Abstract
Chronic inflammation is an important etiology underlying obesity-related disorders such as insulin resistance and type 2 diabetes, and recent findings indicate that the macrophage can be the initiating cell type responsible for this chronic inflammatory state. The mammalian silent information regulator 2 homolog SIRT1 modulates several physiological processes important for life span, and a potential role of SIRT1 in the regulation of insulin sensitivity has been shown. However, with respect to inflammation, the role of SIRT1 in regulating the proinflammatory pathway within macrophages is poorly understood. Here, we show that knockdown of SIRT1 in the mouse macrophage RAW264.7 cell line and in intraperitoneal macrophages broadly activates the JNK and IKK inflammatory pathways and increases LPS-stimulated TNFα secretion. Moreover, gene expression profiles reveal that SIRT1 knockdown leads to an increase in inflammatory gene expression. We also demonstrate that SIRT1 activators inhibit LPS-stimulated inflammatory pathways, as well as secretion of TNFα, in a SIRT1-dependent manner in RAW264.7 cells and in primary intraperitoneal macrophages. Treatment of Zucker fatty rats with a SIRT1 activator leads to greatly improved glucose tolerance, reduced hyperinsulinemia, and enhanced systemic insulin sensitivity during glucose clamp studies. These in vivo insulin-sensitizing effects were accompanied by a reduction in tissue inflammation markers and a decrease in the adipose tissue macrophage proinflammatory state, fully consistent with the in vitro effects of SIRT1 in macrophages. In conclusion, these results define a novel role for SIRT1 as an important regulator of macrophage inflammatory responses in the context of insulin resistance and raise the possibility that targeting of SIRT1 might be a useful strategy for treating the inflammatory component of metabolic diseases.
Keywords: macrophage, insulin resistance
for many years, it has been known that caloric restriction extends life span over a wide range of species, including mammals (27). Silent information regulator 2 (Sir2) is a NAD-dependent deacetylase that is one of the components connecting the metabolic effects of caloric restriction to longevity in yeast, worms, and flies (7). Mammals express 7 homologs of yeast Sir2, identified as the SIRTUIN family, SIRT1–7 (7). SIRT1 has the closest homology to Sir2, and recent data suggest that activation of SIRT1 may be, at least partially, responsible for the extension of life span in mammals (4, 5, 7).
It is now well recognized that in obesity-related diseases, chronic low-grade tissue inflammation is an important etiologic component of insulin resistance, metabolic syndrome, and type 2 diabetes (1, 3, 9, 10, 25, 37). More recent studies (25, 31, 32) have shown that the macrophage plays a central role in orchestrating and initiating obesity-related tissue inflammatory responses. Thus adipose tissue macrophages (ATMs) accumulate in obese adipose tissue where they can elaborate local inflammatory mediators (cytokines) that work in a paracrine fashion to cause the development of tissue insulin resistance. Knockout of monocyte chemotaxic protein-1 (MCP-1), or its receptor CCR2 in mice, impairs macrophage migration and inflammation and improves insulin sensitivity (12, 30), whereas transgenic overexpression of MCP-1 in adipocytes increases infiltration of macrophages and heightens inflammation, producing insulin resistance (11, 12). Other studies have shown that tissue-specific disruption of the macrophage inflammatory pathway by macrophage-specific knockout of IκB kinase (IKK; Refs. 1, 37), c-Jun N-terminal kinase (JNK; Ref. 28), or Cbl-associated protein (CAP; Ref. 14) protects mice from high-fat diet (HFD) or obesity-induced inflammation. Importantly, even though these knockout mice are protected from insulin resistance, they still become fully obese on HFD. Moreover, macrophage peroxisome proliferator activated receptor-γ (PPARγ) normally suppresses inflammatory pathways, and macrophage-specific knockout of PPARγ leads to increased markers of inflammatory responses and insulin resistance (8, 20). Taken together, these findings demonstrate a strong relationship between macrophage inflammatory responses and systemic insulin resistance. Since activation of SIRT1 can lead to improved glucose tolerance and insulin sensitivity (2, 13, 17) and since one of the deacetylated targets of SIRT1 is NF-κB (33), we hypothesized that macrophage SIRT1 could play a role in regulating macrophage inflammatory responses with subsequent effects on insulin sensitivity in insulin target cells.
In the current study, we examined the in vitro effects of SIRT1 in RAW264.7 macrophage/monocytic cells and primary intraperitoneal mouse macrophages. We demonstrate that SIRT1 represses inflammatory pathway activity, gene expression, and release of TNFα from LPS-stimulated macrophages and that pharmacological SIRT1 activators exert broad anti-inflammatory effects. This was recapitulated in vivo, since treatment of obese, insulin-resistant Zucker fatty (ZF) rats led to improved glucose tolerance, enhanced systemic insulin sensitivity, and normalization of tissue markers of inflammation.
MATERIALS AND METHODS
Materials.
Anti-phospho-JNK (Thr183/Tyr185), anti-JNK, anti-phospho-c-JUN (Ser73), anti-c-JUN, anti-phospho-IKKα (Ser180)/IKKβ (Ser181), anti-IKKβ, anti-IκB-α, anti-phospho-NF-κB p65 (Ser536), anti-NF-κB p65, anti-phospho-Akt (Ser 473), and anti-CD11c antibodies were from Cell Signaling (Beverly, MA). Anti-SIRT1, anti-Akt1/2, anti-TLR4, anti-MyD88, anti-TRAF6, anti-TAK1, and anti-β-tubulin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-acetyl-NF-κB antibody was from Abcam (Cambridge, MA). Horseradish peroxidase-linked anti-rabbit, anti-mouse, and anti-goat antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Ultra pure LPS was from InvivoGen (San Diego, CA). DMEM and FCS were obtained from Life Technologies (Grand Island, NY). Low-endotoxin FBS was purchased from Hyclone (Logan, UT). 2-deoxy-[3H]glucose or l-[3H]glucose was from ICN (Costa Mesa, CA). All other reagents and chemicals were purchased from Sigma Chemical (St. Louis, MO).
RAW264.7 and 3T3-L1 culture.
Mouse monocyte/macrophage RAW264.7 cells were obtained from ATCC (American Type Culture Collection, Rockville, MD) and cultured in DMEM supplemented with 10% low-endotoxin FBS. In pretreated cells, the indicated concentration of SRT1720 or resveratrol was added 1 h before the LPS treatment. RAW264.7 cells were then incubated for various periods as indicated in Results (see Fig. 4). 3T3-L1 cells were cultured and differentiated as described previously (34). Experiments in this study utilized mature 3T3-L1 adipocytes cultured for 7 days after completion of the differentiation process as described previously (35).
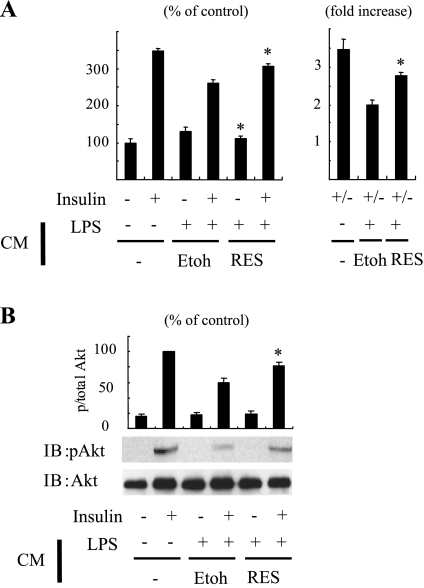
Fig. 4.
Macrophage SIRT1 affects adipocyte insulin sensitivity. A, B: 3T3-L1 adipocytes were incubated with CM, control CM from vehicle (Etoh), or CM from resveratrol-treated RAW cells, diluted 1:250 in DMEM, for 3 h before assays. Cells were stimulated with insulin and then measured 2-deoxyglucose uptake (A) or Akt phosphorylation (B). Graphs show means ± SE. Values are expressed as %control or fold basal (un-stimulated) (n = 4). *P < 0.01, vehicle (Etoh) vs. resveratrol.
Primary macrophage cells culture.
Peritoneal macrophages were isolated from C57BL/6 background mice by peritoneal lavage 4 days after injection of 3 ml of 3% thioglycolate (Difco; BD Diagnostics, Sparks, MD) and plated in 24-well plates at 2 × 105 cells/well.
RNA interference.
The duplexes of small interfering RNA (siRNA), targeting SIRT1 mRNA (target sequence was described previously; Ref. 36), and a negative control (scrambled sequence) were purchased from Dharmacon Research (Lafayette, CO). We electroporated 2 × 107 RAW264.7 cells with 2 nmol of siRNA oligonucleotides to mouse either SIRT1 or scrambled control siRNA, using the XCell Gene Pulser (Bio-Rad, Hercules, CA). Electroporated cells were plated into 12-well tissue culture plates and were incubated for 29 h at 37°C before assays.
RNA isolation and RT-PCR.
Total RNA was isolated and purified from epididymal fat pad or treated cells using TRIzol according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). First-strand cDNA synthesis, quantitative real-time-PCR, and the ArrayPlate assay were performed as previous described (8, 16). The oligonucleotide primers or target sequence used is available upon request.
Western blotting.
Western blotting with indicated antibodies was performed on tissue and cells, as described previously (36).
ELISA.
Conditioned medium (CM) was assayed for mouse TNFα using ELISA kits (Biosource, Camarillo, CA) following the manufacturer's protocol.
Chromatin immunoprecipitated assay.
The chromatin immunoprecipitated (ChIP) assay was performed using the ChIP assay kit from Upstate Biotechnology, as described previously (36). Briefly, RAW 264.7 macrophage were cross-linked, scraped, pelleted, and resuspended. Anti-NF-κB antibody was added to the sonicated chromatin solution and incubated. The chromatin complexes were eluted. After reversal of the cross-linking, the DNA was purified, and input control or ChIP samples were used as a template for PCR using the primer sets for regions containing NF-κB binding sites.
Preparation of CM.
RAW264.7 cells electroporated with control or SIRT1 siRNA were cultured. The cells were treated with or without 50 μM resveratrol for 1 h, followed by the addition of 100 ng/ml LPS or 200 μM palmitate. After 3 h (for LPS) or 6 h (for palmitate), the medium was collected. The control medium and the collected medium were centrifuged. To avoid the effect of medium changes and pH changes, the supernatant was concentrated by Centricon (Millipore Billerica, MA) and was then sterilized.
2-Deoxyglucose uptake.
The procedure for evaluating glucose transport was performed as previously described (35). Fully differentiated 3T3-L1 adipocytes were incubated with control CM or CM obtained from RAW264.7 cells (diluted 1:250 in DMEM with 10% FBS. The ratio 1:250 was the back to the original volume.). Cells were glucose starved for 1 h in HEPES-salt buffer. Cells were stimulated with insulin (100 ng/ml) for 25 min followed by measurement of 2-deoxyglucose uptake. Glucose uptake was assayed in triplicate wells for each condition in four independent experiments.
Preparation of SRT1720 and SRT2379.
The compounds SRT1720 (17) and SRT2379 were provided by Sirtris.
Animals.
Six-week-old, male fatty (fa/fa) Zucker (ZF) rats (Harlan Sprague Dawley) were housed. At 7 wk of age, the animals were randomly assigned to receive either the SIRT1 agonist (SRT2379) at 100 mg per kg of body weight per day or vehicle. Animals were weighed daily, the drug or vehicle was administered by oral gavage on a daily basis for 4 wk, and animals had ad libitum access to food and water. During the second week of drug treatment, we monitored food intake for 3 consecutive days. After 4 wk treatment, rats were anesthetized after an overnight fast, and the epididymal fat pad was removed, rinsed in saline, weighed, and frozen for later analysis. All experimental procedures were approved by the Animal Subjects Committee at the University of Calirfornia, San Francisco, according to NIH guidelines.
Oral glucose tolerance test.
On day 22 of treatment, after a short fast (5 h), rats were orally gavaged (1 g/kg body wt) with dextrose (Hospira). Blood glucose was measured at 0, 15, 30, 60, and 90 min. A blood sample was also taken at 0, 15, 30, and 60 min. This sample was centrifuged, and the plasma was stored for analysis of plasma insulin concentration.
Hyperinsulinemic euglycemic clamp.
Hyperinsulinemic euglycemic clamp was described previously (17). Briefly, 5 days before conducting clamp experiments, animals were cannulated. The evening before the hyperinsulinemic euglycemic clamp animals were fasted overnight for 8 h. At time 0, glucose (variable infusion, 50% dextrose; Hospira) and insulin (25 mU·kg−1·min−1, Humulin R; Eli Lilly) infusions were started simultaneously. Small blood samples (5 μl) were drawn from the carotid artery at 10-min intervals and immediately analyzed for glucose (OneTouch Ultra; LifeScan). The glucose infusion was adjusted to maintain blood glucose at 100 mg/dl. The clamp was completed after blood glucose was at steady state for 30 min (∼120 min).
Histological analysis.
Macrophages in epididymal fat pads were visualized by MAC-2 immunostaining and quantified as described previously (21). The size of adipocyte area was measured using ImageJ software (NIH freeware).
Statistics.
Values are expressed as means ± SE. Statistical significance between two groups was determined by the Student's t-test. Comparisons among several groups were performed by ANOVA, and when the results passed the ANOVA test, we performed Bonferroni's multiple comparison posttests to calculate the relevant P values.
Results
SIRT1 activators inhibit LPS-stimulated inflammatory pathways.
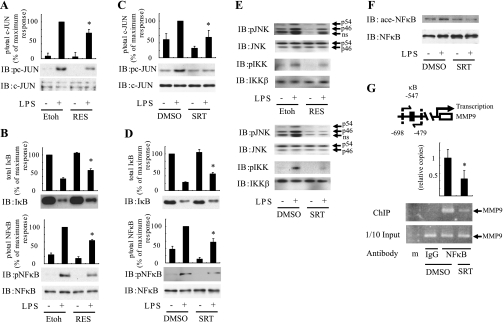
Resveratrol is a natural polyphenolic compound that works, in part, by increasing SIRT1 activity through an allosteric interaction (27). We stimulated the macrophage cell line RAW264.7 with the inflammatory pathway activator LPS in the presence or absence of resveratrol. Pretreatment with resveratrol clearly inhibited LPS-stimulated c-JUN phosphorylation without changing the total level of c-JUN protein (Fig. 1A). Resveratrol treatment also led to decreased LPS-stimulated IκB degradation and NF-κB phosphorylation (Fig. 1B). Since resveratrol is not completely specific for SIRT1, to exclude possible nonspecific effects, we used SRT1720, which is a more specific high affinity activator of the enzyme (17). Consistent with the effects of resveratrol, pretreatment with SRT1720 strongly inhibited LPS-stimulated c-JUN and IκB/NF-κB inflammatory pathways in RAW cells (Fig. 1, C and D) and in primary immunoprecipitated macrophages harvested from C57BL/6 mice (Fig. 1E). We also assessed the acetylation state of NF-κB, a known target of SIRT1 (33). Western blots with anti-acetyl-NF-κB antibody showed that SRT1720 treatment led to decreased NF-κB acetylation (Fig. 1F). To evaluate the functional significance of this change in NF-κB acetylation, we performed ChIP assays. As seen in Fig. 1G, SIRT1 activation by SRT1720 reduced the occupancy of NF-κB on the MMP9 promoter, a known NF-κB target gene. Together, these data show that SIRT1 activity broadly inhibits inflammatory pathway activation in macrophages.
Fig. 1.
SIRT1 activators inhibit LPS-stimulated inflammatory pathways. After pretreatment of 50 μM resveratrol (RES; A, B, and E) or 1 μM SRT1720 (C–G) for 1 h, RAW264.7 cells (A–D, F and G) or peritoneal macrophage (E) were stimulated with (+) or without (−) 100 ng/ml LPS for 10 min (A–E) or 1 h (F and G) and lysed, and immunoblotting (IB) was performed with indicated antibodies. Scanned bar graphs show means ± SE, and values are expressed as %maximum compared with those observed in LPS-stimulated control cells (n = 3). G, top: schematic diagram of the mouse MMP9 promoter region. The positions of κB sites are indicated and pairs of arrows indicate the PCR-amplified regions. Soluble chromatin was immunoprecipitated with anti-NF-κB antibody (NF-κB) or control IgG (IgG). Enrichment of κB containing DNA sequences in the immunoprecipitated DNA pool was visualized by PCR. G, bottom: PCR-amplified MMP9 promoter band from input control. Graph shows mean ± SE from 3 independent experiments, and values are expressed as fold amount of immunoprecipitated promoter DNA copy numbers relative to their corresponding input controls, compared with those observed DMSO-treated cells. *P < 0.05, Etoh or DMSO vs. resveratrol or SRT1720. Etoh or DMSO, vehicle ethanol or DMSO control; p/total, phosphorylation/total; ns, nonspecific; ChIP, chromatin immunoprecipitation; ace, acetylation; m, marker.
SIRT1 knockdown increases inflammatory responses.
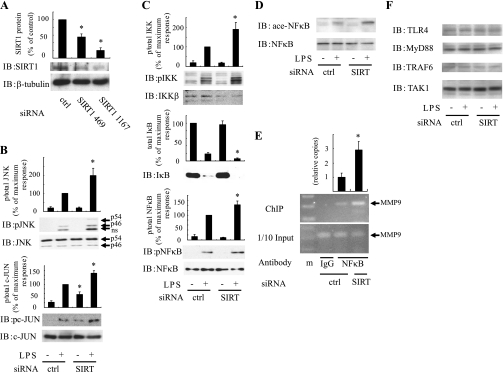
To assess the specificity of SIRT1 mediated anti-inflammatory responses, we utilized siRNAs against SIRT1. Thus RAW264.7 cells were electroporated with SIRT1 siRNA, which led to an ∼80% depletion of SIRT1 protein 48 h later (Fig. 2A). In contrast to SIRT1 activator treatment, SIRT1 knockdown significantly increased LPS-stimulated phosphorylation of JNK and c-JUN (Fig. 2B). LPS-stimulated IKK phosphorylation was also enhanced by SIRT1 knockdown (Fig. 2C), and this was accompanied by greater IκB degradation and enhanced NF-κB activation (as assessed by NF-κB phosphorylation, acetylation, and association with the MMP9 promoter; Fig. 2, C, D, and E). A second independent siRNA against SIRT1 also led to increased phosphorylation of JNK and IKK, showing the absence of off-target effects (data not shown). LPS stimulates inflammation by activating the toll-like receptor 4 (TLR4) pathway. Therefore, we measured expression of TLR4 and several of its downstream effectors and found that SIRT1 knockdown had no effect on protein levels of TLR4, myeloid differentiation marker 88 (MyD88), TNF receptor-associated factor 6 (TRAF6), and TGF-β-activated kinase 1 (TAK1; Fig. 2F).
Fig. 2.
SIRT1 knockdown increases inflammatory responses. A: RAW264.7 macrophages were electroporated with control (ctrl) or SIRT1 (SIRT1) siRNA. Forty-eight hours after electroporation, cell lysates were immunoblotted with indicated antibodies. B–F: cells were electroporated with control or SIRT1 (SIRT) siRNA. Forty-eight hours after electroporation, cells were stimulated with or without 100 ng/ml LPS for 10 min (B, C, and F) or 1 h (D and E), and Western blotting was performed with indicated antibodies. Data are percentages compared with ctrl siRNA electroporated cells and are means ± SE (n = 3). E, top: soluble chromatin was immunoprecipitated with anti-NFκB antibody or control IgG . Enrichment of κB containing DNA sequences in the immunoprecipitated DNA pool was visualized by PCR. E, bottom: PCR-amplified MMP9 promoter band from input control. Graph shows mean ± SE from 3 independent experiments, and values are expressed as fold amount of immunoprecipitated promoter DNA copy numbers relative to their corresponding input controls, compared with those observed DMSO-treated cells. *P < 0.05, control siRNA vs. SIRT1 siRNA.
SIRT1 modulates inflammatory gene expression.
To further define the role of SIRT1 in the regulation of inflammation, we quantified the transcript levels of several inflammatory pathway genes in RAW264.7 cells. SIRT1 knockdown led to an increase in the basal expression levels of TNFα, MCP-1, and KC (Supplemental Fig. 1, inset; supplemental data for this article are available online at the Am J Physiol Endocrinol Metab website). In LPS-stimulated cells, SIRT1 knockdown resulted in enhanced expression of TNFα, IL-1β, MMP9, MCP-1, KC, and IL-6 (Supplemental Fig. 1), consistent with the concept that SIRT1 can broadly suppress inflammatory gene expression.
SIRT1 is the target for the anti-inflammatory effects of SRT1720 and resveratrol.
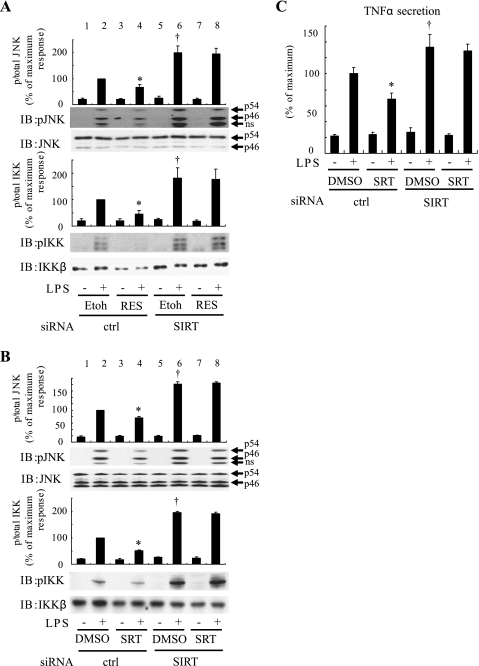
To assess the specificity of the anti-inflammatory effects of resveratrol and SRT1720, we examined the effects of these compounds in SIRT1 knockdown cells. Resveratrol and SRT1720 treatment clearly inhibited LPS-stimulated JNK and IKK phosphorylation in cells electroporated with control siRNA (Fig. 3, A and B, lane 2 vs. 4), while SIRT1 knockdown increased LPS-stimulated phosphorylation of JNK and IKK (Fig. 3, A and B, lane 2 vs. 6). Importantly, in the SIRT1 knockdown cells, pretreatment with resveratrol or SRT1720 was without effect (Fig. 3, A and B, lane 6 vs. 8), indicating that these SIRT1 activators inhibit LPS-stimulated inflammatory responses via effects on SIRT1.
Fig. 3.
SIRT1 is the target for the resveratrol and SRT1720 anti-inflammatory effects. After pretreatment of 50 μM resveratrol (A) or 1 μM SRT1720 (B) for 1 h, RAW264.7 cells electroporated with control or SIRT1 siRNA were stimulated with or without 100 ng/ml LPS for 10 min and then lysed, and immunoblotting was performed with indicated antibodies. Scanned bar graphs show means ± SE, and values are expressed as %maximum in phsphorylation compared with those observed in LPS-stimulated control cells (n = 3). C: RAW264.7 cells, electroporated with control or SIRT1 siRNA, were stimulated with or without 100 ng/ml LPS for 1 h, and conditioned medium (CM) was harvested for ELISA analysis. Secreted TNFα was assayed for mouse TNFα using ELISA assay. Data are presented as percentages of secretion compared with LPS-stimulated control siRNA electroporated cells and represent means ± SE (n = 4). *P < 0.05, Etoh vs. RES or DMSO vs. SRT1720. †P < 0.05, control siRNA vs. SIRT1 siRNA.
Effect of SIRT1 on TNFα secretion.
Since TNFα is a major macrophage-derived proinflammatory cytokine (29), we assessed the role of SIRT1 on TNFα secretion. As seen in Fig. 3C, LPS stimulation led to a large increase in TNFα secretion into the medium, which was inhibited by SRT1720. In SIRT1 knockdown cells, LPS-stimulated TNFα secretion was enhanced, and SRT1720 was now without effect (Fig. 3C).
Macrophage SIRT1 affects adipocyte insulin sensitivity.
To evaluate the role of SIRT1 in the paracrine effects of macrophages to cause insulin resistance in insulin target cells, we measured the effect of CM from RAW264.7 cells on glucose uptake in 3T3-L1 adipocytes. RAW264.7 cells were stimulated with LPS for 3 h, and CM was harvested, filtered to remove cells, concentrated, and then added to fully differentiated 3T3-L1 adipocytes for 18 h (Fig. 4). Exposure to CM from LPS-stimulated cells significantly decreased insulin-stimulated glucose transport by ∼50%, and this was attenuated by pretreating the macrophages with resveratrol (Fig. 4A). Consistent with these changes, CM from LPS-stimulated macrophages caused a reduction in insulin-stimulated Akt phosphorylation in 3T3-L1 adipocytes, which was attenuated by pretreatment of the macrophages with resveratrol (Fig. 4B). Since exogenous administration of fatty acids induces inflammatory responses in macrophages, we also assessed the effect of CM from RAW264.7 cells treated with palmitate on glucose uptake in adipocytes. CM from palmitate-stimulated cells increased basal glucose uptake, as previously reported (24) but also reduced the insulin-stimulated effect, and this was attenuated by resveratrol pretreatment of the macrophages (Supplemental Fig. 2). Control CM containing LPS, as well as unstimulated RAW264.7 CM, was without effect on glucose uptake (data not shown). Taken together, these data suggest that TLR4 ligand stimulation of macrophages results in increased production of macrophage-secreted factors, which can cause insulin resistance through a paracrine mechanism, and that this effect can be inhibited by SIRT1 activation.
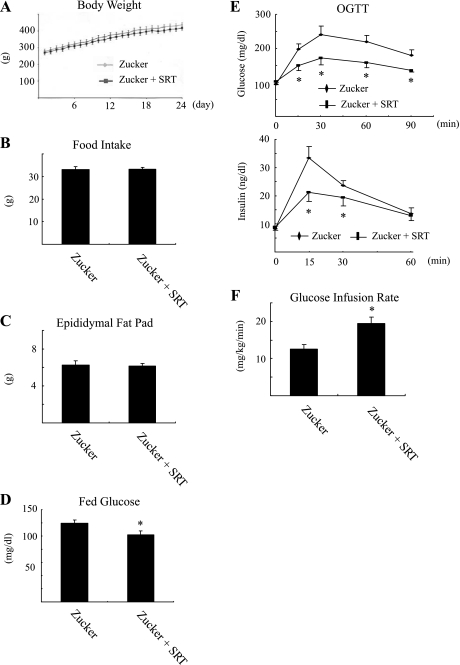
SIRT1 activator improves glucose intolerance and insulin sensitivity in obese ZF rats.
To assess the in vivo effects of SIRT1 activation on insulin sensitivity and tissue inflammation, ZF rats were treated with the SIRT1 activator SRT2379 for 4 wk. As seen in Fig. 5, A-C, body weight, food intake, and epididymal fat pad weight were the same in vehicle and SRT2379-treated animals. Although obesity was comparable between the groups, fed glucose concentration (Fig. 5D) as well as the glucose and insulin (Fig. 5E) responses during oral glucose tolerance tests were significantly improved in SRT2379-treated rats. Consistent with improved glucose tolerance, the glucose infusion rate required to maintain euglycemia during a hyperinsulinemic euglycemic clamp was significantly higher (154%) in SRT2379- vs. vehicle-treated rats (Fig. 5F). Insulin and glucose concentrations during the clamp studies were the same in both groups (data not shown). Taken together, these results demonstrate that treatment of obese rats with a SIRT1 agonist enhances whole body glucose homeostasis and insulin sensitivity.
Fig. 5.
SIRT1 activator improves glucose intolerance and insulin sensitivity in obese Zucker rat. Body weight (A), average daily food intake (B), epididymal fat mass (C), and plasma glucose on ad libitum feeding (D) were measured after Zucker fa/fa rats were placed on normal chow diet (Zucker) or normal chow diet supplemented with SRT2379 at 100 mg per kg of body weight per day (Zucker + SRT) for 24 days (B–D) or indicated time periods (A; n = 6). E: glucose and insulin responses during an oral glucose tolerance test (OGTT) are shown after normal chow diet or normal chow diet with SRT2379 for 24 days (n = 6). G: Insulin sensitivity evaluated through the average glucose infusion rate at equilibrium in a hyperinsulinemic euglycemic clamp (25 mU insulin·kg·−1min−1) in Zucker fa/fa rat treated with or without SRT2379 for 4 wk (n = 4). *P < 0.05, Zucker vs. Zucker + SRT.
SIRT1 treatment leads to reduced CD11c expression in ZF rat epididymal fat.
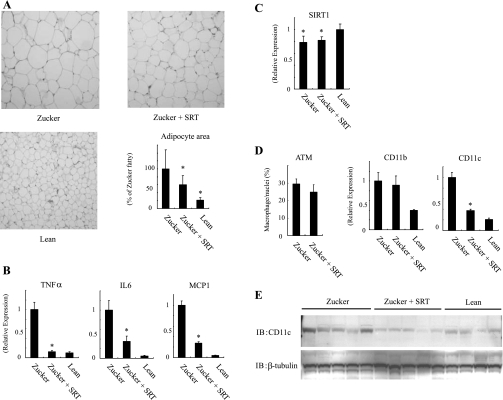
To assess the mechanisms of the insulin-sensitizing effects of SRT2379 treatment, we measured adipocyte size by histological analysis. The average adipocyte size in epididymal fat pads was reduced by SRT2379 treatment (Fig. 6A). We also measured adipose tissue mRNA expression for three proinflammatory cytokines, TNFα, IL-6, and MCP-1. All three were significantly increased in epididymal adipose tissue from ZF rats, and treatment with the SIRT1 activator caused a marked reduction toward levels seen in control rats (Fig. 6B). Similar to earlier findings in HFD mice (36), we also found decreased SIRT1 expression in adipose tissue from ZF rats, which was unchanged by SRT2379 treatment (Fig. 6C). HFD leads to a shift in the activation state of the ATMs from alternatively activated macrophages to classically activated macrophages. Thus we (19) and others (15) have shown that obesity leads to a marked increase in a highly proinflammatory subpopulation of ATMs that display F4/80, CD11b, and CD11c and release cytokines, which can cause cellular insulin resistance. Therefore, we assessed ATMs by MAC-2 immunostaining and also measured CD11c and CD11b expression. Figure 6D shows a striking reduction in adipose tissue CD11c expression in SRT2379-treated ZF rats, while total ATM number and CD11b expression were similar in control and treated animals, indicating that CD11c expression decreases with no change in total ATM content. Consistent with the mRNA expression data, CD11c protein levels were also decreased in the epididymal fat from the SRT2379-treated group (Fig. 6E). Since tissue inflammatory markers were decreased with SRT treatment (Fig. 6B), this suggests that SRT led to a switch in macrophage ATM phenotype to a noninflammatory, CD11c-negative state. To confirm the in vivo data in a direct in vitro system, RAW264.7 cells were treated with SRT1720. This led to decreased CD11c expression (Supplemental Fig. 3A). In contrast to this, SIRT1 knockdown led to increased CD11c expression, and in SIRT1 knockdown cells, SRT1720 was without effect (Supplemental Fig. 3B). Ly6c is another marker of the activated macrophage, and SRT1720 treatment also led to decreased Ly6c expression (Supplemental Fig. 3C).
Fig. 6.
SIRT1 activation leads to a reduction of CD11c expression in epididymal fat. Zucker fa/fa rats were placed on normal chow diet with vehicle (Zucker) or SRT2379 (Zucker + SRT), epididymal fat pad were isolated as described in Materials and Methods. A: representative histologic picture (hematoxylin and eosin staining) of the adipose tissue. Data are presented as percentages of mean area size of adipocyte compared with tissue from Zucker fa/fa rats and represent means ± SE. B–D: total RNA was purified, and then quantitative real-time-PCR was performed. After the mRNA expression differences were normalized to a standard housekeeping gene (GAPDH) mRNA level, data are presented as the relative expression. Error bars represent means ± SE from 4 independent experiments. Adipose tissue macrophage content was measured as described in Materials and Methods. Data are presented as percentages of macrophage per total nuclei and represent means ± SE. E: imunoblotting was performed with indicated antibodies. *P < 0.05, Zucker vs. Zucker + SRT. C: lean vs. Zucker or Zucker + SRT.
DISCUSSION
Insulin resistance is a major pathophysiologic abnormality leading to the metabolic derangements in obesity and type 2 diabetes (18). Chronic inflammation in insulin target tissues is a key etiologic component causing decreased insulin sensitivity, particularly in obesity (1, 3, 9, 10, 37). Substantial evidence has accrued showing that the tissue macrophage is an orchestrating cell type in the initiation and propagation of the inflammatory state, which, in turn, can cause insulin resistance (31, 32). Numerous studies have shown that caloric restriction leads to life span extension in a variety of species (27), and caloric restriction is also a major lifestyle modification used for the treatment of obesity and type 2 diabetes. SIRT1 is a protein deacetylase that is activated by caloric restriction (23), and an important component of the metabolic benefit of caloric restriction is mediated through increased activity of SIRT1 (5). Based on these different lines of evidence, we hypothesized that SIRT1 might play an important role in modulating inflammatory responses in macrophages. Here we report that siRNA-mediated depletion of macrophage SIRT1 broadly activates multiple inflammatory response pathways, whereas treatment of cells with the SIRT1 activators resveratrol or SRT1720 suppresses macrophage inflammatory programs. In addition, activation of SIRT1 in vivo in obese insulin-resistant ZF rats led to improved glucose tolerance, enhanced insulin sensitivity, and decreased adipose tissue inflammatory responses. As such, these studies point to an important role of SIRT1 as a regulator of macrophage inflammatory pathways and show that this effect can be harnessed to produce increased insulin sensitivity.
With respect to the connection between metabolism and SIRT1, most studies (6, 22, 23) have focused on the direct effects of SIRT1 within insulin target tissues. However, based on the established role of macrophages and inflammation in causing insulin resistance, the role of SIRT1 in regulating inflammatory pathways within macrophages is of importance. In this regard, we have found that depletion of macrophage SIRT1 by RNA interference led to broad activation of stress and inflammatory pathway signaling cascades, including increased expression of proinflammatory genes and augmented secretion of cytokines and chemokines. Treatment of cells with resveratrol and the more specific SIRT1 activator SRT1720 (17) inhibited these inflammatory pathways. This latter finding is consistent with previous studies (33) showing that resveratrol can inhibit NF-κB activation, as well as cigarette smoke-induced proinflammatory mediator release in monomac cells. However, resveratrol is a rather nonspecific activator and has several other molecular targets, including AMP kinase (2, 38). Thus studies of resveratrol alone do not clearly demonstrate the involvement of SIRT1. In the current studies, we utilized siRNA knockdown to directly demonstrate the role of SIRT1 as a negative regulator of inflammation and also showed that when SIRT1 is depleted, resveratrol and SRT1720 no longer have anti-inflammatory effects. Taken together with a recent study (26) showing that SIRT1 can regulate LPS- or ethanol metabolite-induced TNFα secretion in cultured macrophages, we believe these data demonstrate a role of SIRT1 as an anti-inflammatory regulatory component within macrophages.
We also used the SIRT activator SRT2379 to assess the potential effects of SIRT1 stimulation in vivo. We found that SRT2379 treatment led to a marked improvement in glucose tolerance, reduced insulinemia, and enhanced insulin sensitivity, with no effect on epididymal fat pad mass or SIRT1 expression, food intake, or body weight in the obese ZF rat model. These beneficial metabolic effects were accompanied by decreased tissue inflammatory responses, as demonstrated by reduced cytokine expression in adipose tissue. We and others have previously shown that the increased ATM content in obesity is characterized by an expanded population of proinflammatory macrophages that are positive for the cell surface markers F4/80, CD11b, and CD11c (15, 19). In contrast, ATMs, which do not displace CD11c, are not proinflammatory and can be characterized as alternatively activated, M2-like macrophages. We found that both CD11b expression and CD11c mRNA expression was markedly increased in ZF rats compared with lean controls, confirming the increased ATM content in these obese insulin-resistant animals. Interestingly, SRT2379 treatment led to normalization of CD11c expression but had no effect on the expression of CD11b. Since CD11b is a generalized marker for macrophages, this result indicates that SIRT1 activation does not change total ATM content but does decrease the proportion of these cells that displace CD11c. Since it is the CD11c-positive macrophages that are proinflammatory, this macrophage population shift accounts for the decreased proinflammatory responses observed both in vivo and in vitro. These findings suggest that SIRT1 activation can direct ATMs toward a more noninflammatory polarization state in which they do not display CD11c or exhibit a proinflammatory phenotype.
Our studies also show that as part of its anti-inflammatory effects, SIRT1 knockdown increased and SIRT1 activator treatment decreased TNFα secretion from macrophages. This is an important finding, since TNFα and other cytokines are thought to mediate the insulin desensitizing effects of activated macrophages by suppressing insulin action in neighboring insulin target cells through paracrine actions (29). Along these lines, we directly evaluated the effect of macrophage SIRT1 depletion on insulin sensitivity in adipocytes. Thus SIRT1-depleted or resveratrol-treated macrophages were stimulated with LPS or palmitate and CM was subsequently harvested from these cells. The CM was then applied to adipocyte cultures, and the ability of insulin to stimulate glucose transport and Akt phosphorylation was measured. We found that SIRT1 depletion augmented the ability of CM to cause cellular insulin resistance, whereas resveratrol attenuated this effect. These results are consistent with the notion that SIRT1 can act within macrophages to exert anti-inflammatory effects, which then lead to improved insulin sensitivity in insulin target tissues.
In summary, these results show a novel anti-inflammatory role of SIRT1 in macrophages that modulates the paracrine-induced effects of macrophage CM on insulin resistance in adipocytes. We show that SIRT1 knockdown increases intracellular inflammatory signaling pathways, secretion of TNFα, and inflammatory gene expression and that SIRT1 activators have opposite effects. Thus activated SIRT1 in macrophages restrains proinflammatory responses, as well as the development of insulin resistance. These results were recapitulated in vivo by showing that SIRT1 activator treatment of ZF rats improves glucose tolerance and insulin sensitivity and causes reduced tissue inflammatory responses. Our findings also raise the possibility that some component of the anti-aging effects of SIRT1 may be related to these anti-inflammatory effects. Together these results suggest that macrophage SIRT1 may play a beneficial role in regulating glucose homeostasis and that pharmacological activation of macrophage SIRT1 might be a useful anti-inflammatory therapeutic strategy for treating insulin resistance.
GRANTS
This study was funded in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to T. Yoshizaki), the Japan Diabetes Foundation (to T. Yoshizaki), the United States and Israel Binational Scientific Foundation #2003238 (to T. Imamura), National Institute of Diabetes and Digestive and Kidney Diseases National Institutes of Health Grants DK-033651 and DK 074868 (to J. M. Olefsky), the Diabetes Endocrinology Research Center (to J. M. Olefsky), and Sirtris. This research was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank High Throughput Genomics for the ArrayPlate assay. We also thank M. T. Audrey Nguyen and Sarah Hosch for scientific advice, Ann-Khoi Nguyen for assistance with rodent experiments, and Elizabeth J. Hansen for editorial assistance.
REFERENCES
- 1.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11: 191–198, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le-Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de-Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 11: 183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science 310: 1641, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de-Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haigis MC, Guarente LP. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPARgamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest 117: 1658–1669, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602–26614, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–5105, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Lesniewski LA, Hosch SE, Neels JG, de-Luca C, Pashmforoush M, Lumeng CN, Chiang SH, Scadeng M, Saltiel AR, Olefsky JM. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med 13: 455–462, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martel RR, Botros IW, Rounseville MP, Hinton JP, Staples RR, Morales DA, Farmer JB, Seligmann BE. Multiplexed screening assay for mRNA combining nuclease protection with luminescent array detection. Assay Drug Dev Technol 1: 61–71, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal C. Novel small molecule activators of SIRT1 as therapeutics for treatment of type 2 diabetes. Nature 450: 712–716, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, mechanisms. Am Heart J 149: 33–45, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447: 1116–1120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8: 301–309, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado-De-Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Samokhvalov V, Bilan PJ, Schertzer JD, Antonescu CN, Klip A. Palmitate- and lipopolysaccharide-activated macrophages evoke contrasting insulin responses in muscle cells. Am J Physiol Endocrinol Metab 296: E37–E46, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118: 2992–3002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol 296: G1047–G1053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126: 987–1002, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6: 386–397, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol 25: 2062–2068, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AWJ. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292: L567–L576, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Mol Cell Biol 27: 5172–5183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshizaki T, Maegawa H, Egawa K, Ugi S, Nishio Y, Imamura T, Kobayashi T, Tamura S, Olefsky JM, Kashiwagi A. Protein phosphatase-2C alpha as a positive regulator of insulin sensitivity through direct activation of phosphatidylinositol 3-kinase in 3T3–L1 adipocytes. J Biol Chem 279: 22715–22726, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol 29: 1363–1374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673–1677, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.