Abstract
Elevated central angiotensin II (ANG II) plays a critical role in the sympathoexcitation of chronic heart failure (CHF) by stimulating upregulated ANG II type 1 receptors (AT1R) in the rostral ventrolateral medulla (RVLM). However, the link between enhanced ANG II signaling and alterations in the electrophysiological characteristics of neurons in the RVLM remains unclear. In the present experiments, we screened for potentially altered genes in the medulla of rats with CHF that are directly related to neuronal membrane conductance using the Rat Genome 230 2.0 Array GeneChip. We found that CHF rats exhibited a 2.1-fold reduction in Kv4.3 gene expression, one of the main voltage-gated K+ channels, in the medulla. Real-time RT-PCR and Western blot analysis confirmed the downregulation of Kv4.3 in the RVLM of CHF rats. In intact animals, we found that microinjection of the voltage-gated potassium channel blocker, 4-aminopyridine, into the RVLM evoked a sympathoexcitation and hypertension in both normal and CHF rats. CHF rats exhibited smaller responses to 4-aminopyridine than did normal rats. Finally, we used a neuronal cell line (CATH.a neurons) to explore the effect of ANG II on Kv4.3 expression and function. We found that ANG II treatment significantly downregulated mRNA and protein expression of Kv4.3 and decreased the A-type K+ current. Employing this cell line, we also found that the ANG II-induced inhibition of Kv4.3 mRNA expression was attenuated by the superoxide scavenger Tempol and the p38 MAPK inhibitor SB-203580. The effects of ANG II were abolished by the AT1R antagonist losartan. We conclude that the sympathoexcitation observed in the CHF state may be due, in part, to an ANG II-induced downregulation of Kv4.3 expression and subsequent decrease in K+ current, thereby increasing the excitability of neurons in the RVLM. The ANG II-induced inhibition of Kv4.3 mRNA expression was mediated by ANG II-AT1R-ROS-p38 MAPK signaling.
Keywords: angiotensin II, K+ channel, reactive oxygen species, p38 mitogen-activated protein kinase
it is well accepted that chronic heart failure (CHF) is characterized by heightened sympathetic outflow (10), which is a compensatory adjustment to a reduction in cardiac function and may be beneficial to provide adequate peripheral tissue perfusion in the early phase of CHF (16). However, this compensatory mechanism gradually becomes more intense and sustained, thereby contributing to the progression of the CHF syndrome (19). During this period, the excessive sympathetic activation not only exacerbates the CHF state, but is also prognostic of sudden death and complications from this syndrome. Indeed, a strong consensus exists as to the adverse influence of sympathetic hyperactivity on the progression and outcome of CHF (4). The central mechanism(s) by which sympathoexcitation occurs in the CHF state, however, is still not completely clear.
A voluminous body of literature has solidly supported the idea that brain angiotensin II (ANG II) mechanisms contribute to the above-mentioned sympathoexcitation in the CHF state (6, 7, 49). Notable findings in CHF animals implicate an enhanced brain renin-angiotensin system, including increase in brain angiotensin converting enzyme (ACE) activity, ANG II type 1 receptor (AT1R) expression and binding, and increased dipsogenic responses to ANG II. Our laboratory's previous studies also clearly show an upregulated AT1R mRNA and protein expression in the rostral ventrolateral medulla (RVLM) of CHF rabbits (12) and an ANG II-induced overexpression of AT1R mRNA and protein in the RVLM of normal rabbits (13). These enhanced ANG II mechanisms are closely related to the sympathoexcitation of CHF, because blockade of central AT1R normalizes sympathetic tone in conscious CHF rabbits and rats (8). Furthermore, reduced sympathetic dysfunction after myocardial infarction has been observed in transgenic rats that lack brain angiotensinogen (43). However, the link between enhanced ANG II signaling and the electrophysiological characteristics of neurons in the RVLM of CHF animals remains unclear. In the present study, we used genetic, electrophysiolgical, and intact animal techniques to define a role for the Kv4.3 channel protein in the RVLM of rats with CHF. We hypothesized that ANG II-induced downregulation of Kv4.3 in the RVLM contributes to the sympathoexcitation in the CHF state.
METHODS
Forty-one male Sprague-Dawley rats, weighing between 320 and 410 g, were used in these experiments. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the American Physiological Society and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rat Model of CHF
CHF was produced by coronary artery ligation, as previously described (11). All rats were anesthetized with ketamine (100 mg/kg ip), intubated, and mechanically ventilated with room air. Under sterile conditions, a left thoracotomy was performed through the fifth intercostal space. After the lung was retracted, the pericardium was opened, and the heart was exteriorized. The left anterior descending coronary artery was ligated near its branch point from the aorta with a 6–0 silk suture that was passed through the superficial layers of myocardium, between the pulmonary artery outflow tract and left atrium. Following these maneuvers, the heart was placed in its original position, and the thorax was closed. The air within the thorax was evacuated, allowing the rats to resume spontaneous respiration and recover from anesthesia. Analgesia (Buprenorphine, Reckitte Benckiser, Hull, UK; 0.1 mg/kg sc) was administered after surgery. Sham-operated rats were prepared in the same manner but did not undergo coronary artery ligation.
The rats were caged in an environment with ambient temperature maintained at 22°C and humidity at 30–40%. Laboratory chow (Purina) and tap water were available ad libitum. The CHF rats used for DNA microarray analysis were studied at 6 mo after coronary ligation to obtain a sufficient amount of tissue. The CHF rats for all other experiments were studied at 6–8 wk after coronary ligation. Cardiac function and the degree of heart failure were determined by echocardiography (Acuson Sequoia 512 C). Under isoflourane anesthesia, a two-dimensional, short-axis view of the left ventricle (LV) was obtained at the level of the papillary muscles. M-mode tracings were recorded through the anterior and posterior LV walls, and anterior and posterior wall thicknesses (end-diastolic and end-systolic) and LV internal dimensions were also measured.
DNA Microarray
Three sham rats and three CHF rats were used for DNA microarray experiments. One sham and one CHF rat constituted a pair that was run on each microarray. The GeneChip (Rat Genome 230 2.0 array) was used to determine differentially expressed genes in the medulla of CHF vs. sham rats. We focused on those genes relevant to neuronal electrophysiological characteristics. The microarrays were carried out according to the standard Affymetrix protocol (Affymetrix Expression Analysis Technical Manual, Santa Clara, CA). Total RNA for DNA microarray was extracted from the medulla with TRIzol (Invitrogen, Carlsbad, CA). RNeasy columns (Qiagen, Valencia, CA) were employed to further purify the extracted total RNA. RNA was then quantified by spectrophotometry (absorbance 260 nm), and the quality of RNA was assessed by electrophoresis through formamide/formaldehyde agarose gel. The arrays were hybridized with biotin-labeled cRNA, prepared as per standard Affymetrix protocol (Affymetrix Expression Analysis Technical Manual). Briefly, total RNA (10 μg) from the right half of the medulla was reverse transcribed using an oligo(dT) primer coupled to a T7 RNA polymerase binding site. Double-stranded cDNA was made, and biotinylated cRNA was synthesized using T7 polymerase. The cRNA was hybridized for 16 h to an array, followed by binding with a streptavidin-conjugated fluorescent marker, and then incubated with a polyclonal anti-streptavidin antibody coupled to phycoerythrin as an amplification step. Following washing, the chips were scanned with a Hewlett-Packard GeneArray laser scanner, and data were analyzed using Gene-Chip software. External standards were included to control for hybridization efficiency and sensitivity.
Using the abovementioned DNA microarray, we found that 56 genes were altered in the medulla of CHF rats compared with that in shams. Among these altered genes, 23 genes were upregulated, and 33 genes were downregulated in the CHF state. The gene encoding the Kv4.3 channel protein was the only gene directly related to neuronal electrophysiological characteristics. In the following experiments, we focused on Kv4.3 K+ to determine its expression, function, and regulation in vivo and in vitro.
Real-time RT-PCR Analysis of Kv4.3 mRNA
We employed real-time RT-PCR to confirm that the decrease in Kv4.3 mRNA also occurred in the RVLM of CHF rats. The RVLM was punched out according to the technique described by Palkovits and Brownstein (28). Briefly, the rat brains were removed and immediately frozen on dry ice, blocked in the coronal plane, and sectioned at 100-μm thickness in a cryostat from 2.0 to 3.0 mm rostral to the obex. The RVLM was then punched using a punch-needle (0.6 mm inside diameter) from 10 sections. The punch site in each section was determined based on the description by Paxinos and Watson (29) and is variable in each individual section. The total span of punches was ∼1 mm, from 1.6 to 2.6 mm lateral to the midline. The punched tissues from the 10 sections were pooled for RNA extraction. The size of the punched tissue from a single RVLM of one rat was ∼0.28 mm3. Total RNA was extracted from bilateral RVLM of rats using RNeasy columns (Qiagen), which was then reverse transcribed into double-stranded cDNA. The RNA product was treated with RNase-free DNase I to remove the potential remaining DNA before it was reverse transcribed into cDNA. Real-time RT-PCR was carried out using a thermocycler (PTC-200 Peltier Thermal Cycler with CHROMO 4 Continuous Fluorescence Detector, BIO-RAD), according to the manufacturer's recommendations. Cycle numbers obtained at the log-linear phase of the reaction were plotted against a standard curve prepared with serially diluted control samples. Expression of target genes was normalized to GAPDH levels. The primers and probes used in this experiment were designed using free software obtained at https://www.genscript.com/ssl-bin/app/primer and synthesized in the Eppley Institute Molecular Biology Core Laboratory on the campus of the University of Nebraska Medical Center. Table 1 shows the gene-specific primers and probes to rat Kv4.3 and GAPDH genes.
Table 1.
Rat gene-specific primers and probes for real-time RT-PCR
| Gene Name (Accession No.) | Forward Primers | Reverse Primers | Probes | Amplicon Size, nt |
|---|---|---|---|---|
| Rat Kv4.3 (NM_031739.1) | CACCACCTGCTACACTGCTT | GCTTCTGGTGGATGGGTAGT | TGCATTGAGCTCTCCATGCAGTTCT | 114 |
| Rat GAPDH (NM_017008) | TCAAGAAGGTGGTGAAGCAG | AGGTGGAAGAATGGGAGTTG | CCGAGGGCCCACTAAAGGGC | 111 |
nt, Nucleotide number.
Western Blot Analysis of Kv4.3 Protein
The RVLM of rats was punched out as described above. Protein was extracted from the RVLM bilaterally using RIPA buffer, the concentration of which was then measured using a protein assay kit (Pierce, Rockford, IL) and was adjusted with equal volumes of 2× 4% SDS sample buffer. The samples were then boiled for 5 min, followed by loading on the 7.5% SDS-PAGE gel (5 μg protein/30 μl per well) for electrophoresis using a Bio-Rad mini gel apparatus at 40 mA/each gel for 45 min. Then the fractionized proteins on the gel were electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore) at 300 mA for 90 min. The membrane was probed with primary antibody (Kv4.3 goat polyclonal antibody, Santa Cruz, 1:1,000) and secondary antibody (rabbit anti-goat IgG-horseradish peroxidase, Santa Cruz, 1:2,500), and then treated with enhanced chemiluminescence substrate (Pierce, Rockford, IL) for 5 min at room temperature. The bands on the membrane were visualized and analyzed using a UVP BioImaging System (Upland, CA).
Acute Animal Experiments
We determined the effects of suppression of A-type K+ current in the RVLM on mean arterial pressure (MAP), heart rate (HR), and renal sympathetic nerve activity (RSNA) in sham and CHF rats. Each rat was anesthetized with urethane (800 mg/kg ip) and α-chloralose (40 mg/kg ip). Supplemental doses of anesthesia were administered at 1/10 of the initial dose per hour. Body temperature was maintained using a heating pad. Through a midline cervical incision, the trachea was cannulated to facilitate mechanical ventilation.
Recording of MAP and HR.
Through a midline incision in the neck, the common carotid artery was exposed and then catheterized with a Millar transducer (model SPR-524, Millar Instruments, Houston, TX) for measurement of MAP. HR was derived from the arterial pressure pulse using the tachometer function of a PowerLab model 16S (AD Instruments, Colorado Springs, CO).
Recording of RSNA.
RSNA was recorded as previously described (11). In brief, the left kidney, renal artery, and nerves were exposed through a retroperitoneal flank incision. The renal sympathetic nerves were placed on a pair of platinum-iridium recording electrodes. When an optimal signal-to-noise ratio was achieved, the electrode and the renal nerve were covered with a fast setting silicone (Kwik-Sil, World Precision Instruments, Sarasota, FL). The signal was amplified with a Grass direct current preamplifier (model P18D, Astro-Med, West Warwick, RI) with low-frequency cutoff set at 30–100 Hz and high-frequency cutoff at 1–3 kHz. The amplified output was monitored on a storage oscilloscope (model 121 N, Tektronix, Beaverton, OR) and then imported to a computer system with the hemodynamic parameters. A voltage integrator (Buxco Electronics, model 1801) was used for quantifying the raw RSNA. The raw nerve activity, integrated nerve activity, arterial pressure, and HR were recorded on a PowerLab model 16S and stored on the disk until analyzed. Noise levels were subtracted from the nerve recording data before percent changes from baseline were calculated. Integrated RSNA was normalized as 100% of baseline in the control period. The unit for the comparison of baseline RSNA between sham and CHF rats was “%max”, the maximum RSNA induced by intravenous infusion of nitroglycerin (25 μg per rat).
RVLM microinjection.
Rats were placed in a stereotaxic apparatus. An occipital craniotomy and partial cerebellar removal were performed to expose the dorsal surface of the brain stem. The dura was opened and retracted to expose the obex, the vertex of which was taken as a basal landmark for stereotaxic coordinates to target the RVLM. The RVLM (coordinates: 2.5–3.0 mm rostral to the obex, 1.8–2.1 mm lateral to midline, and 3.0–3.3 mm ventral to the dorsal surface of brain stem) was chemically identified by a transient pressor response (at least 20 mmHg) to injection of l-glutamate (5 nmol). Microinjections into the RVLM were performed from a three-barreled micropipette (tip diameter 20–50 μm) driven by a micromanipulator using a pressure injection system. The injected volume (50 nl) was measured by observing the movement of the fluid meniscus through a binocular microscope fitted with a calibrated eyepiece graticule. l-Glutamate and 4-aminopyridine (4-AP) were diluted in artificial cerebrospinal fluid.
Measurement of LV end-diastolic pressure (LVEDP) and cardiac infarct size.
At the end of the acute experiment, a Millar pressure catheter was advanced through the carotid artery into the LV to determine LVEDP. The rats were then euthanized with an overdose of pentobarbital, and the hearts were removed to measure infarct size.
In Vitro Experiments
A neuronal cell line (CATH.a) was employed to determine the effects of ANG II on Kv4.3 mRNA and protein expression and the A-type K+ current. CATH.a cells were originally derived from a brain stem catecholaminergic neuronal mouse cell line (38) and displays a differentiated neuronal phenotype and excitable membrane characteristics (20).
CATH.a culture.
CATH.a cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and grown in RPMI 1640 containing 8% horse serum, 4% fetal bovine serum, 1% penicillin-streptomycin, at 37°C in a humidified atmosphere equilibrated with 5% CO2. After subculture, cells were plated on polystyrene tissue culture dishes at a density of 1 × 107 cells/100-mm plate, or 1.5 × 106 cells/well in six-well culture plates with N6,2′-O-dibutyryl adenosine 3′,5′-monophosphate (1 mmol/l, Sigma) to grow for 2 days to obtain differentiated CATH.a cells (2, 9). The cells were treated with ANG II (100 nmol/l, 6 h). Cells were then used in the following experiments to record A-type K+ current by whole cell patch clamp to determine Kv4.3 gene expression by real-time RT-PCR and Western blot and to explore the intracellular signaling pathway mediating the ANG II-induced change in Kv4.3 gene expression.
Immunofluorescence.
CATH.a cells grown on the glass coverslips were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature. The cells then were washed with PBS, followed by incubation with primary antibodies (goat polyclonal IgG anti AT1R and rabbit polyclonal IgG anti-Kv4.3 antibodies; Santa Cruz) overnight at 4°C. On the following day, the cells were washed and incubated with secondary antibody (Alexa Fluor 488 donkey anti-rabbit IgG and Alexa Fluor 594 donkey anti-goat IgG, Molecular Probes; at 1:250 in the same diluent used for the primary antibodies).
Real-time RT-PCR analysis of Kv4.3 mRNA.
Real-time RT-PCR was used to determine the effects of ANG II on Kv4.3 mRNA expression in CATH.a cells. The initial process was the same as described above for intact animals, but the total RNA was extracted from CATH.a cell pellets instead of rat RVLM. In addition, the primers and probes were designed according to the mouse gene sequences. These sequences are shown in Table 2.
Table 2.
Mouse gene-specific primers and probes for real-time RT-PCR
| Gene Name (Accession No.) | Forward Primers | Reverse Primers | Probes | Amplicon Size, nt |
|---|---|---|---|---|
| Mouse Kv4.3 (NM_001039347) | ACACCTGCCCAACTCTAACC | TCAGTCCATCGTCTGCTTTC | CACCACCAGTCGCTCCAGCC | 140 |
| Mouse GAPDH (NM_001001303) | ACAACTTTGGCATTGTGGAA | GATGCAGGGATGATGTTCTG | CATGCCATCACTGCCACCCA | 133 |
Western blot analysis of Kv4.3 protein.
The initial process was the same as that described above for intact animals.
Recording of voltage-gated K+ current.
The whole cell patch-clamp technique was used to determine the effect of ANG II on voltage-gated K+ current in CATH.a cells. This experiment was carried out using a Warner PC-501A patch-clamp amplifier (Warner Instrument, Hamden, CT). Patch pipettes had resistances of 4–6 MΩ when filled with (in mM) 105 potassium-aspartate, 20 KCl, 10 EGTA, 5 Mg-ATP, 10 HEPES, and 25 glucose, pH 7.2. The extracellular solution consisted of the following composition (in mM): 140 NaCl, 5.4 KCl, 0.5 MgCl2, 5.5 HEPES, 11 glucose, 10 sucrose, pH 7.4. Na+ channels were blocked by TTX (0.5 μM). Cell membrane capacitance was determined by integrating the capacitative current evoked by a voltage step from 0 to 5 mV and dividing the resulting charge by the voltage step. Currents were not leak subtracted. Current traces were sampled at 10 kHz and filtered at 5 kHz. Holding potential was −70 mV. Current-voltage relations were elicited by test potentials over the range of −70 mV to +20 mV, with a duration of 400 ms in 10-mV increments (5 s between steps). Peak currents were measured for each test potential. P-clamp 8.1 programs (Axon Instruments) were used for data acquisition and analysis. All experiments were done at 22°C.
Statistical Analysis
Data are expressed as means ± SE. The Student's t-test was employed to compare the differences of gene expression and hemodynamic parameters between sham and CHF rats. A two-way ANOVA with a Bonferroni procedure for post hoc analysis was used in comparisons of K+ currents, RSNA, and Kv4.3 mRNA expression. A P value of <0.05 was considered statistically significant.
Microarray data analysis.
Analyses were conducted with BRB Array Tools developed by Simon and Peng, Probe (low-level) analysis. Low-level analysis, which converts probe level data to a gene level expression data, was done using robust multiarray average (RMA). RMA was implemented using the rma function of the affy package of the Bioconductor project (//www.bioconductor.org/) in the R programming language. RMA does background correction, normalization, and summarization of probe-level data. The background correction method corrects the perfect match probe intensities by using a model based on the assumption that the observed intensities are the sum of signal and noise. Quantile normalization is used to normalize the perfect match probes, and the calculation of summary expression measures was done using the median polish method, which fits a multichip linear model to the data, and gives the expression on a log2 scale. The rats were paired as CHF vs. sham by the day that the arrays were run. The log2 ratio of the CHF rat to the sham rat was computed, giving three ratios. A gene filter was applied before analysis to set a minimum fold change. A gene was excluded from analysis if none of the expression data values had at least a 1.2-fold change in either direction from the gene's median value. For each gene, a paired t-test with a random variance model was used to determine whether there was a significant difference in expression between the groups for that gene. To help control the false discovery rate, an α-level of 0.001 was used for comparison.
RESULTS
Body Weight, Heart Weight, Pulmonary Weight, Baseline Hemodynamics, RSNA, and Echo Data of CHF Rats
The CHF rats used for DNA microarray were 6 mo post-coronary ligation. Before the medulla samples were taken, various physiological parameters were measured and are shown in Table 3. Body weight was lower, but both heart weight and lung weight were higher in CHF rats compared with sham-operated rats. On the other hand, pleural fluid and ascites were found in all of the CHF rats, but not in the sham-operated rats. CHF rats also exhibited a significantly lower MAP and higher RSNA than sham rats. All CHF rats exhibited significantly higher LVEDP, and lower ejection fraction and fractional shortening compared with sham rats. The average infarct size in CHF rats was 41.2 ± 2.6%. The CHF rats used for all other experiments were 6–8 wk post-coronary ligation, whose physiological parameters were similar to our laboratory's previous report (14) (data not shown).
Table 3.
Characteristics of sham and CHF rats (6 mo after coronary artery ligation) that were used for microarray analysis
| Measurements | Sham | CHF |
|---|---|---|
| n | 3 | 3 |
| Body weight, g | 524.2 ± 11.7 | 362.8 ± 24.4* |
| Wet whole heart weight, g | 1.6 ± 0.2 | 2.3 ± 0.3* |
| Wet lung weight, g | 2.3 ± 0.3 | 4.1 ± 0.2* |
| MAP, mmHg | 114.3 ± 8.2 | 89.5 ± 10.6* |
| HR, beats/min | 342.4 ± 18.2 | 396.5 ± 26.3 |
| Infarct size, %LV area | 0 | 41.2 ± 2.6† |
| LVEDP, mmHg | 0.9 ± 1.1 | 14.6 ± 5.4† |
| Baseline RSNA, %maximum | 38.5 ± 2.7 | 66.4 ± 4.6* |
| EF, % | 83.4 ± 2.2 | 47.1 ± 5.1* |
| FS, % | 55.4 ± 1.9 | 21.9 ± 6.2* |
Values are means ± SE; n, no. of rats. CHF, chronic heart failure; MAP, mean arterial pressure; HR, heart rate; LVEDP, left ventricular end-diastolic pressure; RSNA, renal sympathetic nerve activity; EF, ejection fraction; FS, fractional shortening.
P < 0.05 and
P < 0.01 compared with sham.
DNA Microarray Analysis of Medulla
The original purpose of this project was to explore the central mechanisms underlying sympathoexcitation in the CHF state. We hoped to identify altered genes that are involved in modulation of the electrophysiological characteristics of neurons in the RVLM of CHF rats. However, because the amount of total RNA extracted from the RVLM was much less than needed, we used the total RNA from the medulla of each rat for this microarray analysis.
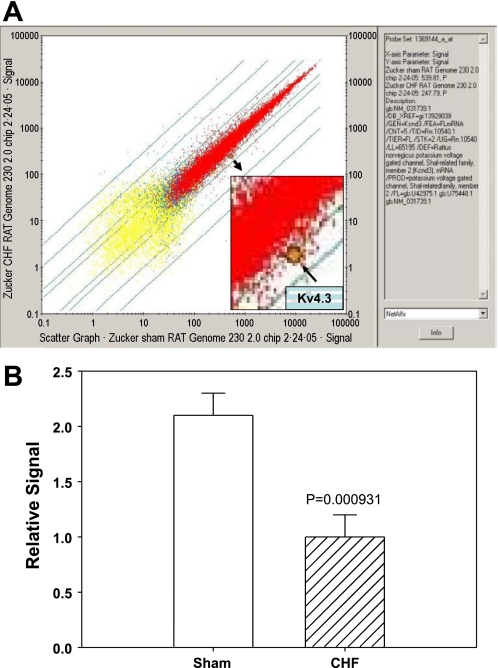
Figure 1A shows original DNA microarray data from one sham and one CHF rat. In this figure, each dot represents one gene. We compared 31,099 genes in the medulla of CHF and sham rats. The yellow dots represent genes that were not expressed in the rat medulla, the red dots represent genes that were expressed in both samples, and the blue dots represent the genes expressed in only one sample, either from sham or from CHF. After the minimum fold change filter was applied, 14,638 genes were available for analysis. A paired analysis found 56 genes that were significantly changed at the 0.001 level. From permutation tests, the probability of getting at least 56 genes significant by chance (at the 0.001 level), if there are no real differences between the groups, is 0.5. Among these altered genes, 23 genes were upregulated and 33 genes downregulated in the CHF state. These genes could be characterized as G protein-coupled receptors, transcription factors, signal transduction proteins, synthases, deaminases, neurotransmitter transporters, and other unknown genes (please see details in the table in the online supplement; the online version of this article contains supplemental data). Among these altered genes, Kv4.3 [the highlighted dot in Fig. 1; gene ID: 1369144; gene bank accession number: NM031739.1; gene information: potassium voltage-gated channel, Shal related family, member 3 (Kcnd3), mRNA] was the only gene that is directly related to neuronal membrane electrophysiological characteristics. Figure 1B shows mean data, indicating that CHF rats expressed reduced Kv4.3 message in the medulla by 2.1-fold.
Fig. 1.
Gene expression analysis of chronic heart failure (CHF) and sham medulla using the Affymetrix microarray chip (rat genome 230). A: scatter plot of gene expression comparisons from one CHF and one sham rat medulla. Each point represents a single gene or expressed sequence tag. Horizontal axis: sham rat; vertical axis: CHF rat; highlighted point represents the Kv4.3 gene, showing the decreased mRNA expression in CHF rat compared with sham. B: group data for microarrays shows the decrease in Kv4.3 gene expression in the medulla of CHF rats compared with sham. Values are means ± SE; n = 3 pairs. *P < 0.05.
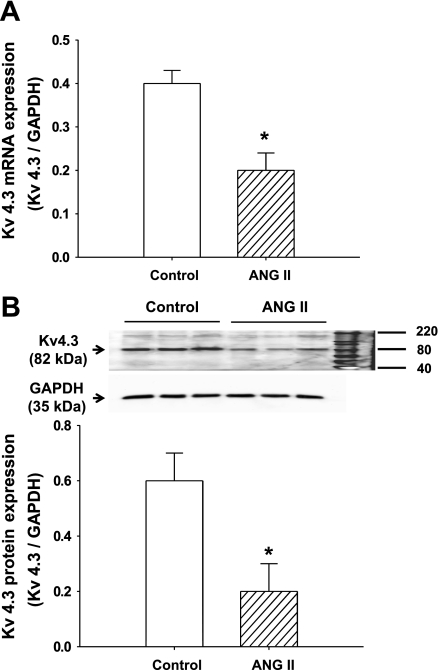
mRNA and Protein Expression of Kv4.3 in RVLM of CHF Rats
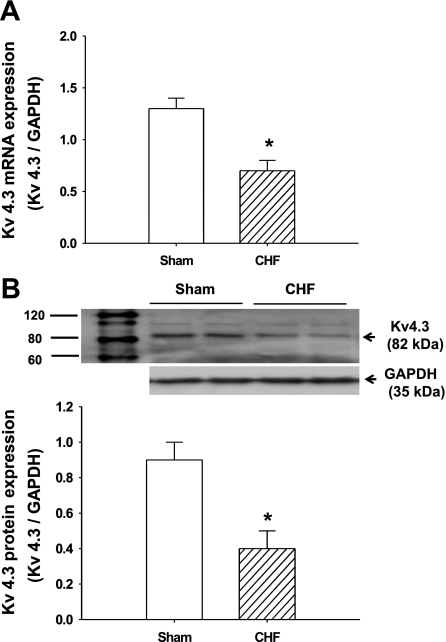
Following microarray analysis showing a significant decrease in the expression of Kv4.3 in the medulla of CHF rats, we then confirmed that this change occurs in the RVLM. The RVLM is a primary nucleus in the medulla that regulates sympathetic outflow by sending direct projections to the intermediolateral cell column of the spinal cord. As expected, both the mRNA (sham: 1.3 ± 0.1, CHF: 0.7 ± 0.1, P < 0.05, n = 5) and protein (sham: 0.9 ± 0.1, CHF: 0.4 ± 0.1, P < 0.05, n = 6) expression of Kv4.3 were significantly downregulated in the RVLM of CHF rats compared with sham (Fig. 2).
Fig. 2.
The expression of Kv4.3 in the rostral ventrolateral medulla (RVLM) of CHF and sham rats. A: group data showing the Kv4.3 mRNA expression of the RVLM from CHF and sham rats by real-time RT-PCR. *P < 0.05 compared with sham. Values are means ± SE; n = 5 each group. B, top: representative Western blots showing the downregulation of Kv4.3 protein expression in the RVLM of CHF rats. Bottom: mean data from densitometric analysis. Values are means ± SE; n = 6 each group. *P < 0.05 compared with sham.
Effect of 4-AP in the RVLM on RSNA
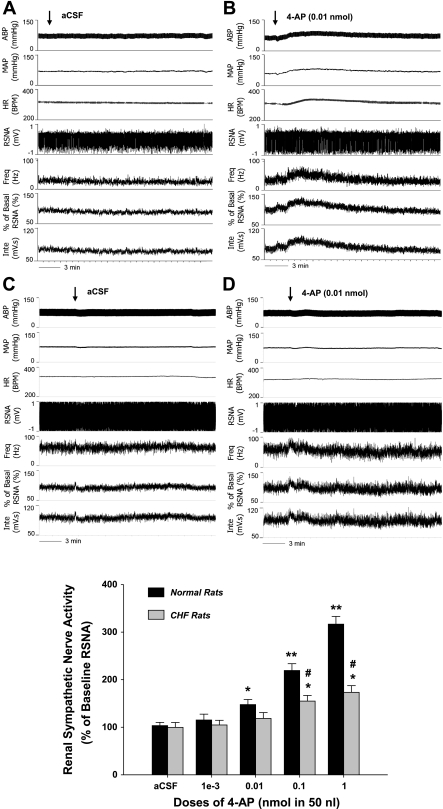
To evaluate the functional significance of the downregulation of Kv4.3 expression in the RVLM of CHF rats, the voltage-gated K+ channel blocker, 4-AP, was unilaterally microinjected into the RVLM of anesthetized sham (n = 7) and CHF rats (n = 6) to determine its influence on RSNA. As indicated in Fig. 3, this treatment resulted in a dose-dependent increase in RSNA in both groups. The initial effective dose was 0.01 nmol for sham rats and 0.1 nmol for CHF rats. We also found that, between 0.1 and 1 nmol, 4-AP evoked smaller sympathoexcitatory responses in CHF rats than in sham rats. The onset latency of these effects was ∼1 min, and the peak effect was reached ∼3 min after administration.
Fig. 3.
The effects of unilateral microinjection of the voltage-gated K+ channel inhibitor, 4-aminopyridine (4-AP), into the RVLM on renal sympathetic nerve activity (RSNA) in anesthetized sham and CHF rats. B and D: representative original recordings showing the increased mean arterial pressure (MAP), heart rate (HR), and RSNA after microinjection of 4-AP (0.01 nmol in 50 nl) into the RVLM of a sham (B) and CHF (D) rat. A and C: the artificial cerebrospinal fluid (aCSF) control recordings. ABP, arterial blood pressure; bpm, beats/min; Inte, ??. Bottom: group data showing a dose-dependent increase in RSNA in response to the RVLM microinjection of 4-AP (50 nl microinjection of 0.02–20 mM). Values are means ± SE; sham rats: n = 7 and CHF rats: n = 6. *P < 0.05 and **P < 0.01 compared with aCSF; #P < 0.05 compared with sham rats at the same dose of 4-AP.
In addition to the RSNA, we found that 4-AP in the RVLM dose-dependently evoked hypertension and tachycardia in both groups. In normal rats, 0.01, 0.1, and 1 nmol 4-AP increased MAP by 9.6 ± 2.4, 16.4 ± 2.1, and 23.7 ± 4.3 mmHg, P < 0.05, and HR by 19.6 ± 3.1, 23.8 ± 5.7, and 29.8 ± 6.3 beats/min, P < 0.05, respectively. However, in CHF rats, only 0.1 and 1 nmol 4-AP significantly elevated MAP by 11.8 ± 2.6 and 13.6 ± 3.2 mmHg, P < 0.05, and HR by 21.6 ± 6.2 and 24.2 ± 7.1 beats/min, P < 0.05, respectively.
Colocalization of AT1R and Kv4.3 in the Membrane of CATH.a Cells
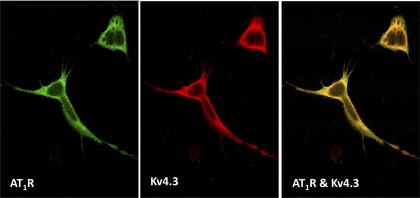
CHF is characterized, in part, by elevated ANG II in both the central nervous system (44) and in peripheral blood (24). On the other hand, evidence has indicated that ANG II downregulates Kv4.3 expression in cardiac myocytes (46). We, therefore, hypothesized that a similar mechanism underlies the downregulation of Kv4.3 expression in neurons. CATH.a cells are a central nervous system catecholaminergic cell line derived from the mouse locus coeruleus and express a variety of pan-neuronal markers, including voltage-gated K+ channels (20, 38). In the present study, we demonstrate the colocalization of the AT1R and Kv4.3 protein in the membrane of CATH.a cells (Fig. 4), suggesting that this neuronal cell line is an appropriate in vitro model to explore the effects of ANG II on Kv4.3 expression and function.
Fig. 4.
Confocal image showing the colocalization (right, yellow) of angiotensin II (ANG II) type 1 receptor (AT1R) immunoreactivity (left, green) and Kv4.3 immunoreactivity (middle, red) in the membrane of the body and process of CATH.a neurons.
Effects of ANG II on mRNA and Protein Expression of Kv4.3 in CATH.a Cells
Real-time RT-PCR and Western blot analysis indicated that ANG II treatment significantly decreases the Kv4.3 mRNA and protein expression. The maximum reductions of mRNA and protein by ANG II (100 nM for 6 h) were −48.6 ± 3.7 and −67.4 ± 8.1%, respectively, when normalized to GAPDH (Fig. 5).
Fig. 5.
The expression of Kv4.3 in CATH.a neurons treated with ANG II. A: group data (real-time RT-PCR) shows the Kv4.3 mRNA expression in CATH.a neurons treated with 100 nM ANG II for 6 h. *P < 0.05 compared with control, repeated 4 times. B, top: representative Western blots showing the downregulation of Kv4.3 protein expression by ANG II (100 nM, 6 h). B, bottom: results of densitometric analysis. *P < 0.05 compared with control, repeated 4 times. Values are means ± SE.
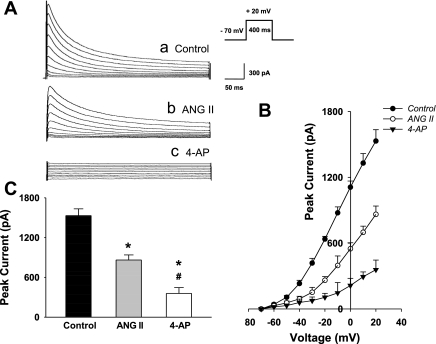
Effects of ANG II on K+ Current in CATH.a Cells
There were no significant differences in the whole cell capacitance between control (10.25 ± 1.04 pF) and ANG II-treated (10.98 ± 1.34 pF) groups. Thus we used absolute currents to reflect current density in the cells.
Figure 6 shows the whole cell patch-clamp data. Figure 6A is the representative K+ current tracings from control cells, ANG II-treated cells, and cells treated with the potassium channel blocker, 4-AP. As can be seen in this panel, the ANG II-treated cell exhibits a smaller, transient K+ current than the control cell does, suggesting that ANG II decreases the neuronal K+ current. The K+ channel blocker, 4-AP, completely abolished this current, providing evidence that this current is primarily mediated by voltage-gated K+ channels. Fig. 6B shows the complete current-voltage relationships. When the test voltage was depolarized above −40 mV from its holding potential (−70 mV), the K+ current was significantly smaller in the ANG II-treated cells compared with the control cells. As can be seen in the bar graphs, at 20 mV of test voltage, there was a clear decrease in K+ current density in the ANG II-treated cells.
Fig. 6.
Effects of ANG II treatment on the voltage-gated K+ current in CATH.a neurons. A: representative whole cell patch-clamp recording showing that the transient K+ current was partially attenuated by ANG II treatment (100 nM for 6 h) and completely inhibited by the voltage-gated K+ channel blocker, 4-AP (1 mM), in CATH.a neurons. B: complete current-voltage relationships in CATH.a neurons from control (n = 12), ANG II-treated (n = 9), and 4-AP-perfused (n = 6) neurons. C: peak K+ current measured in response to a test pulse from −70 to + 20 mV in control, ANG II-treated, and 4-AP-perfused CATH.a neurons. Values are means ± SE. *P < 0.05 compared with control group. #P < 0.05 compared with ANG II group.
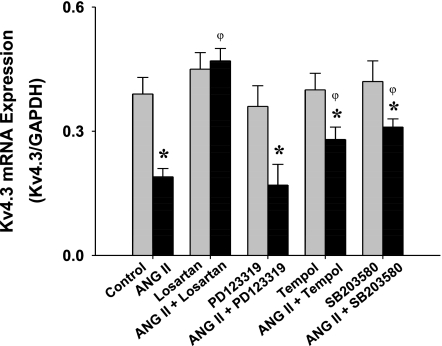
The Intracellular Signaling Pathway Mediating ANG II Induced Downregulation of Kv4.3 Expression
To explore the mechanisms underlying the downregulation of Kv4.3 by ANG II in CATH.a neurons, we employed a selective AT1R antagonist, losartan (10 μM, Merck), a selective AT2R antagonist, PD-123319 (10 μM, Sigma), a superoxide scavenger, Tempol (10 μM, Calbiochem), and the p38 MAPK inhibitor, SB-203580 (20 μM, Calbiochem), to determine the effects of these agents on the ANG II-induced (100 nM, Sigma) decrease in Kv4.3 mRNA expression. As can be seen in Fig. 7, losartan completely abolished the ANG II effect on Kv4.3 mRNA expression. PD-123319, however, had no effect. Both Tempol and SB-203580 partially blocked the inhibitory effect of ANG II on Kv4.3 mRNA expression. Treatment with all of these blockers alone did not exert significant alterations in baseline Kv4.3 mRNA expression.
Fig. 7.
Effects of losartan (10 μM), PD-123319 (10 μM), Tempol (10 μM), and SB-203580 (20 μM) on ANG II (100 nM)-induced downregulation of Kv4.3 mRNA expression. *P < 0.05 compared with control. φP < 0.05 compared with ANG II-treated group, repeated 4 times.
DISCUSSION
The novel finding of this study is the downregulation of Kv4.3 mRNA and protein expression in the RVLM of rats with CHF and the potential role of this decreased Kv4.3 level in the sympathoexcitation in this syndrome. The diversity of potassium channels determines the variety of central neuronal firing patterns and, therefore, physiological function (25, 30). Subthreshold-operating A-type K+ channels are of particular interest because of their roles in regulating firing frequency, spike initiation, and waveform (5, 15). It is believed that Shal-related (Kv4) proteins are the main components of the channels generating many of the subthreshold-operating A-type currents that are recorded in the somatodendritic compartment of neurons in the central nervous system (34, 41). Three distinct genes have been identified that encode mammalian Shal homologs (Kv4.1, Kv4.2, and Kv4.3), of which the latter two are abundant in adult rat brain (33). Decreased Kv4.3 expression implies a lower A-type potassium current and higher neuronal excitability and, therefore, hyperactivity of RVLM neurons in rats with CHF. The RVLM plays a pivotal role in the regulation of sympathetic nerve activity via direct projections to the intermediolateral cell column of the spinal cord. Activation of RVLM will evoke a significant increase in sympathetic nerve activity and blood pressure (3, 18). The downregulation of Kv4.3 expression in the RVLM of CHF rats, thereby, would be, at least in part, responsible for the sympathoexcitation in this syndrome.
To clarify the functional significance of the decreased Kv4.3 expression in the RVLM of CHF rats, we observed the influence of blocking RVLM K+ current on sympathetic outflow in anesthetized rats. Microinjection of 4-AP, a voltage-gated potassium channel blocker, into the RVLM dose-dependently increased RSNA, blood pressure, and HR in both normal and CHF rats. These data provide direct evidence showing that reduction of K+ current in the RVLM evokes sympathetic hyperactivity. Moreover, we observed that CHF rats exhibited smaller sympathoexcitation and pressor responses than sham rats following similar doses of 4-AP, strongly suggesting a suppressed K+ channel function in this syndrome. This alteration in K+ channel function in CHF rats is likely attributed to the above-mentioned changes in Kv4.3 expression. Employing two normal rats, we tested the effects of heteropodatoxin, a more specific blocker of Kv4.x channels (27, 31), given into the RVLM on RSNA. However, we did not find a difference in the sympathoexcitatory responses between heteropodatoxin and 4-AP (data not shown). Kv4.x channels represent the main components of the subthreshold-operating A-type currents in the neurons of the central nervous system (32, 34), and, therefore, it is not likely that significant differences between the inhibition by these two agents would be observed.
Interestingly, Kaab et al. (17) also reported a downregulation of Kv4.3 mRNA in ventricular myocytes of CHF patients, similar to that which has been shown in the hypertrophied myocardium of hypertensive rats by other investigators (21, 40, 45). These data suggest the downregulation of this channel as a common phenomenon in both peripheral and central tissues in the CHF and hypertensive states. However, the precise mechanisms underlying the downregulation of Kv4.3 in these pathological conditions are unknown. In hypertensive rats, Takimoto et al. (40) found that the downregulation of cardiac Kv4.3 mRNA and protein was blocked by ACE inhibition, implicating an involvement of ANG II in this negative control of Kv4.3 gene expression. In cultured neonatal rat cardiac myocytes, Zhang et al. (46) demonstrated that Kv4.3 mRNA and protein were downregulated independently by ANG II and phenylephrine. Their results further indicated that phenylephrine inhibits transcription of the Kv4.3 gene, whereas the effect of ANG II likely involves a destabilization of this channel mRNA, a more rapid mechanism to decrease message. Given the elevated ANG II level in both plasma (24) and cerebrospinal fluid (44) in CHF animals, we, therefore, hypothesized that ANG II plays an important role in the negative regulation of Kv4.3 expression in the central nervous system.
To explore the underlying mechanisms, we employed a neuronal cell line, CATH.a cells, to determine the effects of ANG II on Kv4.3 expression and potassium current in vitro. CATH.a cells were derived from tyrosine hydroxylase-positive tumors that developed in the locus coeruleus of a transgenic mouse carrying the SV40 T-antigen oncogene. These are neuronlike cells, because of the presence of neurofilaments and synaptophysin (38). Using the isotopic ANG II receptor binding technique Sun et al. (37) demonstrated the presence of both AT1R and AT2R in these cells, and, by the immunohistochemical technique, we clearly demonstrated the coexpression of AT1R and Kv4.3 protein in the membrane of differentiated CATH.a cells (Fig. 4). CATH.a cells, therefore, appear to be an appropriate model to determine the influence of ANG II on Kv4.3 expression and function. Employing these neurons, we found that incubation with ANG II for 6 h significantly downregulated the Kv4.3 mRNA and protein expression. These results suggest that, in the CHF state where endogenous ANG II levels are elevated, ANG II may contribute to the downregulation of Kv4.3 gene expression in the RVLM.
In cultured neonatal rat ventricular myocytes, Zhou et al. (48) demonstrated that the 4.9 kb 3′ untranslated region (3′ UTR) of the Kv4.3 channel gene was responsible for this gene's response to ANG II, and the destabilization of the 3′ UTR fully accounts for the downregulation of Kv4.3 mRNA by ANG II. They further found that this regulation is mediated by the AT1R and abolished by NAD(P)H oxidase inhibitors (Apocynin), superoxide dismutase, and the p38 MAP kinase inhibitor SB-239063, suggesting the involvement of AT1R-NAD(P)H-ROS-p38 signaling in this ANG II-induced negative control of Kv4.3 gene expression. Indeed, as shown in Fig. 7, ANG II-induced downregulation of Kv4.3 in CATH.a cells was abolished or attenuated by Losartan, Tempol, or SB-203580, suggesting a similar intracellular signaling pathway mediating ANG II effects in both myocytes and neurons. Interestingly, employing deletion analysis and mutagenesis, Zhou et al. (47) identified an AU-rich element (ARE) in the Kv4.3 3′ UTR as a requirement for ANG II-induced Kv4.3 mRNA destabilization in rat myocytes. They further demonstrated that ANG II could upregulate ARE/poly-(U)-binding/degradation factor 1, which, in turn, binds to the ARE of the Kv4.3 3′ UTR to destabilize this channel mRNA. We postulate that the same mechanisms might also underlie the regulation of Kv4.3 gene expression by ANG II in rat neurons.
Potassium channel gene expression is dynamically controlled in cardiac and neuronal cells by physiological stimuli, pathophysiological conditions, and drugs (23). In addition, the experimental conditions (in vitro vs. in vivo) and cell types (neurons vs. myocardial cells) could also influence potassium channel turnover. In the present study, we found that treatment with ANG II for 6 h significantly decreased Kv4.3 protein. While we do not know the exact half-life of Kv4.3 protein in these differentiated CATH.a cells, based on the data from clonal pituitary cells (39), Kv1.5 protein, another voltage-gated potassium channel, turned over with a half-life less than 4 h. Moreover, Levitan et al. (22) demonstrated that elevating extracellular K+ for 8 h significantly decreased Kv1.5 protein expression. These data suggested a possible short half-life of the potassium channel proteins in vitro.
ANG II has long been known to profoundly influence neuronal electrophysiological characteristics. Employing neonatal rat hypothalamus/brain stem cultured neurons, Wang et al. (42) demonstrated that ANG II perfusion significantly inhibited A-type K+ current and markedly reduced single A-type K+ channel activity by decreasing open probability. Using acutely isolated nodose ganglion neurons from adult rats, Moreira et al. (26) found that ANG II had no effect on total outward K+ current but significantly inhibited fast-activating and fast-inactivating K+ currents, indicating that ANG II can block neuronal A-type K+ current. These results represent an “acute effect” of ANG II on neuronal K+ current, probably via an alteration in the dimensional structure of channel protein. In the present experiment, we found that, after incubation with ANG II for 6 h, CATH.a cells exhibited a significantly lower A-type K+ current compared with the control cells and was concomitant with the downregulation of Kv4.3 protein expression. These results imply a potential “chronic effect” of ANG II on neuronal K+ current.
Based on the current experiments, it is not clear if the alterations in Kv4.3 protein and K+ current in the RVLM are unique to the CHF model, or if these changes in CHF are unique to the RVLM. Sonner et al. (35) described a diminished A-type K+ current in the RVLM-projecting PVN neurons of renovascular hypertensive rats, which contributed to sympathoexcitation and increased blood pressure in hypertensive rats. Employing immunohistochemical techniques, this group further demonstrated that the A-type K+ current in PVN-RVLM neurons of rats is mediated by Kv4.3 and/or Kv1.4 channel subunits (36). On the other hand, the data from Belugin and Mifflin (1) documented a reduced A-type K+ current in NTS neurons from renal wrap hypertensive rats. These reports strongly suggest a close involvement of suppressed A-type K+ current within more universal brain regions involved in sympathoexcitation under pathological conditions. Indeed, our DNA microarray data, which were derived from whole medullary tissue, also implies that the changes of Kv4.3 expression may occur in more regions than just the RVLM in CHF rats. Even though we focused only on the RVLM in the current experiment, we acknowledge that other regions may also be participating in the Kv4.3 response in the CHF state.
In conclusion, the present study unveiled a potential novel mechanism underlying the sympathoexcitation observed in the CHF state, the downregulation of Kv4.3 mRNA, and protein expression in the RVLM. We further demonstrated that, in a brain stem neuron cell line, ANG II downregulated the Kv4.3 mRNA and protein expression and decreased A-type K+ current through the AT1R via ANG II-ROS-p38 MAPK signaling cascade.
GRANTS
This study was supported by American Heart Association Scientist Development Grant 0635007N and National Institutes of Health Grants RO1HL093028, PO1HL62222, and RO1HL038690.
DISCLOSURES
I am not aware of financial conflict(s) with the subject matter or materials discussed in this manuscript with any of the authors, or any of the authors' academic institutions or employers.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the expert technical assistance of Johnnie F. Hackley, Phyllis Anding, and Li Yu. The authors also thank the DNA Microarray Core Facility of University of Nebraska Medical Center for professional assistance in Gene Chip analysis. Finally, we thank Merck and Company for donation of losartan.
REFERENCES
- 1.Belugin S, Mifflin S. Transient voltage-dependent potassium currents are reduced in NTS neurons isolated from renal wrap hypertensive rats. J Neurophysiol 94: 3849–3859, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bundey RA, Jones PG, Kendall DA. An investigation of noradrenaline uptake and release by the CATH.a cell line. J Neurochem 74: 799–806, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Chida K, Miyagawa M, Kawamura H, Takasu T. Effects of chemical stimulation of the rostral ventrolateral medulla on cerebral and renal microcirculation in spontaneously hypertensive rats. J Auton Nerv Syst 70: 51–55, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol 213: 31–53, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dampney RA, Fontes MA, Hirooka Y, Horiuchi J, Potts PD, Tagawa T. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 467–472, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177: 209–218, 2003 [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF, Jones SY, Brooks VL. ANG II receptor blockade and arterial baroreflex regulation of renal nerve activity in cardiac failure. Am J Physiol Regul Integr Comp Physiol 269: R1189–R1196, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Du JQ, Sun CW, Tang JS. Effect of angiotensin II type 1 receptor on delayed rectifier potassium current in catecholaminergic CATH.a cells. Acta Pharmacol Sin 25: 1145–1150, 2004 [PubMed] [Google Scholar]
- 10.Francis GS. Neurohumoral mechanisms involved in congestive heart failure. Am J Cardiol 55: 15A–21A, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension 45: 1173–1181, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 95: 937–944, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52: 708–714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getting PA. Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol 49: 1036–1050, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Just H. Peripheral adaptations in congestive heart failure: a review. Am J Med 90: 23S-–26S., 1991 [DOI] [PubMed] [Google Scholar]
- 17.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98: 1383–1393, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Kapoor V, Minson J, Chalmers J. Ventral medulla stimulation increases blood pressure and spinal cord amino acid release. Neuroreport 3: 55–58, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol 26: 1257–1263, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Lazaroff M, Dunlap K, Chikaraishi DM. A CNS catecholaminergic cell line expresses voltage-gated currents. J Membr Biol 151: 279–291, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Lee JK, Nishiyama A, Kambe F, Seo H, Takeuchi S, Kamiya K, Kodama I, Toyama J. Downregulation of voltage-gated K+ channels in rat heart with right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 277: H1725–H1731, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Levitan ES, Gealy R, Trimmer JS, Takimoto K. Membrane depolarization inhibits Kv1.5 voltage-gated K+ channel gene transcription and protein expression in pituitary cells. J Biol Chem 270: 6036–6041, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Levitan ES, Takimoto K. Dynamic regulation of K+ channel gene expression in differentiated cells. J Neurobiol 37: 60–68, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure. A role for angiotensin II. Circulation 102: 1854–1862, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242: 1654–1664, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Moreira TH, Cruz JS, Weinreich D. Angiotensin II increases excitability and inhibits a transient potassium current in vagal primary sensory neurons. Neuropeptides 43: 193–199, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol 525: 285–298, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain New York: Elsevier, 1988 [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic, 1997 [Google Scholar]
- 30.Rudy B. Diversity and ubiquity of K channels. Neuroscience 25: 729–749, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Sanguinetti MC, Johnson JH, Hammerland LG, Kelbaugh PR, Volkmann RA, Saccomano NA, Mueller AL. Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels. Mol Pharmacol 51: 491–498, 1997 [PubMed] [Google Scholar]
- 32.Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol 72: 1516–1529, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol 79: 1081–1091, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Serodio P, Vega-Saenz dM, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol 75: 2174–2179, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol 586: 1605–1622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonner PM, Stern JE. Functional role of A-type potassium currents in rat presympathetic PVN neurones. J Physiol 582: 1219–1238, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun C, Du J, Raizada MK, Sumners C. Modulation of delayed rectifier potassium current by angiotensin II in CATH.a cells. Biochem Biophys Res Commun 310: 710–714, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Suri C, Fung BP, Tischler AS, Chikaraishi DM. Catecholaminergic cell lines from the brain and adrenal glands of tyrosine hydroxylase-SV40 T antigen transgenic mice. J Neurosci 13: 1280–1291, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takimoto K, Fomina AF, Gealy R, Trimmer JS, Levitan ES. Dexamethasone rapidly induces Kv1.5 K+ channel gene transcription and expression in clonal pituitary cells. Neuron 11: 359–369, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Takimoto K, Li D, Hershman KM, Li P, Jackson EK, Levitan ES. Decreased expression of Kv4.2 and novel Kv43 K+ channel subunit mRNAs in ventricles of renovascular hypertensive rats. Circ Res 81: 533–539, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J Neurosci 15: 1741–1754, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Sumners C, Posner P, Gelband CH. A-type K+ current in neurons cultured from neonatal rat hypothalamus and brain stem: modulation by angiotensin II. J Neurophysiol 78: 1021–1029, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Huang BS, Ganten D, Leenen FH. Prevention of sympathetic and cardiac dysfunction after myocardial infarction in transgenic rats deficient in brain angiotensinogen. Circ Res 94: 843, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Ma R. Cardiac sympathetic afferent reflexes in heart failure. Heart Fail Rev 5: 57–71, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Zhang TT, Cui B, Dai DZ. Downregulation of Kv4.2 and Kv4.3 channel gene expression in right ventricular hypertrophy induced by monocrotaline in rat. Acta Pharmacol Sin 25: 226–230, 2004 [PubMed] [Google Scholar]
- 46.Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circ Res 88: 476–482, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Zhou C, Vignere CZ, Levitan ES. AUF1 is upregulated by angiotensin II to destabilize cardiac Kv4.3 channel. mRNA J Mol Cell Cardiol 45: 832–838, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou C, Ziegler C, Birder LA, Stewart AF, Levitan ES. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res 98: 1040–1047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zucker IH, Liu JL. Angiotensin II–nitric oxide interactions in the control of sympathetic outflow in heart failure. Heart Fail Rev 5: 27–43, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.