Abstract
Variations in circadian rhythms are evident in the incidence of cardiovascular disease, and the risk of cardiovascular events increases when rhythms are disrupted. The suprachiasmatic nucleus is the central circadian pacemaker that regulates the daily rhythm of peripheral organs. Diurnal rhythms have more recently been shown to exist in myocardial tissue and are involved in metabolism and contractile function. Thus we sought to determine whether the functional deletion of the circadian rhythm mouse periodic gene 2 (mPer2) would protect the heart against ischemic injury. Nonreperfused myocardial infarction was induced in anesthetized, ventilated C57 (n = 17) and mPer2 mutant (mPer2-M; n = 15) mice via permanent ligation of the left anterior descending coronary artery. At 4 days post-myocardial infarction, we observed a 43% reduction of infarct area in mPer2-M mice compared with wild-type mice. This is coincident with 25% less macrophage infiltration, 43% higher capillary density, 17% increase in hypertrophy, and 15% less cardiomyocyte apoptosis in the infarct zone. Also, matrix metalloproteinase-9 was expressed in inflammatory cells in both groups, but total protein was 40% higher in wild-type mice, whereas it was not elevated in mPer2-M mice in response to injury. The functional deletion of the mPer2 gene reduces the severity of myocardial infarct injury by limiting the inflammatory response, reducing apoptosis, and inducing cardiomyocyte hypertrophy, thus preserving cardiac function. These findings collectively imply that the disruption of the circadian clock gene mPer2 is protective. Understanding the interactions between circadian rhythm genes and cardiovascular disease may provide insights into potential preventative and therapeutic strategies for susceptible populations.
Keywords: infarct, mouse
circadian rhythms are daily variations of physiological processes that are found in living organisms. In mammals, the circadian rhythms are regulated by the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN synchronizes the circadian rhythms of peripheral organs to each other and to the environmental light-dark cycle via integrated oscillatory expression of multiple circadian clock genes (5, 7, 13, 25). So far, eight core circadian clock genes have been identified in mammals, including a Clock gene, a gene encoding brain-muscle arylhydrocarbon receptor nuclear translocator (Arnt)-like protein 1 (Bmal1) (16), three period genes (Per1, Per2, and Per3) (34, 38, 47), and two cryptochrome genes (Cry1 and Cry2) (12, 33, 40).
Recently, it has been shown that clock genes are found in all peripheral tissues, including the heart. Epidemiologic studies demonstrate the existence of circadian patterns in the incidence of cardiovascular disease. For example, the onset of non-Q-wave angina, unstable angina, myocardial infarctions (MIs), and sudden cardiac death all show marked elevations in the occurrence between the hours of 6:00 am and 12:00 pm, compared with any other time of day (27, 44). A better understanding of the function of circadian genes in the heart and in response to injury may lead to innovative therapies for cardiovascular disease (9, 29, 46).
Cardiac tissue expresses all known isoforms of Cry and Per genes, with Cry2, Per1, and Per2 expressed to the greatest degree (46). However, all of these genes function essentially as reciprocally controlling transcription factors, and in many cases the expression of these genes is monitored by the modulation of many “noncircadian” proteins as readouts. Enzymes regulating cardiac metabolism (57), reactivity of vascular endothelial cells (22, 50, 54), modulation of inflammatory responses (3, 27, 33, 34, 44), bone marrow progenitor cell release (2), and apoptosis (25) all have circadian gene components of control, and all are associated with the myocardial response to coronary artery occlusion. However, the specific relationship between a circadian gene and the inflammatory response and injury associated with early MI has not been determined. Given that circadian rhythms control the cell cycle and that mutations in clock genes have been associated with tumor growth, altered regulation of apoptosis (22), altered contractile function, metabolism, and gene expression in clock gene mutant cardiomyocytes (3), it would appear reasonable to suggest that Per2 may be capable of altering the response to ischemic injury. Therefore, these studies were designed to determine the effect of functional mouse Per2 (mPer2) deletion on early post-MI injury.
METHODS
Animals.
Male wild-type (WT) C57BL/6J mice (aged 8–10 wk) and homozygous mutant mPer2-M mice bred on a C57BL/6J background were obtained from Jackson Laboratories (mPer2-M Brdm1, stock No. 003819; Bar Harbor, ME) (47). Two segments of the PAS domain of the mPer2 gene were deleted, rendering a functional null mutant. RT-PCR indicated that a mutant transcript, if translated, would generate an 87 amino acid protein (47). Mice possessing this ubiquitous mutation are morphologically indistinguishable, have a shorter circadian period, and lose rhythmicity in constant darkness compared with their WT counterparts (47). All animals were individually housed in a light-proof chamber and entrained in a 12-h:12-h light-dark cycle for at least 10 days before surgery. All procedures were approved by the East Carolina University Institutional Animal Care and Use Committee and are in compliance with National Institutes of Health guidelines.
Surgical procedure.
Mice were anesthetized (Avertin, 20 μl/g ip), intubated, and ventilated using a Kent Scientific TOPO ventilator. Briefly, the left anterior descending coronary artery was permanently ligated or sham ligated in controls. The chest cavity was sutured closed, and the animals were permitted to recover in a warming chamber before being returned to the vivarium. No analgesia was used, and all experiments were performed during the light phase of the circadian cycle between zeitgeber time 3 and 9. The surgical procedure is described in more detail elsewhere (19, 30).
At 4 days post-MI, mice were given an injection (0.5 ml ip) of 5-bromodeoxyuridine (BrdU, 5 mg/ml) to label proliferating cells and euthanized 1 h later with an intraperitoneal injection of pentobarbital sodium. The perfused heart and a segment of small intestine (used as a positive control for BrdU+ proliferating cells) were immersed in zinc fixative, and four transversely sectioned slices of equal thickness were processed and embedded in paraffin. Routine histological (hematoxylin and eosin, picrosirius red/fast green, toluidine blue, and congo red) procedures and immunostaining were performed using 5-μm sections.
Morphometry and histology.
For nonreperfused infarct studies, photographs of four hematoxylin and eosin-stained sections per heart (uninjured control and 4 days post-MI, both WT and mPer2-M hearts) were taken at ×20 using a DP70 digital camera. Scion imaging software (Scion, Frederick, MD) was used to trace the infarct zone (granulation tissue and necrosis), necrosis (no myocyte nuclei), and granulation tissue (inflammatory cells, fibroblasts, smooth muscle cells, and endothelial cells).
To assess the myocyte cross-sectional area (MCSA), three images were taken at ×600 from both the epicardial and endocardial surface at the infarct border in two sections containing infarct (12 images total). In each image, three to seven perpendicularly sectioned myocytes with centrally located nuclei were measured and the mean cross-sectional area was calculated.
Immunostaining.
Tissue sections were deparaffinized and endogenous peroxidases quenched. After being rinsed in PBS, the slides were incubated with anti-α-smooth muscle actin (α-SMA; peroxidase conjugated, DAKO, U7033) for myofibroblasts, anti-CD31 (PharMingen, No. 553371; 1:2,000) for endothelial cells in infarcted hearts, isolectin B4 (Vector, No. B-1205) for endothelial cells in control hearts (19), anti-CD45 (PharMingen, No. 550539; 1:2,000) for leukocytes, and anti-Ly6G (BD PharMingen, No. 550291, 1:100) for neutrophils. Biotinylated anti-matrix metalloproteinase (MMP)-9 (R&D Systems, BAF909, 1:3) was used to visualize the expression pattern of MMP-9 in infarcted tissues. The reaction product was visualized with 3,3-diaminobenzidine (DAB; Vector, SK-4100). For BrdU double-labeling of fibroblasts, the slides previously stained with anti-α-SMA were rinsed and incubated with peroxidase-conjugated anti-BrdU (Roche, No. 1585860; 1:25). The reaction product was visualized with Vector VIP (Vector, SK-4600).
Myofibroblast, capillary, macrophage, and neutrophil density was measured in the infarct zone in five fields/section of two sections of infarcted heart per specimen at ×400, and numbers are expressed as n/0.1 mm2. Proliferating cells (DAB+/BrdU+) were counted in random fields throughout the infarct until a total of 500 DAB-positive cells had been counted in each of two sections containing infarct regions. Vascular smooth muscle cells that are clearly part of vessels can easily be seen at this magnification and were thus omitted from these counts. Measurements were expressed as the percentage of double-labeled cells in 1,000 DAB-positive cells ± SE.
For detection of DNA strand breaks in cell nuclei, we used terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) staining, a common means of detecting in situ cell death in tissue sections (32, 45) (Roche, No. 11684817910). All TUNEL-positive (DAB+) cells were counted by inspecting random fields throughout the infarct until a total of 500 nuclei had been counted in each of two sections containing infarct regions, and the data were expressed as a percentage of 1,000 total nuclei. A double stain for TUNEL and anti-α-sarcomeric actin (Sigma, A2172, 1:4,000; visualized with Vector Red, SK-5100) was done to determine the number of apoptotic cardiomyocytes in the border regions of two sections of the heart with infarct regions. The number of double positive cells was expressed as a percentage of 500 total cardiomyocyte nuclei.
Western blot analysis.
Left ventricles (LVs) of mouse hearts were snap frozen in liquid nitrogen at the time of harvest and subsequently homogenized in HEPES buffer containing protease inhibitors. Proteins from control WT and mPer2-M and 4 days post-MI WT and mPer2-M LVs (40 μg) were resolved by SDS polyacrylamide gel electrophoresis (7–15%) and transferred to polyvinylidene difluoride membranes. Chemiluminescence was used for immunodetection. Images of the Western blots were captured using the Typhoon 9410 Imager. Densitometry was performed using ImageQuant TL 1D and array image analysis software. All membranes were subsequently stained with Ponceau S (0.1% wt/vol in 5% acetic acid, Sigma P7170) to confirm equal loading and transfer (26).
RT-PCR.
RNA was isolated from the whole LV according to routine TRIzol method and purified using the RNeasy mini kit (Qiagen; No. 74104). RT was performed using the High-Capacity cDNA RT kit (ABI; 4368814), and 100 ng RNA were amplified with TaqMan Universal Master Mix (ABI; 4364338) using the Applied Biosystems 7900HT Fast Real-Time PCR machine. The following TaqMan primer/probes were purchased from ABI: clock (Mm00455950_m1), bmal1 (Mm00500226_m1), Npas2 (Mm00500848_m1), cry1 (Mm00514392_m1), cry 2 (Mm00546062_m1), mPer1 (Mm00501813_m1), mPer2 (Mm00478113_m1), and GAPDH (Mm99999915_g1) as an internal reference. For each gene, mRNA expression was analyzed in triplicate in three animals.
Hemodynamic determinations.
Uninfarcted mice and mice 4 days after infarction were anesthetized (90 mg ketamine-10 mg xylazine/100 g body wt ip). Echocardiography (Toshiba Nemio 30, Duluth, GA) using a 14-MHz linear array transducer (PLM 1204AT)-derived LV volume was used to calibrate the LV volume signal obtained by the conductance catheter (20). Two-dimensional images of the LV were obtained in the parasternal long-axis and short-axis views, and M-mode images were obtained at the midventricular level in both views, from which internal dimensions of the LV were obtained at end diastole and end systole. LV end-diastolic and end-systolic volumes were determined using the area-length method as validated previously (20).
LV pressure (LVP)-volume measurements were obtained using a 1.2-Fr pressure-volume conductance catheter (Scisense, London, ON, Canada) inserted into the carotid artery and advanced into the LV as described previously (24, 28). Pressure-volume data were recorded (Polyview, Grass Technologies, Warwick, RI) under baseline conditions and after transient occlusion of the inferior vena cava. Pressure-volume loops were subsequently generated and analyzed off-line, using CardioSoft (Sonometrics, London, ON, Canada) data analysis software. Hemodynamic measurements included peak systolic LVP, stroke volume, heart rate, cardiac output, stroke work, maximum rate of LVP development, end-systolic elastance (36), and preload recruitable stroke work (PRSW, linear regression of stroke work vs. end-diastolic volume) (11) and are expressed as means ± SD (Table 1).
Table 1.
Pressure-volume loop data
| C57 Control; n = 6 | mPer2-M Control; n = 6 | C57 4 Days; n = 6 | mPer2-M 4 Days; n = 6 | |
|---|---|---|---|---|
| Heart rate, beats/min | 389 ± 29 | 397 ± 37 | 432 ± 56 | 443 ± 48 |
| Stroke volume, μl | 14.9 ± 2.1 | 15.4 ± 2.0 | 10.8 ± 1.4* | 9.7 ± 2.3* |
| Ejection fraction, % | 57 ± 6 | 58 ± 7 | 28 ± 3* | 26 ± 3* |
| Cardiac output, ml/min | 5.80 ± 1.2 | 6.13 ± 1.3 | 4.67 ± 1.2 | 4.31 ± 1.3 |
| End-diastolic volume, μl | 26 ± 1.2 | 26.5 ± 1.1 | 38 ± 1.6* | 37.2 ± 2.0* |
| LVPsys, mmHg | 101 ± 6 | 100 ± 7 | 67 ± 3* | 79 ± 4*† |
| ESPVR, mmHg/μl | 7.77 ± 0.65 | 7.86 ± 0.83 | 2.23 ± 0.19* | 2.98 ± 0.20*† |
| PRSW, mmHg | 114 ± 7.0 | 119.2 ± 8.2 | 49.1 ± 2.8* | 61.4 ± 2.4*† |
Values are means ± SE. Hemodynamics in C57 wild-type (WT) and mouse periodic gene 2-mutant (mPer2-M) mouse hearts without injury (control) and with chronic infarction 4 days post-myocardial infarction. LVPsys, left ventricular systolic pressure; ESPVR, end-systolic pressure-volume relationship; PRSW, preload recruitable stroke work.
P < 0.05, significantly different from respective control;
P < 0.05, significantly different 4-day WT vs. 4-day mPer2-M.
Statistics.
Data are expressed as means ± SE. Statistical significance between groups was determined by ANOVA, and significance levels were P < 0.05. Statistical analysis of hemodynamic data was performed using two-factor ANOVA, comparing WT and mPer2-M mice at baseline and at 4 days after infarction, and individual subgroup comparisons were made using Tukey's multiple range test (P < 0.05). The mortality between 4-day WT and mPer2 mutants and RT-PCR data were analyzed with a Student's t-test (P < 0.05).
RESULTS
Morphometry and histology.
No significant differences were observed in mortality between the WT and mPer-M mice [survival rates: mPer2-M, 83% (n = 15 of 18); and WT, 85% (n = 17 of 20)]. All mice were included in these analyses, and measurements and counts were done blindly.
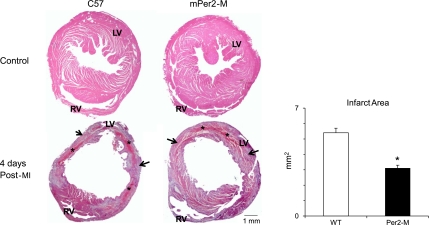
The infarct area was 43% smaller in the mPer2-M mouse hearts (Fig. 1; WT, 5.4 ± 0.3 vs. mPer2-M, 3.1 ± 0.2 mm2; P < 0.05), and as such, there was 48% less residual necrosis (infarct area minus granulation tissue area) in the mPer2-M mice (WT, 2.1 ± 0.2 vs. mPer2-M, 1.1 ± 0.2 mm2; P < 0.05) and 35% less granulation tissue in the mPer2-M mice (WT, 5.1 ± 0.4 vs. mPer2-M, 3.3 ± 0.5 mm2).
Fig. 1.
Mouse periodic gene 2-mutant (mPer2-M) hearts have reduced infarct area at 4 days post-myocardial infarction (post-MI). Left: representative histology of wild-type (WT; top, left) and mPer2-M (top, right) control mouse hearts and WT (bottom, left) and mPer2-M (bottom, right) hearts 4 days after chronic MI. Infarct area (in mm2) was 43% smaller in mPer2-M hearts 4 days post-MI (P < 0.05). Arrows point to granulation tissue; asterisks indicate necrosis. RV, right ventricle; LV, left ventricle. Right: *P < 0.05.
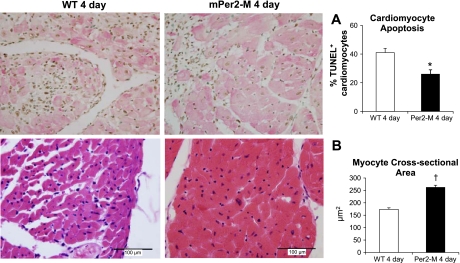
There were 40% less TUNEL-positive apoptotic nuclei (as a percentage of all nuclei) in the mPer2-M infarcts compared with WT mouse hearts (WT, 45 ± 3% vs. mPer2-M, 27 ± 3%; P < 0.05). Specifically, cardiomyocyte apoptosis in 4-day infarcts of mPer2-M mice vs. WT 4-day hearts was significantly less (Fig. 2A; 26 ± 1.9% and 41 ± 1.6%, respectively). The average MCSA was not different between uninjured control hearts of WT and mPer2-M mice (WT, 203 ± 22 vs. mPer2-M, 225 ± 23 μm). There was a nonsignificant trend for MCSA to be higher in the epicardium (220 ± 23 μm) than in the endocardium (186 ± 22 μm) in WT hearts in contrast to mPer2-M hearts that tended to have larger myocytes in the endocardium (247 ± 17 μm) versus the epicardium (208 ± 33 μm). At 4 days post-MI, the average MCSA (both endocardial and epicardial) was increased in mPer2-M hearts, whereas it decreased in WT mouse hearts (Fig. 2B; WT, 174 ± 6 vs. mPer2-M, 262 ± 9 μm). There was no difference between epicardial MCSA versus endocardial MCSA in either WT or mPer2-M hearts at 4 days post-MI.
Fig. 2.
Right: reduced cardiomyocyte apoptosis (A) and increased myocyte cross-sectional area (B) in mPer2-M mouse hearts 4 days after chronic infarction are shown. Left: there were 15% less terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive apoptotic cardiomyocytes observed in mPer2-M mice (top, right) compared with WT (top, left; *P < 0.05). The average myocyte cross-sectional area (in μm2; †P < 0.001) was increased in mPer2-M mice (bottom, right) vs. WT (bottom, left).
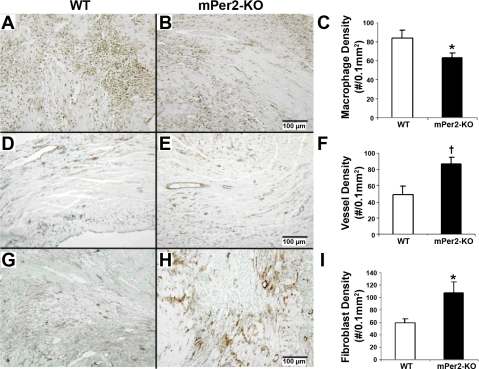
Representative micrographs of immunohistochemical staining for the pan-leukocyte marker CD45 in WT and mPer2-M mouse hearts are shown in Fig. 3, A and B, respectively. Macrophage density was 25% lower in the infarct zone of mPer2-M hearts at 4 days post-MI compared with WT mouse hearts (Fig. 3C; WT, 84 ± 8 vs. mPer2-M, 63 ± 5; P < 0.05). There was no significant difference between neutrophils (WT 4 day, 13 ± 2 vs. mPer2-M 4 day, 12 ± 3). Similarly, eosinophils counts using congo red staining and toluidine blue staining for mast cells were present in very low numbers and thus yielded no significant differences between the groups (data not shown).
Fig. 3.
Pan-leukocyte marker CD45 staining showed reduced CD45-positive inflammatory cells in mPer2-M mice (A) compared with WT mice (B) at 4 days after infarction (C; *P < 0.05). KO, knockout. There was a significant increase in vessel density in mPer2-M (E) hearts compared with WT hearts (D) at 4 days post-MI (F; †P < 0.01). Fibroblast density was also increased in mPer2-M (H) compared with WT (G) mouse hearts at 4 days post-MI (I; *P < 0.05).
There was no difference in the vessel density per 0.1 mm2 in uninfarcted control hearts (WT, 103 ± 8 vs. mPer2-M, 112 ± 4). Representative images of CD31+ endothelial cells in the infarct zone at 4 days post-MI are shown in WT (Fig. 3D) and mPer2-M (Fig. 3E) mouse hearts. We observed a 43% increase in vessel density per 0.1 mm2 in the infarct zone of mPer2-M mice compared with WT (Fig. 3F; WT, 49 ± 10 vs. mPer2-M, 87 ± 8; P < 0.01). Although there was no difference in the average area/vessel in the WT versus mPer2-M control mice or in the vessels in the infarct region, there was a significant difference in the area/vessel in the uninjured tissue regions of the infarcted heart (WT, 6.3 ± 0.8 vs. mPer2-M, 9.7 ± 0.4 μm2; P < 0.01).
Immunohistochemistry for activated fibroblasts was performed using an anti-α-SMA antibody, and representative images of infarct zone of WT and mPer2-M hearts at 4 days post-MI are shown in Fig. 3, G and H, respectively. Fibroblast density in the infarct zone was 44% higher in mPer2-M hearts at 4 days post-MI than in WT mouse hearts (Fig. 3I; WT, 60 ± 6 vs. mPer2-M, 108 ± 17, P < 0.05). There was no difference in fibroblast proliferation rate (SMA+ + BrdU+/SMA+) between the two groups at 4 days post-MI or interstitial fibrosis (data not shown). It is possible that proliferation occurs earlier since there was less injury to the mPer2-M hearts, but this was not determined.
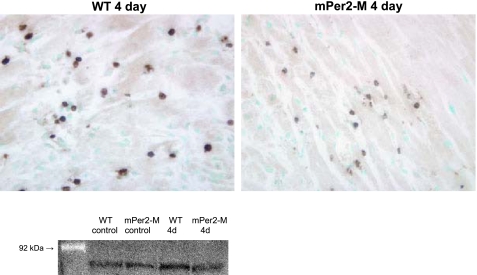
Figure 4 shows representative micrographs for MMP-9 immunohistochemistry in 4-day WT and mPer2-M hearts and a Western blot to demonstrate changes in the expression level. The images demonstrate the presence of this protein in inflammatory cells. One representative sample per group most closely approximating the average of the three per group measured was used for Western blot analysis. Densitometry of the bands shows there is no difference in the expression of MMP-9 between control WT or mPer2-M hearts; however, in contrast to the 40% increase in expression in 4 day WT infarcted hearts compared with control, the expression level does not change in mPer2-M hearts at 4 days post-MI.
Fig. 4.
Matrix metalloproteinase-9 (MMP-9) expression in inflammatory cells is reduced in mPer2-M hearts 4 days (4D) after chronic infarction. MMP-9 is expressed in inflammatory cells in mPer2-M hearts (right) compared with WT (left) at 4 days after infarction. A representative Western blot (bottom) for MMP-9 shows that there is no difference between uninjured WT and mPer2-M hearts. In contrast to the increased expression observed in WT hearts at 4 days post-MI, there is no change in MMP-9 expression in mPer2-M hearts at 4 days post-MI.
RT-PCR.
The level of transcripts as measured by the critical threshold (detection threshold) of each gene in each sample was normalized to the constitutive housekeeping gene GAPDH to control for sample to sample differences. The groups (n = 3) were compared as follows: control mPer2-M versus control WT, 4-day WT versus 4-day control, 4-day mPer2-M versus control mPer2-M, and 4-day mPer2-M versus 4-day WT. There were no differences in the expression levels of any of the seven genes (mPer2 was not measured in mPer2 mutant mice), except for bmal, with a measured 1.54-fold increase in 4-day mPer2-M from 4-day WT mice (P < 0.05).
Cardiac function.
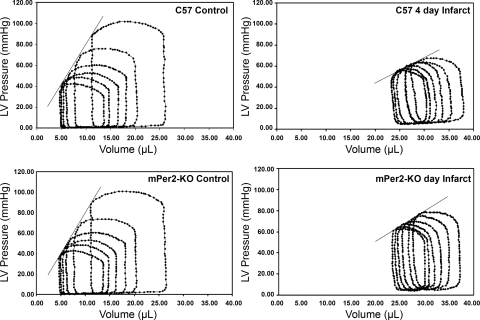
Pressure-volume loops for WT and mPer2-M mice are shown in Fig. 5 (n = 6 per group). There were no differences in ventricular performance between the groups before infarction. After infarction, as expected, the indexes of ventricular performance decreased in both WT and mPer2-M 4 days post-MI (Table 1). However, the loss of ventricular contractile function was significantly attenuated in the mPer2-M animals compared with infarcted WT animals. Peak LVP (WT, 67 ± 3; and mPer2-M, 79 ± 4; P < 0.05), end-systolic pressure-volume relationship (ESPVR; WT, 2.23 ± 0.23; and mPer2-M, 2.98 ± 0.20 mmHg/μl; P < 0.05), and PRSW (WT, 49.1 ± 2.8 mmHg/μl; and mPer2-M, 61.4 ± 2.4 mmHg; P < 0.05) were significantly better in the mPer2 animals, whereas heart rate, cardiac output, ejection fraction, and stroke volume were not different between the groups. Since bradycardia can decrease cardiac output by limiting stroke volume(14), it is possible that anesthesia-induced bradycardia created some degree of ventricular dilatation that partially masked the differences between the groups in measurements of output and stroke volume. However, the ESPVR and PRSW are widely held to be more sensitive measures of performance. Clearly, at 4 days, significant dysfunction compared with control values exists in both groups, but the improved performance in the mPer2-M animals is consistent with the decreased inflammation and reduced apoptosis also described in these animals.
Fig. 5.
mPer2-M mice showed improved LV pressure, end-systolic pressure-volume relationship (ESPVR), and preload recruitable stroke work (PRSW) 4 days after infarction. ESPVR: slope of the best fit line through the end-systolic pressure-volume points in the series of pressure-volume loops. With linear fit, all slopes have regressions r > 0.94. PRSW: r > 0.95 for each of the stroke work/end-diastolic volume slopes.
DISCUSSION
Our data represent novel findings regarding the interaction between the circadian rhythm gene mPer2 and cardiac injury. In summary, we observed significant myocardial protection in mPer2-M mouse hearts as evidenced by a 43% reduction of infarct area, a 43% increase in vascular density, 25% less macrophage infiltration, 17% more hypertrophy, and 15% less cardiomyocyte apoptosis in the infarct zone of mPer2-M mouse hearts compared with WT mouse hearts 4 days post-MI. Our hemodynamic data confirm less dysfunction, as exhibited by the preservation of contractility and indexes of cardiac work such as in ESPVR, PRSW, and LVP in mPer2-M, 4 days post-MI. There was better functional preservation in the mPer2-M animals, although the large difference in infarction was accompanied by modest improvements in indexes of contractility. In part, some of the differences may have been masked by the bradycardia induced by the anesthetic, but more likely, the severity of the infarction and the early time point of these data both contribute to limiting differences that might be observed in functional performance. However, the improvement in performance, combined with the decreased inflammation and volume of necrosis, would be consistent with an expectation of better longer-term recovery once the infarction completely resolves.
In the absence of reperfusion, at 4 days post-MI, granulation tissue comprised of macrophages, endothelial cells, and fibroblasts are at the peak of proliferation and migration to initiate scar formation (43). Mice lacking functional mPer2 protein contained less macrophages, more myofibroblasts, and better preservation of capillary density and displayed decreased total and cardiomyocyte apoptosis compared with matched C57 controls at 4 days post-MI. Previous studies showing that mPer2-M mutant mice failed to show a daily rhythm in levels of IFN-γ, a potent proinflammatory cytokine and activator of macrophages that is secreted by natural killer (NK) cells (1, 23). The decreased responsiveness of the inflammatory cascade may be responsible in part for the decreased apoptosis because of reduced oxidative stress and cytokine elaboration (8, 17, 37). The lack of functional Per2 protein in the heart may be directly responsible for the reduced apoptosis as well, since a previous study showing mPer2 overexpression in mouse Lewis lung carcinoma cells and mammary carcinoma cells (EMT6) resulted in rapid apoptosis by a downregulation of c-Myc, Bcl-X(L), and Bcl-2 and an upregulation of p53 and bax (18). Also, it has previously been shown that in the inflammatory response following an LPS challenge in mPer2 mutant mice, the inflammatory response is blunted because of deficient NK cell function (23). Furthermore, when the circadian system is uncoupled centrally, the rats kept in total darkness during the first 48 h following brain injury exhibited an improved recovery (4, 41, 42). These data coordinately suggest that the reduced injury in the nonreperfused model occurred by decreased immune cell infiltration and function as well as reduced cardiomyocyte apoptosis, leading to the expeditious resolution of infarct repair. An increased myofibroblast density in the infarct zone is also suggestive of faster healing. This notion is further supported by the decreased expression of MMP-9 in mPer2-M hearts at 4 days post-MI. MMP-9 is known to be upregulated early in response to injury and is often found to be expressed by leukocytes (15). Furthermore, MMP-9 null mice exhibit reduced infarct area in response to acute ischemia-reperfusion, and this was attributed to less neutrophil infiltration (31), and in the absence of reperfusion, there was less deleterious remodeling, dilation, and fewer macrophages (6). Indeed, we observed a decreased MMP-9 expression in inflammatory cells, and this, combined with the reduced density of inflammatory cells and reduced cardiomyocyte apoptosis, indicates that less injury is part of the mechanism by which the infarct area is reduced in mPer2-M hearts. Furthermore, the observed decrease in cardiomyocyte apoptosis, increased cardiomyocyte hypertrophy, and reduced infarct size imply that less death and more robust compensation act coordinately to preserve cardiac function.
Mechanistically, Per1, Per2, Cry1, and Cry2 interfere with Clock-Bmal1 activity to repress transcription targets (10, 35). Since Per2 protein is nonfunctional in these mPer2-M mice and bmal1 (arntl) gene expression is increased in response to ischemia in the absence of reperfusion in mPer2-M versus WT mice, this would suggest that the repressor activity normally effected by mPer2 is alleviated and so targets could be upregulated. These targets include endothelin-1, VEGF, and metabolic enzymes known to play a role in hypertrophy and angiogenesis (39). Also, the work by Koyanagi et al. (21) showing that the transfection of tumor cells with Per2 dose-dependently inhibits VEGF induced by hypoxia via the inhibition of hypoxia-inducible factor-1α/ARNT-induced VEGF promoter activity further lends support to the idea that mPer2 mutants probably have increased VEGF levels and hypertrophic mediators in response to hypoxia/ischemia (21, 39). Further investigation into vascular changes, as well as potential mediators of this and the observed increase in cardiomyocyte hypertrophy, is needed.
We postulate that the synchronization between the SCN and peripheral clocks is a continuous process and that the pressure to maintain this synchronization involves signaling mechanisms that are energetically demanding for the peripheral target tissues. When coupled with an underlying pathophysiology that generates a vulnerable substrate, such as coronary artery disease or pressure overload, the pressure to normalize desynchronized rhythms may increase the progression of injury. Additional mechanistic studies are needed to understand the signaling pathways between clock genes and cardiac genes that afford this protection. Long-term studies are also needed to determine whether the observed enhancements in the repair process result in reduced scar formation and consequent ventricular remodeling as well as an amelioration of cardiac dysfunction.
GRANTS
This work was partly supported by National Institute of Neurological Disorders and Stroke Grant NS-047014 (to J. M. Ding).
DISCLOSURES
None.
ACKNOWLEDGMENTS
We acknowledge Dr. Robert G. Carroll for thorough review of this work, Sarath Vijaykumar for mechanistic insights, Fatiha Moukdar for technical support, and Tracy Johnson and Susan Bryant for their assistance.
REFERENCES
- 1.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res 26: 645–649, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bourin P, Ledain AF, Beau J, Mille D, Levi F. In-vitro circadian rhythm of murine bone marrow progenitor production. Chronobiol Int 19: 57–67, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Corwin JV, Vargo JM. Light deprivation produces accelerated behavioral recovery of function from neglect produced by unilateral medial agranular prefrontal cortex lesions in rats. Behav Brain Res 56: 187–196, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Dardente H, Cermakian N. How many pieces to build a circadian clock? [In French.] Med Sci (Paris) 21: 66–72, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvornyk V, Vinogradova O, Nevo E. Origin and evolution of circadian clock genes in prokaryotes. Proc Natl Acad Sci USA 100: 2495–2500, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino Y, Iso H, Tamakoshi A, Inaba Y, Koizumi A, Kubo T, Yoshimura T. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol 164: 128–135, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Gauger MA, Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res 65: 6828–6834, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Hardin PE, Glossop NR. Perspectives: neurobiology. The CRYs of flies and mice. Science 286: 2460–2461, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol 17: 215–253, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hart CY, Burnett JC, Jr, Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol 281: H1938–H1945, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5: 1135–1142, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Honma S, Ikeda M, Abe H, Tanahashi Y, Namihira M, Honma K, Nomura M. Circadian oscillation of BMAL1, a partner of a mammalian clock gene Clock, in rat suprachiasmatic nucleus. Biochem Biophys Res Commun 250: 83–87, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Hori M, Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovasc Res 81: 457–464, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang C, Wang X, Wang Z, Cornelissen-Guillaume G, Halberg F. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci 97: 589–596, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismail JA, Poppa V, Kemper LE, Scatena M, Giachelli CM, Coffin JD, Murry CE. Immunohistologic labeling of murine endothelium. Cardiovasc Pathol 12: 82–90, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Joho S, Ishizaka S, Sievers R, Foster E, Simpson PC, Grossman W. Left ventricular pressure-volume relationship in conscious mice. Am J Physiol Heart Circ Physiol 292: H369–H377, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Koyanagi S, Kuramoto Y, Nakagawa H, Aramaki H, Ohdo S, Soeda S, Shimeno H. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res 63: 7277–7283, 2003 [PubMed] [Google Scholar]
- 22.Lamont EW, James FO, Boivin DB, Cermakian N. From circadian clock gene expression to pathologies. Sleep Med 8: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Mankani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun 74: 4750–4756, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGowan GA, Rager J, Shroff SG, Mathier MA. In vivo alpha-adrenergic responses and troponin I phosphorylation: anesthesia interactions. J Appl Physiol 98: 1163–1170, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Merrow M, Spoelstra K, Roenneberg T. The circadian cycle: daily rhythms from behaviour to genes. EMBO Rep 6: 930–935, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore MK, Viselli SM. Staining and quantification of proteins transferred to polyvinylidene fluoride membranes. Anal Biochem 279: 241–242, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Muller JE. Circadian variation and triggering of acute coronary events. Am Heart J 137: S1–S8, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Nemoto S, DeFreitas G, Mann DL, Carabello BA. Effects of changes in left ventricular contractility on indexes of contractility in mice. Am J Physiol Heart Circ Physiol 283: H2504–H2510, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Portaluppi F, Lemmer B. Chronobiology and chronotherapy of ischemic heart disease. Adv Drug Deliv Rev 59: 952–965, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Reinecke H, Murry CE. Cell grafting for cardiac repair. Methods Mol Biol 219: 97–112, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, Grau E, Mao J, Sathe GM, Ohlstein EH, Yue TL. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res 54: 549–558, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–H1406, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Sancar A. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu Rev Biochem 69: 31–67, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Shimba S, Watabe Y. Crosstalk between the AHR signaling pathway and circadian rhythm. Biochem Pharmacol 77: 560–565, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Suga H, Yamada O, Goto Y. Energetics of ventricular contraction as traced in the pressure-volume diagram. Fed Proc 43: 2411–2413, 1984 [PubMed] [Google Scholar]
- 37.Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci 334: 197–205, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90: 1003–1011, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Thackaberry EA, Gabaldon DM, Walker MK, Smith SM. Aryl hydrocarbon receptor null mice develop cardiac hypertrophy and increased hypoxia-inducible factor-1alpha in the absence of cardiac hypoxia. Cardiovasc Toxicol 2: 263–274, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282: 1490–1494, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Vargo JM, Grachek RA, Rockswold GL. Light deprivation soon after frontal brain trauma accelerates recovery from attentional deficits and promotes functional normalization of basal ganglia. J Trauma 47: 265–272, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Vargo JM, Lai HV, Marshall JF. Light deprivation accelerates recovery from frontal cortical neglect: relation to locomotion and striatal Fos expression. Behav Neurosci 112: 387–398, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol 163: 2433–2440, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willich SN, Kulig M, Muller-Nordhorn J. European survey on circadian variation of angina pectoris (ESCVA) in treated patients. Herz 29: 665–672, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Yoo B, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. Beta1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 297: H1377–H1386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol 290: H1–H16, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400: 169–173, 1999. [DOI] [PubMed] [Google Scholar]