Abstract
The soluble epoxide hydrolase enzyme (SEH) and vascular remodeling are associated with cardiovascular disease. Although inhibition of SEH prevents smooth muscle cell proliferation in vitro, the effects of SEH inhibition on vascular remodeling in vivo and mechanisms of these effects remain unclear. Herein we determined the effects of SEH antagonism in an endothelium intact model of vascular remodeling induced by flow reduction and an endothelium denuded model of vascular injury. We demonstrated that chronic treatment of spontaneously hypertensive stroke-prone rats with 12-(3-adamantan-1-yl-ureido) dodecanoic acid, an inhibitor of SEH, improved the increment of inward remodeling induced by common carotid ligation to a level that was comparable with normotensive Wistar Kyoto rats. Similarly, mice with deletion of the gene responsible for the production of the SEH enzyme (Ephx2−/−) demonstrated enhanced inward vascular remodeling induced by carotid ligation. However, the hyperplastic response induced by vascular injury that denudes the endothelium was unabated by SEH inhibition or Ephx2 gene deletion. These results suggest that SEH inhibition or Ephx2 gene deletion antagonizes neointimal formation in vivo by mechanisms that are endothelium dependent. Thus SEH inhibition may have therapeutic potential for flow-induced remodeling and neointimal formation.
Keywords: endothelium, hypertrophy, hyperplasia, epoxyeicosatrienoic acids, hypertension, mice
pathological remodeling of the vasculature leads to increased arterial stiffness, plaque formation and rupture, or thrombosis, culminating in acute cardiovascular events such as myocardial infarction and stroke. A potential modulator of these chronic vascular responses is the soluble epoxide hydrolase enzyme (SEH). SEH converts cardiovascular protective epoxyeicosatrienoic acids (EETs) into less active diols, attenuating the protective properties of EETs (8, 10, 45, 51). EETs are endothelial-derived hyperpolarizing factors and modulate vascular tone (1, 2, 9, 10, 20, 21, 38); EETs have also been shown to restrain vascular smooth muscle cell (VSMC) proliferation and migration (4, 5, 7, 35) and respond to changes in flow (10, 17, 30). A novel approach to preserve EETs, and perhaps protect from the sequela of hypertension and pathological vascular remodeling via EETs, is to inhibit SEH. SEH inhibition is antihypertensive in angiotensin-dependent, deoxycorticosterone-salt hypertension and spontaneously hypertensive rats (SHR) (22, 32, 49). However, blood pressure was not lowered by SEH inhibitors administered to spontaneously hypertensive stroke-prone (SHRSP) rats (6, 28, 43). Vascular protection conferred by SEH inhibitors includes the prevention of arteriosclerosis in rats and reduction or atherosclerosis in apolipoprotein e-knockout mice (6, 47, 52). VSMC proliferation and migration in vitro are decreased by SEH inhibition (4, 5, 7, 35). In addition, epidemiological studies also indicate that genetic polymorphisms that were found to have reduced SEH activity in vitro are associated with carotid calcification, the stabilized form of an atherosclerotic plaque (36). Single nucleotide polymorphisms and haplotypes associated with plaque calcification were also linked to reduced risk for ischemic stroke in certain subpopulations (11, 13, 50). In Caucasians, the K55R single nucleotide polymorphism, which increases SEH activity, also was associated with an increased risk for incidence of symptomatic coronary artery disease or intravascular intervention (27). As a result, SEH inhibition may be protective against pathological vascular remodeling.
Currently, in vitro studies indicate that SEH inhibition does have antimigratory and antiproliferative effects on VSMCs (4, 5, 7, 35). Other reports indicate that EETs and SEH modulate responses to changes in flow by mechanisms that are mediated by the endothelium (16, 17, 46). Although these studies give indications for potential utility of SEH inhibition in the management vascular remodeling, the effect of SEH inhibition in modulating vascular remodeling in vivo has not been fully characterized. In addition, the mechanisms behind these potential effects have not been deciphered. To determine whether SEH inhibition can modulate the response of the vasculature to changes in flow, inhibit neointimal proliferation, and determine mechanisms behind the protective effects, we conducted studies to assess flow-induced vascular remodeling and a vascular injury.
The carotid ligation technique induces remodeling around a vessel with reduced flow and thus it allows for a comparison of remodeling response between strains under well-controlled conditions. As a result, the carotid ligation technique is often used in genetically altered strains to investigate specific mediators involved in the development of vascular remodeling in vivo (25, 26, 29). Another model for investigating the effects of vascular remodeling is arterial wire injury. This well-characterized model also simulates remodeling, but is a more appropriate model for investigating mediators involved in restenosis of vessels after balloon injury or stent placement (41, 42). The resulting response to the endothelial denudation and outward pressure on vascular wall mimics the circumferential wall stress induced by balloon injury and results in a reduction in medial area and formation of neointima. We employed these two contrasting techniques to examine the effects of SEH inhibition on vascular remodeling.
MATERIALS AND METHODS
Animals.
All animals were housed and fed a normal rat chow in an American Association for the Accreditation of Laboratory Animal Care-approved facility at the Medical College of Georgia. The Institutional Animal Care and Use Committee approved all animal procedures. The following male rat strains were used in the flow reduction studies: 6- to 7-wk-old SHRSP rats (Charles River, Wilmington, MA) and 6- to 7-wk-old Wistar Kyoto (WKY) rats (Harlan, Indianapolis, IN). The following strains of mice were included in the dual injury flow-dependent vascular remodeling and femoral artery catheter injury model: 9-wk-old wild-type (WT) C57BL/6J mice (Jackson Laboratory, Sacramento, CA) and 9-wk-old homozygous Ephx2 gene deleted mice (Ephx2−/−)(44) from Jackson Laboratories that were backcrossed with C57Bl/6J mice for 10 generations. Blood pressure was monitored by tail plethysmography (IITC Life Science, Woodland Hills, CA) during the experimental period in acclimatized conscious rats and mice.
Left carotid artery ligation.
Left common carotid ligation was conducted in the rats and mice as previously described (26, 33, 39, 40). Mice were anesthetized with intraperitoneal injections of 0.2 ml/25 mg of a cocktail of 30 mg/ml ketamine and 4 mg/ml xylazine, and rats were anesthetized with 50 mg/kg of pentobarbital. Careful dissection was used to reveal the bifurcation of the common carotid artery. At the point most proximal to the bifurcation of the external and internal carotid arteries, the left common carotid was ligated using a 8-0 suture in mice and 6-0 suture in the rats, and the surgical incision was closed with a 5-0 suture.
Femoral artery catheter injury.
After performing the left carotid ligation in mice, wire injury of the femoral arteries was also performed (41, 42). To conduct the femoral artery injury, an incision was made into the left thigh to expose the femoral artery, and a wire (0.38 mm in diameter, No. C-SF-15; Cook, Bloomington, IN) was inserted into and withdrawn from the left femoral artery 5–7 times. The incision was closed with a 5-0 suture. All animals were allowed to recover after surgery.
SEH inhibitor treatment.
After surgery, animals were placed in the following experimental groups and on drug regimens. SHRSP and WKY rats were treated with 2 mg/day 12-(3-adamantan-1-yl-ureido) dodecanoic acid (AUDA) for 5 wk prepared in drinking water using vehicle to aid in solublization [500 mg/l of (2-hydroxypropyl)-β-cyclodextrin (Sigma Aldrich, St. Louis, MO) and 0.075% of ethanol] or vehicle. Mice were divided into the following experimental groups: WT-control, Ephx2−/−, and WT mice treated with SEH inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (tAUCB; 0.25 mg/day) in pudding for 6 wk. At the end of the experiment, all animals were anesthetized with pentobarbital (50 mg/kg) for plasma and tissue collection.
Plasma AUBA and tAUCB measurements.
SEH inhibitor tAUCB and 12-(3-adamantyl-ureido)-butyl acid (AUBA), a biologically inactive AUDA metabolite, were assessed in plasma samples by reverse phase HPLC followed by positive mode electron spray ionization and tandem mass spectroscopy (MS-HPLC) as previously described (34). An aliquot of 50 μl is diluted with 50 μl of distilled water. Ten microliters of surrogate 869 (1-(5-butoxypentyl)-3-adamantylurea at 250 ng/ml in methanol) was added (final concentration, 50 ng/ml). After two organic extractions with 200 μl of ethyl acetate, the sample was evaporated under dry N2 gas and then reconstituted in 50 μl of methanol with internal standard 790 (1-adamantylo-3-decyl urea 100 ng/ml; final concentration, 50 ng/ml). An aliquot of 5 μl was analyzed (34, 43). The limit of detection of AUBA and tAUCB are 0.5 ng/ml and 0.25 ng/ml, respectively.
Since AUDA reversibly and competitively inhibits the SEH enzyme, measuring SEH activity in the tissue to determine the degree of SEH inhibition is not feasible (18, 34). As a result, we have previously used SEH inhibitor metabolite levels to indicate inhibitor exposure and SEH inhibitor levels to indicate that the concentrations of inhibitor are sufficient to inhibit enzymatic activity (6, 18). The SEH inhibitor AUDA is metabolized by β-oxidation into an inactive metabolite AUBA. AUBA levels are used as an indication of previous AUDA exposure. We have previously demonstrated that these levels of AUBA correlate with an increase in the epoxide-to-diol ratio (6). The AUBA plasma levels reached 11 ng/ml in the SHRSP rats and 7 ng/ml in the WKY rats treated for 5 wk. These levels were similar to levels we reported previously with AUDA treatment. Plasma levels of tAUCB, a potent SEH inhibitor with increased bioavailability and greater metabolic stability (18), reached 131 ng/ml in the tAUCB-treated WT mice (WT-tAUCB). This inhibitor level should be sufficient to inhibit SEH activity (see supplementary Fig. 1; all supplemental material can be found with the online version of this article) based upon previous studies used to evaluate the efficacy of tAUCB in mice (18).
Fig. 1.
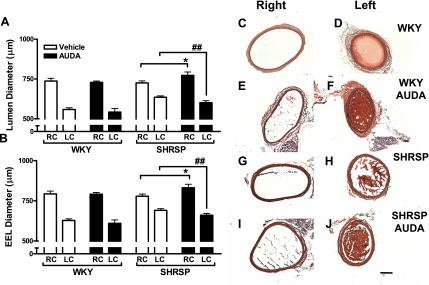
Soluble epoxide hydrolase (SEH) inhibition normalized remodeling in response to changes in flow in spontaneously hypertensive stroke-prone (SHRSP) rats. 12-(3-Adamantan-1-yl-ureido) dodecanoic acid (AUDA; n = 7) treatment did not alter remodeling of the left and right common carotid (LC and RC, respectively) artery induced by ligation in the Wistar Kyoto (WKY; n = 5; A and C–F). AUDA (n = 4) was effective at increasing the response to left common carotid artery ligation in the LC and RC arteries in the SHRSP (n = 4; B and G–J). *P < 0.05 SHRSP RC vs. RC of AUDA-treated SHRSP; ##P < 0.05 SHRSP LC vs. LC of AUDA-treated SHRSP AUDA. Pictures of hematoxylin and eosin (H&E)-stained common carotid arteries were taken at 50× magnification. The black line demarcates 200 μm. White and black bars represent vehicle and AUDA-treated animals, respectively. EEL, external elastic lamina.
Tissue collection and morphometric analysis.
Tissue was collected at the end of the treatment period. Animals were perfused with a vasodilator cocktail containing (in mM/l) 0.3 papaverine, 0.2 adenosine, and 0.2 diltiazem prepared in phosphate-buffered saline (PBS). Parallel sections of the right and left common carotid artery were embedded in optimal temperature cutting media (Tissue Tek, Torrance, CA). Animals for morphometric analysis were perfused with the vasodilator cocktail followed by 10% phosphate-buffered formaldehyde fixation at physiological pressures. For morphometric analysis and VSMC counts in the rats, serial sections of 5 μm thickness were taken along 250 μm of vessel length (∼5 mm from point of ligation). In mice, morphometric analysis and nucleic count were assessed in serial sections of 5 μm thickness in a proximal section (∼4 mm from point of ligation) and a distal section (∼8 mm from point of ligation) of the carotid taken along 50 μm of vessel length (vessels with thrombus formation were excluded). Serial sections of mouse femoral arteries 5 μm thickness were taken along 100 μm of vessel. Photographs of the hematoxylin and eosin (H&E)-stained rat carotid, mouse carotid, and mouse femoral arteries were taken with a light microscope. External elastic lamina (EEL), internal elastic lamina, and luminal perimeters were measured by a blinded reviewer in the H&E-stained vessels via Axiovision 4.0 software (Carl Zeiss Axio Vision Rel.4.6.3; Thornwood, NY). Wall thickness, wall-to-lumen ratio, and neointimal area-to-medial area ratio were calculated using the equation of a circle. VSMC counts were assessed by counting nuclei in the media of H&E-stained common carotid artery cross sections using the Axiovision 4.0 software.
Immunohistochemical staining for proliferating cellular nuclear antigen in rat carotid.
After fixation with 10% formalin and blocking in 10% normal goat serum, frozen 5-μm serial sections of rat left and right common carotid arteries were incubated overnight with proliferating cellular nuclear antigen (PCNA; 1:2,000, ab29; Abcam, Cambridge, MA) at 4°C. Secondary detection was achieved with FITC goat anti-rabbit IgG conjugate (1:200; Zymed, South San Francisco, CA), and diamidino-2-phenylindole (DAPI; 100nM; Molecular Probes) was used to allow visualization of nuclei. Slides were then mounted using Prolong Antifade Reagent Mounting Media (Molecular Probes, Carlsbad, CA). Fluorescence was visualized with a Zeiss Axiophot upright microscope. PCNA positive labeled cells were counted in four pictures taken along 650 μm of the vessel length in both the rat left and right common carotid arteries.
Immunohistochemical staining for 5-bromo-2-deoxyuridine in mice.
To detect proliferating cells in mouse carotid and femoral arteries, animals of each of the three groups were injected intraperitoneally 24 h before tissue collection and again 12 h before tissue collection with 100 mg/kg of 5-bromo-2-deoxyuridine (BrDU; Sigma Aldrich) solubilized in saline. BrDU labeling was detected following the kit instructions supplied by the manufacturer (Becton Dickinson, Franklin Lakes, NJ), substituting the secondary antibody FITC goat anti-rabbit IgG conjugate (1:200; Zymed) for secondary detection. Slides were also incubated with DAPI (100 nM; Molecular Probes) for visualization of nuclei and mounted using Prolong Antifade Reagent Mounting Media (Molecular Probes). Fluorescence was visualized with a Zeiss Axiophot upright microscope. BrDU positive labeled cells were counted in four cross sections along 100 μm of length in the distal and proximal segments of the left common carotid, right common carotid, left femoral, and right femoral arteries.
Statistics.
All data are expressed as means ± SE. Differences were assessed using ANOVA and Student's t-tests with P < 0.05 being statistically significant (Graph Pad Prism 4; GraphPad Software).
RESULTS
Body weight and blood pressure.
The body weight and systolic blood pressure were measured in the animals at baseline and at the end of the study (5 wk in rats and 6 wk in mice, postremodeling/injury; Table 1). In rats, the body weight did not differ between the groups at baseline or the end of the study. However, the systolic blood pressure of the SHRSP rats was higher than that of the WKY animals at baseline and at the end of the study. AUDA treatment did not alter blood pressure in either rat strain. In the mice, body weight was not different at the end of the study. At baseline, there were no differences in systolic blood pressure among the three experimental mice groups (109 ± 3 mmHg WT-control, 104 ± 2 mmHg WT-tAUCB, and 104 ± 3 Ephx2−/−). At the end of the study, the systolic blood pressure of the WT-control mice (117 ± 1 mmHg) was increased in comparison with WT-tAUCB (103 ± 3 mmHg) and Ephx2−/− mice (103 ± 3 mmHg).
Table 1.
Body weight and systolic blood pressure of animals in vascular remodeling study
| Group of Animals | Baseline |
End of Study |
||
|---|---|---|---|---|
| Body Weight, g | Systolic Blood Pressure, mmHg | Body Weight, g | Systolic Blood Pressure, mmHg | |
| WKY | 155.2 ± 2 | 119 ± 11 | 264.0 ± 7 | 146 ± 2 |
| WKY AUDA | 149.1 ± 3 | 125 ± 5 | 248.9 ± 7 | 149 ± 6 |
| SHRSP | 155.5 ± 5 | 139 ± 3* | 258.1 ± 8 | 194 ± 5* |
| SHRSP AUDA | 155.1 ± 11 | 137 ± 2* | 264.3 ± 4 | 183 ± 3* |
| WT-control | 22.0 ± 0.5 | 109 ± 3 | 27.0 ± 1.0 | 117 ± 1 |
| WT-tAUCB | 22.5 ± 0.6 | 104 ± 2 | 25.2 ± 0.4 | 103 ± 3 |
| Ephx2−/− | 25.4 ± 0.5 | 104 ± 3 | 26.0 ± 0.5 | 100 ± 3 |
Values are means ± SE.
P < 0.05, spontaneously hypertensive stroke-prone (SHRSP) vs. Wistar Kyoto (WKY). AUDA, 12-(3-adamantan-1-yl-ureido) dodecanoic acid; WT, wild-type; tAUCB, trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid.
SEH inhibition normalized remodeling in response to changes in flow in SHRSP rats.
We studied vascular remodeling by implementing an established arterial ligation model that preserves the integrity of the endothelium (see Table 2). Specifically, the left common carotid artery is ligated to cause a robust reduction in blood flow, an effect that has been demonstrated in both rats and mice (26, 33, 39). As expected, after 5 wk of left common carotid (LC) ligation, LC lumen and EEL were reduced, whereas right common carotid artery (RC) diameter was increased (Fig. 1), evidence of both inward and outward remodeling of these respective arteries as has been previously shown in rats (33). This manifested as reductions of both the EEL and lumenal diameters (Fig. 1) relative to the unligated RC, which may also outward remodel. Although there was no difference in remodeling of the left and right common carotid arteries in the WKY with AUDA treatment postligation (Fig. 1, A and B, and pictures C–F), the increment of inward remodeling was reduced in the SHRSP rats relative to the WKY rats (Fig. 1, A, B, D, and H; and supplementary figure 2). In WKY, EEL narrowed by 21 ± 2% and lumen diameter by 24 ± 2% relative to the right contralateral arteries (RC). In contrast, the percent reduction of EEL and lumen diameter in the LC versus RC of SHRSP was blunted to only 11 ± 1% and 12 ± 1%, respectively. AUDA treatment increased the adaptive response to LC ligation in the SHRSP, making the percent change in EEL (20 ± 2%) and lumen (22 ± 1%) diameter more similar to that of the WKY rats (Fig. 1, A, B, D, and J). In addition, the RC of SHRSP rats administered AUDA had an increased EEL and lumenal diameter in comparison with untreated SHRSP (Fig. 1, A, B, G, and I). Thus AUDA treatment normalizes the response to common carotid artery ligation.
Table 2.
Morphometric analysis of arteries from rats and mice
| Group of Animals | Right |
Left |
|||
|---|---|---|---|---|---|
| Lumen Diameter, μm | Medial Area, mm3 | Lumen Diameter, μm | Medial Area, mm3 | Neointimal Area, mm3 | |
| WKY | 559 ± 11 | 65 ± 3 | 737 ± 17 | 67 ± 2 | NA |
| WKY AUDA | 579 ± 45 | 52 ± 4 | 729 ± 10 | 65 ± 6 | NA |
| SHRSP | 635 ± 9 | 58 ± 2 | 725 ± 13 | 63 ± 3 | NA |
| SHRSP AUDA | 601 ± 13* | 59 ± 1 | 772 ± 21* | 74 ± 3* | NA |
| Carotid | |||||
| WT-control | 383 ± 21 | 18 ± 2 | 289 ± 34 | 27 ± 2 | 487 ± 18† |
| WT-tAUCB | 356 ± 6 | 16 ± 1 | 279 ± 32 | 34 ± 4 | 17 ± 8 |
| Ephx2−/− | 360 ± 11 | 18 ± 1 | 184 ± 21‡ | 20 ± 1‡ | 11 ± 8 |
| Femoral | |||||
| WT-control | 279 ± 7 | 10 ± 1 | 117 ± 15 | 5 ± 1 | 33 ± 3 |
| WT-tAUCB | 271 ± 5 | 8 ± 1 | 155 ± 22 | 5 ± 1 | 38 ± 7 |
| Ephx2−/− | 258 ± 8 | 8 ± 1 | 138 ± 18 | 5 ± 1 | 39 ± 3 |
Values are means ± SE.
P < 0.05, SHRSP vs. SHRSP AUDA;
P < 0.05, WT mice vs. Ephx2−/−;
P < 0.05, Ephx2−/− vs. WT treated with tAUCB.
SEH inhibition induces cell proliferation in the left carotid of SHRSP rats.
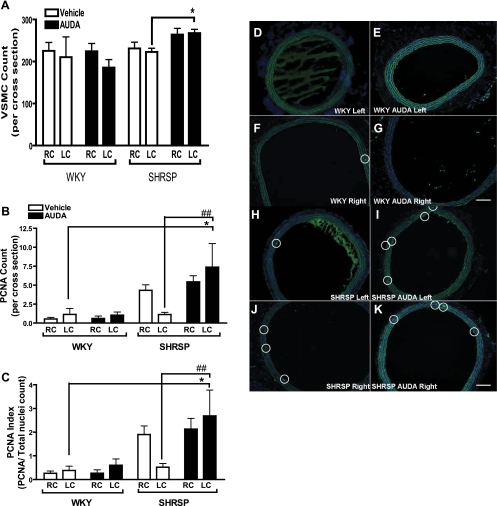
We next quantified cell number by counting cells in cross sections since previous reports indicated that SEH inhibitors decrease VSMC proliferation in vitro (4). There was no significant difference in the total nuclei count per cross section detected between treated and untreated WKY and SHRSP rats by ANOVA (Fig. 2A). However, the number of cells positively stained for PCNA and ratio of PCNA positive cells to total nuclei count was significantly greater in the AUDA-treated SHRSP LC in comparison with the LC in the WKY and SHRSP controls (Fig. 2, B–K).
Fig. 2.
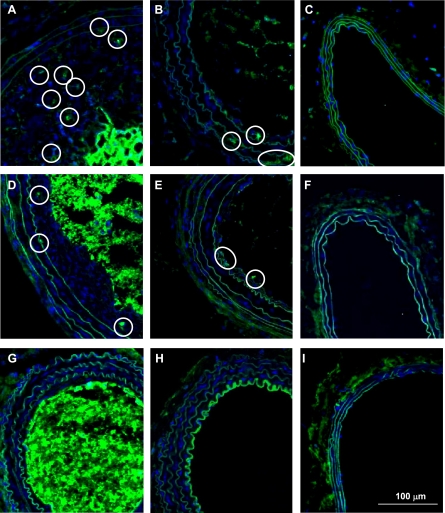
A: vascular smooth muscle cell (VSMC) count in cross sections of rat common carotid arteries. The VSMC count did not differ between the WKY (n = 5), the AUDA-treated WKY (n = 7), and SHRSP (n = 4). PCNA count and index are increased in LC of SHRSP (B and C). Although the PCNA count did not change with AUDA treatment in the WKY, AUDA increased the PCNA count in the SHRSP (n = 4; B; *P < 0.05 LC of AUDA-treated SHRSP vs. LC of WKY; ##P < 0.05 LC of AUDA-treated SHRSP vs. LC of vehicle-treated SHRSP). In addition, this increase was sustained when total nuclei was taken into account (C; *P < 0.05 LC of AUDA-treated SHRSP vs. LC of WKY; ##P < 0.05 LC of AUDA-treated SHRSP vs. LC of vehicle-treated SHRSP). White and black bars represent vehicle and AUDA-treated animals, respectively. D–K are images of common carotid arteries immunofluorescently labeled for PCNA (green; white circle identifies PCNA positive cells) and diamidino-2-phenylindole (DAPI), a nuclear stain (blue) taken at 100× magnification. Presence of PCNA positive cells did not change with AUDA in WKY rats (D–G represent LC WKY, LC WKY AUDA, RC WKY, and RC WKY AUDA, respectively). PCNA positive cells increased in the LC of SHRSP rats treated with AUDA (H–K represent LC SHRSP, LC SHRSP AUDA, RC SHRSP, and RC SHRSP AUDA, respectively).
SEH inhibition and Ephx2 gene deletion (Ephx2−/−) reduces vascular remodeling.
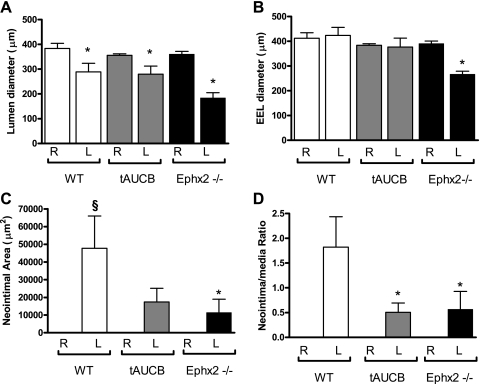
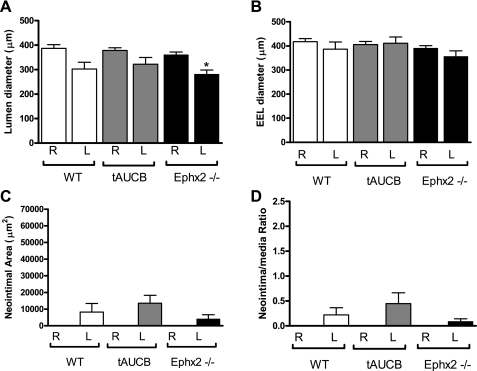
We also conducted experimental studies to assess neointimal formation in mice where EET metabolism and subsequent inactivation is inhibited either by pharmacological SEH inhibition using the tAUCB compound or in mice with genetic disruption of SEH (Ephx2−/−). Since there is some variation in the development of neointima with this rodent model, we assessed remodeling in two areas, proximal and distal to the point of ligation (25, 29). Proximal to the ligation neointimal area was approximately fourfold greater in the WT-control in comparison with the Ephx2−/− mice (Fig. 3, A and C, and Fig. 4C). The neointimal-to-media ratio was threefold greater in the WT-control in comparison with the WT-tAUCB and Ephx2−/− mice (Fig. 4D). Lumen diameter was decreased in all groups in the LC in comparison with the RC (Fig. 3, A–C and G–I, and Fig. 4A). Inward remodeling was enhanced in response to LC ligation in Ephx2−/− mice relative to WT-control as shown by the significant reductions in the EEL and lumenal diameter in the proximal segment (Fig. 4B). In the distal segment of the LC, the EEL was also significantly reduced in the Ephx2−/− mice (Fig. 5A). Distal to the ligation, there were no statistically significant differences in the EEL diameter, neointimal to media area, or neointimal ratio (Fig. 5, B–D).
Fig. 3.
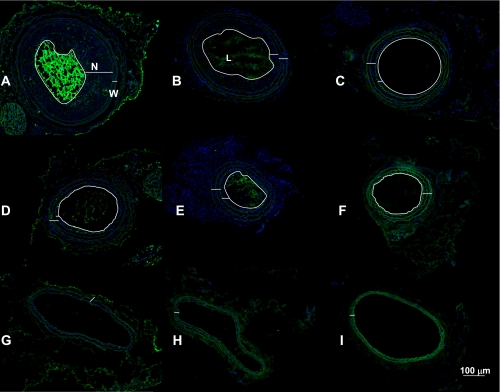
Images of common carotid arteries fluorescently labeled for 5-bromo-2-deoxyuridine (BrDU; green) and DAPI (nuclear stain, blue) depicting vascular protection 6 wk after ligation with Ephx2 gene deletion and trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (tAUCB). Neointimal formation (N) was greater in the wild-type (WT)-control mice (A) in comparison with WT-tAUCB (B) and Ephx2−/− mice (C) in the proximal segment. However, neointima was small in the distal portions of the WT-control (D), WT-tAUCB (E), and Ephx2−/− (F). The wall structure (W) and luminal diameter (L) were largely unchanged in the RC of the WT-control (G), WT-tAUCB (H), and Ephx2−/− (I). The white lines delineate the neointima, wall, and lumen. White line is 100 μm.
Fig. 4.
Neointimal formation was reduced in Ephx2−/− and WT-tAUCB mice proximal to ligation. The lumen diameter decreased significantly in all of the strains after 6 wk of ligation in the proximal portion of the ligated LC in comparison with the RC (A; *P < 0.05). However, EEL diameter decreases significantly only in the Ephx2−/− mice (B; *P < 0.05). Neointimal formation was significantly higher only in the WT-control in comparison with the RC (§P < 0.05), and the neointimal area was significantly lower in the Ephx2−/− in comparison with the WT-control (C; *P < 0.05). Both the WT-tAUCB and Ephx2−/− had significantly less remodeling and is indicated by the neointimal-to-medial ratio (D; *P < 0.05). The white, gray, and black bars represent WT-control (n = 5), WT-tAUCB (n = 5), and Ephx2−/− (n = 8) mice, respectively. R, right; L, left.
Fig. 5.
Neointimal formation distal to ligation was small and unchanged by Ephx2 gene deletion or tAUCB. The lumen diameter decreased significantly only in the Ephx2−/− mice carotid in the distal portion of the ligated LC in comparison with the RC after 6 wk of ligation (A; P < 0.05). EEL diameter (B), neointimal area (C), and neointimal-to-medial ratio was unchanged in all strains (D). The white, gray, and black bars represent WT-control, WT-tAUCB, and Ephx2−/− mice, respectively.
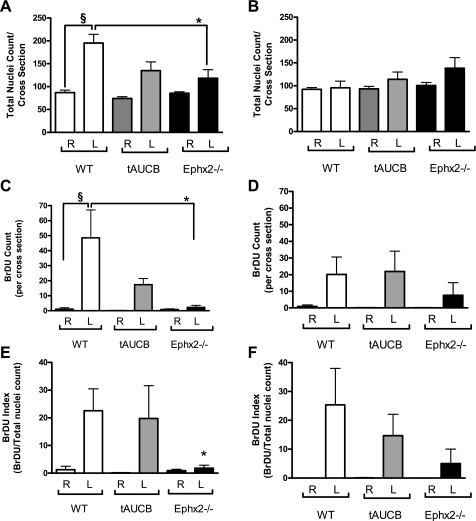
To further quantify the hyperplastic response, total nuclei and BrDU positive cells were counted proximal and distal to the point of ligation (Figs. 6 and 7). The hyperplastic response of the WT-control mice resulted in an increase in total nuclei count proximal to the ligation. Ephx2 gene deletion dampened this response, reducing total nuclei count ∼40%. The hyperplastic response correlated with an increase in cell proliferation as indicated by the increase in the number of BrDU positive nuclei and corresponding BrDU index (BrDU positive cells/total nuclei count) × 100 in WT-control. In the Ephx2−/− mice, the reduction in cellular count also corresponded with a dramatic reduction in BrDU count and index, indicating that the reduction in neointimal formation resulted from mechanisms that restrained cell proliferation. There were no differences in cellularity or proliferation distal to the point of ligation (Fig. 6, B, D, and F, and Fig. 7, A, D, and G).
Fig. 6.
Proliferation was dampened in the Ephx2−/− mice. WT-control mice had an aggressive hyperplastic response to LC ligation as demonstrated by the increase in total nuclei count proximal to the ligation. Ephx2 gene deletion dampens this response (A). The hyperplastic response correlated with an increase in cell proliferation as indicated by the increase in the number of BrDU positive nuclei and corresponding BrDU index in WT-control (C and E). Cell proliferation was dampened in the Ephx2−/− mice (C and E). There was also a trend for reduction BrDU positive cells from WT-tAUCB (P = 0.08; C). There were no differences in cellularity or proliferation distal to the point of ligation (B, D, and F). §P < 0.05 WT-control LC vs. WT-control RC; *P < 0.05 WT-control LC vs. Ephx2−/− LC. White, gray, and black bars represent WT-control, WT-tAUCB, and Ephx2−/−, respectively.
Fig. 7.
BrDU positive cells were more prevalent in proximal WT LC. The following pictures are of mouse carotids immunofluorescently labeled for BrDU and DAPI, a nuclear stain (blue). A–C are the proximal LC, distal LC, and RC of WT-control mice. D–F are the proximal LC, distal LC, and RC of WT-tAUCB. G–I are the proximal LC, distal LC, and RC of Ephx2−/− mice. Images were taken at 100× magnification. White circle identifies BrDU positive cell (green). White line demarcates 100 μm.
SEH inhibition or Ephx2 gene deletion does not alter wire-induced vascular injury.
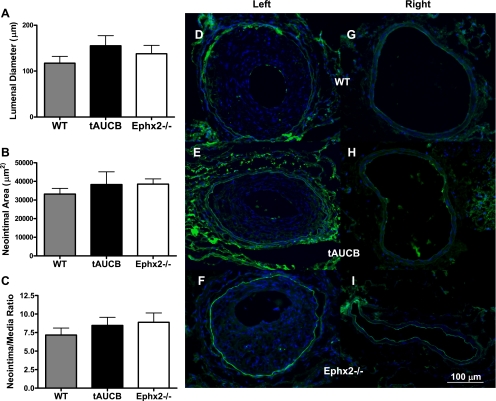
Although the hyperplastic response was restrained by tAUCB and Ephx2 gene deletion in mice undergoing LC ligation, the response to vascular injury induced by mechanical denudation of the vascular endothelium of the femoral artery was unaffected by SEH inhibition or Ephx2 gene deletion (Fig. 8, D–I), resulting in similar lumenal diameters, neointimal area, and intimal-to-media ratios (Fig. 8, A–F). This may reflect the impact and importance of the vascular endothelium, whereby the endothelium remains intact during common carotid artery ligation but is damaged by wire injury. In addition, as shown by the images in supplementary Fig. 4A, there was no difference in the cellularity of the injured left femoral artery. Unlike the remodeled common carotid arteries, there was no difference detected in the count of BrDU positive cells or BrDU index (supplementary Fig. 3, A–F).
Fig. 8.
Ephx2 gene deletion and tAUCB do not prevent hyperplastic response to vascular injury. Lumen diameter (A), neointimal area (B), and neointimal-to-medial ratio were unchanged in femoral arteries following catheter injury in all strains (C). The white, gray, and black bars represent WT-control (n = 10), WT-tAUCB (n = 7), and Ephx2−/− (n = 10) mice, respectively. D–F represent femoral arteries after wire injury, and G–I are right femoral arteries fluorescently labeled for BrDU (green) and DAPI, a nuclear stain (blue) taken at 100× magnification and show that none of the vessels were populated with significant amounts of proliferating cells. White line demarcates 100 μm.
DISCUSSION
Studies were conducted to determine the effect of SEH inhibition and Ephx2 gene deletion on vascular remodeling in vivo and the mechanisms behind these effects. Although SEH inhibition and Ephx2 deletion decreased the hyperplastic response to left common carotid artery ligation, neither abrogated the response to wire-induced vascular injury. These results indicate that SEH inhibition and Ephx2 gene deletion restrains pathological neointimal formation in an endothelium-dependent manner. In contrast, our studies suggest that the antihyperplastic properties of SEH inhibition and Ephx2 gene deletion are not attributed to direct actions of the SEH inhibitors on the VSMCs but depend instead upon actions on the endothelium. In addition, we also found that SEH inhibition normalized the response to changes in blood flow in hypertensive rats.
Herein, we found that inactivation of SEH either by tAUCB in WT or Ephx2 gene deletion inhibited the hyperplastic response to left common carotid ligation in the murine model when the endothelium was intact. The endothelium has been deemed the sensor of changes in hemodynamics of the vasculature (41) and could allow for the integration of these inputs, resulting in varying responses tailored to the changes in the environment. The finding that vascular protection by SEH inhibition and Ephx2 gene deletion was dependent upon the presence the endothelium is in line with previous reports on the role of the epoxygenase pathway, endothelium, and vascular smooth muscle. For example, organ perfusion studies demonstrated that increased shear stress causes EET release from the endothelium, which then aids in flow-induced vasodilation (17). It was also determined that EETs have a more predominate role in flow-mediated vasodilation in the occurrence of nitric oxide deficiency in female endothelial nitric oxide synthase-knockout mice (16) and male estrogen receptor-α knockout mice (46). In addition, laminar flow has been shown to mediate signaling in endothelial cells by EET release and decreased SEH expression in vitro. These effects are augmented with AUDA and decreased with increased endothelial cell SEH expression via transfection (30).
Although SEH inhibitors reportedly inhibit human VSMC proliferation in vitro, we did not see differences in the hyperplastic response to endothelial denudation of the femoral artery. This suggests that the protective effects of the SEH inhibitors, under our experimental conditions, were not solely due to direct actions on VSMCs. A recent report also demonstrates that SEH inhibition significantly attenuated pulmonary artery BrdU incorporation in VSMCs and endothelial cells in response to monocrotaline-induced proliferation (37). Interestingly, there was not a direct effect of EETs or SEH inhibition on proliferation in cultured pulmonary artery VSMCs and it was postulated that the in vivo protective effects on the pulmonary artery were most likely related to indirect actions (37). We speculate that the endothelium may play an important role in the protective effects of SEH inhibition by preventing the degradation of EETs and increasing epoxide bioavailability from the endothelium for paracrine actions on the VSMCs.
Ephx2−/− mice also exhibited a robust inward remodeling response to common carotid artery ligation relative to WT-control. However, unlike the Ephx2 gene deleted mice and the normotensive WKY rats, SEH inhibition in the SHRSP rats resulted in an increase in cell proliferation in the ligated LC of the SHRSP rats. Indeed, the SHRSP is known to exhibit profound endothelial dysfunction in comparison with WKY rats (14, 15, 23, 31), whereas we have found that the Ephx2 mice have enhanced endothelial responses to acetylcholine (32). Furthermore, consistent with the importance of the endothelium in mediating EET responses, we have found that the wire injury response was not affected by pharmacological inhibition of SEH.
We also found that SEH inhibition prevented the hyperplastic response stimulated by flow reduction in Ephx2−/− and WT-tAUCB mice in a model where the endothelium remains intact. Ephx2−/− mice exhibited a robust inward remodeling response to common carotid artery ligation relative to WT-control and WT-tAUCB mice. SEH inhibition was able to normalize the ability of the SHRSP rat to inward remodel in this model of severe flow reduction. Indeed, SHRSP rats have a decreased ability to respond to blood flow changes by mechanisms that are not directly related to hypertension, but may be attributed to endothelial dysfunction (19). As previously stated, the epoxygenase pathway plays a role in the response of the vasculature to flow in nitric oxide deficiency (16, 46). Therefore, preventing the degradation of EETs in these animals may result in an increase in epoxide bioavailability for paracrine actions on the VSMCs. In our study, the most prominent effects of the carotid ligation technique in the mice were noted more proximal to the point of ligation, corresponding to a location more midsection of the carotid artery, and distances from the point of ligation are certainly important for the robustness of the remodeling response.
Noteworthy was the lack of an antihypertensive effect in the SHRSP rats with AUDA treatment, which is consistent with prior observations from our laboratory and others (6, 28, 43). This may be due to the genetic variation in SEH expression and activity, which may influence the contribution of SEH on blood pressure regulation in the SHRSP rats. Indeed, reports have indicated that there are polymorphisms in the Ephx2 gene in some SHR strains (3, 12). Studies investigating the genetic and strain influences over SEH protein levels and activity indicate that the SEH abundance and activity vary among rat strains, particularly WKY, SHR (12), SHRSP, and stroke-resistant SHR (3). However, these changes in abundance of protein or activity of SEH do not positively correlate with regulation of blood pressure (13) or risk for vascular disease (3). In fact, even though SHRSP rats demonstrate a decrease in SEH protein and SEH activity in comparison with stroke-resistant SHR, plasma EET levels are still similar between groups, and the risk for stroke is still higher in the SHRSP rats (3). These varying effects of Ephx2 polymorphisms may partially explain the complexity of the response and resistance to blood pressure reduction by SEH inhibitors.
Previous reports indicate alkanoic acid SEH inhibitors, including AUDA, stimulate peroxisome proliferator-activated receptor (PPAR)-α activity at micromolar concentrations (4) most probably due to its long alkyl fatty acid-like chain (7, 35). Although we cannot exclude the possibility that AUDA acts to stimulate PPAR-α in our studies, the AUBA levels achieved in the present study were in the nanomolar range and are not sufficiently high enough to induce PPAR-α activity. Moreover, we also found protection with the SEH inhibitor tAUCB, which is not an alkanoic acid. We have also shown in hypertensive studies that tAUCB does not lower blood pressure further in deoxycorticosterone salt hypertension, suggesting that tAUCB effects are mediated by SEH inhibition (32). Furthermore, we also found that the Ephx2 gene deleted mice were protected against hyperplastic remodeling induced by common carotid artery ligation. These results suggest that loss or reduction of SEH activity protects from pathological vascular remodeling.
The overall findings of our experimental studies suggest that SEH inhibition may have clinical relevance. Epidemiological studies give evidence that the human SEH polymorphism that decreased SEH activity in vitro (36) correlated with increased incidence of the stabilized form of atherosclerosis (coronary artery calcification) (24). This single nucleotide polymorphism was also shown to decrease the risk for ischemic stroke in the Chinese population (50). A correlation between decreased incidence of ischemic stroke and aortic calcification was also noted with a SEH polymorphism in the Caucasian population (11, 13). The polymorphism associated with increased SEH activity is associated with an increase in coronary artery disease events (27). In fact, in our study, the degree of stenosis induced by carotid ligation was less than the WT-control by 32% in Ephx2 gene deleted mice and 49% in WT-tAUCB. Moreover, the normalization of remodeling in the SHRSP rats provides further evidence for the potential for managing vascular disease with SEH inhibition.
The clinical relevance of SEH inhibition is further shown by the increased risk of coronary artery disease with the K55R polymorphism, which is associated with increased SEH activity (27). Smoking, a risk factor for cardiovascular events, enhances this association (48). This indicates that the patient population most likely afflicted with these vascular diseases and increased risk for a cardiovascular event could benefit from SEH inhibitors. Although we did not find a protective effect with wire-induced vascular injury, these studies suggest that protection can still be conferred by incorporating EETs or EET mimetics into drug-eluting stents to prevent restenosis from intravascular procedures. Overall, this study provides evidence that SEH inhibition modulates vascular remodeling, which may be of utility in the management of vascular diseases.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HL-59699 and F31 HL-087723 and Advancing a Healthier Wisconsin. This work was also supported by NIH Grant R37 E5–02710.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
J. D. Imig is an American Heart Association Established Investigator.
REFERENCES
- 1.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation 107: 769–776, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 78: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Corenblum MJ, Wise VE, Georgi K, Hammock BD, Doris PA, Fornage M. Altered soluble epoxide hydrolase gene expression and function and vascular disease risk in the stroke-prone spontaneously hypertensive rat. Hypertension 51: 567–573, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Davis BB, Morisseau C, Newman JW, Pedersen TL, Hammock BD, Weiss RH. Attenuation of vascular smooth muscle cell proliferation by 1-cyclohexyl-3-dodecyl urea is independent of soluble epoxide hydrolase inhibition. J Pharmacol Exp Ther 316: 815–821, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Davis BB, Thompson DA, Howard LL, Morisseau C, Hammock BD, Weiss RH. Inhibitors of soluble epoxide hydrolase attenuate vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA 99: 2222–2227, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 46: 842–848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang X, Hu S, Watanabe T, Weintraub NL, Snyder GD, Yao J, Liu Y, Shyy JY, Hammock BD, Spector AA. Activation of peroxisome proliferator-activated receptor alpha by substituted urea-derived soluble epoxide hydrolase inhibitors. J Pharmacol Exp Ther 314: 260–270, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, Thompson DA, Hammock BD, Spector AA. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem 276: 14867–14874, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401: 493–497, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s). Pharmacol Res 49: 525–533, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fornage M, Boerwinkle E, Doris PA, Jacobs D, Liu K, Wong ND. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation 109: 335–339, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Fornage M, Hinojos CA, Nurowska BW, Boerwinkle E, Hammock BD, Morisseau CH, Doris PA. Polymorphism in soluble epoxide hydrolase and blood pressure in spontaneously hypertensive rats. Hypertension 40: 485–490, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Fornage M, Lee CR, Doris PA, Bray MS, Heiss G, Zeldin DC, Boerwinkle E. The soluble epoxide hydrolase gene harbors sequence variation associated with susceptibility to and protection from incident ischemic stroke. Hum Mol Genet 14: 2829–2837, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunfeld S, Hamilton CA, Mesaros S, McClain SW, Dominiczak AF, Bohr DF, Malinski T. Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension 26: 854–857, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res 96: 376–383, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem 50: 3825–3840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim J, Miyashiro JK, Berk BC. Shear stress is differentially regulated among inbred rat strains. Circ Res 92: 1001–1009, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res 38: 247–255, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Imig JD, Navar LG, Roman RJ, Reddy KK, Falck JR. Actions of epoxygenase metabolites on the preglomerular vasculature. J Am Soc Nephrol 7: 2364–2370, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension 46: 975–981, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 33: 1353–1358, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Klein LW. Atherosclerosis regression, vascular remodeling, and plaque stabilization. J Am Coll Cardiol 49: 271–273, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined. Circulation 110: 220–226, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 17: 2238–2244, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, Hammock BD, Couper DJ, Heiss G, Zeldin DC. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet 15: 1640–1649, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci 13: 3480–3487, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res 73: 792–796, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA 102: 16747–16752, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma XL, Gao F, Nelson AH, Lopez BL, Christopher TA, Yue TL, Barone FC. Oxidative inactivation of nitric oxide and endothelial dysfunction in stroke-prone spontaneous hypertensive rats. J Pharmacol Exp Ther 298: 879–885, 2001. [PubMed] [Google Scholar]
- 32.Manhiani M, Quigley J, Knight S, Moore T, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal inflammation and injury in DOCA-salt hypertension (Abstract). Hypertension 50: e89, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyashiro JK, Poppa V, Berk BC. Flow-induced vascular remodeling in the rat carotid artery diminishes with age. Circ Res 81: 311–319, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Morisseau C, Hammock BD. Gerry Brooks and epoxide hydrolases: four decades to a phamaceutical. Pest Manag Sci 64: 594–609, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Ng VY, Morisseau C, Falck JR, Hammock BD, Kroetz DL. Inhibition of smooth muscle proliferation by urea-based alkanoic acids via peroxisome proliferator-activated receptor alpha-dependent repression of cyclin D1. Arterioscler Thromb Vasc Biol 26: 2462–2468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, Bradbury JA, Enayetallah AE, Zeldin DC, Grant DF. Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol 64: 482–490, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Revermann M, Barbosa-Sicard E, Dony E, Schermuly RT, Morisseau C, Geisslinger G, Fleming I, Hammock BD, Brandes RP. Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonary hypertension in rats. J Hypertens 27: 322–331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Rudic RD, Brinster D, Cheng Y, Fries S, Song WL, Austin S, Coffman TM, FitzGerald GA. COX-2-derived prostacyclin modulates vascular remodeling. Circ Res 96: 1240–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Rudic RD, Sessa WC. Nitric oxide in endothelial dysfunction and vascular remodeling: clinical correlates and experimental links. Am J Hum Genet 64: 673–677, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 101: 731–736, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J Mol Cell Cardiol 32: 2097–2104, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol 174: 2086–2095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem 275: 40504–40510, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res 43: 55–90, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Sun D, Yan C, Jacobson A, Jiang H, Carroll MA, Huang A. Contribution of epoxyeicosatrienoic acids to flow-induced dilation in arteries of male ER-α knockout mice: role of aromatase. Am J Physiol Regul Integr Comp Physiol 293: R1239–R1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol 52: 314–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DR, Siscovick DS, Fornage M. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis 190: 26–34, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, Parker C, Graham L, Engler MM, Hammock BD, Zeldin DC, Kroetz DL. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res 87: 992–998, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Ding H, Yan J, Hui R, Wang W, Kissling GE, Zeldin DC, Wang DW. Genetic variation in cytochrome P450 2J2 and soluble epoxide hydrolase and risk of ischemic stroke in a Chinese population. Pharmacogenet Genomics 18: 45–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27: 1931–1940, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 15: 1244–1253, 2004. [PubMed] [Google Scholar]