Abstract
Anterior pituitary cells express cation-conducting P2X receptor channels (P2XRs), but their molecular identity, electrophysiological properties, cell-specific expression pattern, and physiological roles have been only partially characterized. In this study, we show by quantitative RT-PCR that mRNA transcripts for the P2X4 subunit are the most abundant in rat anterior pituitary tissue and confirm the P2X4R protein expression by Western blot analysis. Single-cell patch-clamp recordings show that extracellular ATP induced an inward depolarizing current in a majority of thyrotropin-releasing hormone-responsive pituitary cells, which resembled the current profile generated by recombinant P2X4R. The channels were activated and desensitized in a dose-dependent manner and deactivated rapidly. Activation of these channels led to stimulation of electrical activity and promotion of voltage-gated and voltage-insensitive Ca2+ influx. In the presence of ivermectin, a specific allosteric modulator of P2X4Rs, there was an approximately fourfold increase in the maximum amplitude of the ATP-induced inward current, accompanied by an increase in the sensitivity of receptors for ATP, slowed deactivation of receptors, and enhanced ATP-induced prolactin release. These results indicate that thyrotropin-releasing hormone-responsive cells, including lactotrophs, express homomeric and/or heteromeric P2X4Rs, which facilitate Ca2+ influx and hormone secretion.
Keywords: adenosine 5′-triphosphatase, adenosine 5′-triphosphatase-gated receptor channels, calcium, lactotrophs, prolactin, ivermectin
in addition to its intracellular functions, ATP can be released by excitable and nonexcitable cells and act as an extracellular messenger through the stimulation of P2Y receptors (P2YRs) and P2X receptors (P2XRs). To date, eight mammalian P2YRs have been identified and are denoted as P2Y1R, P2Y2R, P2Y4R, P2Y6R, P2Y11R, P2Y12R, P2Y13R, and P2Y14R (12). Four of these receptors (P2Y1R, P2Y2R, P2Y4R, and P2Y6R) signal through a Gq/11-dependent pathway, leading to the activation of phospholipase C and the generation of inositol trisphosphate (InsP3) and diacylglycerol. P2YRs also have been reported to activate the MAP kinase and phospholipase D signaling pathways, both of which are secondary to the activation of protein kinase C. They also have been found to couple to the Gi/o (P2Y13R) and Gs (P2Y11R) signaling pathways (10). On the other hand, seven identified P2XRs comprise the family of trimeric channels that use the energy of ATP binding to initiate a depolarizing flux of cations, including Ca2+, through the pore of channels. In excitable cells, P2XR-induced depolarization also facilitates Ca2+ influx through voltage-gated Ca2+ (Cav) channels. Prolonged application of ATP causes a decrease in the conductivity of some P2XRs, a process termed receptor desensitization, whereas removal of the agonist leads to deactivation of the receptors. The rates of P2XR activation, desensitization, and deactivation are receptor specific. The efficacy of ATP and its analogs in channel activation is also receptor specific (29). The extracellular actions of ATP are terminated by ectonucleotidases, leading to the generation of ADP, a primary agonist for some P2YRs, and adenosine, the common agonist for adenosine subtypes of receptor (ARs) (32).
Several lines of evidence have suggested that anterior pituitary cells release ATP and express the ectonucleotidases eNTPDase 1-3 (4, 14, 43). The expression of P2YRs and ARs is also well documented in these cells (34, 40). The initial characterization of P2YRs was done using inositol phosphate measurements in mixed populations of anterior pituitary cells (9, 44) and single-cell Ca2+ measurements in identified pituitary cell types (3, 5, 7). Chen et al. (6) cloned the UTP-activated P2Y2R from a rat pituitary complementary DNA library (6), and our recent studies (13) have shown the expression of a Ca2+-mobilizing P2Y1R that can be activated by ATP and ADP, but not UTP, in lactotrophs and other unidentified pituitary cells. Anterior pituitary cells express three subtypes of adenosine receptors, A1R, A2AR, and A2BR, and activation of these receptors leads to modulation of Ca2+ influx through Cav channels (27, 33, 34).
Secretory anterior pituitary cells also express functional P2XR channels, as documented using single-cell Ca2+ measurements (8, 24, 30, 43, 46). Characterization of these depolarizing channels is of physiological relevance, because in all pituitary secretory cell types, the depolarization-induced electrical activity generates Ca2+ transients of sufficient amplitude to trigger hormone secretion (39, 41). Molecular studies have indicated that several P2X2Rs and their splice variants are expressed in these cells, and the cloned channels were initially characterized using different expression systems (20–22). More recent electrophysiological studies have revealed that gonadotrophs express these channels and that native P2X2Rs have the capacity to depolarize the plasma membrane, leading to initiation of the firing of action potentials (APs) in quiescent cells as well as an increase in the intracellular Ca2+ concentration ([Ca2+]i) (23, 50). Single-cell [Ca2+]i measurements also raised the possibility that other subtypes of P2XR are present in pituitary cells (8, 24, 30, 46); however, their molecular structure, biophysical properties, expression among pituitary cells, and physiological roles have not been clarified. In this study, we focused on the expression of P2X4Rs and their electrophysiological characterization in thyrotropin-releasing hormone (TRH)-responsive cells.
MATERIALS AND METHODS
Animals and cell cultures.
Experiments were performed on anterior pituitary cells from postpubertal (8-wk-old) female Sprague-Dawley rats obtained from Taconic Farms (Germantown, MD). Pituitary cells were dispersed and cultured in medium 199 (M199) containing Earle's salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) (all from Gibco Invitrogen, Carlsbad, CA) as previously described (24, 45). Experiments were approved by National Institute of Child Health and Human Development Animal Care. GT1 cells were cultured with Dulbecco's modified Eagle's culture medium [DMEM/F-12, 1:1, with l-glutamate, pyridoxine hydrochloride, 2.5 g/l sodium bicarbonate, 10% heat-inactivated FBS, and 100 mg/ml gentamicin (Invitrogen, Carlsbad, CA)].
Electrophysiological recordings.
For electrophysiological recordings, dissociated cells were diluted to 0.6 × 106 cells/ml, seeded onto the bottom of 35-mm culture dishes covered with poly-l-lysine, and kept overnight in Earle's salts-containing M199 at 37°C. Plasma membrane voltage potential and whole cell currents were measured using the nystatin-perforated patch-clamp technique at room temperature. Cells were continuously perfused with an extracellular solution containing 150 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose; pH was adjusted to 7.3 with NaOH. Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) and polished by heat to a tip resistance of 5–7 MΩ. Pipette solution contained 70 mM K-aspartate, 70 mM KCl, 3 mM MgCl2, and 10 mM HEPES; pH was adjusted to 7.2 with KOH. Before measurement, nystatin and the dispersing agent Pluronic F-127 from stock solutions were added to the intracellular solution to obtain final concentrations of 250 and 500 μg/ml, respectively. Recordings were performed 10 min after seal formation. The plasma membrane potential was held at −60 mV. Current-clamp and voltage-clamp recordings were performed using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Data were captured and stored using the pClamp 8 software packages in conjunction with the Digidata 1322A analog-to-digital converter (Axon Instruments). No series resistance compensation was used, and the corrections of membrane potential for the Donnan potential and liquid junction potential were ignored. Solutions were delivered to the recording chamber by a gravity-driven microperfusion system (ALA Scientific Instruments, Westbury, NY). The application tip was routinely positioned 50 μm above the recorded cell. Less than 200 ms were required for the exchange of solutions around the patched cells.
Single-cell Ca2+ measurements.
For measurements of [Ca2+]i, cells were incubated in Krebs-Ringer medium supplemented with 2 μM fura-2 AM (Invitrogen, Carlsbad, CA) at room temperature for 60 min. Coverslips with cells were then washed and mounted on the stage of an Axiovert 135 microscope (Carl Zeiss, Oberkochen, Germany) and examined under a ×40 oil-immersion objective during exposure to alternating 340- and 380-nm light beams. The intensity of light emission at 520 nm was measured. The images were captured using a digital C4742-95-12ER camera (Hamamatsu, Bridgewater, NJ) and were processed and analyzed using MetaFluor software (Molecular Devices, Sunnyvale, CA). The ratio of emission intensities (F340/F380), which reflects changes in [Ca2+]i, was simultaneously followed in several single cells.
Prolactin measurements.
Prolactin (PRL) secretion was monitored using cell column perifusion experiments. Briefly, 1.2 × 107 cells were incubated with preswollen Cytodex-1 beads in 60-mm petri dishes for 18 h. The beads were then transferred to 0.5-ml chambers and perifused with Hanks’ M199 containing 25 mM HEPES, 0.1% BSA, and penicillin (100 U/ml)-streptomycin (100 μg/ml) for 2.5 h at a flow rate of 0.8 ml/min and at 37°C to establish stable basal secretion. Fractions were collected at 1-min intervals and later assayed for PRL contents using radioimmunoassay. The primary antibody and standard for the PRL assay were purchased from the National Pituitary Agency and Dr. A. F. Parlow (Harbor-UCLA Medical Center, Torrance, CA), and 125I-labeled PRL was purchased from PerkinElmer Life Sciences (Boston, MA).
RNA extraction and RT-PCR.
Total RNA was extracted from rat anterior pituitary tissues using the Trizol reagent (Invitrogen) and treated with RNase-free DNase I (Invitrogen) for 15 min at room temperature. The extracted RNA was considered to be of high quality when the 260-nm/280-nm ratio was above 1.85. Total RNA (2 μg) was reverse-transcribed to cDNAs with the SuperScript III First-Strand kit (Invitrogen). The gene-specific and intron-spanning primers for amplification of rat P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7 (NCBI sequence accession nos. NM_012997, NM_053656, NM_031075, NM_031594, NM_080780, NM_012721, and NM_019256, respectively) were designed using Primer3Plus online software (Table 1). The sizes of PCR-amplified products from pituitary cDNA were verified in a 2% agarose gel under UV illumination. The gel bands containing the target DNA were excised and purified with the QIAquick gel extraction kit. The specificity of products generated by primer pairs was further confirmed by DNA sequencing.
Table 1.
Primers used for detection of P2XR mRNA transcripts
| Receptor | Primers, 5′→3′ | Size, bp |
|---|---|---|
| P2X1 | (f): GTGTTTGGGATTCACTTTGATA | 169 |
| (r): TCTGCTTGTAGTAGTGCCTCTT | ||
| P2X2 | (f): AGAAGAGTGACTACCTCAAGCA | 158 |
| (r): ACAGTTCCAGTTGATGATGACT | ||
| P2X3 | (f): CCTCACCGACAAGGACATA | 158 |
| (r): ACACCCAGCCGATCTTAAT | ||
| P2X4 | (f): TCCTGATAAGACCAGCATTT | 208 |
| (r): CAAGAGGGTGAAGTTTTCTG | ||
| P2X5 | (f): CAAATCTCTACTGTCCCATCTT | 157 |
| (r): TAGTAGTGTGGGTTGCATTTAG | ||
| P2X6 | (f): GTTCTTCCTGGTAACCAACTT | 158 |
| (r): CACACTGACCAGTCTTTATGC | ||
| P2X7 | (f): TCGGAGAGAACTTTACAGAGG | 150 |
| (r): ACAGGGACTCATTGGTGTACT |
P2XR, P2X receptors; f, forward primers; r, reverse primers.
Quantitative real-time RT-PCR.
Absolute quantification of P2XR mRNA expression was performed using a LightCycler 2.0 instrument (Roche Diagnostics, Mannheim, Germany) and glass capillaries containing in the total volume of 20 μl the following: FastStart DNA master mix SYBR green I (Roche), 3 mM MgCl2, 2 μl of 10× diluted cDNA or plasmid, and 0.5 μM forward and reverse primers. Calibration curves were generated from the serial dilution of linearized pIRES2-EGFP recombinant plasmids containing the coding region of P2XR cDNAs. The mean cycle threshold (CT) values were plotted against the copy number to establish calibration curves and to determine the concentration of unknown pituitary samples as described previously (49). Briefly, the concentration of each plasmid DNA was measured with a spectrophotometer (Nanodrop ND-1000), and the number of plasmid copies per unit volume was calculated. Mass of a single plasmid molecule was calculated using the equation m = n(1.0958e−21 g/bp), where n is the size of the plasmid in bp. Plasmids were linearized with NheI restriction enzyme (Roche) for 1 h at 37°C, and plasmid dilutions of 5 × 106, 5 × 105, 5 × 104, 5 × 103, 5 × 102, and 5 × 101 copies/μl were used to generate standard curves for absolute quantification of P2X mRNA expression in the samples of anterior pituitary. The results were analyzed with LightCycler software version 3.5 with the sample CT values calculated using the second derivative maximum algorithm and normalized to the initial amounts of total RNA used in reverse transcription. The linearity of standard curves was expressed as the square of the Pearson correlation coefficient (R2), and the real-time PCR amplification efficiencies were calculated from the slope (S) values estimated by the LightCycler 3.5 software and the equation E = 10(−1/S) − 1 × 100 (%) (38). For all standard curves, the R2 coefficients were above 0.98, indicating a high degree of linearity between the two parameters (31), and the PCR amplification efficiencies (E) calculated from the slope values generated by LightCycler 3.5 analysis software were 92 ± 3%, which indicated good quality of the PCR amplification.
Western blot analysis.
Recombinant P2XRs were used as controls for the expression of these channels in pituitary tissue. For that purpose, we used P2X2, P2X3, P2X4, P2X6, and P2X7 subunits cloned into the expression vector pcDNA3.1 (Invitrogen) containing a V5 epitope at the COOH terminus. Transfection was conducted 24 h after plating the cells. Plasmids were diluted into serum-free Opti-MEM and transfected into GT1 cells using Lipofectamine 2000 reagent (Invitrogen), according to the manufacture's recommendation. After 4.5 h of incubation, the transfection mixture was replaced with normal culture medium, and cells were cultured for an additional 24 h. Cells were washed with cold phosphate-buffered saline (PBS) twice, scraped, resuspended in radioimmunoprecipitation buffer (Boston Bio Products, Worcester, MA) containing a protease inhibitor cocktail (Calbiochem, NJ) on ice for 1 h, and centrifuged at 13,000 rpm for 30 min. Rat pituitary tissue was collected and washed with cold PBS twice to remove blood residue. The tissue was then homogenized in radioimmunoprecipitation buffer with a glass homogenizer supplemented with a protease inhibitor (Calbiochem) and centrifuged at 14,000 rpm at 4°C for 1 h. Cell lysates were supplemented with 2× SDS-PAGE sample buffer, separated on 8–16% SDS-PAGE gels, and transferred onto polyvinylidene difluoride membrane (Invitrogen). The membrane was blocked for 1 h at room temperature with PBS with Tween 20 buffer containing 10 mM sodium phosphate (pH 7.5), 150 mM NaCl, and 0.1% Tween 20 in 5% BSA fraction V and then incubated overnight at 4°C with primary anti-P2X2, -P2X3, -P2X4, -P2X6, and -P2X7 antibodies at a 1:1,000 dilution (Alomone Labs, Jerusalem, Israel). After washing, positive signals of individual blots were visualized by incubating the membrane with peroxidase-conjugated goat anti-rabbit or secondary antibody (1:10,000; KPL), subsequent treatment with SuperSignal West Pico Luminol/Enhanced solution (Pierce), and exposure to X-ray film (Kodak, Rochester, NY).
Data analysis.
Concentration-response data were fitted, using a nonlinear curve-fitting program (Kaleidagraph; Synergy Software, Reading, PA), to a three-parameter logistic equation I/Imax= 1/[1 + (EC50/C)nH, where I is the peak current evoked by ATP concentration C, Imax is the peak current induced by 300 μM ATP, nH = 1.3 is the apparent Hill coefficient, and the adjustable parameter EC50 is the concentration of ATP that produces a half-maximal response. This program was also used for evaluating statistical significances, applying one-way analysis of variance and Tukey's post hoc test or a Student's t-test. The ATP-induced current amplitudes were measured using the Clampfit 8 software (Axon Instruments). The rate of desensitization and the kinetics of current decay evoked by washout of agonists (deactivation) were fitted by a single-exponential function y = A · exp(−t/τ), where A is the amplitude and τ is a time constant. P values of <0.05 were considered significant.
RESULTS
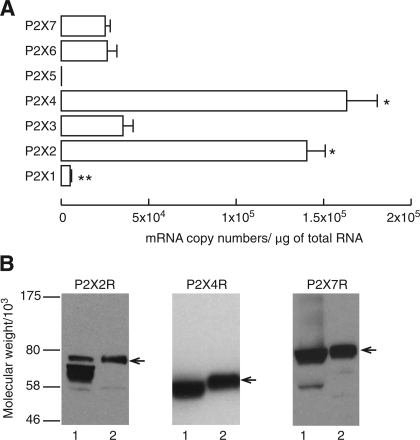
Absolute quantification of P2X mRNA transcripts in anterior pituitary tissue was done by RT-PCR using total RNA extracted from anterior pituitary tissue and primers specific for these receptors. This analysis revealed that, with the exception of P2X5, expression of all P2X mRNAs was detected in anterior pituitary tissue but that the levels of expression were significantly different among them. The number of mRNA transcript copies per 1 μg of total RNA for P2X4 (163 ± 17 × 103) and P2X2 (140 ± 10 × 103) was significantly higher than the number of transcripts for all other members of this family of channels. The expression of P2X3 (35 ± 6 × 103), P2X6 (26 ± 5 × 103), and P2X7 (25 ± 3 × 103) mRNAs was approximately four- to sevenfold lower than that of P2X2 and P2X4 subunit mRNAs, whereas the expression of P2X1 (5 ± 1 × 103) was significantly lower than that of P2X3, P2X6, and P2X7 (Fig. 1A).
Fig. 1.
Expression of P2X receptor channels (P2XRs) in the anterior pituitary gland. A: absolute quantification of P2X mRNA expression in anterior pituitary tissues. Data are means ± SE from 4 independent experiments and are normalized to reflect the number of mRNA copies of P2Xs per 1 μg of total RNA initially used in reverse transcription. For details, see materials and methods. *P < 0.01, significantly higher than P2X1, P2X3, P2X6, and P2X7. **P < 0.01, significantly lower than any other P2X mRNAs. P2X5 was not included in the statistical comparison. B: Western blot analysis of P2XR expression: 1, rat pituitary tissue; 2, recombinant channels expressed in GT1 cells. Arrows indicate the corresponding P2XRs. Note that receptors were tagged with epitopes at the COOH terminus, resulting in larger molecule sizes compared with endogenously expressed P2XRs on SDS-PAGE. Immunoblotting was done with anti-P2X2, -P2X4, and -P2X7 antibodies at a 1:1,000 dilution.
To verify the expression of these receptors at the protein level, we used Western blot analysis of proteins from anterior pituitary tissue and GT1 cells expressing recombinant P2XRs. Figure 1B shows results for P2X2R, P2X4R, and P2X7R expression. The existence of two bands for P2X2Rs in pituitary tissue is consistent with the presence of the full-length receptor, termed P2X2aR, and the splice variant of this receptor, termed P2X2bR, which was dominant. Specific single bands for P2X4R and P2X7R were also identified in pituitary tissue. The antibodies used to recognize P2X3R and P2X6R also recognized other products in both pituitary tissue and GT1 cells, so the presence of these two receptors at protein level could not be confirmed.
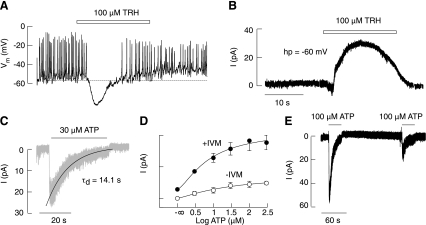
In previous work, the electrophysiological properties of native P2XRs have been characterized in gonadotropin-releasing hormone (GnRH)-responsive anterior pituitary cells (23, 50). In this study, we focused on TRH-responsive subpopulations of anterior pituitary cells. The majority of these cells fired APs spontaneously; some fired single APs, and others exhibited the plateau-bursting type of electrical activity (Figs. 2 and 3). In spontaneously firing cells, application of 100 μM TRH led to a transient hyperpolarization of the cell membrane and the abolition of APs (Fig. 2A), due to activation of Ca2+-controlled K+ channels (Fig. 2B). A large majority of TRH-responsive cells (45 of 49) clamped at −60 mV responded to the application of ATP with an inward current (Fig. 2C). The current exhibited a rapid rise, followed by a gradual decay in amplitude during the sustained agonist application (desensitization) and a rapid decay of current after the washout of the agonist (deactivation). The maximum amplitude of current ranged from 2 to 60 pA; on average, spontaneously firing cells responded to the application of 100 μM ATP with a peak amplitude of ∼15 pA, and the estimated EC50 value for ATP was ∼27 μM (Fig. 2D and Table 2). Receptors desensitized at a mean rate constant of ∼11 s and deactivated at a rate constant of ∼0.3 s (Table 2). Recovery from desensitization was only 33% of the initial peak amplitude after a 3-min washout period (Fig. 2E). These results indicate that depolarizing P2XRs are expressed in TRH-responsive cells and that these receptors desensitize during sustained agonist application.
Fig. 2.
Characterization of ATP-induced inward currents in pituitary cells. A and B: identification of thyrotropin-releasing hormone (TRH)-responsive cells under current-clamped (A) and voltage-clamped conditions (B). C: an example recording of an ATP-induced current in a TRH-responsive cell. Shaded trace indicates the experimental recording, and the solid line illustrates the monoexponential fitting curve for the decay of the current in the presence of ATP (desensitization). D: concentration-dependent effects of ATP on the peak current during the initial agonist application in the presence and absence of ivermectin (IVM). Data are means ± SE. E: partial recovery of the current amplitude in response to washout and repetitive agonist application.
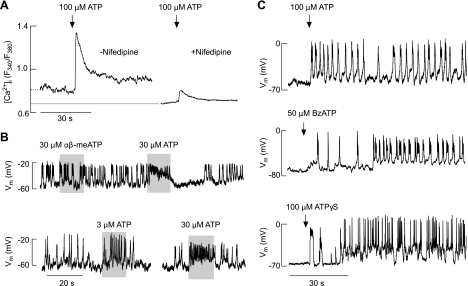
Fig. 3.
Agonist-induced changes in Ca2+ signaling and electrical activity in TRH-responsive cells. A: ATP induced an elevation in intracellular Ca2+ concentration ([Ca2+]i) in cells bathed in medium containing MRS2500, a specific inhibitor of P2Y1Rs. Experiments were done in Ca2+-containing medium in the absence (left trace) and presence (right trace) of nifedipine, a specific blocker of L-type voltage-gated Ca2+ channels during the initial agonist application. Nifedipine (1 μM) was kept for 10 min before ATP application. Traces shown are mean values for several cells in 2 dishes (with and without nifedipine) in 1 of several experiments. Dotted line denotes basal [Ca2+]i in the presence and absence of nifedipine. The averaged basal [Ca2+]i (expressed as the fluorescence intensity ratio F340/F380) was 0.77 ± 0.05 (n = 13) and 0.63 ± 0.04 (n = 12) in the absence and presence of nifedipine, respectively (P < 0.05). ATP-induced peak [Ca2+]i response was 1.29 ± 0.12 (n = 13) and 0.79 ± 0.05 (n = 12) in the absence and presence of nifedipine, respectively (P < 0.05). B: modulation of patterns of electrical activity in spontaneously firing cells. Note the lack of effects of αβ-methylene ATP (αβ-meATP), a specific agonist for P2X1R and P2X3R, in an ATP-responsive cell (top) and dose-dependent effects of ATP on the frequency of firing (bottom). Vm, membrane voltage potential. C: initiation of firing of action potentials in quiescent cells by ATP (top), 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-trisphosphate (BzATP; middle), and adenosine 5′-O-(3-thiophosphate) (ATPγS; bottom).
Table 2.
Characterization of the ATP-induced inward current in TRH-responsive rat pituitary cells
| Treatment | Amplitude, pA | τd, s | τoff, s | EC50, μM | Recovery, % |
|---|---|---|---|---|---|
| Controls | 15 ± 3.4 | 11 ± 2 | 0.31 ± 0.03 | 27 ± 5 | 33 ± 5 |
| +IVM | 64 ± 11* | 28 ± 3* | 20 ± 1.8* | 7.4 ± 1.9* |
Data are means ± SE from 45 (controls) and 26 (with ivermectin, +IVM) recordings in thyrotropin-releasing hormone (TRH)-responsive cells. IVM (3 μM) or control solution was applied for 2 min before agonist application. Cells were stimulated with 100 μM ATP to estimate maximum amplitude. Cells were stimulated with 1, 5, 10, 50, and 100 μM ATP to estimate EC50 values. Recovery was considered the percentage of the amplitude observed during the secondary agonist application after a 3-min washout period. τd, Desensitization time constant; τoff, deactivation time constant.
P < 0.05, control vs. IVM-treated cells.
To study the depolarizing nature of these channels, in further experiments we stimulated cells with ATP in the presence of 10 μM MRS2500, a highly specific antagonist of P2Y1R (16). This G protein-coupled receptor is present in a large fraction of TRH-responsive cells (13), and its inhibition allows P2XR activation alone to be examined. In cells treated with 10 μM MRS2500, ATP-induced a biphasic [Ca2+]i response composed of an early spike response and a sustained plateau response (Fig. 3A, left trace). This was observed in 24 of 26 TRH-responsive cells, confirming the expression of P2XRs in these cells. In cells treated with both MRS2500 and the L-type Cav channel blocker nifedipine, the amplitude of ATP-induced [Ca2+]i responses decreased by 40% (Fig. 3A, right trace), indicating that P2XR activation in these cells leads to facilitation of Ca2+ influx through Cav channels in addition to promoting Ca2+ influx through the pore of the channel. In cells with blocked Cav channels, there was also a decrease in basal [Ca2+]i levels for ∼20%, further indicating the relevance of spontaneous Ca2+ influx through these channels to cellular Ca2+ homeostasis (Fig. 3A, dotted line).
Extracellular ATP also changed the pattern of electrical activity in TRH-responsive cells. In spontaneously firing cells, ATP induced depolarization in a dose-dependent manner and increased the firing frequency of APs from ∼0.3 to 3 Hz (Fig. 3B, bottom). In quiescent cells, short application of ATP initiated APs (Fig. 3C), indicating that P2XRs could play a role as pacemaking channels in these cells. The stimulatory action of ATP on electrical activity was mimicked by 100 μM adenosine 5′-O-(3-thiophosphate) and 50 μM 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-trisphosphate (Fig. 3C), but not by 100 μM ADP (data not shown) or 30 μM αβ-methylene ATP, a specific agonist for P2X1R and P2X3R (Fig. 3B, top).
The pharmacological properties of agonist-induced currents and the rates of receptor desensitization and recovery from desensitization were consistent with the expression of P2X4R and/or P2X2R in these cells (29). To determine which of these two receptor subtypes are expressed in TRH-responsive cells, we used ivermectin (IVM), a specific allosteric modulator of P2X4Rs (17). In cells expressing recombinant rat P2X4R, IVM has three types of effects: 1) it increases the sensitivity of receptors for ATP by ∼8-fold; 2) it augments the peak amplitude of current in response to supramaximal agonist concentration by ∼2-fold; and 3) it slows deactivation of receptors by ∼60-fold (52). Among mammalian P2XRs, the actions of IVM are specific for homomeric and heteromeric P2X4Rs (17).
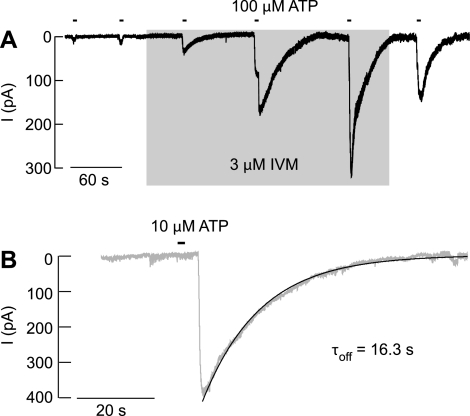
In our experiments, IVM modulated the ATP-induced current in 26 of 27 TRH-responsive cells. Figure 4A shows the progressive increase in the peak amplitude of current in response to repetitive application of 100 μM ATP, Fig. 4B shows slow decay of current after the washout of ATP in the presence of 3 μM IVM, and Fig. 2D shows a leftward shift in the sensitivity of receptors to ATP in the presence of IVM. On average, there was an ∼4-fold increase in the sensitivity of receptors to ATP, an ∼4-fold increase in the maximum peak amplitude of current in response to 100 μM ATP, and an ∼60-fold increase in the time constants of receptor deactivation (Table 2).
Fig. 4.
Effects of IVM, an allosteric modulator of P2X4R, on the ATP-induced current in TRH-responsive cells. A: the time courses of the peak current amplitude augmentation during IVM application and attenuation after IVM washout. Shaded area indicates duration of IVM treatment, and horizontal bars above traces indicate duration of ATP treatments. B: representative recordings of current deactivation induced by washout of ATP in cells bathed in the presence of IVM. Shaded traces illustrate experimental records, and solid lines illustrate the fitted curves. τoff, Deactivation time constants, derived from a monoexponential fitting.
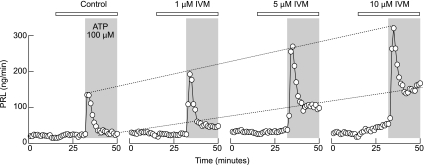
Two major fractions of anterior pituitary cells express functional TRH receptors, lactotrophs and thyrotrophs. To study the contribution of P2X4R in lactotroph functions, we perifused cells with variable IVM concentrations (1–10 μM) or solvent (control) for 30 min and applied 100 μM ATP in the presence of IVM. Under these experimental conditions, there was also a concentration-dependent increase in the amplitudes of the peak and plateau PRL release in cells perifused with 100 μM ATP for 20 min. At the highest (10 μM) concentrations, IVM itself slightly increased basal PRL release (Fig. 5). These experiments indicate that pituitary lactotrophs express functional P2X4R and that their activation elevates [Ca2+]i sufficiently to stimulate hormone secretion.
Fig. 5.
Concentration-dependent effects of IVM on 100 μM ATP-induced prolactin (PRL) release by perifused pituitary cells. Horizontal bars indicate duration of IVM treatment, and shaded areas indicate duration of ATP application. Cells were perifused at rate of 0.8 ml/min, and samples were collected every minute. Notice that the highest concentration of IVM (10 μM) increased basal PRL release in the absence of ATP.
DISCUSSION
Single-cell Ca2+ measurements have suggested that several subtypes of functional P2XR channels are expressed in secretory pituitary cells (8, 24, 30, 43, 46). However, this method is of limited use for the specific identification of receptor subtypes, especially in characterizing the rapidly desensitizing homomeric and heteromeric P2XRs (15). More conclusive evidence for the expression of multiple P2XR subtypes was obtained by qualitative RT-PCR analyses (24). In this study we have shown by quantitative RT-PCR analysis that mRNAs for P2X2 and P2X4 subunits are the most abundantly expressed, and the Western blot analysis confirmed the presence of these channels at protein level in pituitary tissue. The expression of mRNA transcripts for P2X3R, P2X6R, and P2X7R was four- to sevenfold lower than that of P2X2R and P2X4R but was sufficient to generate detectable levels of P2X7R, whereas further analysis is required to clarify the status of P2X3R and P2X6R protein expression.
The presence of two major subtypes of P2XRs in pituitary tissue raised the questions what are the functions of these receptors and in which cell types they are expressed. Earlier electrophysiological studies have shown that the majority of GnRH-responsive cells express functional P2XRs, which could operate as pacemaking channels, leading to facilitation of voltage-gated Ca2+ influx, modulation of InsP3-dependent Ca2+ mobilization, and stimulation of LH release (50). In this study we have shown that ATP generates a depolarizing inward current in at least 90% of TRH-responsive cells, with a capacity to promote Ca2+ influx through the P2XR pore and to initiate AP firing in quiescent cell and increase the firing frequency in spontaneously active cells, leading to stimulation of voltage-sensitive Ca2+ influx and PRL secretion. Thus, in both cell types, endogenously expressed P2XRs have the capacity to initiate and modulate electrical activity and promote Ca2+ influx of sufficient amplitude to trigger the secretory pathway.
In general, the biophysical properties of P2XRs, specifically the rates of receptor desensitization, and the pharmacological properties of these receptors allow for the possibility for distinction of some receptor subtypes expressed in a particular cell type (29). For native P2XR subtypes expressed in GnRH- (50) and TRH-responsive cells (present data), the desensitization rates of receptors were similar and comparable to those observed in cells expressing recombinant P2X2bR (51) and P2X4R (48) and different from that observed in cells expressing recombinant P2X7R (47). Because pituitary cells express splice variants of P2X2R (21, 23), similarity in the rates of receptor desensitization in GnRH- and TRH-responsive cells should not be used as supporting evidence that these cells express the same type of P2XRs.
In contrast to rates of receptor desensitization, P2X2R and P2X4R exhibit different pharmacological profiles when expressed as recombinant channels. The P2X2R but not P2X4R is sensitive to blockade by the conventional antagonists suramin, pyridoxalphosphate-6-azophenyl-2,4-disulfonic acid (PPADS), and reactive blue-2 (29). The most useful distinguishing feature of P2X4R is its potentiation by IVM, not seen in cells expressing P2X2Rs and other subtypes of these receptors (17, 52). Thus the rapid blockade of the ATP-induced inward current and Ca2+ signaling by suramin, PPADS, and reactive blue-2 and the insensitivity of these receptors to IVM treatment are consistent with the expression of P2X2Rs in GnRH-responsive cells (43, 50). On the other hand, in this study we have shown that IVM enhanced the amplitude of the current response and significantly slowed deactivation of P2XRs in TRH-responsive cells, indicating that TRH-responsive cells express the P2X4R subtype. The higher augmentation of the response amplitude of native receptors in the presence of IVM compared with the heterologously expressed recombinant rat P2X4R further raised the possibility that the receptors expressed in TRH-responsive cells are P2X4R heteromers. The ability of high IVM concentrations to slightly increase basal PRL release could also suggest the direct opening of P2X4R channels by this compound, as is observed with other ligand-gated channels (25).
Earlier studies also have revealed that TRH-responsive cells and PRL-secreting GH3 immortalized pituitary cells express the Gq/11-coupled P2Y1Rs, leading to the activation of phospholipase C and generation of InsP3 and diacylglycerol (13), as well as A1Rs that signal through Gi/o heterotrimeric proteins (28, 33). In the hypothalamopituitary system, the expression of P2Y1R, P2X4R, and/or A1R is also observed in other cell types. TRH-insensitive anterior pituitary cells (13), neurons in the ventromedial, lateral (18, 35), and posterior hypothalamic nuclei (36), and astrocytes in the supraoptic nucleus (11) also express P2Y1Rs. The P2X4R is also expressed in GnRH neurons in olfactory placode cultures from rhesus monkeys, where they contribute to the synchronization of [Ca2+]i transients (42), as well as in supraoptic and paraventricular nuclei (37). In the posterior pituitary, A1Rs are expressed in the nerve endings of vasopressinergic neurons together with P2X2Rs and inhibit Cav channels (19).
ATP is released by normal and immortalized anterior pituitary cells at resting conditions, and such release is enhanced in cells treated with ARL67156, an inhibitor of ectonucleotidases (14). Agonist-stimulated gonadotropin release is also accompanied by elevation in basal ATP release, raising the possibility that ATP is stored in the secretory vesicles of these cells (43). This is consistent with an earlier study showing Ca2+ dependence of ATP release (4) and modulation of ATP release by PRL secretagogues (30). However, basal PRL release is not coupled with ATP release (14), suggesting that ATP also could be released by another mechanism. In other tissues, ABC-binding cassette transporters, hemichannels, and P2X7R, among others, have been suggested to participate in nonvesicular ATP release (1). Interestingly, pituitary cells express functional multidrug resistance proteins (2, 26), and in this study we showed the presence of P2X7R, both of which could contribute to ATP release. Furthermore, the presence of functional ectonucleotidases eNTPDase 1–3 in pituitary cells (14) is consistent with the sequential mode of ATP action. In that scenario, endogenously released ATP initially stimulates P2X4R and P2Y1R; its hydrolysis to ADP should shut off P2X4R activity while extending P2Y1R activity. On the other hand, the breakdown of ADP to AMP and adenosine provides a pathway for activation of A1Rs, which silence electrical activity.
In summary, we have shown that mRNA transcripts for all P2XRs other than P2X5R are expressed in anterior pituitary and that, of these, P2X4R mRNA transcripts are the most abundant. We also have shown the presence of P2X4R proteins in pituitary cells and identified native receptors in TRH-responsive cells. The activation of these receptors caused depolarization, which was sufficient to increase the frequency of APs and to initiate firing of APs in quiescent cells, as well as to facilitate Ca2+ influx through Cav channels. Ca2+ influx through the pore of P2X4Rs also contributes to ATP-induced Ca2+ signaling. PRL secretory studies clearly indicate that P2X4Rs are present in lactotrophs, and further studies should clarify whether thyrotrophs also express these channels. We clarified earlier that GnRH-responsive cells express P2X2R, and further work should be focused on identification of receptor subtypes expressed in other secretory cells types as well as in nonsecretory folliculostrellate cells.
GRANTS
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health, the Grant Agency of the Czech Republic (305/07/0681), and Centrum for Neuroscience Grant LC554.
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci 32: 19–29, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology 147: 3435–3445, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Carew MA, Wu ML, Law GJ, Tseng YZ, Mason WT. Extracellular ATP activates calcium entry and mobilization via P2U-purinoceptors in rat lactotrophs. Cell Calcium 16: 227–235, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Chen ZP, Kratzmeier M, Levy A, McArdle CA, Poch A, Day A, Mukhopadhyay AK, Lightman SL. Evidence for a role of pituitary ATP receptors in the regulation of pituitary function. Proc Natl Acad Sci USA 92: 5219–5223, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen ZP, Kratzmeier M, Poch A, Xu S, McArdle CA, Levy A, Mukhopadhyay AK, Lightman SL. Effects of extracellular nucleotides in the pituitary: adenosine triphosphate receptor-mediated intracellular responses in gonadotrope-derived alpha T3–1 cells. Endocrinology 137: 248–256, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology 137: 1833–1840, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Chen ZP, Levy A, McArdle CA, Lightman SL. Pituitary ATP receptors: characterization and functional localization to gonadotropes. Endocrinology 135: 1280–1283, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca2+]i changes and depolarization in GH3 cells. Br J Pharmacol 130: 1843–1852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson JS, Wakefield IK, Sohnius U, van der Merwe PA, Millar RP. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology 126: 80–87, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflügers Arch 452: 552–562, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Espallergues J, Solovieva O, Techer V, Bauer K, Alonso G, Vincent A, Hussy N. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience 148: 712–723, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem 14: 2429–2455, 2007 [DOI] [PubMed] [Google Scholar]
- 13.He ML, Gonzalez-Iglesias AE, Stojilkovic SS. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem 278: 46270–46277, 2003 [DOI] [PubMed] [Google Scholar]
- 14.He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells. Purinergic Signal 1: 135–144, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He ML, Zemkova H, Koshimizu TA, Tomic M, Stojilkovic SS. Intracellular calcium measurements as a method in studies on activity of purinergic P2X receptor channels. Am J Physiol Cell Physiol 285: C467–C479, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther 316: 556–563, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khakh BS, Proctor WR, Dunwiddie TV, Labarca C, Lester HA. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci 19: 7289–7299, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kittner H, Franke H, Harsch JI, El-Ashmawy IM, Seidel B, Krugel U, Illes P. Enhanced food intake after stimulation of hypothalamic P2Y1 receptors in rats: modulation of feeding behaviour by extracellular nucleotides. Eur J Neurosci 24: 2049–2056, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Knott TK, Marrero HG, Fenton RA, Custer EE, Dobson JG JR, Lemos JR. Endogenous adenosine inhibits CNS terminal Ca2+ currents and exocytosis. J Cell Physiol 210: 309–314, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Koshimizu T, Koshimizu M, Stojilkovic SS. Contributions of the C-terminal domain to the control of P2X receptor desensitization. J Biol Chem 274: 37651–37657, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Koshimizu T, Tomic M, Koshimizu M, Stojilkovic SS. Identification of amino acid residues contributing to desensitization of the P2X2 receptor channel. J Biol Chem 273: 12853–12857, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Koshimizu T, Tomic M, Van Goor F, Stojilkovic SS. Functional role of alternative splicing in pituitary P2X2 receptor-channel activation and desensitization. Mol Endocrinol 12: 901–913, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Koshimizu TA, Kretschmannova K, He ML, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. Carboxyl-terminal splicing enhances physical interactions between the cytoplasmic tails of purinergic P2X receptors. Mol Pharmacol 69: 1588–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Koshimizu TA, Tomic M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology 141: 4091–4099, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Krusek J, Zemkova H. Effect of ivermectin on gamma-aminobutyric acid-induced chloride currents in mouse hippocampal embryonic neurones. Eur J Pharmacol 259: 121–128, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Kucka M, Kretschmannova K, Murano T, Wu CP, Zemkova H, Ambudkar SV, Stojilkovic SS. Dependence of multidrug resistance protein-mediated cyclic nucleotide efflux on the background sodium conductance. Mol Pharmacol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumari M, Buckingham JC, Poyser RH, Cover PO. Roles for adenosine A1- and A2-receptors in the control of thyrotrophin and prolactin release from the anterior pituitary gland. Regul Pept 79: 41–46, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Mollard P, Guerineau N, Chiavaroli C, Schlegel W, Cooper DM. Adenosine A1 receptor-induced inhibition of Ca2+ transients linked to action potentials in clonal pituitary cells. Eur J Pharmacol 206: 271–277, 1991 [DOI] [PubMed] [Google Scholar]
- 29.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Nunez L, Villalobos C, Frawley LS. Extracellular ATP as an autocrine/paracrine regulator of prolactin release. Am J Physiol Endocrinol Metab 272: E1117–E1123, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 33.Rees DA, Scanlon MF, Ham J. Adenosine signalling pathways in the pituitary gland: one ligand, multiple receptors. J Endocrinol 177: 357–364, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Rees DA, Scanlon MF, Ham J. Novel insights into how purines regulate pituitary cell function. Clin Sci (Lond) 104: 467–481, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Seidel B, Bigl M, Franke H, Kittner H, Kiess W, Illes P, Krugel U. Expression of purinergic receptors in the hypothalamus of the rat is modified by reduced food availability. Brain Res 1089: 143–152, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Sergeeva OA, Klyuch BP, Fleischer W, Eriksson KS, Korotkova TM, Siebler M, Haas HL. P2Y receptor-mediated excitation in the posterior hypothalamus. Eur J Neurosci 24: 1413–1426, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol 514: 351–367, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stahlberg A, Aman P, Ridell B, Mostad P, Kubista M. Quantitative real-time PCR method for detection of B-lymphocyte monoclonality by comparison of kappa and lambda immunoglobulin light chain expression. Clin Chem 49: 51–59, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Stojilkovic SS, Izumi S, Catt KJ. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem 263: 13054–13061, 1988 [PubMed] [Google Scholar]
- 40.Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab 12: 218–225, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Stojilkovic SS, Zemkova H, Van Goor F. Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab 16: 152–159, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol 19: 2736–2747, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem 271: 21200–21208, 1996 [DOI] [PubMed] [Google Scholar]
- 44.van der Merwe PA, Wakefield IK, Fine J, Millar RP, Davidson JS. Extracellular adenosine triphosphate activates phospholipase C and mobilizes intracellular calcium in primary cultures of sheep anterior pituitary cells. FEBS Lett 243: 333–336, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Vanecek J, Klein DC. A subpopulation of neonatal gonadotropin-releasing hormone-sensitive pituitary cells is responsive to melatonin. Endocrinology 133: 360–367, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Villalobos C, Alonso-Torre SR, Nunez L, Garcia-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am J Physiol Cell Physiol 273: C1963–C1971, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol 132: 563–573, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z, Liang Z, Obsil T, Stojilkovic SS. Participation of the Lys313-Ile333 sequence of the purinergic P2X4 receptor in agonist binding and transduction of signals to the channel gate. J Biol Chem 281: 32649–32659, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Yu Y, Lee C, Kim J, Hwang S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89: 670–679, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol 20: 1423–1436, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Zemkova H, He ML, Koshimizu TA, Stojilkovic SS. Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J Neurosci 24: 6968–6978, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zemkova H, Yan Z, Liang Z, Jelinkova I, Tomic M, Stojilkovic SS. Role of aromatic and charged ectodomain residues in the P2X4 receptor functions. J Neurochem 102: 1139–1150, 2007 [DOI] [PubMed] [Google Scholar]