Abstract
Normal childhood growth is determined by ultradian and infradian variations in GH secretion, yet GH treatment of children with short stature is restricted to daily fixed doses. We have used GH-deficient dwarf rats to determine whether variable GH dose regimens promote growth more effectively than fixed doses. Animals were treated with saline or 4.2 mg of recombinant bovine GH given as 1) 700 μg/wk in 100 μg/day doses, 2) alternating weekly doses of 966 (138 μg/day) or 434 μg (62 μg/day), or 3) 700 μg/wk in randomized daily doses (5–250 μg/day). Body weight and length were measured weekly. Femur and tibia lengths and internal organ, fat pad, and muscle weights were recorded at the end of the study (6 wk); blood was collected for IGF axis measurements. GH promoted femur [F(3,60) = 14.67, P < 0.05], tibia [F(3,60) = 14.90, P < 0.05], muscle [F(3,60) = 10.37, P < 0.05], and organ growth [liver: F(3,60) = 9.30, P < 0.05; kidney: F(3,60) = 2.82, P < 0.05] and an increase in serum IGF-I [F(3,60) = 9.18, P < 0.05] and IGFBP-3 [F(3,60) = 6.70, P < 0.05] levels. IGF-I levels correlated with final weight (r = 0.45, P < 0.05) and length (r = 0.284, P < 0.05) in the whole cohort, but within each group, growth parameters correlated with serum IGF-I only in animals treated with random GH doses. The variable regimens promoted femur length (P < 0.05) and muscle (P < 0.05) and kidney (P < 0.05) weight more effectively than treatment with the fixed regimen. This study demonstrates that aspects of growth are improved following introduction of infradian variation to GH treatment in a GH-deficient model. The data suggest that varying the pattern of GH doses administered to children may enhance growth performance without increasing the overall GH dose.
Keywords: insulin-like growth factor, growth hormone deficiency, infradian, insulin-like growth factor-binding protein, acid-labile subunit
growth hormone (GH) is the major regulator of postnatal body growth (46). Consequently, GH deficiency (GHD) during childhood results in severe growth retardation and, if untreated, marked impairment of adult height. Therefore, children with GHD are offered replacement therapy, and, if given in accordance with the published guidelines of 25–50 μg·kg−1·day−1 (31), many achieve an adult height within the normal range. However, this is not always the case; in a group of children with idiopathic GHD, treatment with biosynthetic GH resulted in a mean height standard deviation score of −0.7, which was 0.45 SD less than the parental target height of −0.25 (7).
GH is also used in the management of other growth problems, for example, in idiopathic short stature and Turner syndrome. Overall, therapy is advantageous, but in these groups there is also considerable variability in the response to GH, and some children remain short even with treatment (33). Thus these studies demonstrate that although GH administration is beneficial for some children, the therapeutic potential of GH is not fulfilled in all patients.
Current treatment regimens are based on daily injections of GH at a constant dose determined by body weight or surface area. However, this mode of administration may hamper the efficacy of treatment since it does not mimic the physiological profile of GH secretion, which varies considerably; levels fluctuate within 1 day (ultradian rhythms) (27) and also, as shown by our previous work, from day to day and over weeks and months (infradian rhythms) (55). Our analysis of childhood growth in relation to urinary GH excretion (as a surrogate for daily GH secretion) over the same time period suggests that the amount of GH is only one determinant of growth velocity. Other attributes of GH output are also important since the relationship between the pattern of growth and GH production is best described by an asymptotic function (28). Hence, there is a rapid increase in growth rate as GH secretion increases from very low levels, but thereafter, large changes in GH have a much smaller influence. We also found that the variation in GH output that occurs throughout the year is positively correlated with height (28), implying that large changes in GH output from week to week are associated with tall stature, whereas short stature is linked to less variation in GH production. Moreover, changes of constant magnitude were correlated with growth rate such that tall children with a high growth velocity had large but constant changes in GH. Further analysis revealed that an irregular temporal pattern to the changes in GH also contributed to good growth (29). Taken together, our data suggest that to optimize growth, GH replacement therapy should be tailored to mimic the underlying variability in normal GH output. Therefore, in this study we have used a rat model of GHD to investigate whether administering GH by a regimen that more closely imitates the natural infradian rhythms in GH release will promote growth more effectively than schedules based on a fixed GH dose. Our data demonstrate that, for a given amount of GH, it is possible to improve outcome, as measured by a number of growth parameters, by introducing an element of variability to the pattern of GH delivery.
MATERIALS AND METHODS
Animals and experimental protocol.
Two pairs of GH-deficient dwarf rats maintained on a Lewis background (10) were obtained from the National Institute for Medical Research (Mill Hill, London, UK) and bred in Manchester to establish the colony from which the 6-wk-old male rats used in this study (n = 64) were drawn. Animals (8/cage) were housed in temperature- and light-controlled rooms (21–22°C, 12-h light) with ad libitum access to food and water. At the start of the study, rats were randomly allocated to one of four treatment groups (16 animals/group): group 1, daily subcutaneous (sc) injection of 0.9% saline; group 2, daily sc injection of 100 μg of recombinant bovine (rb)GH (fixed dose; a generous gift from Monsanto, St. Louis, MO); group 3, daily sc injection of 138 μg of rbGH for 1 wk alternated with a daily dose of 62 μg of rbGH the following week (square-wave dose); group 4, daily sc injection of rbGH at a dose chosen at random from the following possibilities: 5, 15, 50, 80, 130, 170, or 250 μg (random dose). The regimens used in groups 3 and 4 were designed to mimic the features of GH secretion that we have previously identified as being important for growth: overall week-to-week variation (group 3), dose-to-dose variation (groups 3 and 4), and irregularity (group 4). Importantly, however, by the end of the experiment (6 wk) the animals in groups 3 and 4 received the same total amount of GH (4.2 mg) as the rats in group 2. Body weight and length (nose to anus) were measured at the start of the experiment, and thereafter, weight was recorded three times/wk and length (reported as the average of 3 readings/animal) measured weekly. All measurements were performed on conscious animals. Five days before the end of the experiment, all animals were given a single intraperitoneal injection of oxytetracycline (10 mg/kg; Sigma, Dorset, UK) to allow subsequent analysis of bone deposition, and then at 6 wk, all animals were euthanized by carbon dioxide asphyxiation. The wet weight of the retroperitoneal and epididymal fat pads, right gastrocnemius muscle, kidneys, lungs, heart, and liver were recorded for each animal, and the femur and tibia from each hind leg were also collected. The liver and muscle from each animal were flash-frozen in liquid nitrogen and stored at −80°C for subsequent analysis of gene expression. Blood was harvested, processed, and serum stored (−20°C) for subsequent analysis of components of the IGF axis. All experimental procedures were performed after review and approval by the Home Office UK and in accordance with the licence granted under the Home Office Animals (Scientific Procedures) Act of 1986.
Bone analysis.
The femur and tibia were stripped of all muscle and cartilage, and then the length of each bone was measured using a digital Vernier calliper (accuracy ± 0.1 mm; CamLab). Bone growth during the final week of the study was assessed by analyzing oxytetracycline labeling of the upper growth plate of the right tibia, as described previously (26).
IGF/IGF-binding protein/acid-labile subunit measurements.
Serum IGF-I was measured using a rat/mouse IGF-I two-site immunoenzymometric assay (IDS Diagnostic, Tyne and Wear, UK) in accordance with the manufacturer's instructions. The concentration of serum acid-labile subunit (ALS) was determined using the previously reported radioimmunoassay for rat ALS (4). The serum IGF-binding protein (IGFBP) profile was assessed by our standard protocol of Western ligand blotting with 125I-IGF-I (20). The sera from animals within each of the four groups were pooled, and then the four samples were analyzed by Western ligand blot on four separate occasions and the resultant bands semiquantitatively assessed by densitometry.
Analysis of liver and muscle mRNA expression.
RNA was extracted from each sample by homogenizing tissue with a TissueRuptor probe (Qiagen) in accordance with the manufacturer's instructions. RNA samples were then pooled according to organ type and treatment group. Five nanograms of total RNA from each pool was reverse transcribed, and then IGF-I, IGFBP-3, ALS, or GH receptor (GHR) mRNA expression was quantified in triplicate by quantitative PCR using 50–100 ng of cDNA, iQ SYBR Green supermix (BioRad), and a Chromo4 thermal cycler running Opticon 3.1 software (Bio-Rad Ltd); primer pairs are given in Table 1. Amplification products were electrophoresed on agarose gels and then visualized by ethidium bromide staining. All reactions resulted in a single product of the expected size.
Table 1.
Primer pairs used in the amplification of IGF-I, IGFBP-3, ALS, and GHR mRNA
| Gene | Primer Sequence | Amplicon Size |
|---|---|---|
| IGF-I | ||
| Forward | CTTGAGCAACCTGCAAAACA | 80 |
| Reverse | GGAAATGCCCATCTCTGAAA | |
| GHR | ||
| Forward | AAAACGATGAGCCCGATATG | 106 |
| Reverse | TTTTCGAAGCTCCGTTGTCT | |
| IGFBP-3 | ||
| Forward | GCTATGACACCAAGGGGAAA | 136 |
| Reverse | AGCTGCTGATCACGTTGTTG | |
| ALS | ||
| Forward | GGGCTTGTTCACACACACAC | 93 |
| Reverse | GAGGTCCCAAAGGTGACTGA | |
| RpL13a | ||
| Forward | ACAAGAAAAAGCGGATGGTG | 167 |
| Reverse | TTCCGGTAATGGATCTTTGC |
IGFBP-3, IGF-binding protein-3; ALS, acid-labile subunit; GHR, growth hormone receptor; RpL13a, ribosomal protein L13a.
Expression of the genes of interest was quantified relative to the expression of ribosomal protein L13A, which was shown in preliminary experiments to be unaffected by treatment with GH, and subsequently to the control group to generate a fold change in response to GH treatment; an at least twofold change in expression was considered meaningful.
Analysis of STAT5b, suppressor of cytokine signaling 1–3, and cytokine-inducible SH2 protein 1 in liver and muscle.
Protein was extracted from each sample by homogenizing tissue (100 mg) in lysis buffer containing protease and phosphatase inhibitors using a TissueRuptor (Qiagen). Samples were then pooled according to organ type and treatment group. Protein from each sample was resolved by SDS-PAGE and transferred to nitrocellulose membranes for Western blotting with antiserum specific for phospho-STAT5 (Tyr694; Cell Signaling Technology), total STAT5b (Zymed Laboratories), suppressor of cytokine signaling (SOCS)1, SOCS2, SOCS3, or cytokine-inducible SH2 protein (CIS)1 (AnaSpec). Immune complexes were visualized by probing with a horseradish peroxidase-linked secondary antibody followed by enhanced chemiluminescence.
Statistical analysis.
Differences between groups were evaluated using one-way ANOVA with planned contrast analysis of statistically significant parameters [saline vs. GH treatment, fixed vs. variable (square-wave and random doses) GH dose, square-wave vs. random GH dose, fixed vs. square-wave GH dose, and fixed vs. random GH dose]. The reported results include the F ratio (the ratio of systematic variance to unsystematic variance), the degrees of freedom from which it was calculated, and the significance value (1-tailed). The relationships between parameters were investigated using Pearson product-moment correlation coefficient.
RESULTS
Effect of GH treatment on body weight and length.
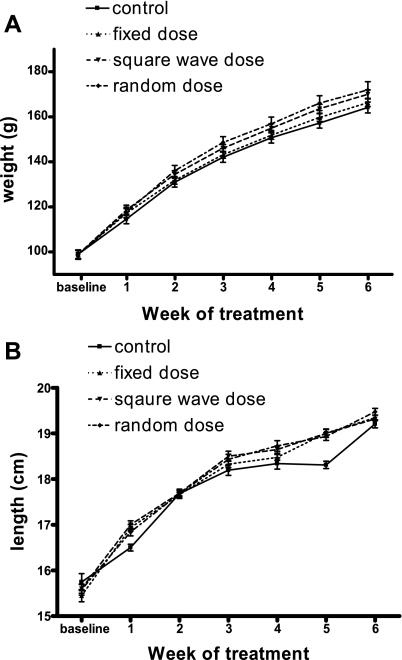
During the first week of the study, all animals treated with GH gained significantly more weight [F(3,60) = 15.60, P < 0.05] and length [F(3,60) = 18.15, P < 0.05] than rats receiving saline (Table 2), which supports previous data on the short-term (5- to 7-day) treatment of young GH-deficient animals (10, 27). However, during week 2 the control animals grew more, both in terms of weight and length, than the rats treated with GH (Table 2), although within the GH-treated animals, the variable GH treatment regimens had a significantly greater effect than the fixed-dose schedule. Similarly, in week 5, saline-treated animals gained significantly more length than GH-treated rats (data not shown), and in general, there was a greater variability in the measurements of length of the control animals compared with those of the GH-treated rats. Consequently, by the end of the study (6 wk of treatment) there was no significant difference in the absolute weight (Fig. 1A) or length (Fig. 1B) of the rats across the four groups. However, the gain in weight by animals treated with GH was significantly greater than in saline-treated rats [F(3,60) = 6.28, P < 0.05], and treatment with a variable GH regimen was more effective in stimulating weight gain than was administering a fixed daily dose [F(3,60) = 3.87, P < 0.05].
Table 2.
The effect of different GH treatment regimens on weight and length gain during weeks 1 and 2 of treatment
| Gain in Weight, g |
Gain in Length, cm |
|||
|---|---|---|---|---|
| Group | Week 1 | Week 2 | Week 1 | Week 2 |
| 1 (Control) | 15.64 ± 0.83 | 16.33 ± 0.70 | 0.76 ± 0.15 | 1.18 ± 0.08 |
| 2 (Fixed GH dose) | 18.46 ± 0.46a | 14.63 ± 0.94 | 1.49 ± 0.12a | 0.72 ± 0.09 |
| 3 (Square-wave GH dose) | 19.70 ± 0.92a | 15.80 ± 1.08b | 1.40 ± 0.10a | 0.69 ± 0.09b |
| 4 (Random GH dose) | 18.79 ± 0.63a | 18.38 ± 0.95b | 1.26 ± 0.12a | 0.84 ± 0.12b |
GH-deficient rats were treated with either 1) 0.9% saline (control) or 2) 700 μg of recombinant bovine GH (rbGH), administered as detailed in the legend to Fig. 1. The weight and length (nose to anus) of each animal was recorded at the start of the study and then again after 7 and 14 days of treatment. Gain in weight and length are presented as means ± SE, and 1-way analysis of variance with planned contrasts was used to assess significant differences (P < 0.05):
GH-treated vs. saline-treated groups;
variable vs. fixed GH treatment groups.
Fig. 1.
The effect of different growth hormone (GH) treatment regimens on the weight and length of GH-deficient rats. GH-deficient rats were treated for 6 wk with either 1) 0.9% saline (control), 2) 700 μg of recombinant bovine GH (rbGH)/wk administered as a fixed dose of 100 μg/day (fixed dose), 3) 966 μg of rbGH/wk (138 μg/day) alternated with 434 μg rbGH/wk (62 μg/day, square-wave dose), or 4) 700 μg rbGH/wk administered as daily doses of 5, 15, 50, 80, 130, 170, and 250 μg, which were given in a random order each week (random dose). There were 16 animals in each group, and the weight (A) and length (B) of each animal was measured at the start of the study and then weekly thereafter. Data are presented as means ± SE.
Effect of GH treatment on the IGF axis.
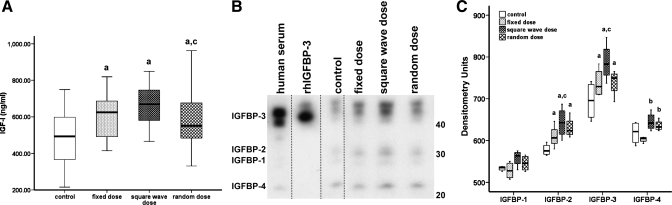
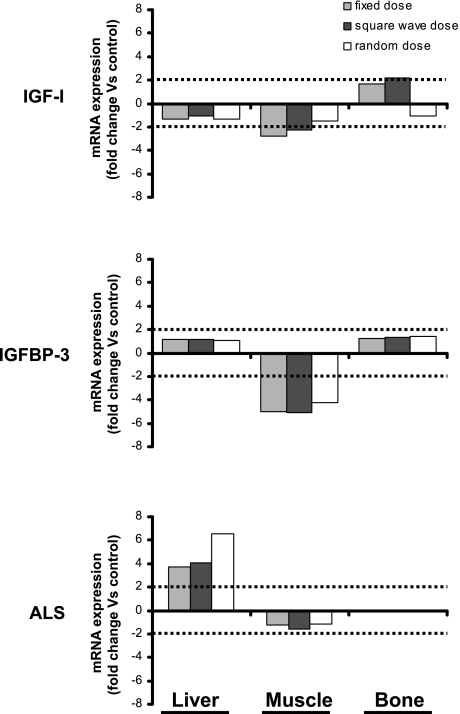
At the end of the study period, the concentrations of serum IGF-I and ALS were measured using rat-specific immunoassays, and the serum IGFBP profile was assessed by Western ligand blot with 125I-IGF-I. Compared with treatment with saline, administration of GH significantly increased serum IGF-I levels [F(3,60) = 9.18, P < 0.05]. IGF-I levels were highest in those animals treated with the square-wave GH regimen, and although they were significantly different from the level of IGF-I in sera from rats in the random GH dose group, this increase in IGF-I (35% vs. saline) was similar to that seen in rats treated with a fixed GH dose (21% increase vs. saline; Fig. 2A). Liver is thought to produce the majority of circulating IGF-I; however, hepatic expression of IGF-I was similar in all four groups (Fig. 3), as was the expression of GHR mRNA (data not shown), which may suggest treatment-induced downregulation of receptors. GH regulates hepatic IGF-I expression through the interaction of its downstream signaling molecule, STAT5B, with the igf1 promoter. However, Western blot analysis of protein isolated from liver and muscle demonstrated that neither the expression nor activation of STAT5B was consistently altered by any of the GH treatment regimens (data not shown). Similarly, there were no striking differences in the expression of the negative regulators of GH receptor signaling, SOCS1–3 and CIS1, in either the liver or muscle of animals from the four treatment groups (data not shown).
Fig. 2.
The effect of different GH treatment regimens on the serum concentration of IGF-I and the IGF-binding protein (IGFBP) profile. GH-deficient rats were treated for 6 wk, as detailed in the legend to Fig. 1, and blood was harvested from each animal (n = 64, 16/group) at the end of the study. A: the median (range) IGF-I concentration was determined using a rat-specific immunoassay. B: The IGFBP profile was assessed by Western ligand blot analysis of 2 μl of pooled serum with 125I-IGF-I. Two microliters of human serum and 50 ng of recombinant human IGFBP-3 (rhIGFBP-3) served as positive control samples. The blot depicted is representative of results obtained from 3 independent experiments. Within each experiment, all samples were run on the same gel; however, we have rearranged some lanes (indicated by dotted line) from the resultant image of the autoradiograph in order to present the data in the sequence used to report all other results: control, fixed dose, square-wave dose, and random dose. C: the densitometric analysis (median ± range) of the bands with molecular mass corresponding to IGFBP-3 (44/40 kDa), IGFBP-2 (30 kDa), IGFBP-1 (28 kDa), and IGFBP-4 (24 kDa) is presented. One-way analysis of variance with planned contrasts was used to assess significant (P < 0.05) differences between the groups: aGH vs. saline treatment; bvariable (square-wave and random) vs. fixed GH dose; crandom vs. square-wave GH dose.
Fig. 3.
The effect of different GH treatment regimens on IGF-I, IGFBP-3, and acid-labile subunit (ALS) mRNA expression by liver, bone, and muscle. GH-deficient rats were treated for 6 wk, as detailed in the legend to Fig. 1, and the organs and bones were harvested from each animal (n = 64, 16/group) at the end of the study. RNA was extracted from each sample and then pooled according to organ type and treatment group. Total RNA from each pool was reverse transcribed, and then IGF-I, IGFBP-3, or ALS mRNA expression was measured in triplicate. Expression was quantified relative to the expression of ribosomal protein L13A and subsequently to the control group to generate a fold change in response to GH treatment. Data represent the mean expression from multiple independent experiments (liver, n = 4; muscle and bone, n = 2).
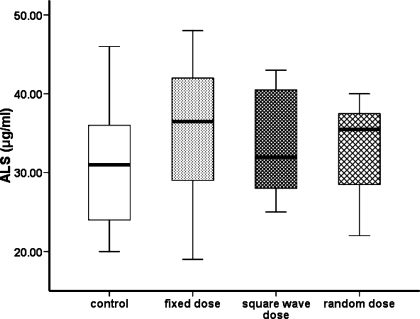
Densitometric analysis of Western ligand blot data (Fig. 2B) demonstrated that, compared with control animals, the intensity of bands representing proteins with molecular weights corresponding to that of IGFBP-3 [44/40 kDa; F(1,12) = 6.70, P < 0.05] and IGFBP-2 [30 kDa; F(1,12) = 10.34, P < 0.05] were increased by GH treatment, with the greatest effect observed in the animals receiving alternating weekly (square-wave) GH doses compared with the other regimens (Fig. 2C). However, hepatic expression of IGFBP-3 mRNA did not differ between the groups (Fig. 3). The intensity of the band representing IGFBP-4 was significantly greater in animals treated with a variable rather than a fixed GH regimen [24 kDa; F(1,12) = 27.89, P < 0.05]. None of the GH treatment schedules affected the intensity of the band representing IGFBP-1. Analysis of hepatic ALS mRNA suggested that, compared with the saline regimen, expression was increased by GH treatment (Fig. 3), although the concentration of ALS measured in the serum of each of the four groups of animals was similar (Fig. 4).
Fig. 4.
The effect of different GH treatment regimens on the serum concentration of ALS. GH-deficient rats were treated for 6 wk, as detailed in the legend to Fig. 1, and blood was harvested from each animal (n = 64, 16/group) at the end of the study. The ALS concentration was determined using a rat-specific immunoassay and is presented as median and range. One-way analysis of variance with planned contrasts demonstrated that there was no significant difference between the groups.
Serum IGF-I levels were correlated with final weight (r = 0.445, n = 64, P < 0.05) and length (r = 0.284, n = 64, P < 0.05) when all animals were included in the analysis. However, when each treatment group was considered individually, only the body growth of animals treated with the random GH dose regimen was correlated significantly with serum IGF-I levels (weight: r = 0.587, n = 16, P < 0.05; length: r = 0.609, n = 16, P < 0.05).
Variable GH treatment regimens are better than a fixed daily dose of GH at promoting growth of the femur.
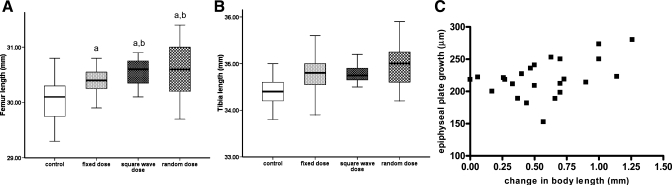
The lengths of both the femur and tibia were significantly increased by treatment with GH [F(3,60) = 14.67, P < 0.05, and F(3,60) = 14.90, P < 0.05, respectively; Fig. 5, A and B] and correlated with serum IGF-I concentrations (femur: r = 0.351, P < 0.05; tibia: r = 0.297, P < 0.05). Both variable GH treatment regimens induced better growth of the femur (∼2-fold greater gain) than that achieved in animals receiving a fixed dose of GH [F(3,60) = 3.06, P < 0.05], although length of the tibia did not differ between the three GH treatment groups. Tibial expression of IGF-I, IGFBP-3, and GHR mRNA was similar in saline- and GH-treated animals, and the different GH treatment regimens had no obvious effect on mRNA expression (Fig. 3). Bone growth during the final week of the study, as measured by oxytetracycline labeling of the proximal tibial growth plate, reflected the gain in body length over the same time period (r = 0.462, P < 0.05; Fig. 5C).
Fig. 5.
The effect of different GH treatment regimens on the length of the femur and tibia. GH-deficient rats were treated for 6 wk, as detailed in the legend to Fig. 1. There were 16 animals in each group, and the length of the femur (A) and tibia (B) measured by digital callipers at the end of the study is presented as median and range. One-way analysis of variance with planned contrasts was used to assess significant (P < 0.05) differences between the groups: aGH vs. saline treatment; bvariable (square-wave and random) vs. fixed GH dose. C: bone growth (μm) during the final week of the study, assessed by analyzing oxytetracycline labeling of the upper growth plate of the right tibia, was correlated (r = 0.462, P < 0.05) with the change in body length (mm) during the same period.
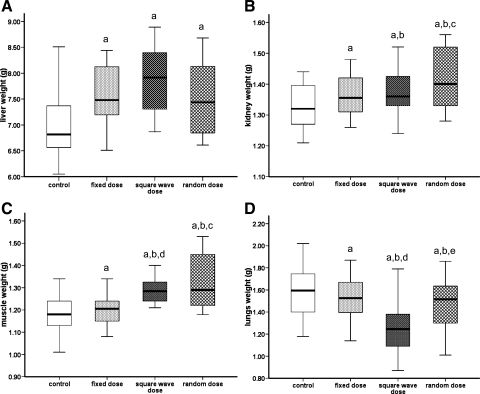
Both kidney and muscle respond best to variable GH treatment regimens.
None of the GH treatment regimens affected the weight of the heart or either of the fat pads analyzed, although epididymal fat pad weights were correlated with serum IGF-I concentrations (r = 0.267, n = 64, P < 0.05). GH treatment did increase the weight of the gastrocnemius muscle [F(3,60) = 10.37, P < 0.05], liver [F(3,60) = 9.30, P < 0.05], and kidneys [F(3,60) = 2.82, P < 0.05] (Fig. 6), and the weights of these organs correlated with serum IGF-I levels (liver: r = 0.497, kidney: r = 0.276; P < 0.05). In contrast, the weight of the lungs from GH-treated animals was significantly lower than that of rats treated with saline [F(3,60) = 5.81, P < 0.05; Fig. 6]. Further analysis demonstrated that lung weight was affected only by the square-wave GH dose regimen, since the lungs of these animals weighed significantly less than those treated with either the fixed or random GH dose regimen [F(3,60) = 10.69, P < 0.05, and F(3,60) = 6.20, P < 0.05, respectively].
Fig. 6.
The effect of different GH treatment regimens on the weights of liver, kidney, gastrocnemius muscle, and lung. GH-deficient rats were treated for 6 wk, as detailed in the legend to Fig. 1. There were 16 animals in each group, and the weight of the liver (A), kidneys (B), gastrocnemius muscle (C), and lungs (D) harvested from each animal at the end of the study is presented as median and range. One-way analysis of variance with planned contrasts was used to assess significant (P < 0.05) differences between the groups: aGH vs. saline treatment; bvariable (square-wave and random) vs. fixed GH dose; crandom vs. fixed GH dose; dsquare-wave vs. fixed GH dose; erandom vs. square-wave GH dose.
Mode of GH administration did not affect liver weight; however, compared with treatment with a fixed dose of GH, both the square-wave and random GH dose regimens increased muscle weight [F(3,60) = 6.55 and 8.24, respectively, P < 0.05], and the latter also increased kidney weight [F(3,60) = 5.38, P < 0.05]. Muscle expression of IGF-I, ALS, and GHR mRNA was not affected by GH treatment. IGFBP-3 mRNA levels were lower in animals receiving GH, although the mode of GH administration appeared to have no effect.
DISCUSSION
There is considerable evidence to suggest that the ultradian rhythm of GH is an important determinant of GH action; in response to the prevailing GH-releasing hormone, ghrelin, and somatostatin milieu, the anterior pituitary releases GH in discrete pulses, causing peaks and troughs in GH levels (57). Analysis of 24-h GH profiles from adults has indicated that peak GH levels influence the concentration of the GH effector hormone IGF-I (35), and in rats the sexual dimorphism in GH secretion parallels the differences in growth rate (36). Male rats secrete GH in discrete pulses separated by low trough levels and grow at a faster rate than females that exhibit high basal GH levels and less pulsatility. However, little consideration has been given to the significance of long-term infradian variation in GH levels (over days, weeks, and months), although it is known that monthly changes in childhood weight and body mass index correlate with the levels of IGF-I and its main serum carrier protein IGFBP-3 (23) and that antler growth in deer is associated with GH output over the preceding month (53). These studies clearly suggest that information of relevance to the growth process can be stored in the infradian pattern of hormone output, and there are now clues to the molecular mechanisms by which this is achieved. Gerbert et al. (24) have demonstrated that the activation of STAT5b, one of GH's key intracellular signaling molecules, is regulated by the temporal pattern of GH stimulation. STAT5b is involved in mediating both the direct and, through its activation of the igf1 promoter (14, 58), the indirect actions of GH. At the end of our study, expression of STAT5b in both liver and muscle was similar in the four groups of animals, and there were no striking differences in the activation of this signaling molecule. Previous in vivo work has demonstrated that chronic exposure of rats to GH results in desensitization of the JAK2/STAT5b signaling pathway (22, 44). This phenomenon has been attributed to GH-induced changes in the expression of the negative regulators of GH signaling, the SOCS, and the CIS proteins (18). However, in our study, rats treated with GH had SOCS1–3 and CIS expression profiles in both liver and muscle that were similar to rats treated with saline. We did not investigate the expression of the potentially relevant protein-tyrosine phosphatases SH2 domain-containing protein tyrosine phosphatase (SHP)-1 and SHP-2 (18), but it is possible that major differences in tissue expression of all of these signaling molecules may not be detectable at the end of a 6-wk exposure to GH.
Comparison of growth in igf1-null mutant mice and GH-deficient animals suggests that the direct actions of GH also contribute to postnatal growth (2); however, the influence of GH patterning on the direct actions of GH has not been explored. Data from studies using hypophysectomized rats suggest that the pattern of GH delivery can influence IGF-I production, because intermittent GH administration was better than continuous GH infusion at increasing serum IGF-I levels (42). In contrast, experiments with the spontaneous dwarf rat, which is also GH deficient, suggest continuous GH infusion as the more effective stimulant of IGF-I (21). Our study, which used a relatively low total dose of GH to allow a wide range of individual doses in the random regimen, supports the previous finding that GH treatment of GH-deficient dw/dw rats promotes increased serum IGF-I and IGFBP-3 levels (12), although in this regard, neither of the variable GH dose regimens were more effective than the fixed-dose schedule. A clinical study of GH administration to deficient adults found that a twice-daily treatment schedule was better than a single injection at increasing serum IGF-I (40), although data from numerous studies in children demonstrate that circulating IGF-I levels are not affected by the pattern of GH administration (8, 16, 49). Taken together, these data suggest that serum IGF-I, which is produced primarily in the liver, is more sensitive to the presence rather than the pattern of GH administration (5, 27).
Circulating IGF-I exists mostly in a 150-kDa complex with IGFBP-3 and ALS; the latter is produced in the liver and is thought to be regulated by GH. Consequently, children and adults with GH deficiency have reduced serum ALS levels (1, 39), which can be restored by GH therapy. Similarly, GH-deficient dw/dw rats are reported to have 75% less serum ALS than control animals (4), and hepatic ALS mRNA levels are reduced markedly by hypophysectomy (47), although there is still an excess of unbound circulating ALS in these animals (41). In our study, treatment with GH caused a four- to sixfold increase in hepatic ALS mRNA expression. However, GH did not increase the concentration of circulating ALS, and levels in the 12-wk-old saline-treated and GH-treated animals were similar to levels found in normal rats (4). Other researchers have also noted a discrepancy between hepatic ALS mRNA and ALS protein levels. In a study of the GH-deficient Snell dwarf mouse (48), the expression of ALS protein by the livers of dwarf mice was similar to that of livers from control animals despite significant differences in mRNA levels, which led these authors to speculate that ALS is posttranscriptionally regulated to maintain the protein pool at normal levels. Nonetheless, our study suggests that the pattern of GH exposure is not an important determinant of ALS production.
We did not investigate the effect of the various GH treatment regimens on the level of circulating insulin, because previous studies of the dw/dw rat have shown that the plasma concentration of both insulin and glucose in nonfasted animals administered with GH is similar to that of untreated rats (50) and that GH treatment does not have any effect on in vitro insulin stimulation of glucose transport in skeletal muscle (13). These findings are in keeping with clinical studies of patients with GH deficiency in which insulin resistance induced by short-term GH therapy is decreased by chronic treatment (19, 37, 45).
Short-term (9-day) administration of GH at a fixed daily dose has been reported to have no effect on organ weight of dw/dw rats (52). However, similar treatment of the spontaneous dwarf rat increased the weight of the liver, lungs, and kidneys but not the heart (21). In our study, prolonged (6-wk) treatment with GH also led to an increase in the weight of liver and kidney, and, like other studies, the weight of the heart was unaffected. However, the weight of the lungs appeared to be lower in GH-treated rats, although further statistical analysis demonstrated that this could be explained by the effect of the square-wave pattern of GH administration. Lung growth in the rat is thought to be relatively independent of pituitary function (30). However, GH is known to stimulate the production of IGFBP-2 by rat lung (3). In our study, the square-wave mode of GH delivery promoted the greatest increase in serum IGFBP-2, and if this effect was mirrored in lung, it is possible that the reduced lung weight of animals treated with the square-wave GH dose regimen reflects reduced IGF-I bioavailability, as numerous studies have demonstrated the importance of IGF-I for lung development and growth (52a). GH treatment also increased the weight of gastrocnemius muscle, which is in keeping with the findings of previous studies on the administration of GH to hypophysectomized rats (17). Interestingly, both of the variable GH dose regimens were more effective than treatment with a fixed dose.
Previous studies of dw/dw rats have shown that GH therapy promotes longitudinal bone growth (52), and in the current study also, the lengths of both the femur and tibia were increased significantly in animals receiving GH compared with saline-treated rats. Moreover, femur length was greatest in animals treated with the variable GH-dosing schedules, suggesting that the pattern of GH delivery can influence this growth parameter. Tibial length was not affected by mode of GH administration, but this may reflect the relative maturity of this bone, as studies of craniofacial development in GH-deficient dwarf rats suggest that the effect of GH deficiency and also the outcome of GH therapy depend on the amount of growth remaining for a particular bone (51). Other researchers have noted disparities in the response of the femur and tibia to different GH treatment regimens; treatment of growth hormone-releasing hormone-knockout mice with one dose of GH/day normalized (compared with wild-type animals) both tibia and femur length. However, when the null animals were treated with two GH injections/day (same overall dose), only the length of the femur was significantly greater than that of wild-type mice (1a). Bone-to-bone variation in responsiveness to GH has also been observed in hypophysectomized rats, where the effect of GH on the expression of IGF-I and a number of bone markers differs between the femur and humerus (6).
In humans, the length of the long bones is an important determinant of height, whereas in rats, length (nose to anus) is dependent on the axial skeleton and skull, which may explain why treatment with the relatively low dose of GH used in this study had no significant effect on length. Length measurements are also likely to be affected, at least to a degree, by muscle tone and strength. The latter is likely to be lower in the saline-treated animals compared with GH-treated animals. This may have contributed to the greater variability in the measurements of length recorded for these animals. There was also no significant difference between the final weight of animals treated with GH and those administered with saline, although gain in weight over the study was greater in GH-treated animals. In rats, feeding behavior is thought to be related to ghrelin production by the stomach (56) rather than the endogenous GH profile (54). Indeed, the intake of food by dw/dw rats is normal (15); because chronic GH administration does not affect ghrelin production (15), it is unlikely that, in our study, food intake by the GH-treated animals would be different from that of the control rats, although this parameter was not assessed directly. Other studies of the dw/dw rat have reported an increase in body weight following GH treatment; however, these studies were performed over relatively short time periods (<14 days; Table 3). Indeed, we also noted a GH-induced increase in body weight during the first week of our study, and so it is possible that our finding of similar final weights reflects increased subcutaneous lipolysis in response to prolonged GH treatment.
Table 3.
Comparison of a number of studies investigating the effect of GH treatment on various growth parameters of the GH-deficient dw/dw rat
| Overall Effect of Treatment On |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Age at Start, wk | Length of Treatment, days | GH Regimen | Weight | Length | IGF axis | Muscle weight | Organ weight | Bone length | Ref. No. |
| Male dw/dw rats | 6 | 7 | #Bovine GH, 700 μg/wk | ↑∗ | ↑ ∗ | Current study | ||||
| 42 | #Bovine GH, 700 μg/wk | = | = | IGF-I↑∗ | GN↑∗ | liver↑∗ | Fem↑∗ | |||
| IGFBP-3↑∗ | lung↓∗ | Tib↑∗ | ||||||||
| ALS=∗ | kidney↑∗ | |||||||||
| heart=∗ | ||||||||||
| Female dw/dw rats | 6 | 7 | Human GH, 144 μg/day iv | ↑ | NR | NR | NR | NR | NR | 25 |
| 16 | 14 | Human GH, 200 μg/day sc infusion | ↑ | NR | NR | NR | NR | NR | ||
| Male dw/dw rats | 12 | 9 | Human GH, 66 μg/day continuous iv infusion | ↑ | NR | IGF-I= | Liver= | Tib↑ | 52 | |
| lung= | ||||||||||
| kidney= | ||||||||||
| Male dw/dw rats | 25–27 | 10 | Human GH, 250 μg/kg sc twice daily | ↑ | NR | IGF-I↑ | Sol↑ EDL↑ |
NR | NR | 13 |
| Male dw/dw rats | 5 | 14 | Human GH, 1,250 μg/kg sc twice daily | ↑ | NR | NR | NR | NR | Fem↑ | 43 |
| Male and female dw/dw rats | NR | 5 | Ovine GH, 100 μg twice daily | Male↑ | NR | NR | NR | NR | Male and female Tib↑ |
10 |
| Female↑ | Tib↑ | |||||||||
NR, not reported; Sol, soleus muscle; EDL, extensor digitorum longus muscle; GN, gastrocnemius muscle; Tib, tibia; Fem, femur. ↑Increased compared with control; ↓decreased compared with control; =no difference compared with control. #GH-deficient rats were treated with either 1) 0.9% saline (control) or 2) 700 μg of rbGH, administered as detailed in the legend to Fig. 1. ∗See text for details of the specific effect caused by each mode of GH administration.
The effect of GH treatment over 6 wk on the length, and in fact many other growth parameters, of dw/dw rats has not been reported (Table 3). We do recognize that initiation of GH treatment in dw/dw rats at 6 wk of age may not provide the optimum model for studying every aspect of GH action. Nevertheless, our study is the first to perform such a comprehensive analysis of growth parameters within a single model of GH deficiency and highlights the differing tissue and molecular sensitivity to GH treatment.
One possible explanation for the overall lack of effect of GH on length could be due to the development of antibodies to the bovine GH used in our study. However, previous studies have shown that bovine GH has a similar antigenicity to rat GH (34), and it does not induce anti-bovine GH antibodies after infusion of a relatively high dose of bovine GH, 250 μg·day−1·rat−1 over 14 days (9). Additionally, GH was shown to have a number of significant effects on other end points, such as femur length and organ weight. We consider that this makes the production of anti-GH antibodies attenuating GH action over the course of our study most unlikely.
In summary, we have shown for the first time that introducing an element of variability to even relatively low-dose GH treatment schedules can enhance outcomes since muscle, kidney, and bone growth were all improved by regimens designed to mimic important features (week-to-week variation, dose-to-dose variation, and irregularity) of endogenous secretion compared with administration of the same amount of GH via a fixed daily dose. The differential responses to variable GH dosing (no effect on serum IGF-I but marked effect on muscle weight) indicate that infradian patterns in GH secretion are likely to influence tissue sensitivity to GH. This has important implications to the design of long-acting GH preparations, where the serum profile over the week after administration is very different from the pattern of endogenous GH secretion (38), especially since STAT5b is desensitized following chronic exposure to GH (11).
Fixed-dose GH is a very successful treatment for GH deficiency, but long-term growth responses to GH are highly variable in non-GH-deficient conditions. Data obtained in the current study demonstrate that altering the method of delivering the same amount of GH influences several aspects of GH function, although the additional benefit gained from variable vs. fixed GH regimens, in actual terms, was modest. Indeed, it would be unrealistic to expect a very large difference. Our findings suggest that if relative gains were sustained in response to GH treatment throughout childhood and adolescence, implementing a variable GH treatment regimen could translate into a very useful increase in growth (of the order of several cm) over a child's growing years. Therefore, we feel that clinical investigations to assess the impact of modifying fixed-dose GH treatment regimens are warranted.
GRANTS
This project was funded by a Pharmacia International Translational Award (open competition); the research groups are supported by the National Institute for Health Research Biomedical Research Funding Scheme.
DISCLOSURES
The authors have nothing to declare.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge technical assistance from Reena Morjaria.
REFERENCES
- 1.Aguiar-Oliveira MH, Gill MS, de A Barretto ES, Alcântara MR, Miraki-Moud F, Menezes CA, Souza AH, Martinelli CE, Pereira FA, Salvatori R, Levine MA, Shalet SM, Camacho-Hubner C, Clayton PE. Effect of severe growth hormone (GH) deficiency due to a mutation in the GH-releasing hormone receptor on insulin-like growth factors (IGFs), IGF-binding proteins, and ternary complex formation throughout life. J Clin Endocrinol Metab 84: 4118–4126, 1999 [DOI] [PubMed] [Google Scholar]
- 1a.Alba M, Fintini D, Salvatori R. Effects of recombinant mouse growth hormone treatment on growth and body composition in GHRH knock out mice. Growth Horm IGF Res 15: 275–282, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75: 73–82, 1993 [PubMed] [Google Scholar]
- 3.Batchelor DC, Lewis RM, Breier BH, Gluckman PD, Skinner SJ. Fetal rat lung epithelium has a functional growth hormone receptor coupled to tyrosine kinase activity and insulin-like growth factor binding protein-2 production. J Mol Endocrinol 21: 73–84, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Baxter RC, Dai J. Purification and characterisation of the acid-labile subunit of rat serum insulin-like growth factor binding protein complex. Endocrinology 134: 848–852, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Bick T, Hochberg Z, Amit T, Isaksson OG, Jansson JO. Roles of pulsatility and continuity of growth hormone (GH) administration in the regulation of hepatic GH-receptors, and circulating GH-binding protein and insulin-like growth factor-I. Endocrinology 131: 423–429, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Bikle DD, Harris J, Halloran BP, Currier PA, Tanner S, Morey-Holton E. The molecular response of bone to growth hormone during skeletal unloading: regional differences. Endocrinology 136: 2099–2109, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Blethen SL, Baptista J, Kuntze J, Foley T, LaFranchi S, Johanson A. Adult height in growth hormone (GH)-deficient children treated with biosynthetic GH. The Genentech Growth Study Group. J Clin Endocrinol Metab 82: 418–420, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Cavallo L, De LF, Bernasconi S, Russo R, Zecchino C, Arrigo T. Subcutaneous growth hormone administration in growth-hormone-deficient children. Continuous plus pulsatile overnight versus single daily injection: effects on growth rate velocity. Horm Res 42: 86–89, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Chandrashekar V, Bartke A. The role of growth hormone in the control of gonadotropin secretion in adult male rats. Endocrinology 139: 1067–1074, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Charlton HM, Clark RG, Robinson IC, Porter-Goff AE, Cox BS, Bugnon C, Bloch BS. Growth hormone-deficient dwarfism in the rat: a new mutation. J Endocrinol 119: 51–58, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Choi HK, Waxman DJ. Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology 140: 5126–5135, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Crawford BA, Dobbie P, Bass JJ, Lewitt MS, Baxter RC, Handelsman DJ. Growth hormone (GH) regulation of circulating insulin-like growth factor-I levels during sexual maturation of the GH-deficient dwarf (dw/dw) male rat. J Endocrinol 141: 393–401, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Daugaard JR, Laustsen JL, Hansen BS, Richter EA. Insulin action in growth hormone-deficient and age-matched control rats: effect of growth hormone treatment. J Endocrinol 160: 127–135, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology 142: 3836–3841, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Davies JS, Gevers EF, Stevenson AE, Coschigano KT, El-Kasti MM, Bull MJ, Elford C, Evans BA, Kopchick JJ, Wells T. Adiposity profile in the dwarf rat: an unusually lean model of profound growth hormone deficiency. Am J Physiol Endocrinol Metab 292: E1483–E1494, 2007 [DOI] [PubMed] [Google Scholar]
- 16.De Schepper J, Craen M, Massa G, Heinrichs C, Maes M, Du Caju M, Rausin L, Bourguignon JP. Growth hormone therapy in Turner's syndrome: one versus two daily injections. J Clin Endocrinol Metab 79: 489–494, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Everitt AV, Terry V, Phillips MJ, Kerry HM, Shorey CD. Morphometric analysis of gastocnemius muscle fiber size and fiber proportions in the hypophysectomised rat after prolonged administration of growth hormone or thyroxine. Growth Dev Aging 60: 85–93, 1996 [PubMed] [Google Scholar]
- 18.Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol Endocrinol 20: 241–253, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Fowelin J, Attvall S, Lager I, Bengtsson BA. Effects of treatment with recombinant human growth hormone on insulin sensitivity and glucose metabolism in adults with growth hormone deficiency. Metabolism 42: 1443–1447, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Freeth JS, Ayling RM, Whatmore AJ, Towner P, Price DA, Norman MR, Clayton PE. Human skin fibroblasts as a model of growth hormone (GH) action in GH receptor-positive Laron's syndrome. Endocrinology 138: 55–61, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Gargosky SE, Tapanainen P, Rosenfeld RG. Administration of growth hormone (GH), but not insulin-like growth factor-I (IGF-I), by continuous infusion can induce the formation of the 150-kilodalton IGF-binding protein-3 complex in GH-deficient rats. Endocrinology 134: 2267–2276, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Gebert CA, Park SH, Waxman DJ. Down-regulation of liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol Endocrinol 13: 213–227, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Gelander L, Blum WF, Larsson L, Rosberg S, Albertsson-Wikland K. Monthly measurements of insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in healthy prepubertal children: characterization and relationship with growth: the 1-year growth study. Pediatr Res 45: 377–383, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Gerbert CA, Park SH, Waxman DJ. Regulation of stat5b activation by the temporal pattern of growth hormone stimulation. Mol Endocrinol 11: 400–414, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Gevers EF, Loveridge N, Robinson IC. Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology 143: 4065–4073, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Gevers EF, Wit JM, Robinson IC. Effect of gonadectomy on growth and GH responsiveness in dwarf rats. J Endocrinol 145: 69–79, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Gevers EF, Wit JM, Robinson IC. Growth, growth hormone (GH)-binding protein, and GH receptors are differentially regulated by peak and trough components of the GH secretory pattern in the rat. Endocrinology 137: 1013–1018, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Gill MS, Thalange NK, Foster PJ, Tillmann V, Price DA, Diggle PJ, Clayton PE. Regular fluctuations in growth hormone (GH) release determine normal human growth. Growth Horm IGF Res 9: 114–122, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Gill MS, Tillmann V, Veldhuis JD, Clayton PE. Patterns of GH output and their synchrony with short term height increments influence stature and growth performance in normal children. J Clin Endocrinol Metab 86: 5860–5863, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Glasscock GF, Hein AN, Miller JA, Hintz RL, Rosenfeld RG. Effects of continuous infusion of insulin-like growth factor I and II, alone and in combination with thyroxine or growth hormone, on the neonatal hypophysectomized rat. Endocrinology 130: 203–210, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Growth Hormone Research Society Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab 85: 3990–3993, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Guyda HJ. Four decades of growth hormone therapy for short children: what have we achieved? J Clin Endocrinol Metab 84: 4307–4316, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Hayashida T, Contopoulos AN. Immunological studies with rat pituitary growth hormone. I. Basic studies with immunodiffusion and antihormone tests. Gen Comp Endocrinol 9: 217–226, 1967 [DOI] [PubMed] [Google Scholar]
- 35.Hindmarsh PC, Fall CH, Pringle PJ, Osmond C, Brook CG. Peak and trough growth hormone concentrations have different associations with the insulin-like growth factor axis, body composition and metabolic parameters. J Clin Endocrinol Metab 82: 2172–2176, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Jansson JO, Eden S, Isaksson OG. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6: 128–150, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Jørgensen JO, Thuesen L, Müller J, Ovesen P, Skakkebaek NE, Christiansen JS. Three years of growth hormone treatment in growth hormone-deficient adults: near normalization of body composition and physical performance. Eur J Endocrinol 130: 224–228, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Kemp SF, Fielder PJ, Attie KM, Blethen SL, Reiter EO, Ford KM, Marian M, Dao LN, Lee HJ, Saenger P. Pharmacokinetic and pharmacodynamic characteristics of a long-acting growth hormone (GH) preparation (nutropin depot) in GH-deficient children. J Clin Endocrinol Metab 89: 3234–3240, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Labarta JI, Gargosky SE, Simpson DM, Lee PD, Argente J, Guevara-Aguirre J, Rosenfeld RG. Immunoblot studies of the acid-labile subunit (ALS) in biological fluids, normal human serum and in children with GH deficiency and GH receptor deficiency before and after long-term therapy with GH or IGF-I respectively. Clin Endocrinol (Oxf) 47: 657–666, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Laursen T, Jørgensen JO, Christiansen JS. Metabolic effects of growth hormone administered subcutaneously once or twice daily to growth hormone deficient adults. Clin Endocrinol (Oxf) 41: 337–343, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Lewitt MS, Saunders H, Phuyal JL, Baxter R. Complex formation by human insulin-like growth factor binding protein-3 and human acid-labile subunit in growth hormone-deficient rats. Endocrinology 134: 2404–2409, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Maiter D, Underwood LE, Maes M, Davenport ML, Ketelslegers JM. Different effects of intermittent and continuous growth hormone (GH) administration on serum somatomedin-C/insulin-like growth factor I and liver GH receptors in hypophysectomized rats. Endocrinology 123: 1053–1059, 1988 [DOI] [PubMed] [Google Scholar]
- 43.Martinez DA, Orth MW, Carr KE, Vanderby R, Jr, Vailas AC. Cortical bone growth and maturational changes in dwarf rats induced by recombinant human growth hormone. Am J Physiol Endocrinol Metab 270: E51–E59, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Miquet JG, Sotelo AI, Bartke A, Turyn D. Desensitization of the JAK2/STAT5 GH signaling pathway associated with increased CIS protein content in liver of pregnant mice. Am J Physiol Endocrinol Metab 289: E600–E607, 2005 [DOI] [PubMed] [Google Scholar]
- 45.O'Neal DN, Kalfas A, Dunning PL, Christopher MJ, Sawyer SD, Ward GM, Alford FP. The effect of 3 months of recombinant human growth hormone (GH) therapy on insulin and glucose-mediated glucose disposal and insulin secretion in GH-deficient adults: a minimal model analysis. J Clin Endocrinol Metab 79: 975–983, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev 19: 55–79, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Ooi G, Cohen FJ, Tseng LY, Rechler MM, Boisclair Y. Growth hormone stimulates transcription of the gene encoding the acid-labile subunit (ALS) of the circulating insulin-like growth factor binding protein complex and ALS promoter activity in rat liver. Mol Endocrinol 11: 997–1007, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Papaconstantinou J, Deford JH, Gerstner A, Hsieh CC, Boylston WH, Guigneaux MM, Flurkey K, Harrison DE. Hepatic gene and protein expression of primary components of the IGF-I axis in long lived Snell dwarf mice. Mech Ageing Dev 126: 692–704, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Phillip M, Hershkovitz E, Belotserkovsky O, Leiberman E, Limoni Y, Zadik Z. Once versus twice daily injections of growth hormone in children with idiopathic short stature. Acta Paediatr 87: 518–520, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Robinson KA, Willi SM, Bingel S, Buse MG. Decreased hexosamine biosynthesis in GH-deficient dwarf rat muscle. Reversal with GH, but not IGF-I, therapy. Am J Physiol Endocrinol Metab 276: E435–E442, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Singleton DA, Buschang PH, Behrents RG, Hinton RJ. Craniofacial growth in growth hormone-deficient rats after growth hormone supplementation. Am J Orthod Dentofacial Orthop 130: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Skottner A, Clark RG, Fryklund L, Robinson IC. Growth responses in a mutant dwarf rat to human growth hormone and recombinant human insulin-like growth factor I. Endocrinology 124: 2519–2526, 1989 [DOI] [PubMed] [Google Scholar]
- 52a.Stiles AD, D'Ercole AJ. The insulin-like growth factors and the lung. Am J Respir Cell Mol Biol 3: 93–100, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Suttie JM, Fennessy PF, Corson ID, Laas FJ, Crosbie SF, Butler JH, Gluckman PD. Pulsatile growth hormone, insulin-like growth factors and antler development in red deer (Cervus elaphus scoticus) stags. J Endocrinol 121: 351–360, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Tannenbaum GS, Martin JB, Colle E. Ultradian growth hormone rhythm in the rat: effects of feeding, hyperglycemia, and insulin-induced hypoglycemia. Endocrinology 99: 720–727, 1976 [DOI] [PubMed] [Google Scholar]
- 55.Thalange NK, Gill MS, Gill L, Whatmore AJ, Addison GM, Price DA, Clayton PE. Infradian rhythms in urinary growth hormone excretion. J Clin Endocrinol Metab 81: 100–106, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Tolle V, Bassant MH, Zizzari P, Poindessous-Jazat F, Tomasetto C, Epelbaum J, Bluet-Pajot MT. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology 143: 1353–1361, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Veldhuis JD, Carlson ML, Johnson ML. The pituitary gland secretes in bursts: appraising the nature of glandular secretory impulses by simultaneous multiple-parameter deconvolution of plasma hormone concentrations. Proc Natl Acad Sci USA 84: 7686–7690, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woelfle J, Chia DJ, Rotwein P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278: 51261–51266, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.