Abstract
Clinical efficacy of probiotics in treating various forms of diarrhea has been clearly established. However, mechanisms underlying antidiarrheal effects of probiotics are not completely defined. Diarrhea is caused either by decreased absorption or increased secretion of electrolytes and solutes in the intestine. In this regard, the electroneutral absorption of two major electrolytes, Na+ and Cl−, occurs mainly through the coupled operation of Na+/H+ exchangers and Cl−/OH− exchangers. Previous studies from our laboratory have shown that Lactobacillus acidophilus (LA) acutely stimulated Cl−/OH− exchange activity via an increase in the surface levels of the apical anion exchanger SLC26A3 (DRA). However, whether probiotics influence SLC26A3 expression and promoter activity has not been examined. The present studies were, therefore, undertaken to investigate the long-term effects of LA on SLC26A3 expression and promoter activity. Treatment of Caco-2 cells with LA for 6–24 h resulted in a significant increase in Cl−/OH− exchange activity. DRA mRNA levels were also significantly elevated in response to LA treatment starting as early as 8 h. Additionally, the promoter activity of DRA was increased by more than twofold following 8 h LA treatment of Caco-2 cells. Similar to the in vitro studies, in vivo studies using mice gavaged with LA also showed significantly increased DRA mRNA (∼4-fold) and protein expression in the colonic regions as assessed by Western blot analysis and immunofluorescence. In conclusion, increase in DRA promoter activity and expression may contribute to the upregulation of intestinal electrolyte absorption and might underlie the potential antidiarrheal effects of LA.
Keywords: probiotics, Caco-2, DRA (downregulated in adenoma), diarrhea
probiotics have been used in clinical trials for the prevention and treatment of various forms of diarrhea such as acute infectious diarrhea, antibiotic-associated diarrhea, and diarrhea-predominant irritable bowel syndrome (9). Probiotics are reported to have trophic effects on gut mucosa, e.g., enhancement of intestinal epithelial barrier function, increase in cell survival, and growth and stimulation of mucin synthesis and secretion (22). A number of mechanisms have been proposed to account for these beneficial effects of probiotics, e.g., reduction in luminal pH, inhibition of bacterial adherence, and secretion of some antibacterial compounds, bacteriocins, to remove pathogenic bacteria. Studies have also suggested that probiotics produce trophic factors such as spermine and spermidine (6) and short-chain fatty acids such as butyrate that upregulate the expression of advantageous genes (2, 27). Most of these studies have emphasized the beneficial effects of probiotics; however, their mechanism of action at the molecular level, with respect to their antidiarrheal effects, is not fully understood.
Diarrhea is considered to be a multifactorial event presented owing to either increased secretion of fluid and electrolytes, decreased absorption, or both. Electroneutral absorption of two main electrolytes, Na+ and Cl−, occurs via the coupled operation of sodium hydrogen and anion exchangers localized to the apical membrane of the intestinal epithelial cells. NHE2 and NHE3 are the two main sodium hydrogen exchangers on the apical surface whereas DRA (downregulated in adenoma) and putative anion transporter-1 (PAT-1) are the chloride hydroxyl exchangers. Among these, DRA appears to be the major intestinal anion exchanger since alterations in the function and expression of DRA have been implicated in diarrheal disorders; e.g., mutations in DRA are associated with a rare disorder congenital chloridiarrhea (14), presented by voluminous watery diarrhea with an extremely high chloride content. This pathology has been attributed to the lack of chloride absorption from the colon, a major site of chloride uptake in the body (11).
Lactic acid bacteria, particularly lactobacilli, are one of the predominant commensal bacteria in the gut microflora and are most commonly used probiotics for the prevention and treatment of diarrheal disorders (35). Lactobacillus acidophilus (LA) has been shown to prevent enteroinvasive Escherichia coli-mediated disruption of intestinal epithelial barrier function in Caco-2 cells (26). We have previously demonstrated stimulation of Cl−/OH− exchange activity via enhanced DRA function in response to short-term (3 h) LA treatment of Caco-2 cells (3). In the clinical trials, probiotics are generally given for a number of days for the treatment of diarrhea; however, nothing is known about the long-term effects of probiotics on the function and expression of intestinal chloride transporters. We hypothesized that, in addition to the short-term benefits (3), clinical efficacy of probiotics may also involve upregulation of genes involved in electrolyte absorption in the intestine. Therefore, the studies were designed to examine the long-term effects of LA on SLC26A3 and SLC26A6 expression in both the in vitro and in vivo models. Our data show that LA enhances the DRA expression in the intestine via a transcriptional mechanism.
MATERIALS AND METHODS
Materials.
Caco-2 cells were procured from ATCC. Radionucleotide 36Cl was obtained from PerkinElmer. RNeasy kits for RNA extraction were obtained from Qiagen, and real-time quantitative RT-PCR (qRT-PCR) kits were from Stratagene. 4,4′-Diisothiocyanate-stilbene-2,2′-disulfonic acid (DIDS) and 2-(N-morpholino)ethanesulfonic acid (MES) were procured from Sigma-Aldrich (St. Louis, MO). Common reagents for SDS-PAGE such as ammonium persulfate, acrylamide, and bis-acrylamide were from Fisher Scientific (Pittsburgh, PA).
Cell culture.
Caco-2 cells were grown in T-75 cm2 culture flasks at 37°C in a 5% CO2-95% O2 incubator. The medium in which the cells were grown consisted of minimum essential medium, 20% FBS (fetal bovine serum), 20 mM HEPES, 100 IU/ml penicillin, and 100 mg/ml streptomycin; 2 × 104 cells were plated per well in a 24-well culture plate and were used between passages 25 and 45. Fully differentiated confluent monolayers were used for the experiments (10–12 days postplating).
Bacterial culture.
LA was procured from ATCC (4357). Bacteria were grown overnight in MRS broth at 37°C in a 5% CO2-95% O2 incubator. The next day, bacteria were spun down by centrifuging at 3,000 rpm for 10 min. For in vitro studies, culture supernatant was separated from spun-down bacteria, filtered through a 0.22-μm filter, and mixed with cell culture media for further use; for in vivo studies, 3 × 109 colony-forming units (CFUs) of bacteria were gavaged per animal for 14 to 24 h.
Cl−/OH− exchange activity.
Cl−/OH− exchange activity was assessed as 36Cl− uptake carried out in well-differentiated polarized Caco-2 cells as described previously (13). After treatment of Caco-2 cells with LA culture supernatant diluted 50 times in culture media (LA-CM) for different time intervals, cells were incubated in base medium containing 20 mM HEPES pH 8.5 at room temperature. After 30 min, base medium was removed and the cells were rapidly washed with 1 ml tracer-free mannitol uptake buffer containing 260 mM mannitol and 20 mM Tris-MES, pH 7.0. This was followed by incubation with or without 600 μM DIDS in uptake buffer for 5 min since this time period is within the linear range of Cl− uptake in cells. The radioactive 36Cl (2.9 mM) in hydrochloric acid (specific activity 17.12 mCi/g) was added to the uptake buffer at a concentration of 1.4 mCi/ml. Uptake was stopped by removing the radioactivity-containing buffer and washing the cells rapidly two times with ice cold phosphate-buffered saline (PBS), pH 7.2. Furthermore, the cells were solubilized by 0.5 N NaOH for 4 h and protein concentration was measured by the Bradford method (4). Radioactivity was measured by a Packard Tri-Carb 1600 TR liquid scintillation analyzer (Packard Instruments; PerkinElmer). The Cl−/OH− activity was then calculated as DIDS-sensitive 36Cl− uptake, and the specific activity is expressed as nanomoles per milligram protein per 5 min.
Real-time PCR.
RNA was extracted from LA-treated and untreated Caco-2 cells and mice samples by use of Qiagen RNeasy kits. RNA was reverse transcribed and amplified with Brilliant SYBR Green qRT-PCR Master Mix kit (Stratagene). Human DRA (accession no. BC025671) was amplified with gene-specific primers (sense primer 1250 bp, 5′-TTCAGTTGCCAGCGTCTATTC-3′; antisense primer 1416 bp, 5′-GTGTTTTGCCTCCTGTGCTCT-3′). Human histone was amplified as an internal control utilizing gene-specific primers (sense primer, 5′-ACCGACCTTCGTTTCCAGAG-3′; antisense primer, 5′-CTTGGCGTGAATAGCACAGA-3′). Mouse DRA (accession no. BC139273) was amplified with gene-specific primers (sense primer 454 bp, 5′-TGGTGGGAGTTGTCGTTACA-3′; antisense primer 607, 5′-CCCAGGAGCAACTGAATGAT-3′). Mouse histone was amplified as an internal control by utilizing gene-specific primers (sense primer, 5′-GTACTGTGGCACTCCGTGAA-3′; antisense primer, 5′-CAAAAAGGCCAACCAGAAAG-3′). Human PAT-1 was amplified with the gene-specific primers (sense primer, 5′-AGATCCCCCACTACTCTGTCCT-3′; antisense primer, 5′-ATCCACACCACACCTCTGCTT-3′), and mouse PAT-1 was amplified with gene-specific primers (sense primer, 5′-GAAATGGAGCTGCAGAGGA-3′; antisense primer, 5′-GCT GGAGCAGAAGAGAATGG-3′). Relative levels of DRA and PAT-1 mRNA were expressed as percent of control normalized to histone.
Transfections.
Caco-2 cells were transfected with DRA promoter (p-1183/+114) fragment cloned upstream of the luciferase reporter gene in pGL2-Basic and β-galactosidase expression vector by electroporation using Amaxa Nucleofactor System as described previously (2). Activities of firefly luciferase and β-galactosidase were measured according to the manufacturer's instructions (Promega). DRA promoter activity was expressed in terms of relative luciferase activity normalized to β-galactosidase activity.
In vivo studies.
In vivo studies performed in C57Bl/6J mice were approved by the Animal Care Committee of University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. Mice were gavaged with LA (3 × 109 CFUs) or sterile PBS as vehicle for 14 to 24 h. Intestines were resected and mucosa was scraped for RNA and protein extraction. A part (∼2 cm) of the different regions of intestine (ileum and colon) was immediately snap frozen in optimal cutting temperature embedding medium (Tissue-Tek OCT compound; Sakura) for immunofluorescence studies. RNA was extracted and real-time qRT-PCR was performed as described above.
Western blotting.
Tissue lysates were prepared from the scraped mucosa of ileum and colon by use of cell lysis buffer (Cell Signaling). Lysates were run on a 8% gel and then transferred onto nitrocellulose membrane. Immunoblotting was carried out with anti-DRA affinity purified antibody as previously described (3).
Immunofluorescence.
Snap-frozen tissues were cut into 5-μm sections on the slides by use of a cryostat and were preserved at −80°C until further use. For immunostaining, these sections were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and permeabilized with 0.5% Nonidet P-40 in PBS for 5 min, followed by blocking in 5% normal goat serum (NGS) for 120 min at room temperature. Sections were then incubated with primary antibodies anti-DRA (1:100) and anti-villin (1:100) in 1% NGS for 120 min. After being washed with 1% NGS, sections were incubated with secondary antibodies, Alexa Fluor 568-conjugated goat anti-rabbit IgG and Alexa Fluor 488-conjugated goat anti-mouse IgG, for 60 min and then mounted with slow-fade DAPI by using coverslips. Microscopy was performed with a Zeiss LSM 510 laser scanning confocal microscope equipped with a ×63 water immersion objective.
Statistical analysis.
Data are presented as means ± SE of three to five independent experiments. Difference between control vs. treated was analyzed by one-way analysis of variance with Tukey's test. Differences were considered significant at P ≤ 0.05.
RESULTS
Long-term (6–24 h) LA exposure increases Cl−/OH− exchange activity in Caco-2 cells.
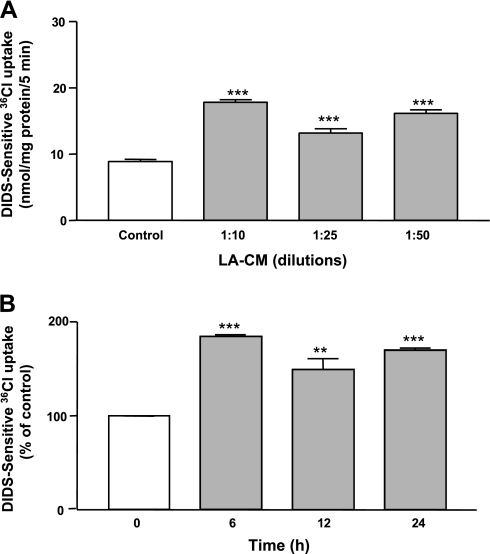
Previous studies from our laboratory have shown that short-term exposure to LA for 3 h increased the Cl−/OH− exchange activity in Caco-2 cells. To examine the long-term effects of LA, Caco-2 monolayers were treated with LA-CM (different dilutions) for 6–24 h and DIDS sensitive 36Cl− uptake was measured after base loading the cells. As shown in Fig. 1A, Cl−/OH− exchange activity was significantly increased at all the dilutions of LA-CM used. In addition, Cl−/OH− exchange activity was significantly enhanced at all time points in response to LA-CM treatment ranging from 6 to 24 h (Fig. 1B).
Fig. 1.
Cl−/OH− exchange activity (DIDS-sensitive 36Cl− uptake) in Caco-2 cells in response to treatment for 24 h with different dilutions (A) and for indicated time periods with 1:50 dilutions of Lactobacillus acidophilus (LA) culture supernatant (B). LA-CM, LA culture supernatant in culture media. Values are means ± SE of 3 independent experiments performed in triplicate. Differences between groups, control vs. LA treated, are statistically significant at ***P < 0.001, **P < 0.01.
LA-mediated stimulation of Cl−/OH− exchange activity occurs via increased levels of DRA but not PAT-1.
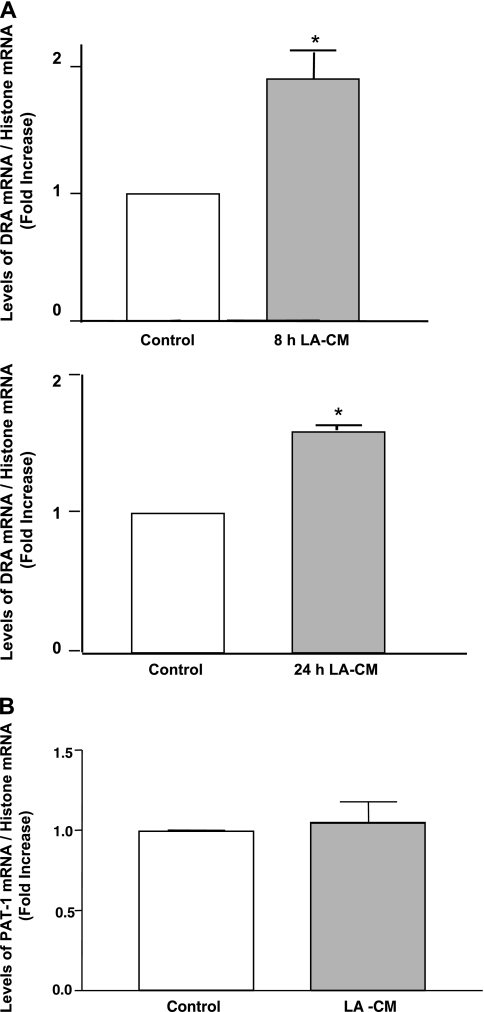
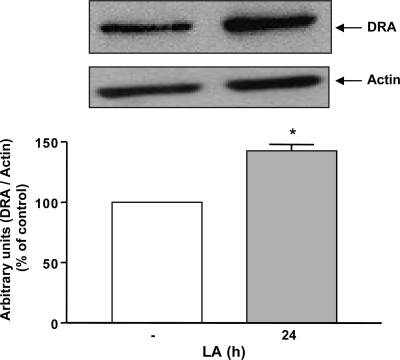
Two members of the SLC26 gene family, DRA (SLC26A3) and PAT-1 (SLC26A6) have been implicated as candidate genes for apical Cl−/OH−(HCO3−) exchange activity in the mammalian intestine (11). Therefore, to examine the effect of LA on DRA and PAT-1 mRNA, Caco-2 monolayers were treated with LA-CM (1:50 dilution) for 8 and 24 h and mRNA levels of DRA and PAT-1 were determined by real-time quantitative PCR. As shown in Fig. 2A, mRNA levels of DRA were found to be significantly increased as early as at 8 h of LA-CM treatment and remained elevated until 24 h. PAT-1 mRNA levels, however, did not change significantly following LA-CM 24 h treatment as shown in Fig. 2B. Furthermore, levels of DRA protein were also elevated by about ∼1.5 fold in Caco-2 cells after 24 h of LA treatment as revealed by the densitometry analysis of the Western blot (Fig. 3).
Fig. 2.
Changes in downregulated in adenoma (DRA; A) and putative anion transporter-1 (PAT-1; B) mRNA levels in Caco-2 cells in response to treatments with LA culture supernatant for indicated time periods. Total RNA from control and treated Caco-2 cells were extracted and used for real-time quantitative RT-PCR. Values of mRNA levels for DRA and PAT-1 were normalized against histone mRNA levels. Results represent means ± SE of 3 independent experiments performed in triplicate. Differences between groups, control vs. LA treated, are statistically significant at *P < 0.05.
Fig. 3.
Changes in DRA protein levels in Caco-2 lysates in response to LA-CM treatment for 24 h. Total lysates were extracted from the Caco-2 cells with and without LA-CM treatment and used for Western blot analysis. Densitometry was performed to measure the intensity of DRA protein normalized to actin. Results represent means ± SE of 4 different experiments. Differences between groups, control vs. LA treated, are statistically significant at *P < 0.01.
LA increases DRA promoter activity.
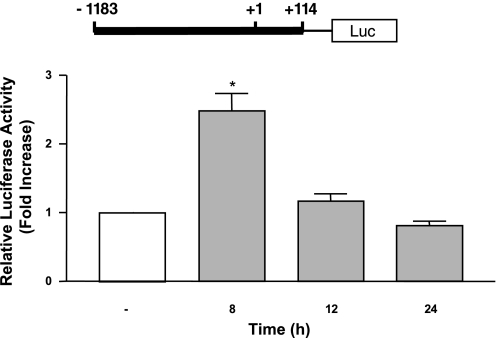
We next investigated whether the increase in the DRA mRNA expression was through a transcriptional mechanism involving increased promoter activity. Caco-2 cells were transfected with DRA promoter reporter plasmid, 24 h posttransfection, cells were treated with LA-CM (1:50 dilution) for different time intervals and DRA promoter activity was assessed. DRA promoter activity was markedly increased by more than twofold in response to LA-CM following 8 h of treatment without any change at the later time points studied (Fig. 4). These results demonstrate that long-term LA treatment increases DRA expression through a transcriptional mechanism in human intestinal epithelial cells.
Fig. 4.
Changes in DRA promoter activity in response to treatments with LA culture supernatant. Caco-2 cells were transiently transfected with a 1.3 kb fragment of the promoter region of DRA gene cloned into pGL2. After 24 h, cells were treated with 1:50 dilution of LA culture supernatant for another 8, 12, or 24 h. Cells were then harvested, and luciferase activity (Luc) was measured and normalized with β-gal activities. The results are shown as fold increase in normalized luciferase activity compared with control. Results represent means ± SE of 4 independent experiments. Differences between groups, control vs. LA treated, are statistically significant at *P < 0.001.
In vivo studies.
Our in vitro studies in Caco-2 cells clearly demonstrated that LA increased Cl−/OH− (HCO3−) exchange activity by increasing DRA expression. However, in vitro studies do not give a comprehensive idea of these absorptive processes in native intestine. Therefore, we next investigated the effects of LA on DRA expression in an in vivo mouse model.
LA increases DRA but not PAT-1 mRNA levels.
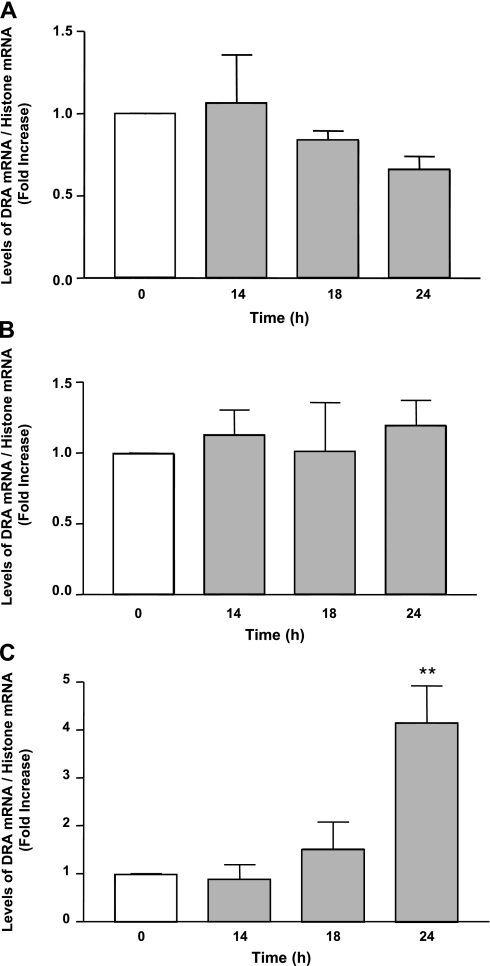
DRA mRNA levels were assessed by real-time qRT-PCR in samples from the mice gavaged with LA. Jejunum (Fig. 5A) and ileum (Fig. 5B) did not show a significant change in the mRNA expression of DRA compared with the controls; however, colon showed a highly significant increase of about fourfold (P < 0.01 vs. control) after 24 h of LA treatment as shown in Fig. 5C. However, PAT-1 mRNA levels remained unchanged in all the intestinal regions following LA treatment (Table 1).
Fig. 5.
Changes in DRA mRNA levels in mice samples in response to treatment with LA (3 × 109 CFUs) for indicated time periods. Total RNA from different intestinal regions, i.e., jejunum (A), ileum (B), and colon (C) of control and treated mice, was extracted and used for real-time quantitative RT-PCR. Values of mRNA levels for DRA were normalized against histone mRNA levels. Results represent means ± SE of 5 different experiments. Differences between groups, control vs. LA treated, are statistically significant at **P < 0.01.
Table 1.
PAT-1 levels remain unchanged following Lactobacillus acidophilus exposure in mice
| LA, h | Jejunum | Ileum | Colon |
|---|---|---|---|
| 0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| 14 | 1.58 ± 0.39 | 0.83 ± 0.24 | 1.17 ± 0.17 |
| 18 | 1.09 ± 0.22 | 1.01 ± 0.35 | 1.01 ± 0.18 |
| 24 | 1.01 ± 0.14 | 0.84 ± 0.18 | 0.97 ± 0.13 |
Results represent means ± SE of 5 independent experiments. Values of mRNA levels for putative anion transporter (PAT-1) were normalized against histone mRNA levels and further compared to 0 h treatment considered as 1.0 ± 0.0. LA, Lactobacillus acidophilus; PAT-1, putative anion transporter-1.
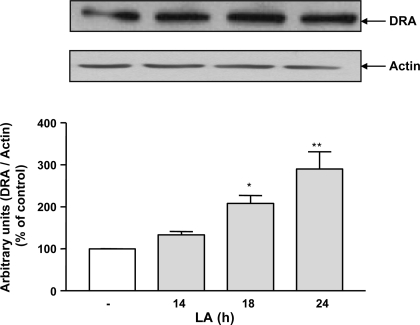
LA induces DRA protein expression.
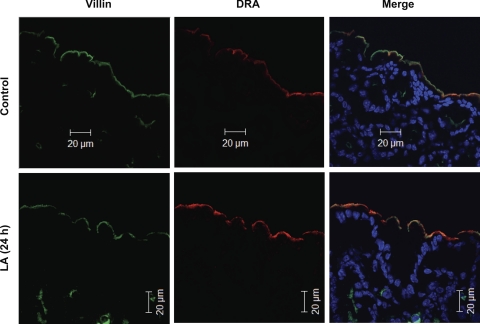
Since we observed an increase in DRA mRNA levels in the colon, we next assessed DRA protein expression upon LA treatment. Western blot analysis revealed that DRA protein expression was enhanced in the LA-treated colonic tissues at all time points with a maximal increase observed at 24 h. Furthermore, densitometric analysis revealed that there was a significant increase in the DRA protein levels (∼3-fold) in LA-treated samples compared with the controls (Fig. 6). These results were further corroborated by immunofluorescence imaging. As shown in Fig. 7, significant increase in the expression of DRA protein can be clearly visualized on the apical surface of colonic sections from LA-gavaged mice compared with those from control mice. DRA has been labeled in red color and villin in green color. A sharp increase in the intensity of red color (i.e., DRA) as well as yellow color in the merged pictures was observed in the sections from mice gavaged with LA.
Fig. 6.
Changes in DRA protein levels in mice lysates in response to LA (3 × 109 colony-forming units) treatment for indicated time periods. Total lysates were extracted from the colons of control mice and mice treated with LA and used for Western blot analysis. Densitometry was performed to measure the intensity of DRA protein normalized to actin. Results represent means ± SE of 5 different experiments. Differences between groups, control vs. LA treated, are statistically significant at **P < 0.01, *P < 0.05.
Fig. 7.
In vivo effects of LA on DRA expression. Immunofluorescent localization of DRA (red) and villin (green) in the colonic epithelium of control mice and 24-h LA-treated mice. Control colon shows a modest expression of DRA on the epithelial surface whereas LA-treated colon shows enhanced expression of the DRA as visualized from the increased intensity of yellow color in the merge picture. Scale bar: 20 μm. A representative of 5 different experiments has been shown.
DISCUSSION
Recently, an upsurge has been observed regarding the reports on beneficial effects of probiotics. Many probiotic strains are being used in clinical trials for the improvement of intestinal health. For example, LA has been shown to reduce antibiotic-associated diarrhea whereas L. plantarum and Bifidobacterium breve are helpful in irritable bowel syndrome, inflammatory bowel disease, and diarrhea (23, 24). However, the mechanisms for their action at a molecular and cellular level still remain elusive. In this regard, we have previously demonstrated that the probiotic LA stimulated Cl−/OH− exchange activity in human intestinal epithelial Caco-2 cells within 3 h. This effect was dependent on PI-3 kinase activity and involved increased surface levels of DRA, the major apical Cl−/OH− exchanger (3). This was the first report showing involvement of a signaling pathway in the short-term stimulation of Cl− absorption by probiotics. Under in vivo conditions, intestinal epithelial cells are continuously exposed to probiotic strains and hence their secreted factors are available to these cells for longer periods of time. To the best of our knowledge, currently there are no reports regarding the long-term effects of probiotics on intestinal ion absorptive mechanisms. Therefore, the present studies were designed to examine the long-term effects of LA utilizing both in vitro and in vivo models.
Probiotics B. infantis, E. coli 1917, and L. plantarum improve epithelial barrier function by increasing the expression of tight junction proteins such as zonula occludens (ZO)-1, ZO-2, and occludin (30, 36). In the present study we found that Cl−/OH− exchange activity was also enhanced after 24-h LA treatment, which suggested that some additional mechanisms might be operating, most probably through increased transcription of SLC26A3 gene. Interestingly, DRA promoter activity was found to be increased after 8 h of LA treatment and DRA mRNA expression was enhanced between 8–24 h without any change in the PAT-1 mRNA expression. However, DRA promoter activity returned back to normal at 12 and 24 h, implying that an increase in the promoter activity at 8 h was sufficient to sustain enhanced mRNA and protein expression observed until 24 h. This increase in DRA expression by LA is an exciting observation considering the fact that DRA is most likely the major luminal Cl−/OH− exchanger in colon, since mutations in this gene give rise to congenital chloride diarrhea (10) and DRA knockout mice also exhibit similar symptoms (29). In contrast, PAT-1 knockout mice have not been shown to produce chloride diarrhea (33). It is also possible that PAT-1 (i.e., SLC26A6) is not regulatable by LA via transcriptional mechanism. Based on our combined results from previous and present studies, it can be inferred that LA can upregulate Cl−/OH− exchange activity via both posttranslational and transcriptional mechanisms. When the intestinal epithelial cells are exposed to the secreted factors of LA for a short time (i.e., 3 h), Cl−/OH− exchange activity is increased owing to the increased levels of DRA at the apical surface, most probably through trafficking, and, when the cells are treated with secreted factors of LA on a long-term basis as in the present study (8–24 h), DRA expression is also enhanced, secondary to the increased transcription.
We further validated our in vitro results of Caco-2 cells utilizing an in vivo mouse model. Consistent with the in vitro results, in vivo data also showed that DRA mRNA expression was significantly enhanced in the colon of LA-gavaged mice whereas mRNA levels of DRA were unchanged in jejunum and ileum. This could be due to the relative expression of DRA and its functional role along the length of mouse intestine (2, 18, 20). Colonic regions have been shown to express significantly higher DRA, which plays an important role in electroneutral Cl− absorption in colonic regions compared with the small intestine (21). It could also be due to the fact that Lactobacilli strains predominantly colonize in the colon compared with the small intestine (1, 32) and hence they can affect the gene expression in colon either directly or through their secreted factors (7, 12). Another reason for observed effects in colon could be the rapid transit of probiotics from the small intestine compared with colon where they stay for a longer period of time. Levels of PAT-1, which is predominantly expressed in jejunum and ileum, remained unchanged following LA exposure, which could again be due to the preferential colonization or slow transit of LA in colonic regions compared with small intestine. It is possible that increased mRNA levels might not necessarily translate into increased protein expression because of potential regulation by specific micro-RNAs or changes in mRNA stability (8). However, increased DRA protein expression as assessed by Western blotting and confocal immunofluorescence clearly demonstrated that increased mRNA levels did translate into protein.
The mechanism of action of probiotics is not well characterized at the molecular level. Some studies using microarray analysis reported up- and downregulation of various genes involved in inflammation, cell signaling, cell cycle, and lipid metabolism following probiotics exposure (17, 25, 31). We report for the first time that expression of the chloride transporter gene, DRA, is enhanced in response to LA treatment; however, the identity of secreted bioactive factors is unclear at present. Few recent studies have suggested that probiotics secrete some bioactive trophic factors such as spermine and spermidine that act as growth promoters, resulting in overall increased mucosal mass and increased expression of brush border enzymes such as sucrase and alkaline phosphatase (5, 6). Conjugated linoleic acids produced by Lactobacillus are shown to be effective in Helicobacter pylori-infected gastric epithelial cells (16) and some soluble proteins derived from Lactobacillus rhamnosus GG have been reported to regulate intestinal epithelial cell survival and growth (34). Another report has shown that conditioned media from some of the individual strains of VSL#3 (a probiotics mixture) improved epithelial barrier function both in vitro and in vivo (19). Probiotics are well known to produce short-chain fatty acids such as butyrate from complex carbohydrates by bacterial fermentation, which in turn are reported to stimulate NHE3 (15) and DRA (2) expression, Therefore, the stimulation of DRA expression as observed in our present studies could arise from any known bioactive factors documented above or could be due to some unidentified bioactive molecules secreted by these bacteria. We speculate that stimulation of DRA expression in our in vivo studies could be partly via the production of short-chain fatty acids by probiotics. Since DRA is one of the most critical transporters involved in coupled electroneutral NaCl absorption from intestine, our findings hold significance in contributing to the molecular mechanism for the beneficial effects of probiotics in treatment of diarrheal disorders. Further studies are needed to examine the potential beneficial effects of probiotic strains on sodium absorption as well as to delineate signaling mechanisms used by the bioactive factors of probiotics that elicit these beneficial effects.
GRANTS
These studies were supported by the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 54016 (P. K. Dudeja), DK 81858 (P. K. Dudeja), Program Project Grant DK 067887 (P. K. Dudeja and K. Ramaswamy), DK 74459 (R. K. Gill), and CCFA Grant 1942 (S. Saksena).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Ahmed S, Macfarlane GT, Fite A, McBain AJ, Gilbert P, Macfarlane S. Mucosa-associated bacterial diversity in relation to human terminal ileum and colonic biopsy samples. Appl Environ Microbiol 73: 7435–7442, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293: G923–G934, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Broekaert IJ, Nanthakumar NN, Walker WA. Secreted probiotic factors ameliorate enteropathogenic infection in zinc-deficient human Caco-2 and T84 cell lines. Pediatr Res 62: 139–144, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Buts JP, De Keyser N. Effects of Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci 51: 1485–1492, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res 58: 1185–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chen XM, Splinter PL, O'Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem 282: 28929–28938, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collado MC, Isolauri E, Salminen S, Sanz Y. The impact of probiotic on gut health. Curr Drug Metab 10: 68–78, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Dorwart MR, Shcheynikov N, Baker JM, Forman-Kay JD, Muallem S, Thomas PJ. Congenital chloride-losing diarrhea causing mutations in the STAS domain result in misfolding and mistrafficking of SLC26A3. J Biol Chem 283: 8711–8722, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudeja PK, Ramaswamy K. Intestinal anion absorption. In: Physiology of the Gastrointestinal Tract (4th ed.), edited by Johnson LR, Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD. Burlington, MA: Elsevier Academic, 2006, p. 1881–1916. [Google Scholar]
- 12.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kere J. Overview of the SLC26 family and associated diseases. Novartis Found Symp 273: 2–11; discussion 11–18, 261–264, 2006 [PubMed] [Google Scholar]
- 15.Kiela PR, Kuscuoglu N, Midura AJ, Midura-Kiela MT, Larmonier CB, Lipko M, Ghishan FK. Molecular mechanism of rat NHE3 gene promoter regulation by sodium butyrate. Am J Physiol Cell Physiol 293: C64–C74, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kim JM, Kim JS, Kim YJ, Oh YK, Kim IY, Chee YJ, Han JS, Jung HC. Conjugated linoleic acids produced by Lactobacillus dissociates IKK-gamma and Hsp90 complex in Helicobacter pylori-infected gastric epithelial cells. Lab Invest 88: 541–552, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Lin PW, Nasr TR, Berardinelli AJ, Kumar A, Neish AS. The probiotic Lactobacillus GG may augment intestinal host defense by regulating apoptosis and promoting cytoprotective responses in the developing murine gut. Pediatr Res 64: 511–516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(−)/HCO(3)(−) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J Biol Chem 274: 22855–22861, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296: G1140–G1149, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol 276: G185–G192, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Musch MW, Arvans DL, Wu GD, Chang EB. Functional coupling of the downregulated in adenoma Cl−/base exchanger DRA and the apical Na+/H+ exchangers NHE2 and NHE3. Am J Physiol Gastrointest Liver Physiol 296: G202–G210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15: 300–310, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Ouwehand AC, Nermes M, Collado MC, Rautonen N, Salminen S, Isolauri E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J Gastroenterol 15: 3261–3268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouwehand AC, Salminen S, Isolauri E. Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82: 279–289, 2002 [PubMed] [Google Scholar]
- 25.Panigrahi P, Braileanu GT, Chen H, Stine OC. Probiotic bacteria change Escherichia coli-induced gene expression in cultured colonocytes: implications in intestinal pathophysiology. World J Gastroenterol 13: 6370–6378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52: 988–997, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol 587: 4169–4174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saksena S, Dwivedi A, Singla A, Gill RK, Tyagi S, Borthakur A, Alrefai WA, Ramaswamy K, Dudeja PK. Characterization of the 5′-flanking region and regulation of expression of human anion exchanger SLC26A6. J Cell Biochem 105: 454–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Shen TY, Qin HL, Gao ZG, Fan XB, Hang XM, Jiang YQ. Influences of enteral nutrition combined with probiotics on gut microflora and barrier function of rats with abdominal infection. World J Gastroenterol 12: 4352–4358, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shima T, Fukushima K, Setoyama H, Imaoka A, Matsumoto S, Hara T, Suda K, Umesaki Y. Differential effects of two probiotic strains with different bacteriological properties on intestinal gene expression, with special reference to indigenous bacteria. FEMS Immunol Med Microbiol 52: 69–77, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Voltan S, Castagliuolo I, Elli M, Longo S, Brun P, D'Inca R, Porzionato A, Macchi V, Palu G, Sturniolo GC, Morelli L, Martines D. Aggregating phenotype in Lactobacillus crispatus determines intestinal colonization and TLR2 and TLR4 modulation in murine colonic mucosa. Clin Vaccine Immunol 14: 1138–1148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker NM, Simpson JE, Yen PF, Gill RK, Rigsby EV, Brazill JM, Dudeja PK, Schweinfest CW, Clarke LL. Down-regulated in adenoma Cl/HCO3 exchanger couples with Na/H exchanger 3 for NaCl absorption in murine small intestine. Gastroenterology 135: 1645–1653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care 9: 717–721, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 9: 804–816, 2007 [DOI] [PubMed] [Google Scholar]