Abstract
The sensitivity of baroreflex control of sympathetic nerve activity (SNA) represents the responsiveness of SNA to changes in blood pressure. In a slightly different analysis, the baroreflex threshold measures the probability of whether a sympathetic burst will occur at a given diastolic blood pressure. We hypothesized that baroreflex threshold analysis could be used to estimate the sensitivity of the sympathetic baroreflex measured by the pharmacological modified Oxford test. We compared four measures of sympathetic baroreflex sensitivity in 25 young healthy participants: the “gold standard” modified Oxford analysis (nitroprusside and phenylephrine), nonbinned spontaneous baroreflex analysis, binned spontaneous baroreflex analysis, and threshold analysis. The latter three were performed during a quiet baseline period before pharmacological intervention. The modified Oxford baroreflex sensitivity was significantly related to the threshold slope (r = 0.71, P < 0.05) but not to the binned (1 mmHg bins) and the nonbinned spontaneous baroreflex sensitivity (r = 0.22 and 0.36, respectively, P > 0.05), which included burst area. The threshold analysis was also performed during the modified Oxford manipulation. Interestingly, we found that the threshold analysis results were not altered by the vasoactive drugs infused for the modified Oxford. We conclude that the noninvasive threshold analysis technique can be used as an indicator of muscle SNA baroreflex sensitivity as assessed by the modified Oxford technique. Furthermore, the modified Oxford method does not appear to alter the properties of the baroreflex.
Keywords: modified oxford method, baroreflex threshold, spontaneous baroreflex
the baroreflex is a key modulator of tonic sympathetic nerve activity (28), so that sympathetic bursts are synchronized with transient reductions in arterial pressure and are silenced during increased pressure (9). The baroreflex influence on sympathetic nerve activity can be assessed by measuring baroreflex sensitivity, defined as the responsiveness or gain in the reflex during transient changes in arterial pressure.
The most well-accepted method to examine baroreflex sensitivity involves a drug-induced manipulation of blood pressure. This approach was first developed to evaluate the cardiac baroreflex by Smyth et al. (23) in Oxford, England, who infused angiotensin to generate acute transient increases in arterial pressure and then measured resulting reflex decreases in heart rate. Subsequently, investigators have used a modified approach (sequential boluses of nitroprusside and phenylephrine) to determine baroreflex sensitivity for muscle sympathetic nerve activity (MSNA). This so-called “modified Oxford technique” involves measurements of both the number of MSNA bursts and their area (7, 10) and is currently accepted as the “gold standard” method for the evaluation of the sympathetic baroreflex sensitivity (22).
There are, however, limitations to the modified Oxford technique. Since the technique is relatively invasive, its use is limited in some laboratories. In addition, nitroprusside exerts its depressor (vasodilating) effect via nitric oxide, which may also inhibit the central nuclei responsible for sympathetic outflow in animals (5, 13, 16) and may also alter the responsiveness of the blood vessels to MSNA (3). Therefore, some argue that the estimation of baroreflex sensitivity using the modified Oxford technique may underestimate sensitivity because of the central neural effects of nitric oxide (16). In view of such concerns, a reliable noninvasive approach for assessing baroreceptor sensitivity would be desirable.
Sympathetic baroreflex function can also be assessed by associating spontaneous changes in MSNA to natural oscillations in blood pressure. In an earlier study, Sundlöf and Wallin (25) assessed the baroreflex threshold by calculating the percent occurrence of sympathetic bursts at different diastolic blood pressures. The resulting threshold diagram, associating burst occurrence to diastolic blood pressure, was defined by the blood pressure value at which burst occurrence was 50% (the T50 value) and the slope of the regression line. This slope provides a measure of the range of variability of the threshold. In addition, Sundlöf and Wallin (25) determined baroreflex sensitivity by relating, for each burst, its strength (burst area) to the diastolic blood pressure of the heart beat that generated the burst. More recently, Kienbaum et al. (15) compared the two measures of baroreflex function and found that all subjects had a significant blood pressure threshold relationship, whereas burst strength was significantly related to blood pressure in only 55% of the subjects. The poor relationship between blood pressure and burst strength has also been reported by Rudas et al. (22). Kienbaum et al. (15) concluded that there are distinct mechanisms regulating the occurrence and strength of sympathetic bursts. Since the first report of the baroreflex threshold analysis by Sundlöf and Wallin (25) and the subsequent analysis by Keinbaum et al. (15), other authors have used this method to estimate baroreflex sensitivity but called the analysis the “burst incidence” method (4, 14). However, it remains unknown whether the threshold analysis gives a valid estimate of baroreflex sensitivity as measured by the modified Oxford method.
In the present study, we addressed two main hypotheses. First, we hypothesized that a steep slope of the threshold diagram not only will indicate a narrow range of variability of threshold but will also be a reflection of high baroreflex sensitivity. In an analogous way, a flat slope would reflect low baroreflex sensitivity. We also compared traditional spontaneous baroreflex sensitivity analysis with the modified Oxford analysis. For the latter analyses, we evaluated whether using “binning” in the spontaneous total activity-blood pressure relationships would improve the accuracy of this analysis.
Our second main goal was to evaluate whether the vasoactive drugs infused during the modified Oxford affected the sensitivity of the sympathetic baroreflex. We investigated this by comparing the slope of the threshold analysis and the diastolic blood pressure where burst occurrence was 50% (T50) at rest and during a modified Oxford.
METHODS
Participants
All records used in this study were retrospectively analyzed from baseline and modified Oxford trials from other studies completed in our laboratory (6, 7). The investigations from which the data were obtained had ethical approval from the Institutional Review Board of the Mayo Clinic. Twenty-five healthy individuals (16 men) gave their informed consent to participate in the specific studies (means ± SE; age, 24 ± 1.5 yr; body mass, 75.3 ± 1.76 kg; height, 1.76 ± 0.02 m, and body mass index, 24.5 ± 0.9 kg/m2). The subjects were recreationally physically active nonsmokers with no history of cardiovascular or other chronic diseases.
Participants were asked not to consume anything except small volumes of water within 2 h of the experiment and were asked to abstain from caffeine or alcohol consumption 24 h before the study. To minimize the effects of the reproductive hormones on autonomic control or cardiovascular function, all women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use (21).
Measurements
All studies were performed in a clinical research laboratory at the Mayo Clinic. On arrival to the laboratory, the subjects rested in the supine position during instrumentation. Following local anesthesia with 2% lidocaine, a 5-cm, 20-gauge catheter was inserted into the brachial artery of the nondominant arm, using aseptic technique. The catheter was connected to a pressure transducer and interfaced with a personal computer to monitor arterial pressure. A three-lead ECG was used for continuous monitoring of heart rate.
Multiunit MSNA was measured from the right peroneal nerve at the fibular head using tungsten microelectrodes. A muscle sympathetic fascicle was identified when taps on the muscle belly or passive muscle stretched evoked mechanoreceptive impulses (27). The recorded signal was amplified 80,000-fold, band-passed filtered (700 to 2,000 Hz), rectified, and integrated (time constant, 0.1 s) by a nerve traffic analyzer.
Protocol
Baseline heart rate, arterial pressures, and MSNA were measured over a 5-min period of quiet supine rest. This was followed by a modified Oxford trial to estimate baroreflex sensitivity. As previously described, sodium nitroprusside (100 μg) was infused through a venous catheter inserted into an antecubital vein in the dominant arm, which was followed by phenylephrine (150 μg) 60 s later (7, 22). Data were collected for a further 2 min.
Data Analyses
Data were analyzed only from control periods, i.e., before any manipulations associated with the specific study. Data were sampled at 240 Hz and stored on a personal computer for off-line analysis. Mean arterial pressure was calculated as the time integral over the pressure pulse. Systolic, diastolic, and mean blood pressures; heart rate; and MSNA were taken from the 5-min period immediately preceding the nitroprusside infusion.
Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program (15); burst identification was then corrected by inspection by a single observer (ECH). The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle.
Baroreflex Sensitivity
We compared four different methods that estimated baroreflex sensitivity. First, we used the “gold standard” modified Oxford method. Second, we used spontaneous baroreflex analysis with no blood pressure binning. Third, we used spontaneous baroreflex analysis that involved binning diastolic blood pressure, and finally, we used the threshold analysis reported by Sundlöf and Wallin (25). The latter three were measured during the quiet supine rest preceding the modified Oxford drug infusions. The threshold analysis was also performed during the modified Oxford manipulation.
Modified oxford analysis.
An index of baroreflex control of sympathetic outflow was provided by the relationship between MSNA and diastolic blood pressure during the drug boluses (7, 8, 22). To perform a linear regression between the total MSNA activity (total burst area) and pressure, values for MSNA from baroreflex trials were first signal averaged over 3-mmHg pressure ranges (“bins”) via custom software (11). This pooling procedure reduces the statistical impact of the inherent beat-by-beat variability in nerve activity because of nonbaroreflex influences. A window of nerve activity that was 1.0 s in length and synchronized by the R wave of the electrocardiogram was signal averaged. The window was time shifted to account for the latency between R waves and sympathetic bursts. The duration of the shift was varied as needed from subject to subject. Any cardiac cycle not followed by a burst was assigned a total integrated activity of zero. Diastolic blood pressure was used because MSNA correlates more closely with diastolic blood pressure than with systolic pressure (25). The sensitivity of the baroreflex measured by the modified Oxford method was defined as the slope of the linear regression between MSNA (total activity) and the means of the diastolic blood pressure bins. For the linear regression analysis, all data were weighted for the number of cardiac cycles in each diastolic blood pressure bin.
Spontaneous baroreflex analysis.
Another index of baroreflex control of sympathetic nerve activity was also measured from spontaneous changes in arterial pressure during the 5-min baseline period preceding the modified Oxford. We assessed this relationship in two ways. First, we performed linear regression analysis between the area of sympathetic bursts and the corresponding diastolic blood pressure. This will be referred to as “spontaneous nonbinning analysis.” The slope of the linear regression line was used an index of the baroreflex sensitivity.
We then performed linear regression analysis between the MSNA and baseline diastolic blood pressures that were binned and will be referred to as “spontaneous binning analysis.” Specifically, sympathetic burst areas during the 5-min baseline period were signal averaged in diastolic blood pressure bins of 1, 2, and 3 mmHg via the custom software designed by Halliwill (11). In line with the modified Oxford analysis, any cardiac cycle that was not followed with a burst was given a total integrated nerve activity value of zero. Linear regression analysis was then performed on the relationship between the mean of each diastolic blood pressure bin and the total integrated MSNA for each bin. For the linear regression analysis, all data were weighted for the number of cardiac cycles in each diastolic blood pressure bin. The slope of the linear regression was used as another index of baroreflex sensitivity.
Baroreflex threshold analysis.
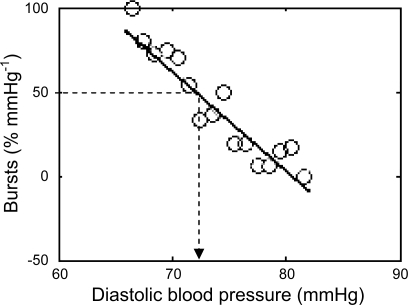
The baroreflex threshold analysis method examines how diastolic blood pressure relates to the occurrence of a sympathetic burst in a steady-state baseline period. To produce a baroreflex threshold diagram (Fig. 1), diastolic blood pressures are grouped into bins of 1 mmHg and within these bins the percentage of heart beats associated with a burst is determined. To generate a baroreflex threshold slope, we used an algorithm written in Matlab (The MathWorks, Natick, MA), which returned the results in a Matlab format and graph illustrated in Fig. 1. The unpublished algorithm was written my T. Karlsson and is provided as a supplemental file (note: supplemental material can be found posted with the online version of this article). Since a low diastolic blood pressure is more likely to be associated with a burst than a high pressure, an inverse relationship is produced (Fig. 1). We then compared this “threshold slope” with our other indexes of baroreflex sensitivity. The diastolic blood pressure at which 50% of the diastoles were associated with a burst (Fig. 1) was defined as the T50 value.
Fig. 1.
Example of a threshold diagram from a female participant. The individual circles represent the mean diastolic blood pressure in each 1-mmHg blood pressure bin. For each of these bins the percentage of heart beats associated with a burst is plotted against the mean of the pressure in that bin. The dotted line represents the diastolic blood pressure associated with 50% bursts (T50). In this particular female the T50 is 72 mmHg.
Statistics
We examined whether the slope of the baroreflex measured during the modified Oxford was similar to those assessed by spontaneous and threshold analysis. First, we assessed whether significant correlation coefficients existed between the baroreflex slopes and performed a least-squares linear regression analysis on this relationship.
However, when we evaluated whether one method agrees with another, the correlation analysis can produce some erroneous results (2). Therefore, we used the method of differences to compare the spontaneous and threshold methods to the established modified Oxford methods (2). Bland-Altman plots were generated using GraphPad Prism 4 (La Jolla, CA). The bias is defined as the mean difference between the established method and the new method, and a small bias indicates agreement between the two methods. The limits of this agreement are defined by the 95% confidence intervals. In some cases, the bias between the two methods can become a proportional bias (i.e., the difference between the 2 methods changes as the mean of both values becomes higher or lower). A proportional bias can result in an under- or overestimation of the actual error. A proportional bias exists if a linear regression line fitted to the Bland-Altman plot is significantly different from 0 (18, 20). We found a proportional bias for both of our comparisons (Fig. 4, left, solid line). Therefore, to ensure that we were not under- or overpredicting the agreement between the two methods, we calculated the prediction interval of the slope of the regression line (12). This gives an estimate of the area within which 95% of the population are expected to fall; consequently, we could predict whether future differences between the two methods of measuring baroreflex sensitivity will be within acceptable limits (12). This allowed us to more comprehensively report the agreement between the two methods over the range of values observed. The significance for all tests was set at P < 0.05, and all data are reported as means ± SE.
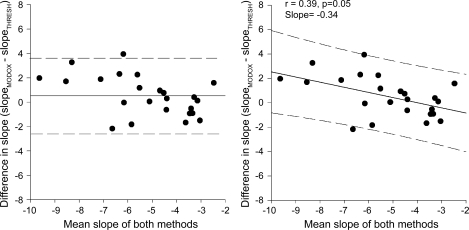
Fig. 4.
Bland-Altman plots to predict the limits of agreement between the baroreflex slope measured by the modified Oxford method and the baroreflex slope estimated by the baroreflex threshold analysis (n = 25 participants). Left: fixed bias (solid line) between 2 methods (the mean difference between the 2 methods) and the dashed lines represent the 95% confidence limits. The bias is low when the slope of the modified Oxford is compared with the slope calculated from the baroreflex threshold analysis (bias = 0.4); therefore, both methods are producing similar results. Right: there is a proportional bias between the methods (the slope of the relationship between the difference between both methods and the mean of both methods is significant from 0, solid line). The dashed lines represent the prediction interval calculated from the slope of the regression line. These intervals suggest that 95% of all data points will lie within these limits.
RESULTS
Cardiovascular Hemodynamics
Mean resting MSNA for all subjects was 35 ± 3 bursts/100 hb (range 17–67 bursts/100 hb) and 19 ± 2 bursts/min (range, 10 to 38 bursts/min). Resting heart rate and systolic and diastolic blood pressures for the group were 57 ± 2 beats/min and 131 ± 3 and 71 ± 2 mmHg, respectively.
During the 5-min resting period from which the spontaneous and threshold analyses were made, the spontaneous decreases in diastolic blood pressure from the mean diastolic pressure were on average 8 ± 1 mmHg (range, 5 to 13 mmHg). Corresponding increases were on average 11 ± 1 mmHg (range, 3 to 15 mmHg).
For the modified Oxford assessment, nitroprusside and phenylephrine were infused sequentially to decrease and then increase blood pressure. During the modified Oxford, diastolic blood pressure decreased by an average of 15 ± 1 mmHg (range, 6 to 24 mmHg) after the nitroprusside bolus and increased by an average of 19 ± 1 mmHg (range, 3 to 21 mmHg) following phenylephrine administration.
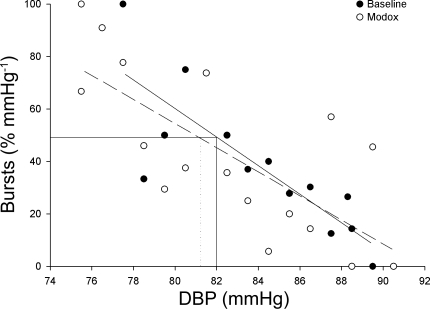
The T50 (midpoint of the threshold analysis curve) was similar during periods of rest and the modified oxford analysis (65.7 ± 1.7 vs. 65.2 ± 2.0 mmHg, P > 0.05), suggesting that the overall set point for baroreflex control of MSNA was not influenced by nitroprusside or phenylephrine administration. Furthermore, a correlation analysis of individual data showed that the T50 calculated during rest was significantly related to the T50 during the modified oxford (r = 0.88, P < 0.01). Figure 2 shows an individuals baroreflex curve analyzed by the threshold analysis at baseline and during the modified Oxford. This further indicates that the slope of the threshold and the T50 were not affected by the vasoactive drugs infused during the modified Oxford (threshold slope at baseline, −5.4%/mmHg vs. threshold slope in modified Oxford −4.6%/mmHg).
Fig. 2.
Threshold analysis of an individual male participants baroreflex sensitivity at baseline (solid line, black circles) and during a modified Oxford (Modox; dashed line, white circles). There were no changes in the sensitivity of the baroreflex (T50: baseline, 82 mmHg, solid line vs. modified Oxford; 82 mmHg, dotted line).
Sympathetic Baroreflex Sensitivity
Results from the analysis of sympathetic baroreflex sensitivity with the four different methods are given in Table 1. In short, spontaneous nonbinning analysis gave a mean r value of −0.39, where baroreflex slopes were significant for only 8 of the 25 subjects. With spontaneous binning analysis (in bins of 1 mmHg, see Table 1 for bins 2 and 3 mmHg), 16 of 25 subjects had significant slopes and the mean r value was −0.70 with a baroreflex sensitivity of −4.8 ± 1.0 arbitrary units (AU)·beat−1·mmHg−1. The baroreflex slopes calculated from the spontaneous baroreflex analysis when blood pressure was binned into 2 and 3 mmHg was not different from the 1-mmHg binned slope (−3.2 ± 0.6 and −2.1 ± 0.6 AU·beat−1·mmHg−1, respectively; P > 0.05). With the modified Oxford analysis, the mean r value was −0.79, where baroreflex slopes were significant in all subjects with an average baroreflex sensitivity of −5.0 AU·beat−1·mmHg−1. Finally, with the baroreflex threshold analysis, the mean r value was −0.81, where all participants had a significant baroreflex slope with an average baroreflex slope of −5.4 ± 0.5% bursts/mmHg. We also evaluated whether there was an effect of sex on the sensitivity of the baroreflex. There was no difference in sympathetic baroreflex slope between men and women when the modified Oxford method (−4.7 ± 0.4 vs. −5.4 ± 0.7 AU·beat−1·mmHg−1) or the threshold method (−4.9 ± 0.7 vs. 6.2 ± 0.9% bursts/mmHg) was used.
Table 1.
Analysis of baroreflex sensitivity by four different methods
| Method of Analysis | Mean Correlation Coefficient | Range of Correlation Coefficient | Individuals with Significant Slope, % |
|---|---|---|---|
| Modified Oxford | −0.79 ± −0.03 | −0.46 to −0.95 | 100 |
| Spontaneous nonbinning | −0.39 ± −0.03 | −0.33 to −0.52 | 32 |
| Spontaneous binning | |||
| 1 mmHg | −0.70 ± −0.04 | −0.44 to −0.92 | 64 |
| 2 mmHg | −0.79 ± −0.04 | −0.44 to −0.98 | 72 |
| 3 mmHg | −0.84 ± −0.04 | −0.55 to −0.99 | 64 |
| Threshold | −0.81 ± −0.02 | −0.42 to −0.95 | 100 |
Values are means ± SE of only the slopes with linear regression that were significant from zero (P < 0.05). Only the threshold and the modified Oxford analyses were successful in all participants.
Agreement Between Methods
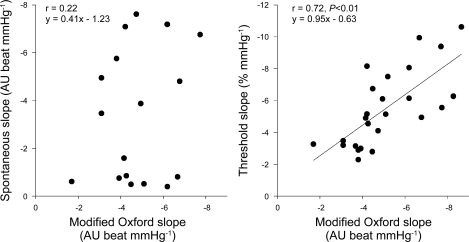
Figure 3 demonstrates that there was a strong positive relationship between the MSNA baroreflex sensitivity estimated by the modified Oxford analysis and the baroreflex threshold analysis (r = 0.71, P < 0.01). In contrast, there was no relationship between sensitivities obtained with the modified Oxford analysis and the spontaneous binning analysis when blood pressure was in 1, 2, or 3 mmHg bins (r = 0.06, 0.22, and 019, respectively; P > 0.05). Moreover, there was no relationship between the baroreflex sensitivities measured by the spontaneous nonbinning analysis and the modified Oxford analysis (r = 0.36, P > 0.05). There was also no correlation between the T50 value and the sensitivity obtained with the modified Oxford analysis (r = 0.02, P > 0.05).
Fig. 3.
Relationship of sympathetic baroreflex slope estimated by the modified Oxford method to the slope measured by the spontaneous baroreflex in 2-mmHg blood pressure bins (left, n = 18 participants) and the slope measured by the threshold method (right, n = 25 participants). The slope measured by the modified Oxford method was not related to the slope estimated by the spontaneous baroreflex method but was strongly related to the slope calculated by the threshold analysis. AU, arbitrary units.
The comparison of the agreement between the methods by way of Bland-Altman plots indicated that only a small bias (average difference between 2 methods) existed when data from the modified Oxford analysis were compared with data from the baroreflex threshold analysis (bias, 0.4% bursts/mmHg). The limits of agreement between these two methods were −2.8 and 3.6. Therefore, 95% of the estimated baroreflex sensitivity measured by either method will lie within these two values. We did not calculate the limits of agreement between the slopes measured by the modified Oxford and the spontaneous analyses (binned and unbinned) as there was no correlation between the slopes measured by these methods.
Since the bias and the 95% confidence intervals were small between the baroreflex sensitivity measured by the threshold and the modified Oxford analysis, there is good agreement between these two methods. However, because Fig. 4, right, indicates that there is a proportional bias, we also calculated the prediction interval of the slope of the regression line. This gives an estimate of the area in which one would expect 95% of all data points to fall. The prediction interval was 3.8 and −3, which means that 95% of future baroreflex slopes measured by the two methods will lie within these limits. This further indicates that the threshold analysis is in agreement with the modified Oxford analysis compared with either of the spontaneous baroreflex analyses (binned or unbinned), which did not correlate with the modified Oxford baroreflex slope.
DISCUSSION
The major findings of the present study are that 1) baroreflex threshold analysis predicts the baroreflex sensitivity determined with the modified Oxford method and 2) the vasoactive drugs used in the modified Oxford method do not appear to affect the sensitivity of the sympathetic baroreflex.
Comparison of Methods to Measure Baroreflex Sensitivity
Our present comparisons suggest that the baroreflex threshold analysis, originally developed by Sundlöf and Wallin (25), which was later modified by Kienbaum et al. (15), can be used during a resting baseline period to estimate baroreflex sensitivity as assessed by the “gold standard” modified Oxford method. One major conceptual difference between the two methods is that the threshold analysis relates to the occurrence of bursts, whereas the modified Oxford method also incorporates burst area to give an index of total activity at each level of blood pressure. The agreement between these two methods supports our hypothesis that that the baroreflex threshold analysis provides reliable information about the responsiveness of the baroreflex to buffer changes and its control of sympathetic nerve activity. This is important in situations where the modified oxford cannot be used to estimate baroreflex function (4, 14).
A criticism of the spontaneous baroreflex approaches is that the correlation between MSNA and spontaneous changes in blood pressure may not reflect baroreflex causality. For example, MSNA may actually contribute to the changes in blood pressure rather than responding to them. Another issue with estimating baroreflex sensitivity from spontaneous type analyses is that there are numerous inputs other than baroreflex control that affect sympathetic nerve activity. Therefore, these nonbaroreflex inputs may limit the interpretation of the observed reflex changes in sympathetic nerve activity to alterations in blood pressure. These issues may explain the lack of correlation between the baroreflex sensitivity measured by the spontaneous approaches and the modified Oxford analysis. However, it is possible that the threshold analysis agrees with that of the modified Oxford analysis because it deals with the possibility of whether a burst occurs or not at a given blood pressure on a probabilistic basis. Our observation that the threshold slope gives similar information to that gained from a modified Oxford suggests that the probability of burst occurrence at a given diastolic pressure may be less affected by nonbaroreflex influences compared with total activity of that burst.
We also found that using a total activity analysis on baseline data (spontaneous binning and nonbinning analysis) resulted in baroreflex sensitivity values that were extremely variable and not related to modified Oxford-derived slopes from the same individuals a few minutes later. This is consistent with the conclusions of a previous study (22) regarding comparisons between modified Oxford and spontaneous slopes for MSNA baroreflex sensitivity. These data, together with the similarity observed between results obtained with baroreflex threshold and modified Oxford analyses, suggest that the confounding factors (i.e., respiration and other nonbaroreflex influences) related to evaluating baroreflex sensitivity in a resting steady state may be affecting burst area more than the occurrence of a burst. This is consistent with the conclusions of Kienbaum et al. (15) regarding gating mechanisms for burst occurrence versus burst area or total activity. Other investigators have successfully used the threshold analysis to examine changes in sympathetic baroreflex sensitivity under different physiological conditions (4, 14). These authors also used the spontaneous binning type analysis on their data. They found that both analyses showed similar changes in baroreflex function for a given condition; however, these authors did not directly compare whether both types of analysis agreed with each other within an individual.
Vasoactive Drugs and Their Effect on the Baroreflex
It has been well established that nitric oxide is involved in central neural signaling processes that alter sympathetic nerve outflow (19). Some investigators, therefore, have some concerns that systemic nitric oxide infusions may alter fundamental characteristics of the baroreflex via central neural influences (5, 13) and altered vessel responsiveness to MSNA (3). Our present data and analysis suggest that these conclusions are not well founded in humans. This is supported by the fact that T50 and the slope calculated by threshold analysis were similar at rest and during modified Oxford drug infusions (Fig. 2). Consequently, our data support the idea that the amount of nitroprusside used in the modified Oxford does not substantially alter neuronal signaling within the baroreflex and, therefore, does not alter sympathetic baroreflex sensitivity.
However, these conclusions contrast with those of a previous study demonstrating that the slope of a baseline threshold analysis did not correlate well with the threshold slope calculated following the administration of nitroprusside only (16). The major difference between our present analysis and those of Kienbaum and Peters (16) appears to be that these investigators analyzed data over a smaller range of blood pressures and a hypotensive period of only 30 s. These differences may explain the different conclusions between their study and ours.
Limitations
In the present study we assessed sympathetic baroreflex sensitivity in young healthy participants. Therefore, the findings of this study can only be applied to young normotensive individuals. Whether baroreflex threshold analysis predicts baroreflex sensitivity (as measured by the modified Oxford) in aging and hypertensive individuals remains to be elucidated in further studies.
Studinger et al. (24) indicated that the hysteresis occurs in the sympathetic component of the baroreflex, such that the sympathetic baroreflex is less responsive to increases in arterial pressure than it is to decreases in arterial pressure. While hysteresis is a recognized characteristic of the baroreflex (22, 24, 26), we did not split the baroreflex into increasing and decreasing pressure phases because this reduces the amount of data included in the regression analysis for the estimation of each individuals baroreflex sensitivity. For example, Rudas et al. (22) indicated that once the baroreflex analysis was split into increasing and decreasing blood pressure phases, there were few significant baroreflex slopes. Therefore, although it is possible that splitting the 3-min modified Oxford test into increasing and decreasing phases may address the potential confound of ignoring hysteresis, it often adds the relatively major limitation of not having enough data points for a meaningful correlational analysis. Consequently, we measured baroreflex sensitivity using both the increasing and decreasing arterial pressure phases in one relationship.
In summary, the major findings of this study are twofold. First, we found that a spontaneous method, the threshold analysis, can be used as a reliable marker of sympathetic baroreflex sensitivity. This offers an important alternative method in circumstances where these drugs cannot be used. Second, a traditional assessment of spontaneous sympathetic baroreflex sensitivity does not reflect sensitivity assessed by the modified Oxford. These first two findings support the idea that, during spontaneous changes in blood pressure, the occurrence of a burst is more strongly linked to blood pressure than is the size of that burst (15). Taken together, these findings support the conclusions of Kienbaum et al. (15) that there is a differential regulation of the generation of a burst and the area of the burst. Under spontaneous resting conditions, nonbaroreflex inputs may be more dominant factors in deciding the size of a burst. However, when blood pressure is pushed over a wider range, the change in blood pressure becomes more dominant in whether a burst occurs and the size of the burst. Finally, we believe the agreement between the modified Oxford and the threshold analysis suggests that the vasoactive drugs infused during the modified Oxford do not alter baroreflex function.
GRANTS
This study was supported by National Institutes of Health (NIH) Grants HL-83947 and DK-082424, American Heart Association Grant 070036Z, and by Swedish Medical Research Council Grant 12170. This project was also supported by National Center for Research Resources (NCRR) Grant 1-UL1-RR-024150, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Additional support came from the Mayo Foundation including a philanthropic gift from the Caywood family and the Mayo Clinic Department of Anesthesia.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Shelly Roberts, Karen Krucker, Shirley Kingsley-Berg, Nicholas Strom, and Jessica Sawyer for assistance in conducting the studies. Finally, we thank the subjects for their participation.
All authors either contributed toward the conception and design (E. C. Hart, N. Charkoudian, B. G. Wallin, M. J. Joyner, and T. B. Curry), analysis and interpretation of data (E. C. Hart, N. Charkoudian, T. Karlsson, and T. B. Curry), drafting the article (E. C. Hart and N. Charkoudian) and/or revising it critically for important intellectual content (E. C. Hart, N. Charkoudian, B. G. Wallin, M. J. Joyner, and T. B. Curry), and final approval of the version to be published (E. C. Hart, N. Charkoudian, T. B. Curry, T. Karlsson, B. G. Wallin, and M. J. Joyner).
REFERENCES
- 1.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol Heart Circ Physiol 254: H377–H383, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]
- 3.Bucher B, Ouedraogo S, Tschopl M, Paya D, Stoclet JC. Role of the l-arginine-NO pathway and of cyclic GMP in electrical field-induced noradrenaline release and vasoconstriction in the rat tail artery. Br J Pharmacol 107: 976–982, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapleau MW, Hajduczok G, Sharma RV, Wachtel RE, Cunningham JT, Sullivan MJ, Abboud FM. Mechanisms of baroreceptor activation. Clin Exp Hypertens 17: 1–13, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. Am J Physiol Heart Circ Physiol 289: H2456–H2460, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. J Physiol 552: 635–644, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 287: H1658–H1662, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972 [DOI] [PubMed] [Google Scholar]
- 10.Ebert TJ. Differential effects of nitrous oxide on baroreflex control of heart rate and peripheral sympathetic nerve activity in humans. Anesthesiology 72: 16–22, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol 88: 767–773, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hamilton C, Stamey JD. Using a prediction approach to assess agreement between two continuous measurements. J Clin Monit Comput 23: 311–314, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Hogan N, Kardos A, Paterson DJ, Casadei B. Effect of exogenous nitric oxide on baroreflex function in humans. Am J Physiol Heart Circ Physiol 277: H221–H227, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol 573: 445–451, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kienbaum P, Peters J. Muscle sympathetic baroreflex sensitivity is different at rest and during evoked hypotension. Basic Res Cardiol 99: 152–158, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Legramante JM, Raimondi G, Massaro M, Cassarino S, Peruzzi G, Iellamo F. Investigating feed-forward neural regulation of circulation from analysis of spontaneous arterial pressure and heart rate fluctuations. Circulation 99: 1760–1766, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lipman RD, Salisbury JK, Taylor JA. Spontaneous indices are inconsistent with arterial baroreflex gain. Hypertension 42: 481–487, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Liu JL, Murakami H, Zucker IH. Effects of NO on baroreflex control of heart rate and renal nerve activity in conscious rabbits. Am J Physiol Regul Integr Comp Physiol 270: R1361–R1370, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Ludbrook J. Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol 29: 527–536, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ Res 24: 109–121, 1969 [DOI] [PubMed] [Google Scholar]
- 24.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 587: 2049–2057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundlof G, Wallin BG. Muscle-nerve sympathetic activity in man. Relationship to blood pressure in resting normo- and hyper-tensive subjects. Clin Sci Mol Med Suppl 4: 387s–389s, 1978 [DOI] [PubMed] [Google Scholar]
- 27.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272: 383–397, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallin BG, Eckberg DL. Sympathetic transients caused by abrupt alterations of carotid baroreceptor activity in humans. Am J Physiol Heart Circ Physiol 242: H185–H190, 1982 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.