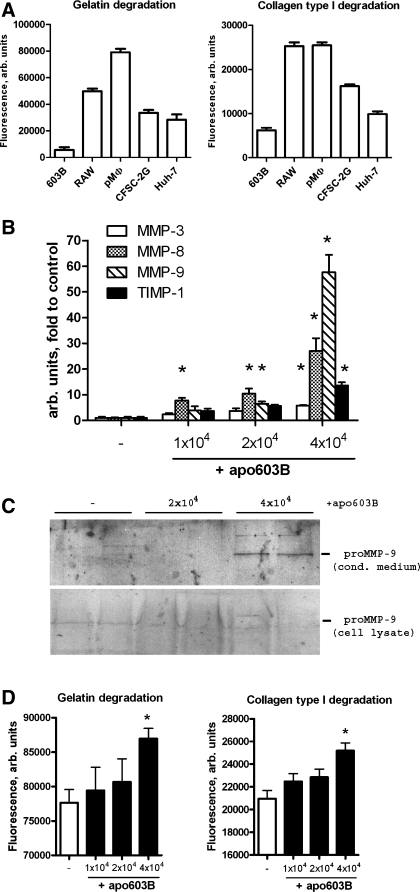
Macrophages Acquire A Fibrolytic Phenotype Upon Engulfment of Apoptotic Cholangiocytes In Vitro
We then explored whether, indeed, macrophages clearing apoptotic cholangiocyte were responsible for the increased MMP expression and fibrolytic activities observed in vivo during fibrosis reversal. First, we compared in vitro total matrix degrading (gelatinolytic and collagenolytic) activities of several cells and cell lines representing major liver cell types at basal, unstimulated conditions. Freshly isolated rat peritoneal macrophages and the mouse macrophage cell line RAW demonstrated the highest basal capacity to degrade both collagen and gelatin, followed by HSCs (CFSC-2G) and hepatocytic cells (Huh-7), whereas 603B cholangiocytes displayed the lowest activity (Fig. 6A). Next we mimicked the cellular events during biliary fibrosis reversal by coculturing apoptotic 603B cholangiocytes with rat peritoneal macrophages to study the effect of their engulfment on candidate MMP expression and matrix-degrading activities. Addition of apoptotic 603B cells markedly and dose dependently induced MMP-3, -8, and -9 transcripts (up to 6-, 28-, and 57-fold, respectively) in macrophages (Fig. 6B), whereas the major tissue inhibitor of MMPs, TIMP-1, was induced 14-fold. Interestingly, macrophage-associated MMP-12 and -13 transcripts remained unchanged (not shown). In addition, we confirmed the prominent expression of MMP-9 at the protein level by Western blotting. MMP-9 was apparently rapidly secreted from macrophages upon phagocytosis because the increase of pro-MMP-9 was detected in conditioned media but not in cell lysates of macrophages exposed to apoptotic cholangiocytes (Fig. 6C). Importantly, cholangiocyte phagocytosis also lead to a significant increase in the net gelatinolytic and collagenolytic activities in macrophages (Fig. 6D).
Fig. 6.
Engulfment of apoptotic cholangiocytes induces MMP expression and matrix-degrading activities in macrophages. A: high intrinsic gelatinase and collagenase activity in macrophage vs. nonmacrophage cell cultures. Freshly isolated rat peritoneal macrophages (pMΦ), the murine cholangiocyte cell line (603B), the rat hepatic stellate cell line CFSC-2G, and human hepatoma cells (Huh-7) were cultured at a density of 30 × 104 cells/well in the presence of self-quenched DQ-gelatin or DQ-collagen substrates for 16 h to measure live cell gelatinase and collagenase activities as described in materials and methods. B: dose-dependent induction of MMP expression in macrophages upon engulfment of apoptotic cholangiocytes. Apoptotic 603B cholangiocytes (apo603B) were prepared as described in materials and methods, and 1–4 × 104 cells were added to freshly isolated rat peritoneal macrophages (3 × 104 cells/well). Relative mRNA levels of MMP-3, -8, and -9 and TIMP-1 were determined by quantitative RT-PCR 12 h after addition of apoptotic cells. C: corresponding increase in secreted MMP-9 protein levels upon phagocytosis as detected by Western blotting of conditioned macrophage supernatants; no MMP-9 detected in the corresponding cell lysate. D: phagocytosis-associated MMP induction results in a net increase in macrophage matrix-degrading activity. Collagenolytic and gelatinolytic activities were determined as outlined above over 16 h after addition of apoptotic 603B cholangiocytes at increasing concentrations. Data are expressed as means ± SE, with each bar representing 3 (B) or 6–8 (A and D) wells per experimental condition. All in vitro experiments were repeated at least 3 times. *P < 0.05 compared with the control group.
DISCUSSION
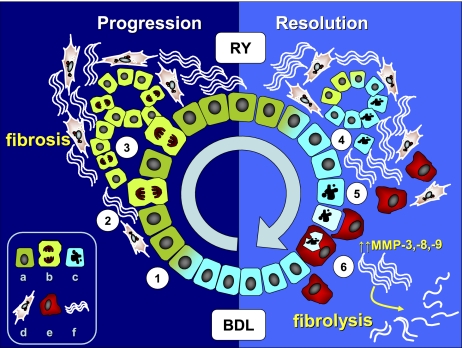
Our results show that reversal of biliary fibrosis occurs via rapid downregulation of multiple profibrogenic genes and upregulation of ECM-degrading activities that reach a maximum after 4 wk of RY-anastomosis and that cholangiocyte apoptosis is a remarkable feature and driving force of biliary fibrosis reversal. We provide evidence that programmed cell death of activated cholangiocytes 1) eliminates potent paracrine profibrogenic stimuli that activate HSC/MF, and 2) triggers recruitment of CD68+ macrophages into the scar tissue to remove the apoptotic cholangiocytes via phagocytosis, a process that we propose is instrumental in fibrolytic ECM remodeling (summarized in Fig. 7). We identify a specific subset of fibrolytic MMPs that can be linked to fibrosis reversal in vivo, i.e., MMP-3, -8, -9, -12, and -14. Finally, we demonstrate in vitro that engulfment of apoptotic cholangiocytes by macrophages upregulates macrophage MMP-3, -8, and -9 transcripts and their matrix-degrading (gelatinolytic and collagenolytic) activities.
Fig. 7.
Proposed pathophysiology of extracellular matrix remodeling during biliary fibrosis progression and reversal. Cholestasis (BDL) triggers cholangiocyte activation and proliferation (1), which upregulate profibrogenic αvβ6 integrin and soluble factors, e.g., TGF-β, PAI-1 and connective tissue growth factor, leading to paracrine myofibroblast activation (2) and enhanced collagen synthesis. Aberrant ductular proliferation (3) further amplifies HSC/MF recruitment and fibrous matrix deposition, leading to fibrosis and loss of normal liver architecture. Upon restoration of bile flow by RY-anastomosis (RY), activated cholangiocytes undergo rapid deactivation (4) and apoptosis (5), which removes the profibrogenic stimuli acting on myofibroblasts. Cholangiocyte apoptosis triggers recruitment of CD68+ macrophages into scarred portal tracts to remove apoptotic cholangiocytes via phagocytosis (6) and to upregulate MMP-3, -8, and-9 to remodel the scar, leading to dissolution of fibrous septa and restoration of normal liver architecture. a, activated; b, proliferating cholangiocyte; c, apoptotic cholangiocyte; d, myofibroblast; e, macrophage; f, fibrotic matrix.
Prior data suggest that apoptosis plays a key role in the progression and reversal of liver fibrosis, but the nature of its involvement in fibrogenesis vs. fibrolysis remains complex and far from being completely understood. However, a clearer picture emerges when our present study is evaluated in the context of prior research, which indicated that a distinction must be made between the cell types undergoing apoptosis (e.g., parenchymal cells, hepatocytes vs. nonparenchymal cells, HSC/MF or cholangiocytes). Furthermore, it appears critical which cell type is responsible for apoptotic cell removal [e.g., professional phagocytes (macrophages) vs. nonprofessional phagocytes such as HSC/MF]. Thus it was demonstrated that hepatocyte apoptosis drives fibrogenesis in the BDL fibrosis model in vivo (5), and that engulfment of apoptotic hepatocytes by the myofibroblastic human hepatic stellate cell line LX-1 in vitro leads to profibrogenic (and anti-inflammatory) effects via autoinduction of TGF-β1 and enhanced procollagen type I expression (6). In contrast, HSC/MF apoptosis was shown to promote reversal of CCl4-induced liver fibrosis resolution (22), and macrophage (Kupffer cell)-mediated phagocytosis of apoptotic hepatocytes in vitro stimulated release of the proinflammatory (and potentially profibrolytic) cytokine TNF-α (4). Thus both the cells undergoing apoptosis and the cells that clear them determine whether a profibrogenic or profibrolytic response will result. In vivo, macrophages continuously monitor cell viability and ingest and remove dying cells. If macrophage-mediated phagocytosis is compromised, apoptotic cells will be engulfed by neighboring cells, e.g., activated (myo)-fibroblasts (such as HSC/MF), which are also able to ingest apoptotic cells, although at a much slower rate than macrophages (26). This will consequently lead to a prolonged exposure of the organism to apoptotic cells and leakage of toxic cell contents, causing persistence of inflammation, autoimmune reactions (25), and fibrogenic activation of HSC/MF by apoptotic bodies (6). It is temping to speculate that this scenario may be responsible for the failure of resolution in advanced CCl4-induced liver fibrosis when macrophages were depleted during the recovery phase (12). However, in this study the persistence of apoptotic cells upon macrophage depletion was not measured.
Our findings indicate that clearance of proliferating bile ductular structures is a dynamic process that is characteristic of and central to the reversal of secondary biliary (BDL-induced) fibrosis, with apoptosis of cholangiocytes, by far exceeding that of nonbiliary cells. This is particularly evident at the peak of reversal, where dramatic increase in cholangiocyte apoptosis coincides with a maximum of MMP activity and pronounced histological signs of septal fragmentation and architectural remodeling (Figs. 1, 3, and 5). Interestingly, although we were not able to detect an increase in net MMP activity in the early peak of cholangiocyte apoptosis (3 days after RY, Fig. 3), this was nonetheless followed by a significant decrease in total collagen content (Fig. 1C) and MMP-3 induction at the mRNA level (Fig. 5C). It is likely that analysis of crude liver homogenates, where MMPs and their inhibitors are mixed together, does not allow the detection of local increase in MMP activity at the cellular level because of the persistent surplus of MMP inhibitors such as TIMP-1 (Fig. 2B). We cannot exclude that the proposed MMP-dependent macrophage mechanism might also be operative at that early time point at the local microenvironment level, where MMPs and metalloproteinase inhibitors are spatially separated. Proliferating (activated) cholangiocytes are a prominent source of several key profibrogenic cytokines that activate ECM-producing HSC/MF in a paracrine manner (30, 44), recapitulating early developmental programs of ductal plate formation. They also express integrin αvβ6, which contributes to fibrogenesis by binding and activating the profibrogenic cytokine TGF-β1 (37, 46). Of note, those profibrogenic transcripts, which are abruptly downregulated immediately following RY-anastomosis, are predominantly expressed by activated bile ductular cells, which undergo rapid deactivation/apoptosis after RY-anastomosis. Thus prior in situ hybridization and immunohistochemistry studies demonstrated prominent cholangiocyte expression of CTGF (44), procollagen-α2(IV) (29), TGF-β2 (30), and integrin-β6 (37, 46)(Fig. 2A; Supplemental Fig. S1).
Another distinct group of profibrogenic transcripts showed a pattern of slow decline during reversal and can be attributed to longer persistence and slower deactivation of HSC/MF, such as TGF- β1(30), procollagen-α2(1) (29), TIMP-1 (22), and PDGFR-β (34) (Fig. 2B; Supplemental Fig. S1). We cannot rule out entirely the contribution of HSC/MF apoptosis to biliary fibrosis reversal because a small fraction of apoptotic cells in our study could not be attributed either to cholangiocytes or hepatocytes. However, our findings overall do not support a critical role of HSC/MF apoptosis in biliary fibrosis reversal as suggested in a prior study by Issa et al. (23). This discrepancy cannot be fully explained by minor differences of the model used (BDL for 4 instead of 3 wk, follow up during resolution of 12 vs. 6 wk), but the major drawback appears to be the lack of a robust and universal HSC/MF marker that would allow tracking of “deactivated” HSC during reversal. Thus we were unable to utilize α-smooth muscle actin (SMA), a common marker for a subset of activated HSC, because α-SMA was rapidly downregulated after RY-anastomosis (Supplemental Fig. S1) and became almost undetectable in septal cells as early as 3 days after reversal (not shown). This must be seen in the light of an apparent persistence of (partly) activated HSC/MF throughout reversal, as evidenced by the slow decline of “myofibroblast-specific” profibrogenic transcripts such as procollagen-α1(I) (Fig. 2B). Our data confirm prior reports that apoptosis is a rare event in obstructive cholestatic injury (15) and that remarkable cholangiocyte apoptosis occurs after relief of biliary obstruction in rats (8).
In addition, our findings suggest that activated cholangiocytes are an attractive target for antifibrotic therapies (including proapoptotic approaches targeting these cholangiocytes) not only in biliary fibrosis but also in essentially any etiologies of liver fibrosis where enhanced cholangiocyte proliferation is a hallmark of progression, e.g., fibrosis attributable to hepatitis B infection (10), alcohol (39), hepatitis C (7) or nonalcoholic steatohepatitis (40). Recently, we and others have shown that a small molecule antagonist or an antibody against the cholangiocyte-specific integrin αvβ6 attenuated fibrosis progression in secondary biliary fibrosis and in Mdr2−/− mice (37, 46).
Another important result of our study is the definition of the profibrolytic subset of MMPs that were differentially regulated at the peak of fibrolytic remodeling and ECM degradation. Although the multilevel regulation of MMP activity is very complex (38), prior experimental data strongly suggest that certain MMPs, such as MMP-2, have a profibrogenic role (31, 36), whereas others, such as MMP-3, -8, -9, and -13 may participate in fibrosis reversal (14, 35, 36). In our present study, correlative analysis of expression patterns during biliary fibrosis reversal in vivo suggested that MMP-3, -8, -9, -12, and -14, but not MMP-2 or -13, might play a role in removal of the fibrotic matrix. Three of these proteases (MMP-3, -8, and -9) were induced in macrophages upon phagocytosis of apoptotic cholangiocytes, strongly implicating infiltrating macrophages in the resolution of fibrosis via novel, hitherto unexplored mechanisms that result in the induction of fibrolytic MMPs. The exact role and contribution of each MMP to fibrosis progression or reversal needs to be established in further experiments using fibrosis models in mice with genetic MMP deletions, which are presently underway. Sorting out “profibrogenic” from “profibrolytic” MMPs in liver fibrosis is relevant because drug design is moving toward more selective MMP inhibitors (20). Moreover, it is increasingly recognized that MMPs cleave and thus activate or deactivate many substrates other than ECM molecules, such as cytokines and chemokines (28). Together with recent advances in understanding the structural basis for this substrate “flexibility” of a given MMP, such as MMP-9 (41), this may permit not only the inhibition of specific MMPs but also the modulation of their enzymatic activity toward selected substrate(s) while sparing activity toward others.
Importantly, the process of phagocytosis, which links apoptosis and macrophage-mediated fibrosis reversal, can be modulated therapeutically. Better understanding of the mechanisms and the molecules critically involved in phagocytosis have already led to the emergence of experimental strategies that modulate the clearance of apoptotic cells in vivo (26), e.g., via the soluble form of the Mer receptor tyrosine kinase (42).
In summary, by dissecting the events that occur during resolution of biliary fibrosis in vivo, we found that apoptosis of activated cholangiocytes and their subsequent macrophage-mediated clearance are instrumental in fibrosis reversal. Apoptosis of cholangiocytes eliminates fibrogenic stimuli, and their phagocytosis by macrophages triggers a fibrolytic program characterized by a unique temporal pattern of MMP expression. For the first time, we provide evidence that the process of phagocytosis by macrophages functionally contributes to fibrosis reversal via induction of subsets of MMPs that result in effective fibrolysis, thus linking apoptosis to fibrolytic macrophage activation. Finally, our findings further support the notion that activated cholangiocytes are prime targets for antifibrotic therapies in liver fibrosis.
GRANTS
This work was supported by Grant Schu 646/14-1 by the German Research Council (Deutsche Forschungsgemeinschaft), an appointment grant by the Beth Israel Deaconess Medical Center, and a grant by the National Institutes of Health (NIH 1 R21 DK076873-01A1) to D. Schuppan. Y. Popov was a recipient of a Sheila Sherlock fellowship of the European Association for the Study of the Liver, G. Millonig of a Schroedinger-Fellowship from the Austrian Science Fund, and S. Krahenbuhl of a grant from the Swiss National Science Foundation (310000-112483).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
REFERENCES
- 1.Abdel-Aziz G, Lebeau G, Rescan PY, Clement B, Rissel M, Deugnier Y, Campion JP, Guillouzo A. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol 137: 1333–1342, 1990 [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson DC, Chamuleau RA, Frederiks WM, Gooszen HG, Heijmans HS, James J. Reversibility of cholestatic changes following experimental common bile duct obstruction: fact or fantasy? J Hepatol 18: 85–95, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther 308: 1191–1196, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology 38: 1188–1198, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology 123: 1323–1330, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Canbay A, Taimr P, Torok N, Higuchi H, Friedman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest 83: 655–663, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 41: 809–818, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Costa AM, Tuchweber B, Lamireau T, Yousef IM, Balabaud C, Rosenbaum J, Desmouliere A. Role of apoptosis in the remodeling of cholestatic liver injury following release of the mechanical stress. Virchows Arch 442: 372–380, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Dan Z, Popov Y, Patsenker E, Preimel D, Liu C, Wang XD, Seitz HK, Schuppan D, Stickel F. Hepatotoxicity of alcohol-induced polar retinol metabolites involves apoptosis via loss of mitochondrial membrane potential. FASEB J 19: 845–847, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Davies SE, Portmann BC, O'Grady JG, Aldis PM, Chaggar K, Alexander GJ, Williams R. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology 13: 150–157, 1991 [PubMed] [Google Scholar]
- 11.Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol 40: 860–867, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115: 56–65, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80: 1298–1307, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol 178: 5288–5295, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol 42: 378–385, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1: 98–105, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Friedman SL, Bansal MB. Reversal of hepatic fibrosis—fact or fantasy? Hepatology 43: S82–S88, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest 65: 644–653, 1991 [PubMed] [Google Scholar]
- 19.Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, Ueno T, Ueno Y, Ishii M, Sakisaka S, Sata M. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int 23: 3–11, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 6: 480–498, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest 117: 539–548, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest 102: 538–549, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut 48: 548–557, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126: 1795–1808, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Krysko DV, D'Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 11: 1709–1726, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Krysko DV, Vandenabeele P. From regulation of dying cell engulfment to development of anti-cancer therapy. Cell Death Differ 15: 29–38, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Lang C, Berardi S, Schafer M, Serra D, Hegardt FG, Krahenbuhl L, Krahenbuhl S. Impaired ketogenesis is a major mechanism for disturbed hepatic fatty acid metabolism in rats with long-term cholestasis and after relief of biliary obstruction. J Hepatol 37: 564–571, 2002 [DOI] [PubMed] [Google Scholar]
- 28.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore!. Curr Opin Cell Biol 13: 534–540, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Milani S, Herbst H, Schuppan D, Kim KY, Riecken EO, Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology 98: 175–184, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Milani S, Herbst H, Schuppan D, Stein H, Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol 139: 1221–1229, 1991 [PMC free article] [PubMed] [Google Scholar]
- 31.Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest 108: 1369–1378, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 57: 1275–1282, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology 135: 660–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinzani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG, Gentilini P. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrogenesis. Am J Pathol 148: 785–800, 1996 [PMC free article] [PubMed] [Google Scholar]
- 35.Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem 281: 15090–15098, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol 43: 1045–1054, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, Kolb A, Friess H, Schuppan D. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol 48: 453–464, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol 26: 587–596, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray MB, Mendenhall CL, French SW, Gartside PS. Bile duct changes in alcoholic liver disease. The Veterans Administration Cooperative Study Group. Liver 13: 36–45, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133: 80–90, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Rosenblum G, Van den Steen PE, Cohen SR, Grossmann JG, Frenkel J, Sertchook R, Slack N, Strange RW, Opdenakker G, Sagi I. Insights into the structure and domain flexibility of full-length pro-matrix metalloproteinase-9/gelatinase B. Structure 15: 1227–1236, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109: 1026–1033, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 371: 838–851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol 158: 1239–1244, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci USA 93: 3942–3946, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang B, Dolinski BM, Kikuchi N, Leone DR, Peters MG, Weinreb PH, Violette SM, Bissell DM. Role of alphavbeta6 integrin in acute biliary fibrosis. Hepatology 46: 1404–1412, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winwood PJ, Schuppan D, Iredale JP, Kawser CA, Docherty AJ, Arthur MJ. Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology 22: 304–315, 1995. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.