Abstract
This study sought to test the hypothesis that orthostasis-induced cerebral hypoperfusion would be less severe in physically active elderly humans (ACT group) than in sedentary elderly humans (SED group). The peak O2 uptake of 10 SED (67.1 ± 1.4 yr) and 9 ACT (68.0 ± 1.1 yr) volunteers was determined by a graded cycling exercise test (22.1 ± 1.2 vs 35.8 ± 1.3 ml·min−1·kg−1, P < 0.01). Baseline mean arterial pressure (MAP; tonometry) and middle cerebral arterial blood flow velocity (VMCA; transcranial Doppler) were similar between the groups (SED vs. ACT group: 91 ± 3 vs. 87 ± 3 mmHg and 54.9 ± 2.3 vs. 57.8 ± 3.2 cm/s, respectively), whereas heart rate was higher and stroke volume (bioimpedance) was smaller in the SED group than in the ACT group. Central hypovolemia during graded lower body negative pressure (LBNP) was larger (P < 0.01) in the ACT group than in the SED group. However, the slope of VMCA/LBNP was smaller (P < 0.05) in the ACT group (0.159 ± 0.016 cm/s/Torr) than in the SED group (0.211 ± 0.008 cm/s/Torr). During LBNP, the SED group had a greater augmentation of cerebral vasomotor tone (P < 0.05) and hypocapnia (P < 0.001) compared with the ACT group. Baseline MAP variability and VMCA variability were significantly smaller in the SED group than in the ACT group, i.e., 0.49 ± 0.07 vs. 1.04 ± 0.16 (mmHg)2 and 1.06 ± 0.19 vs. 4.24 ± 1.59 (cm/s)2, respectively. However, transfer function gain, coherence, and phase between MAP and VMCA signals (Welch spectral estimator) from 0.08–0.18 Hz were not different between SED (1.41 ± 0.18 cm·s−1·mmHg−1, 0.63 ± 0.06 units, and 38.03 ± 6.57°) and ACT (1.65 ± 0.44 cm·s−1·mmHg−1, 0.56 ± 0.05 units, and 48.55 ± 11.84°) groups. We conclude that a physically active lifestyle improves the intrinsic mechanism of cerebral autoregulation and helps mitigate cerebral hypoperfusion during central hypovolemia in healthy elderly adults.
Keywords: cerebral vasomotor tone, hypocapnia, transfer function gain, lower body negative pressure
cerebral hypoperfusion, indicated by a decrease in middle cerebral arterial blood flow velocity (VMCA), occurs with orthostasis-induced central hypovolemia during upright standing (10, 11, 14, 18, 20, 23, 40), head-up tilt (21, 29, 30, 38), or lower body negative pressure (LBNP) (13, 15, 26, 53). Deterioration of cerebral autoregulation or cerebral perfusion may be associated with orthostatic intolerance with or without pathophysiological conditions (14, 15, 20, 29, 53). Since healthy senescence (i.e., primary aging) is commonly accompanied by chronic physical inactivity (i.e., secondary aging), we hypothesized that an improved physical fitness level as a result of a physically active lifestyle or chronic physical activity would help mitigate the age-related cardiovascular dysfunction and protect cerebral perfusion during orthostatic stress in elderly adults. However, there were scant data with regard to the effect of physical fitness on cerebral blood flow in relation to the systemic hemodynamic response during orthostatic challenge in the elderly population. A previous study (9) indicated that the response of VMCA during orthostatic stress simulated by LBNP was not different between fit and unfit older adults, because there was no change in VMCA observed in either group during steady-state LBNP (9). Based on previous observations (4, 5, 13, 18, 23, 26, 50), we postulated that there would be a cerebral hypoperfusion during upright or LBNP-simulated orthostatic stress in older subjects as a result of central hypovolemia and hyperventilation-elicited hypocapnia.
The present study was designed to compare cerebral hemodynamic responses in relation to the change of systemic cardiovascular function during the challenge of mild to moderate LBNP between healthy sedentary elderly adults (SED group) and physically active elderly adults (ACT group). Although LBNP only simulates orthostasis, it allows graded changes in central blood volume without alteration of the hemodynamic reference point and isolates the cardiovascular responses without additional stimulus of the balance function or vestibular mechanism. During the study, changes in VMCA were continuously monitored using transcranial Doppler (TCD) sonography along with cardiac output (Q) and forearm blood flow (FBF) responses and breath-by-breath end-tidal Pco2 (PetCO2). In addition, baseline dynamic cerebral autoregulation was assessed by transfer function gain between mean arterial pressure (MAP) variability and VMCA variability, which was compared between the groups.
METHODS
Subjects.
Ten healthy, average fit sedentary (6 men and 4 women; SED group) and 9 physically active (6 men and 3 women; ACT group) elderly adults voluntarily participated in the study after giving their informed consent and passing a physical examination (see Table 1 for their physical characteristics). Although group age was not different between the sedentary (67.1 ± 1.4 yr) and physically active (68.0 ± 1.1 yr) subjects, body mass index was greater (P < 0.05) in the SED group (29.5 ± 1.6) than in the ACT group (22.9 ± 0.7). The study protocol was approved by the Institutional Review Board for the Protection of Human Subjects of the University of North Texas Health Science Center (Fort Worth, TX). All subjects were clinically confirmed to be free of cardiovascular, metabolic, renal, and pulmonary diseases and symptoms before the exercise stress test. Before the medical examination, ∼10% of subjects who had given their consent and medical/health history were excluded from the study because of taking medications, such as adrenergic blockers or diuretics, which could directly interfere with arterial baroreflex function or cardiovascular homeostasis. About 25% of the elderly subjects failed to pass the medical examination. Physical fitness was determined by 1) the subject's peak O2 uptake (VacuMed Vista VO2 Lab, Ventura, CA), measured during graded maximal cycling exercise on a stationary bicycle; 2) the physical activity history from the subject's self-report in the medical/health history questionnaire; or 3) both. Elderly subjects with a peak O2 uptake of ≤28 or ≥30 ml·min−1·kg−1 were considered sedentary or physically active, respectively. Peak O2 uptake was significantly different (P < 0.0001) between the groups, ranging from ∼18 to ∼28 ml·min−1·kg−1 (average of 22.1 ml·min−1·kg−1) in the SED group and ranging from ∼30 to ∼40 ml·min−1·kg−1 (average of 35.8 ml·min−1·kg−1) in the ACT group, respectively. Two SED subjects described themselves as exercise trained or physically active. No ACT subject reported himself/herself as physically inactive or untrained (see Table 1). Before the experimental testing, all subjects had an orientation in the laboratory to familiarize with themselves with the experimental procedures and measurements applied in the study. During the orientation visit, ∼70% of healthy elderly subjects were able to produce a desirable VMCA signal.
Table 1.
Physical fitness and physical activity information of the individual subjects
| Sex | Age, yr | Weight, kg | Height, m | Peak O2 Consumption, ml·min−1·kg−1 |
Self-Report |
||||
|---|---|---|---|---|---|---|---|---|---|
| Lifestyle | Training duration | Exercise type | Exercise frequency, days/wk | Exercise time, min/day | |||||
| SED group | |||||||||
| M | 75 | 69.0 | 1.69 | 22.80 | SED | 0 | 0 | 0 | 0 |
| M | 71 | 65.0 | 1.59 | 21.95 | SED | 0 | 0 | 0 | 0 |
| M | 68 | 64.0 | 1.67 | 26.01 | ACT | ≥1 yr | Walking | 3–4 | 20–40 |
| M | 64 | 86.5 | 1.65 | 22.19 | SED | 0 | 0 | 0 | 0 |
| M | 63 | 87.5 | 1.83 | 26.18 | ACT | ≥1.5 yr | Walking and swimming | 2–3 | 20–40 |
| M | 63 | 90.0 | 1.75 | 27.98 | SED | 0 | 0 | 0 | 0 |
| F | 72 | 86.6 | 1.73 | 18.90 | SED | 0 | 0 | 0 | 0 |
| F | 69 | 88.0 | 1.65 | 18.50 | SED | 0 | 0 | 0 | 0 |
| F | 63 | 81.0 | 1.52 | 18.10 | SED | 0 | 0 | 0 | 0 |
| F | 63 | 97.0 | 1.59 | 18.32 | SED | 0 | 0 | 0 | 0 |
| ACT group | |||||||||
| M | 72 | 76.0 | 1.77 | 38.22 | ACT | >35 yr | Running and weightlifting | 4–5 | 41–60 |
| M | 72 | 73.2 | 1.72 | 30.10 | ACT | >20 yr | Cycling and weightlifting | 4–5 | 61–90 |
| M | 68 | 79.5 | 1.84 | 39.40 | ACT | >25 yr | Running and weightlifting | 4–5 | 41–60 |
| M | 66 | 81.8 | 1.80 | 37.61 | ACT | >35 yr | Running and weightlifting | 4–5 | 61–90 |
| M | 64 | 86.4 | 1.88 | 40.33 | ACT | >26 yr | Running and weightlifting | 4–5 | 61–90 |
| M | 64 | 66.0 | 1.75 | 33.53 | ACT | >50 yr | Running and weightlifting | 6–7 | 41–60 |
| F | 71 | 63.5 | 1.69 | 30.96 | ACT | >16 yr | Yoga and running | 3–4 | 61–90 |
| F | 70 | 48.3 | 1.50 | 38.94 | ACT | >50 yr | Running and weightlifting | 6–7 | 41–60 |
| F | 65 | 51.0 | 1.65 | 33.41 | ACT | >27 yr | Running | 6–7 | 41–60 |
Subjects were divided into physically active/exercise-trained (ACT) and sedentary/untrained (SED) groups. M and F indicate male and female, respectively. Lifestyle, duration of exercise training, type of physical exercise, exercise frequency, and time were reported by the subjects.
Measurements.
During the experiment, each subject's heart rate (HR) was monitored from a standard lead of the ECG. Radial arterial blood pressure (ABP) was measured using a tonometer (model 7000 Tonometry Colin, San Antonio, TX). This method has been previously validated to be accurate for the noninvasive measurement of ABP compared with directly measured ABP (34, 49). Thoracic impedance was monitored by four tetra polar electrodes, 3/4-in.-wide Mylar tape strips, placed around the neck and lower chest (EBI100C, Biopac, Santa Barbara, CA). Previous data have confirmed that a change in the thoracic bioimpedance is a reliable index of changes in central blood volume or stroke volume (SV) (7, 32, 47). FBF was measured by venous occlusion plethysmography using a double-stranded mercury-in-Silastic strain gauge (Hokanson, Bellevue, WA), the measurement of which has been repeatedly applied in our previous studies (41–43). VMCA was determined by TCD sonography using a 2-MHz probe (EZ-Dop DWL System) placed on the left temple window of the head. Regional cerebral tissue oxygenation (RcO2) of the prefrontal cortex was monitored using near-infrared spectroscopy by a sensor placed on the right side of the forehead (4100 INVOS Cerebral Oximeter, Somanetics, Troy, MI). The position and angle of the TCD probe was fixed to the head using a custom-made ring held by a Velcro band throughout the test as previously described (13). Systemic arterial O2 saturation (SaO2) was measured by an arterial pulse oximeter (OXY100C, Biopac). The fraction of end-tidal CO2 (FetCO2) in a subgroup of the SED (n = 8) and ACT (n = 7) groups was continuously monitored via a nasal cannula using a mass spectrometer (1100 Medical Gas Analyzer, Perkin-Elmer, St. Louis, MO). PetCO2 was calculated from the product of ambient barometric pressure (corrected with vapor pressure) and FetCO2. All these analog data were digitized at 400 Hz and continuously monitored by a computer interfaced with a data-acquisition system (Biopac). Total peripheral resistance (TPR), forearm vascular resistance (FVR), and the index of cerebral vascular resistance (CVR) were derived offline from the ratios of MAP to Q, MAP to FBF, and MAP to VMCA, respectively.
Procedures.
The experiment was carried out with the subject's lower body supported in an airtight LBNP box in the supine position at a room temperature of 24–25°C. After ≥20 min of supine rest, the subject's baseline HR, SV, ABP, VMCA, RcO2, SaO2, and FetCO2 data were continuously recorded for 6 min; meanwhile, four to five FBF curves were collected after ∼45 s of the wrist cuff being inflated and maintained at ∼200 mmHg to arrest the circulation of the hand. After the baseline data collection, graded LBNPs of −10, −15, −20, −30, −40, and −50 Torr were continuously applied, and each grade of LBNP was maintained for 6 min. Two SED subjects and 1 ACT subject did not complete the whole LBNP ramp because of the appearance of presyncope. Cardiovascular variables and FetCO2 were continuously monitored during LBNP.
Data analyses.
A section of 5-min continuous VMCA and MAP data under the supine resting condition was selected for transfer function analysis using the Welch spectral estimator (35). The transfer function between MAP-VMCA signals [i.e., H(f)] was computed from the cross-spectrum (CS) between MAP-VMCA variabilities [CS(MAP)(VMCA)(f)] and autospectrum (AS) VMCA variability [AS(MAP)(f)] as follows: H(f) = [CS(MAP)(VMCA)(f)]/[AS(MAP)(f)]. The real and imaginary components of H(f) [HR(f) and HI(f), respectively] were used to calculate the magnitude or gain, i.e., H(f)G as follows: H(f)G = {[HR(f)]2 + [HI(f)]2}1/2. The time relationship or phase [θ(f)] between MAP and VMCA signals was calculated as follows: θ(f) = arctan[HI(f)/HR(f)]. The coherence [C(f)] was calculated as follows: C(f) = [CS(MAP)(VMCA)(f)]2/[AS(MAP)(f) × AS(VMCA)(f)], where AS(VMCA) is the autospectrum VMCA variability. Transfer function magnitude, phase, and coherence between the 0.08- and 0.18-Hz spectrum (where the group average coherence was ≥0.5) along with VMCA variability and MAP variability were extracted and compared between the groups. This spectral range (i.e., 0.08–0.18 Hz) of the transfer function data between MAP and VMCA variabilities is below normal breathing frequency and has been selected for the assessment of dynamic cerebral autoregulation (3, 51, 53). In addition, baseline low-frequency (LF; 0.05–0.15 Hz) and high-frequency (HF; 0.16–0.40 Hz) HR variability were analyzed as described in our previous studies (8, 49).
Differences in baseline data between the groups were determined by the two-tailed t-test with two independent samples. Two-way ANOVA for repeated measures was applied to determine the significance of LBNP and fitness factors on cardiovascular and respiratory responses. Assessment of the relative changes (i.e., the responses) allowed us to minimize the influence of individual variance between or within the groups and to normalize the responses with different units. Post hoc analysis with Tukey's option was applied when the major factor reached significance (i.e., P values of ≤0.05). Simple linear regression was applied for assessment of the relationship (i.e., slope) between two variables. A general linear model procedure was applied for the comparison of the slopes between SED and ACT subjects and for the assessment of the interaction between LBNP and fitness factors. All data are reported as group means ± SE. Statistic Analysis System softward (Cary, NC) was applied for statistical analyses.
RESULTS
Baseline data.
Baseline ABP was not significantly different between the two elderly groups. However, HR was lower (P = 0.010) and SV was greater (P = 0.011) in ACT subjects than in SED subjects (Table 2). Baseline VMCA and RcO2 were statistically similar between SED and ACT groups. The breathing rate at rest was faster (P = 0.025) in SED subjects than in ACT subjects, whereas baseline PetCO2 was not significantly different between the groups (Table 2).
Table 2.
Baseline physiological characteristics
| Variables | SED Group | ACT Group | P Value |
|---|---|---|---|
| Heart rate, beats/min | 65 ± 4 | 52 ± 2 | 0.010 |
| Stroke volume, ml | 44.8 ± 3.9 | 73.9 ± 8.7 | 0.011 |
| Cardiac output, l/min | 2.83 ± 0.19 | 3.77 ± 0.39 | 0.053 |
| Systolic blood pressure, mmHg | 132 ± 5 | 126 ± 3 | 0.26 |
| Diastolic blood pressure, mmHg | 70 ± 3 | 67 ± 4 | 0.62 |
| Mean arterial pressure, mmHg | 91 ± 3 | 87 ± 3 | 0.53 |
| Pulse pressure, mmHg | 62 ± 4 | 58 ± 4 | 0.39 |
| Total peripheral resistance, units | 33.1 ± 1.9 | 25.4 ± 2.9 | 0.047 |
| Forearm blood flow, ml·100 g−1·min−1 | 5.90 ± 0.92 | 4.14 ± 0.57 | 0.13 |
| Forearm vascular resistance, units | 20.9 ± 4.2 | 24.8 ± 3.6 | 0.49 |
| Middle cerebral artery blood flow velocity, cm/s | 54.9 ± 2.3 | 57.8 ± 3.2 | 0.47 |
| Cerebral vascular resistance, units | 1.69 ± 0.09 | 1.55 ± 0.10 | 0.33 |
| Regional cerebral tissue oxygenation, % | 59.8 ± 0.9 | 64.6 ± 2.9 | 0.14 |
| Arterial O2 saturation, % | 94.9 ± 0.3 | 96.3 ± 0.3 | 0.012 |
| End-tidal Pco2, mmHg | 38.75 ± 0.83 | 38.95 ± 0.60 | 0.85 |
| Respiratory rate, cycles | 15.2 ± 0.9 | 12.6 ± 0.5 | 0.025 |
Values are means ± SE. P values were the outcome of a t-test with two independent samples.
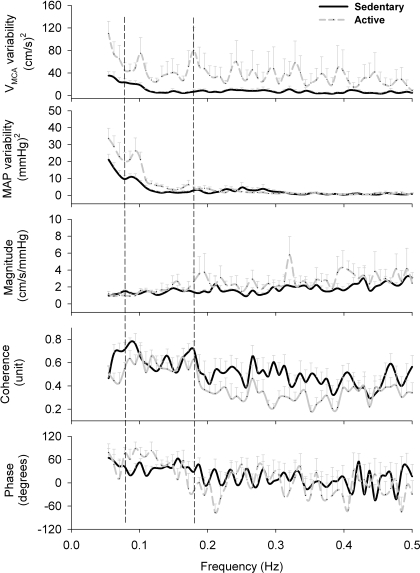
The baseline power spectral density (between 0.08 and 0.18 Hz) of VMCA variability and MAP variability were significantly smaller in the SED group than in the ACT group, i.e., 1.06 ± 0.19 vs. 4.24 ± 1.59 (cm/s)2 (P = 0.051) and 0.49 ± 0.07 vs. 1.04 ± 0.16 (mmHg)2 (P = 0.004), respectively. However, their transfer function magnitude (or gain), coherence, and phase were not significantly different between the groups (Fig. 1). Both LF (0.05–0.15 Hz) and HF (0.16–0.40 Hz) HR variability at rest were significantly greater in the ACT group than in the SED group, i.e., ACT vs. SED: 1.57 ± 0.44 vs. 0.39 ± 0.12 (beats/min)2 (P = 0.0142) and 1.73 ± 0.71 vs. 0.21 ± 0.06 (beats/min)2 (P = 0.0381), respectively.
Fig. 1.
Baseline power spectral data (from top to bottom) of middle cerebral arterial blood flow velocity (VMCA) variability, mean arterial pressure (MAP) variability, and their transfer function magnitude, coherence, and phase between VMCA-MAP signals. Baseline transfer function analysis data were not significantly different between the two groups, i.e., the sedentary group (SED) vs. the physically active (ACT) group: 1.41 ± 0.18 vs. 1.65 ± 0.44 cm·s−1·mmHg−1 (P = 0.62), 0.63 ± 0.06 vs. 0.56 ± 0.05 units (P = 0.111), and 38.03 ± 6.57 vs. 48.55 ± 11.84° (P = 0.44), respectively. However, both VMCA variability and MAP variability were significantly greater in ACT subjects than in SED elderly subjects. The two vertical dashed lines define the spectrum between 0.08 and 0.18 Hz from where the frequency-domain data were extracted and compared between the groups. Two SED subjects and two ACT subjects have coherence < 0.5; their data were excluded from transfer function analysis. Therefore, the number of subjects in the data of VMCA variability and MAP variability was n = 10 and 9, whereas for the transfer function magnitude, coherence, and phase, the number of the subjects was n = 8 and 7, in the SED and ACT groups, respectively.
LBNP effects.
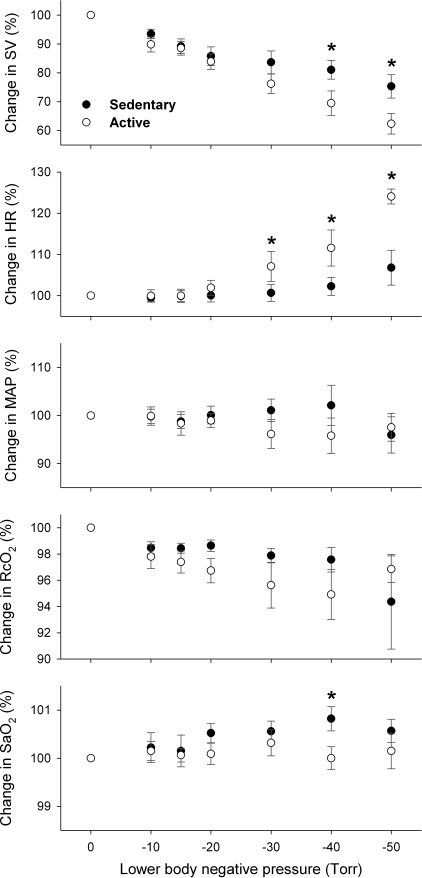
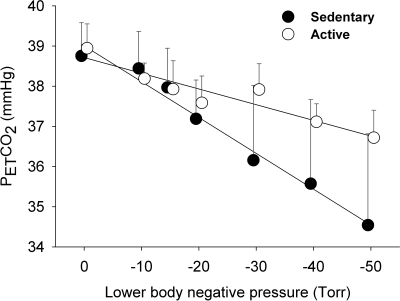
In both SED and ACT groups, SV was progressively decreased (P = 0.0001) with graded LBNP (Fig. 2), indicating central hypovolemia. HR was progressive increased above the LBNP of −20 Torr as a result of unloading arterial baroreceptors. Decreases in Q, VMCA, and FBF were significantly (P < 0.05) correlated with LBNP or central hypovolemia, which were associated with significant increases in TPR, FVR, and CVR, respectively, in both subject groups (Fig. 3). However, LBNP-elicited central hypovolemia did not cause significant changes in MAP (P > 0.05) in either group (Fig. 2), which was likely compensated for by a vasoconstriction stimulated by the baroreflex-mediated sympathoexcitation (22, 36). Neither systolic blood pressure nor diastolic blood pressure was statistically affected by LBNP. Although SaO2 was not significantly altered (P > 0.05), RcO2 was significantly decreased (P < 0.05) in both SED and ACT groups during LBNP (Fig. 2). Hypocapnia was observed, as indicated by a progressive decrease in PetCO2 during graded LBNP (Fig. 4). Significant decreases in PetCO2 appeared at −20 Torr of LBNP in both SED (−1.57 ± 0.44 mmHg) and ACT (−1.36 ± 0.39 mmHg) groups. The decreases in PetCO2 were augmented with further increases in LBNP, e.g., −4.07 ± 1.29 and −2.23 ± 0.64 mmHg at −50 Torr in SED and ACT subjects, respectively.
Fig. 2.
Changes in stroke volume (SV), heart rate (HR), MAP, regional cerebral tissue oxygenation (RcO2), and systemic arterial O2 saturation (SaO2) during lower body negative pressure (LBNP). Graded decreases in SV were associated with progressive increases in LBNP (P = 0.0001), suggesting central hypovolemia in response to LBNP-simulated orthostatic challenge. This decrease in SV appeared more substantial in the ACT group than in the SED group (P = 0.0006). *Significant difference between the groups. A tachycardiac response occurred from a LBNP of −30 Torr (P = 0.0001). This baroreflex-mediated HR was significantly more sensitive in the ACT group in than the SED group (P = 0.0003). MAP was similarly maintained during LBNP in both fitness groups (P = 0.89). RcO2 was diminished during LBNP (P = 0.0187), which was not significantly different between the groups (P = 0.19). The change in SaO2 was not significantly altered by LBNP (P = 0.35), which appeared to become higher in the SED group compared with the ACT group (P = 0.0241).
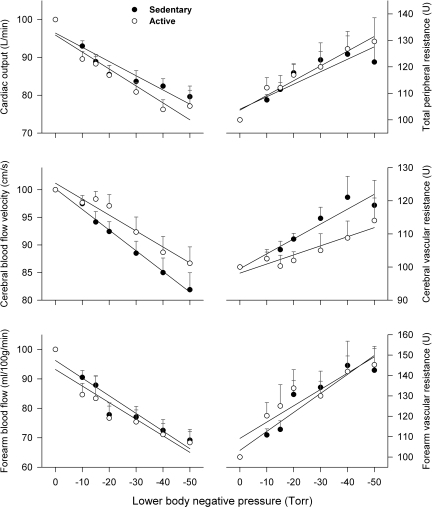
Fig. 3.
Cardiac output (Q), total peripheral resistance (TPR), cerebral blood flow VMCA, cerebral vascular resistance (CVR), forearm blood flow (FBF), and forearm vascular resistance (FVR) during graded LBNP. The slopes of Q/LBNP (top left) and TPR/LBNP (top right) were not different between the two groups, i.e., SED vs. ACT groups: 0.376 ± 0.062%/Torr (R2 = 0.88) vs. 0.448 ± 0.068%/Torr (R2 = 0.90, P = 0.45) and −0.471 ± 0.100%/Torr (R2 = 0.82) vs. −0.557 ± 0.058%/Torr (R2 = 0.95, P = 0.48). However, the rate of decrease in VMCA during LBNP (middle left) was significantly more severe (P = 0.0247) in the SED group (slope: 0.375 ± 0.014%/Torr, R2 = 0.99) than in the ACT group (slope: 0.289 ± 0.029 %/Torr, R2 = 0.95), whereas the rate of increase in CVR during LBNP (middle right) was significantly greater (P = 0.039) in the SED group (slope: −0.449 ± 0.057%/Torr, R2 = 0.90) than in the ACT group (slope: −0.275 ± 0.046%/Torr, R2 = 0.88). Neither the slopes of FBF/LBNP (bottom left) nor the slopes of FVR/LBNP (bottom right) were statistically different between the two groups, i.e., SED vs. ACT group: 0.600 ± 0.085%/Torr (R2 = 0.91) vs. 0.564 ± 0.102%/Torr (R2 = 0.86, P = 0.79 for FBF/LBNP) and −0.931 ± 0.142%/Torr (R2 = 0.89) vs. −0.800 ± 0.151%/Torr (R2 = 0.85, P = 0.54), respectively.
Fig. 4.
The rate of decreases in end-tidal Pco2 (PetCO2) in terms of per unit LBNP was more substantial (P = 0.0008) in the SED group (0.088 ± 0.008 mmHg/Torr, R2 = 0.96, P = 0.0001) than in the ACT group (0.039 ± 0.006 mmHg/Torr, R2 = 0.88, P = 0.0016).
Fitness effects.
Decreases in SV during LBNP were significantly more severe in the ACT group than in the SED group (Fig. 2), whereas the tachycardiac responses were significantly greater in the ACT group than in the SED group, indicating sensitized baroreflex control of HR in the exercise-trained elderly adults. The slopes of Q/LBNP and TPR/LBNP were not different between SED and ACT subjects. However, the rate of decreases in VMCA per unit change in LBNP were greater (P = 0.025) in SED subjects (slope: 0.375 ± 0.014%/Torr, R2 = 0.99) than in ACT subjects (slope: 0.289 ± 0.029%/Torr, R2 = 0.95). In addition, the rate of increases in CVR per unit change in LBNP were significantly greater (P = 0.039) in SED subjects (slope: −0.449 ± 0.057%/Torr, R2 = 0.90) than in ACT subjects (slope: −0.275 ± 0.046%/Torr, R2 = 0.88). Neither the slopes of FBF/LBNP nor the slopes of FVR/LBNP were statistically different between SED and ACT subjects (see Fig. 3).
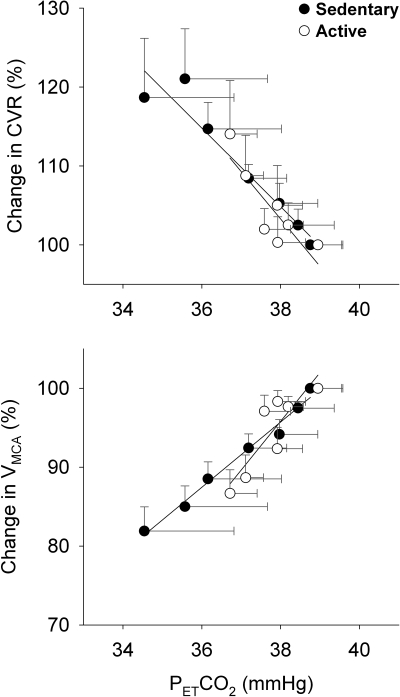
During graded LBNP, the rate of decreases in PetCO2 (i.e., the slope) was significantly greater (P = 0.0008) in SED subjects (0.088 ± 0.008 mmHg/Torr, R2 = 0.96) than in ACT subjects (0.039 ± 0.006 mmHg/Torr, R2 = 0.88; see Fig. 4). However, the slopes of CVR and VMCA in response to PetCO2 during LBNP were not significantly different between the two groups (Fig. 5).
Fig. 5.
Slopes of %ΔCVR/PetCO2 (top) and %ΔVMCA/PetCO2 (bottom) during graded LBNP. Although hypocapnia was greater in the SED subjects than in the ACT subjects, neither the slopes of %ΔCVR/PetCO2 (P = 0.5519, −4.98 ± 0.64%/mmHg, R2 = 0.92, vs. −6.01 ± 1.65%/mmHg, R2 = 0.73) nor the slopes of %ΔVMCA/PetCO2 (P = 0.1442, 4.14 ± 0.24%/mmHg, R2 = 0.98, vs. 6.19 ± 1.58%/mmHg, R2 = 0.75) were statistically different between the two groups.
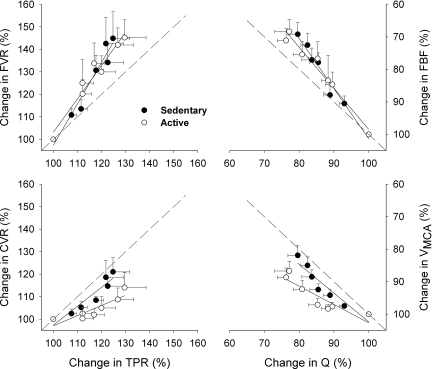
Although the slopes of %ΔFVR/%ΔTPR during graded LBNP were not significantly different (P = 0.166) between SED (1.83 ± 0.18, R2 = 0.95) and ACT (1.49 ± 0.15, R2 = 0.95) groups (Fig. 6, top left), the rate of augmented CVR in terms of per unit increase in TPR (i.e., the slope of %ΔCVR/%ΔTPR) was significantly greater (P = 0.0476) in SED subjects (0.84 ± 0.14, R2 = 0.88) than in ACT subjects (0.45 ± 0.11, R2 = 0.77; Fig. 6, bottom left). The slopes of %ΔFBF/%ΔQ (Fig. 6, top right) and %ΔVMCA/%ΔQ (Fig. 6, bottom right) tended to be greater in the SED group than in the ACT group.
Fig. 6.
Relationships between the changes in regional and systemic vascular resistances (left) and between the changes in regional and systemic blood flows (right) during graded LBNP. Although the slopes of %ΔFVR/%ΔTPR were not statistically (P = 0.166) different between the two groups, i.e., SED vs. ACT groups: 1.84 ± 0.18 (R2 = 0.95) vs. 1.48 ± 0.15 (R2 = 0.95), the slope of %ΔCVR/%ΔTPR was greater (P = 0.0476) in SED subjects (0.84 ± 0.14, R2 = 0.88) than in ACT subjects (0.45 ± 0.11, R2 = 0.77). The slopes of %ΔVMCA/%ΔQ and %ΔFBF/%ΔQ showed a tendency to be greater in the SED group than in the ACT group [slopes of%ΔVMCA/%ΔQ: 0.91 ± 0.15 (R2 = 0.88) vs. 0.56 ± 0.12 (R2 = 0.81, P = 0.1015) and slopes of %ΔFBF/%ΔQ: 1.55 ± 0.12 (R2 = 0.97) vs. 1.26 ± 0.11 (R2 = 0.96, P = 0.1081)]. The dashed lines indicate the identity line or slope = 1. In both SED and ACT groups, the slopes of %ΔFVR/%ΔTPR and %ΔFBF/%ΔQ were >1.0 or above the identity line, whereas the slopes of %ΔCVR/%ΔTPR and %ΔVMCA/%ΔQ were <1.0 or below the identity line, indicating a preferential protection against excessive cerebral vasoconstriction or cerebral hypoperfusion mediated by the intrinsic or regional mechanisms of cerebral autoregulation during LBNP. However, the slopes of %ΔCVR/%ΔTPR and %ΔVMCA/%ΔQ were more parallel with the identity line in the SED group compared with the ACT group, indicating a diminished function of cerebral autoregulation during central hypovolemia.
DISCUSSION
The present study provides two novel findings. First, the cerebral hypoperfusion that occurred during mild to moderate LBNP was less severe in ACT elderly subjects compared with SED elderly subjects. This finding appears to suggest that an enhanced cardiovascular-respiratory fitness of healthy seniors resulting from a physically active lifestyle or chronic physical activity may help protect the brain against hypoperfusion under orthostatic stress. Second, baseline MAP variability and VMCA variability were significantly smaller in SED elderly subjects than ACT elderly subjects, although the transfer function magnitude or gain between MAP and VMCA signals was not different between the two groups under the resting condition.
During orthostatic challenge, there are three factors likely involved in a subsequent cerebral hypoperfusion or reduction of cerebral blood flow. First, the reduction of venous return and resulting reduction of Q restrict the systemic availability of circulating blood flow (5, 13, 18, 26). Second, baroreflex-mediated sympathoexcitation may increase cerebral vasomotor tone along with an augmented TPR (2, 13, 21, 26). This neurogenically augmented CVR would reduce VMCA in the presence of stable or falling MAP. Third, hypocapnic hyperventilation, as indicated by a decrease in PetCO2, stimulates cerebral vasoconstriction (21, 24, 38). This chemogenically augmented CVR would also cause a diminution of cerebral blood flow.
Since the LBNP-induced reductions of SV and Q tended to be greater in the ACT group than in the SED group in the present study, the altered systemic flow availability was not likely a responsible mechanism for the difference in cerebral hypoperfusion observed between the two groups. During graded LBNP, TPR appeared to be similarly augmented in both SED and ACT elderly subjects. These data suggested that the sympathoexcitation-mediated augmentation of total peripheral vasomotor tone was comparable between the groups. Therefore, a difference in sympathoexcitation and its neurogenic influence during LBNP was unlikely to be the factor responsible for the difference in cerebral hypoperfusion between the groups. However, in terms of per unit increase in TPR, the rate of the augmented CVR during LBNP was significantly greater in SED than in ACT subjects (Fig. 6). These data implied that the function of cerebral autoregulation could be diminished in the SED group and that the cerebral regional mechanism could be less effective to protect cerebral perfusion against the augmented cerebral vasomotor tone during LBNP. Alternatively, cerebral vasomotor tone could have been stimulated by a greater chemogenic influence (i.e., decrease in PetCO2) in the SED group during LBNP.
The present study indicated that the decrease of PetCO2 or LBNP-elicited hypocapnia was greater in SED than in ACT subjects (see Fig. 4). Hypocapnia manifested by decreases in PetCO2 or arterial Pco2 during orthostasis has been reportedly to be partially explained by a reduction of Q (11, 38) or sympathetic activation (21). However, the changes in Q and TPR (an index of sympathetic-stimulated vasomotor tone) during LBNP were not different between the groups (Fig. 3). Although the sensitivity of increase in CVR and decrease in VMCA in response to hypocapnia during orthostatic challenge (based on the slopes of %ΔCVR/PetCO2 and %ΔVMCA/PetCO2) were not significantly different between SED and ACT subjects (Fig. 5), whether any acute adaptation to hypocapnia (19) occurred during LBNP and whether this adaption affected the subjects differently based on fitness level are not known. Since graded hypocapnia occurred with progressive increases in LBNP intensity (Fig. 4), which was significantly correlated with the changes in CVR or VMCA in both groups (Fig. 5), our data suggested that hypocapnia remained potent on cerebral vasomotor tone in the present experimental setting. Based on the data that the diminished PetCO2 was significantly smaller in the ACT group compared with the SED group, we suggested that a difference in hypocapnia elicited by LBNP was likely a mechanism responsible for the different cerebral hypoperfusion between SED and ACT groups, in addition to the function of cerebral autoregulation and the reduction of venous return. Previously, Ide et al. (16) observed a peak slope of VMCA/PetCO2 of ∼2.5%/mmHg during hypocapnia-elicited changes in VMCA without central hypovolemia, which was much smaller than the slopes of VMCA/PetCO2 in both the SED (4.3%/mmHg) and ACT (6.2%/mmHg) elderly groups in the present study. Collectively, these data implied that a portion of the ∼50% decrease in VMCA or increase in CVR during LBNP in the present study could be explained by central hypovolemia or its reflex sympathoexcitation. An alternative explanation for the difference in cerebral hypoperfusion between the two elderly groups during LBNP in the present study may involve a cerebral regional or intrinsic mechanism that differently counteracts the augmentation of cerebral vasomotor tone induced by hypocapnia or sympathoexcitation. Evidence that this cerebral regional mechanism was effective is manifested by a smaller increase in CVR compared with TPR in both SED and ACT subjects during LBNP (Fig. 6) with the presence of hyperventilation-elicited hypocapnia. However, in terms of per unit increase in TPR, the rate of augmentation of CVR was significantly smaller in ACT than in SED subjects, indicating a potential difference in the activation of a cerebral regional mechanism.
The transfer function magnitude between beat-to-beat MAP and VMCA variabilities has been considered as a gain of dynamic cerebral autoregulation (28, 40, 52, 53). Under the supine resting condition, there were no differences in transfer function gains between SED and ACT groups (Fig. 1). However, the beat-to-beat oscillations in both MAP and VMCA spectral power were significantly smaller in the SED group compared with the ACT group in the present study. These data suggested that the beat-to-beat MAP variability and VMCA variability of the SED group oscillated within a much narrower range, although the gain or sensitivity of the dynamic cerebral autoregulation at rest was not different between the two elderly groups (Fig. 1). The mechanism for a smaller baseline MAP variability observed in the SED group is not fully understood. It may be related to a greater stiffness of arterial blood vessels associated with aging, which appears to be favorably alleviated or reversed after chronic exercise training in older adults (6, 37, 46). Another possibility for the smaller MAP variability in the SED group may be related to a diminished HR variability, which was significantly smaller in the SED group compared with their ACT cohort. Although ABP variability precedes HR variability, the latter may also conversely exert its influence on beat-to-beat ABP oscillation in the closed cardiovascular system when MAP is less affected by sympathetic nerve activity under the supine resting condition. This postulation seems to be supported by the observation that ABP variability is significantly diminished after the impairment of HR variability with vagal-cardiac blockade using atropine or glycopyrrolate (49).
Cerebral hypoperfusion is one mechanism responsible for orthostatic intolerance (14, 15, 20, 26, 29, 48). For example, patients with idiopathic orthostatic intolerance show a greater decrease in VMCA and a greater increase in CVR compared with age- and sex-matched healthy adults during graded orthostatic stress simulated by head-up tilt (20). The better maintained cerebral perfusion observed in the elderly ACT group compared with the elderly SED cohort in the present study seems to be contrary to the belief that “trained man can run, but they cannot stand” (12). More incidence of orthostatic intolerance in high-fit younger subjects has been observed compared with their age-matched healthy, average-fit counterparts, which has been reportedly related to a greater cardiac compliance (27) that compromises SV output (25) and a diminished baroreflex sensitivity that compromises tachycardiac and vasoconstrictive responses (33). As a result, the fitness-related cardiovascular alternations in younger athletes may compromise their orthostatic tolerance and cerebral perfusion during orthostatic stress. However, the present study (Fig. 2) and our previous data (44) have indicated that baroreflex function appeared to be enhanced in elderly subjects with a physically active lifestyle. This observation was opposite to the difference in arterial baroreflex sensitivity between younger high-fit and average-fit subjects, which has been shown to be diminished after exercise training based on both cross-sectional and longitudinal comparisons (31, 33, 45). Although left ventricular compliance is greater in master athletes than in healthy sedentary seniors, a difference in compliance is not distinguishable between elderly high-fit and young average-fit subjects (3). Collectively, these data suggest that exercise training prevents the impact of the normal aging process on the heart and on baroreflex sensitivity. This may help maintain the cardiovascular function of physically active seniors at a level comparable with that of healthy sedentary younger subjects. Therefore, we postulate that the impact of physical fitness on the regulation of cerebral blood flow during orthostasis is age specific. Chronic physical activity counteracts secondary aging and provides more protection against cerebral hypoperfusion during orthostatic challenge in elderly people. However, a previous study (9) did not detect a fitness-related difference in VMCA during steady-state LBNP in healthy elderly subjects. An important factor in this discrepancy is that VMCA was constantly maintained during mild to moderate LBNP in both old fit and old unfit subjects in the previous study (9), whereas VMCA was progressively decreased with central hypovolemia in both SED and ACT subjects in the present study (Fig. 2). Our data suggested that the intrinsic mechanism of cerebral autoregulation could be diminished in the SED group compared with their ACT cohort. Also, greater hypocapnia in the SED group was a factor causing greater cerebral hypoperfusion during central hypovolemia.
The present study has some potentially important implications. Habitual exercise not only provides a beneficial influence on overall cardiovascular conditioning but also mitigates cerebral hypoperfusion in elderly adults during an orthostatic challenge. Physiologically, this provides a margin of safety against orthostatic intolerance. Nonetheless, the present study was limited to a cross-sectional comparison. A longitudinally designed study is needed to more definitively distinguish the impact of secondary aging from primary aging on cerebral hypoperfusion during central hypovolemia. Also, only mild to moderate LBNP was applied to simulate orthostasis in the present study, which could not produce the true orthostatic impact on venous return (or ventilation) and orthostatic intolerance. Another limitation of the study was that the change in PetCO2 was determined for hypocapnia during LBNP. Although this noninvasive method was commonly applied in previous studies (1, 11, 16) and highly correlated with arterial blood CO2 (18, 19, 39), direct systemic hypocapnia may be overestimated by PetCO2 (18, 38).
In conclusion, improved physical fitness resulting from a physically active lifestyle or chronic physical activity in elderly adults mitigates cerebral vasoconstriction and hypoperfusion during central hypovolemia. Our data indicate that the intrinsic mechanism of cerebral autoregulation may be improved, along with the sensitized arterial baroreflex control of HR, in the physically active elderly compared with the sedentary elderly adults. This adaptive change may help prevent orthostatic intolerance in physically active elderly people, which is different from the observation made in their younger counterparts.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-65613.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors sincerely thank all volunteer subjects for the cheerful participation in the study as well as Partrick Chanthovang, Hong Guo, and Dhanashri Kohok for the help during the subject recruitment and data collection.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. Differential responses to CO2 and sympathetic stimulation in the cerebral and femoral circulations in humans. J Physiol 566: 613–624, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation 110: 1799–1805, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bondar RL, Kassam MS, Stein F, Dunphy PT, Fortney S, Riedesel ML. Simultaneous cerebrovascular and cardiovascular responses during presyncope. Stroke 26: 1794–1800, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Brown CM, Dutsch M, Hecht MJ, Neundorfer B, Hilz MJ. Assessment of cerebrovascular and cardiovascular responses to lower body negative pressure as a test of cerebral autoregulation. J Neurol Sci 208: 71–78, 2003 [DOI] [PubMed] [Google Scholar]
- 6.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ebert TJ, Eckberg DL, Vetrovec GM, Cowley MJ. Impedance cardiograms reliably estimate beat-by-beat changes of left ventricular stroke volume in humans. Cardiovasc Res 18: 354–360, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Formes K, Wray DW, OYurvati A, Weiss M, Shi X. Sympathetic cardiac influence and arterial blood pressure instability. Auton Neurosci 118: 116–124, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Franke WD, Allbee KA, Spencer SE. Cerebral blood flow responses to severe orthostatic stress in fit and unfit young and older adults. Gerontology 52: 282–289, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gisolf J, van Lieshout JJ, van Heusden K, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol 560: 317–327, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gisolf J, Wilders R, Immink RV, van Lieshout JJ, Karemaker JM. Tidal volume, cardiac output and functional residual capacity determine end-tidal CO2 transient during standing up in humans. J Physiol 554: 579–590, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenleaf JE, Sciaraffa D, Shvartz E, Keil LC, Brock PJ. Exercise training hypotension: implications for plasma volume, renin, and vasopressin. J Appl Physiol 51: 298–305, 1981 [DOI] [PubMed] [Google Scholar]
- 13.Guo H, Tierney N, Schaller F, Raven PB, Smith SA, Shi X. Cerebral autoregulation is preserved during orthostatic stress superimposed with systemic hypotension. J Appl Physiol 100: 1785–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Harms MP, Colier WN, Wieling W, Lenders JW, Secher NH, van Lieshout JJ. Orthostatic tolerance, cerebral oxygenation, and blood velocity in humans with sympathetic failure. Stroke 31: 1608–1614, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hesse B, Mehlsen J, Boesen F, Schmidt JF, Andersen EB, Waldemar G, Andersen AR, Paulson OB, Vorstrup S. Regulation of cerebral blood flow in patients with autonomic dysfunction and severe postural hypotension. Clin Physiol Funct Imaging 22: 241–247, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Ide K, Eliasziw M, Poulin M. Relationship between middle cerebral artery blood velocity and end-tidal Pco2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol 95: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Immink RV, Secher NH, Roos CM, Pott F, Madsen PL, van Lieshout JJ. The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol 96: 609–614, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Immink RV, Truijen J, Secher NH, Van Lieshout JJ. Transient influence of end-tidal carbon dioxide tension on the postural restraint in cerebral perfusion. J Appl Physiol 107: 816–823, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Jacob G, Atkinson D, Jordan J, Shannon JR, Furlan R, Black BK, Robertson D. Effects of standing on cerebrovascular resistance in patients with idiopathic orthostatic intolerance. Am J Med 106: 59–64, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I. Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension 36: 383–388, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Joyner MJ, Shepherd JT, Seals DR. Sustained increases in sympathetic outflow during prolonged lower body negative pressure in humans. J Appl Physiol 68: 1004–1009, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Bogert LW, Immink RV, Harms MP, Colier WN, van Lieshout JJ. Effects of aging on the cerebrovascular orthostatic response. Neurobiol Aging In press [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy S, Wang X, Bhakta D, Bruce E, Evans J, Justice T, Patwardhan A. Dynamic cardiorespiratory interaction during head-up tilt-mediated presyncope. Am J Physiol Heart Circ Physiol 287: H2510–H2517, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Levine BD, Buckey JC, Fritsch JM, Yancy CW, Jr, Watenpaugh DE, Snell PG, Lane LD, Eckberg DL, Blomqvist CG. Physical fitness and cardiovascular regulation: mechanisms of orthostatic intolerance. J Appl Physiol 70: 112–122, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90: 298–306, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84: 1016–1023, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke 31: 1897–1903, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 29: 104–111, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876–1881, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Ogoh S, Volianitis S, Nissen P, Wray DW, Secher NH, Raven PB. Carotid baroreflex responsiveness to head-up tilt-induced central hypovolaemia: effect of aerobic fitness. J Physiol 551: 601–608, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pawelczyk J, Matzen S, Friedman D, Secher N. Cardiovascular and hormonal responses to central hypovolaemia in humans. In: Blood Loss and Shock, edited by Secher N, Pawelczyk J, Ludbrook J. London: Arnold, 1994, p. 25–36 [Google Scholar]
- 33.Raven PB, Rohm-Young D, Blomqvist CG. Physical fitness and cardiovascular response to lower body negative pressure. J Appl Physiol 56: 138–144, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Sato T, Nishinaga M, Kawamoto A, Ozawa T, Takatsuji H. Accuracy of a continuous blood pressure monitor based on arterial tonometry. Hypertension 21: 866–874, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol Heart Circ Physiol 261: H1231–H1245, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Seals DR. Sympathetic neural adjustments to stress in physically trained and untrained humans. Hypertension 17: 36–43, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501–507, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Serrador JM, Hughson RL, Kowalchuk JM, Bondar RL, Gelb AW. Cerebral blood flow during orthostasis: role of arterial CO2. Am J Physiol Regul Integr Comp Physiol 290: R1087–R1093, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Serrador JM, Schlegel TT, Black FO, Wood SJ. Cerebral hypoperfusion precedes nausea during centrifugation. Aviat Space Environ Med 76: 91–96, 2005 [PMC free article] [PubMed] [Google Scholar]
- 40.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol 98: 151–159, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Shi X, Crandall CG, Raven PB. Hemodynamic responses to graded lower body positive pressure. Am J Physiol Heart Circ Physiol 265: H69–H73, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Shi X, Gallagher KM, SASM , Bryant KH, Raven PB. Diminished forearm vasomotor response to central hypervolemic loading in aerobically fit individuals. Med Sci Sports Exerc 28: 1388–1395, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Shi X, Gallagher KM, Welch-O'Connor RM, Foresman BH. Arterial and cardiopulmonary baroreflexes in 60- to 69- vs. 18- to 36-yr-old humans. J Appl Physiol 80: 1903–1910, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Shi X, Schaller FA, Tierney N, Chanthavong P, Chen S, Raven PB, Smith ML. Physically active lifestyle enhances vagal-cardiac function but not central autonomic neural interaction in elderly humans. Exp Biol Med (Maywood) 233: 209–218, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Shi X, Stevens GH, Foresman BH, Stern SA, Raven PB. Autonomic nervous system control of the heart: endurance exercise training. Med Sci Sports Exerc 27: 1406–1413, 1995 [PubMed] [Google Scholar]
- 46.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Thomas SH. Impedance cardiography using the Sramek-Bernstein method: accuracy and variability at rest and during exercise. Br J Clin Pharmacol 34: 467–476, 1992 [PMC free article] [PubMed] [Google Scholar]
- 48.Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol 94: 833–848, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Wray DW, Formes KJ, Weiss MS, OYurvati AH, Raven PB, Zhang R, Shi X. Vagal cardiac function and arterial blood pressure stability. Am J Physiol Heart Circ Physiol 281: H1870–H1880, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Zhang R, Levine BD. Autonomic ganglionic blockade does not prevent reduction in cerebral blood flow velocity during orthostasis in humans. Stroke 38: 1238–1244, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zhang R, Witkowski S, Fu Q, Claassen JA, Levine BD. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension 49: 1149–1155, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106: 1814–1820, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol 85: 1113–1122, 1998 [DOI] [PubMed] [Google Scholar]