Abstract
Oxidative stress has been shown to suppress endothelial nitric oxide synthase expression through activation of the transcription factor forkhead box O 1 (FOXO1) in cultured endothelial cells. We previously reported that circulating kallistatin levels are markedly reduced in rats with chronic oxidative organ damage. In this study, we investigated the potential role of oxidative stress in suppression of kallistatin expression via FOXO1 activation. In Dahl salt-sensitive (DSS) rats, we found that high salt intake induced a time-dependent correlation of increased thiobarbituric acid reactive substances (TBARS, an indicator of lipid peroxidation) with reduced serum kallistatin levels. Moreover, salt loading provoked an elevation of in situ aortic superoxide formation in association with reduced kallistatin levels. Expression of kallistatin was identified in cultured endothelial cells by immunocytochemistry and flow cytometry; however, H2O2 dose-dependently lowered kallistatin mRNA and protein levels as determined by real-time PCR and Western blot, respectively. Downregulation of kallistatin synthesis by oxidative stress was restored by knockdown of FOXO1 expression with small-interfering RNA. H2O2 rapidly induced FOXO1 nuclear translocation, but the effect was blocked by c-Jun NH2-terminal kinase (JNK) inhibitor. Inhibition of JNK by pharmacological inhibitor or small-interfering RNA reversed H2O2's effect on kallistatin expression in endothelial cells. This study demonstrates that an inverse relationship exists between oxidative stress and kallistatin levels in the circulation and blood vessels and that kallistatin expression is negatively regulated by oxidative stress via JNK-dependent FOXO1 activation in cultured endothelial cells.
Keywords: kallistatin, Forkhead box O 1, oxidative stress, c-Jun NH2-terminal kinase, blood vessel
kallistatin, a plasma protein that belongs to the serine protease inhibitor (serpin) family, is widely expressed in organs such as liver, kidney, and blood vessel (9, 11, 14, 38). Previous studies have demonstrated kallistatin to be a potent anti-inflammatory agent. For example, kallistatin reduced lipopolysaccharide-induced inflammation and lethality in kallistatin transgenic mice (15). Kallistatin administration via gene delivery significantly decreased neutrophil accumulation and joint swelling in a rat model of arthritis (36). Moreover, kallistatin improved cardiac function and diminished oxidative stress, cardiomyocyte apoptosis, and inflammatory cell accumulation after acute myocardial ischemia-reperfusion (13). Kallistatin also reduced myocardial infarct size, inflammation, and ventricular remodeling after myocardial infarction and attenuated salt-induced renal damage, inflammation, and fibrosis in association with reduced oxidative stress and increased nitric oxide (NO) levels (18, 33).
Kallistatin is a negative acute phase protein, since its levels are rapidly decreased after endotoxin shock and experimental inflammation (10, 27). Moreover, circulating kallistatin levels are reduced in NO-deficient and spontaneously hypertensive rats as well as in normotensive rats with ischemic cerebral injury and gentamicin-induced kidney damage (7, 8, 11). Increasing evidence indicates that reactive oxygen species (ROS) are the common factors underlying the pathogenesis of hypertension, inflammation, and cardiovascular and renal diseases (1, 32, 34). These combined findings suggest that oxidative stress may play an important role in the suppression of kallistatin synthesis. However, the molecular mechanism underlying negative regulation of kallistatin expression has not been explored.
Forkhead box O (FOXO) transcription factors promote a variety of cellular responses, including cell differentiation and stress response by modulating a series of specific target gene expression (19, 21). Accumulating evidence has shown that growth factor-activated protein kinase B (Akt) and stress-activated c-Jun N-terminal kinase (JNK) have opposing effects on FOXO: Akt prevents FOXO nuclear localization and inhibits its activity, whereas JNK increases FOXO activity by promoting its import in the nucleus (4, 17, 37). Thus the survival pathway and the stress pathway appear to be in a tight balance to regulate FOXO activation. FOXO1, the most abundant FOXO isoform in endothelial cells, has been shown to bind to the consensus sequences in endothelial NO synthase (eNOS) promoter and suppress eNOS expression in response to oxidative stress (30). Exposing endothelial cells to hypoxia resulted in significant reduction in eNOS expression (22, 25). Because both kallistatin and eNOS levels are reduced in rats with salt-induced hypertension (33), it is likely that FOXO1 may also be involved in the negative regulation of kallistatin expression under oxidative stress. Our present study was designed to determine the role and mechanism of oxidative stress in suppressing kallistatin expression in cultured endothelial cells.
MATERIALS AND METHODS
Animal treatments.
All procedures complied with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). The protocol for our animal study was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. Four-week-old, male Dahl salt-sensitive (DSS) rats (Sprague-Dawley Harlan, Madison, WI) were fed either with a normal salt (0.4% NaCl) or a high-salt (4% NaCl) diet for 6 wk. Blood was collected each week for thiobarbituric acid reactive substances (TBARS) and rat kallistatin measurements. On the day of death, serum was collected by cardiac puncture, and kidneys and aortas were removed for histological and biochemical analyses.
Cell culture.
Human umbilical vein endothelial cells (HUVECs) were acquired from Cambrex Bioscience and cultured in endothelial cell basal medium-2 supplemented with EGM-2 singleQuots kit (Lonza, Allendale, NJ).
Analyses of kallistatin expression.
Kallistatin levels in rat serum and in kidney extracts were determined using an enzyme-linked immunosorbent assay specific for rat kallistatin as previously described (24). Kallistatin expression in rat aorta was detected by immunohistochemistry. Briefly, aortic sections were incubated at 4°C overnight with specific antibody against rat kallistatin (1:250). Immunohistochemistry was performed using the Vectastain Universal Elite ABC Kit (Vector Laboratories, Burlingame, CA), following the supplied instructions. Expression of human kallistatin in HUVECs was detected by immunocytochemistry using a specific monoclonal antibody (12). For flow cytometric analysis of human kallistatin expression in HUVECs, cells were incubated with 3 μg/ml anti-human kallistatin monoclonal antibody followed by 10 μg/ml fluorescein isothiocyante-conjugated anti-mouse IgG at 4°C for 1 h. After washing and centrifugation, the cells were resuspended in 0.4 ml PBS for immediate acquisition by flow cytometry.
Assays for TBARS and superoxide formation.
Serum lipid peroxidation, an indicator of oxidative stress, was determined by measuring circulating TBARS levels at 535 nm using malondialdehyde standards (0–3 μM) (23). Superoxide production was measured in kidney extracts by ferricytochrome c reduction assay using a modified protocol (5).
In situ detection of superoxide in rat aorta.
Aortic ring segments were immediately placed in optimum-cutting temperature embedding medium at the time of death and stored at −80°C until analysis. Aortic ring segments were cut into 30-μm-thick sections and incubated with 2 μM fluorescent dye hydroethidine, which is oxidized to ethidium in the presence of superoxide to produce red fluorescence. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min (26). Images were obtained with a laser-scanning confocal microscope equipped with a krypton/argon laser. Laser settings were identical for acquisition of images for all slides. Fluorescence was detected with a 585-nm long-pass filter.
RNA extraction and quantitative PCR.
Total RNA was isolated from cultured cells with Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Total RNA was reverse-transcribed using the High Capacity cDNA Reverse Transcription Kit. Real-time quantitative RT-PCR was performed with the TaqMan Gene Expression Assay and was normalized against 18S RNA using an ABI 7300 real-time PCR System (Applied Biosystems, Foster City, CA). The assay Hs00167166_ml was used for detection of eNOS. Kallistatin expression was analyzed using SYBR Green Supermix with custom primers: 5′-GCATCTTCCCAAGTTCTCCATT-3′ and 5′-ATGCCGGATAAGTCAG CCCA-3′.
Fluorescence microscopy analysis of FOXO1.
HUVECs were pretreated with SP-600125 (SP; a JNK inhibitor, 2 μM) (Calbiochem, San Diego, CA) for 30 min followed by treatment with 200 μM H2O2 for 3 h. FOXO1 cellular localization was detected by immunofluorescence labeling as previously described (4, 16). HUVECs were fixed with 4% formaldehyde, permeabilized with 0.2% Triton X-100, and stained with an anti-FOXO1 antibody (Cell Signaling, Danvers, MA) and Cy3-conjugated anti-rabbit IgG secondary antibody. Cells were counterstained with 1 μg/ml 4′-6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO) to visualize the nucleus. A matched control IgG was used as a negative control to demonstrate specificity. Images were acquired using a fluorescence microscope. Fluorescence intensity was measured separately within the nucleus and cytoplasm. To calculate relative nuclear fluorescence, nuclear fluorescence was divided by the total amount of cellular fluorescence. All quantitative values represent averages of at least 30 cells from five independent experiments.
Small-interfering RNA transfection.
Expression of FOXO1 and JNK in HUVECs was inhibited by small-interfering RNA (siRNA) oligonucleotides. Knockdown was performed by transfection of siRNA into cells using the DharmaFECT1 transfection reagent according to the manufacturer's instructions. As a control, cells were also transfected with scrambled siRNA. Knockdown efficiency was verified by real-time PCR and Western blot. HUVECs with or without siRNA transfection were treated with H2O2 for 24 h, and kallistatin expression was determined by real-time PCR and Western blot.
Western blot analysis.
Total cell lysates (50 μg) were loaded on a SDS-polyacrylamide gel. Nonspecific binding sites were blocked with a blocking solution containing 5% nonfat dry milk in Tris-buffered saline (pH 7.6) with 0.1% Tween 20 (TBST) at room temperature for 1 h. The membrane was then incubated overnight at 4°C with a kallistatin monoclonal antibody (1:3,000 dilution) or JNK and phospho-JNK antibodies (1:1,000 dilution; Cell Signaling) in TBST containing 5% BSA. After three washes, the membrane was then incubated at room temperature for 1 h with horseradish peroxidase-conjugated anti-mouse IgG (1:3,000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) in the blocking solution. Immunoreactive bands were visualized using the enhanced chemiluminescence detection system (Perkin-Elmer, Waltham, MA), and chemiluminescence was detected with Kodak x-ray films. Densitometry was then analyzed using Scion Image (Scion, Frederick, MD). All densitometry data are expressed as folds of control.
Statistical analysis.
All data are presented as means ± SE. Comparison between groups was made using one-way ANOVA with the Fisher multiple-comparison test. A probability value of P < 0.05 was considered statistically significant. All experiments were performed in triplicates in at least three separate occasions.
RESULTS
Inverse relationship between kallistatin levels and oxidative stress in DSS rats.
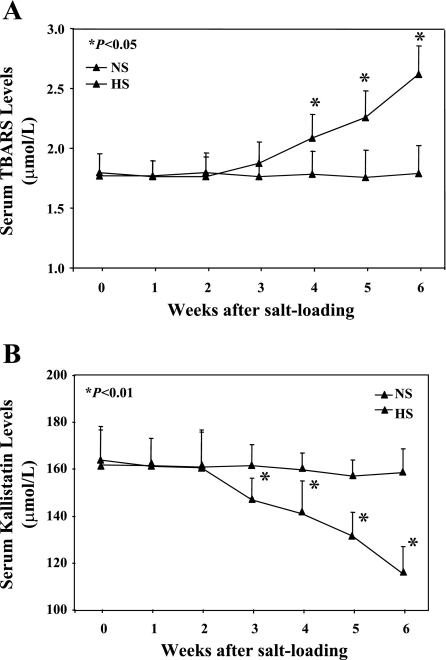
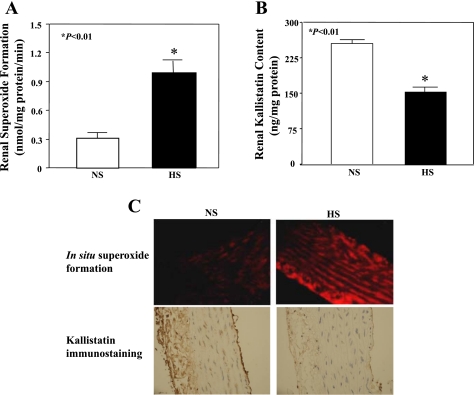
Circulating TBARS levels, an index of lipid peroxidation, are caused by oxygen-free radical interaction with the polyunsaturated fatty acids of cell membranes. No difference in TBARS levels of DSS rats was observed between groups before high salt loading. Elevation of serum TBARS levels was detected starting 3 wk after high salt diet, with a significant increase by 6 wk (2.70 ± 0.21 vs. 1.77 ± 0.01 μmol/l, n = 6–8, P < 0.01; Fig. 1A). Conversely, a time-dependent decrease of serum kallistatin levels beginning at 3 wk after high-salt diet correlated with the rise in TBARS levels (149.3 ± 8.2 vs. 272.4 ± 5.3 ng/mg protein, n = 6–8, P < 0.01; Fig. 1B). These results indicate that oxidative stress is inversely associated with circulating kallistatin levels under pathological conditions. A similar reduction of kallistatin levels was found after high-salt diet in the kidney and aorta, with a concomitant elevation of superoxide formation (Fig. 2).
Fig. 1.
Time-dependent increase in oxidative stress is inversely associated with reduced kallistatin levels in salt-loaded Dahl salt-sensitive (DSS) rats. A: serum thiobarbituric acid reactive substances (TBARS) levels. FITC, fluorescein isothiocyanate. B: serum kallistatin levels. NS, normal salt diet; HS, high-salt diet.
Fig. 2.
Oxidative stress is inversely associated with kallistatin levels in the kidney and aorta of in hypertensive DSS rats. A: renal superoxide formation. B: kallistatin levels in renal extracts of DSS rats. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. C: representative images for in situ superoxide production and kallistatin immunostaining in rat aorta.
H2O2-induced suppression of kallistatin expression in endothelial cells.
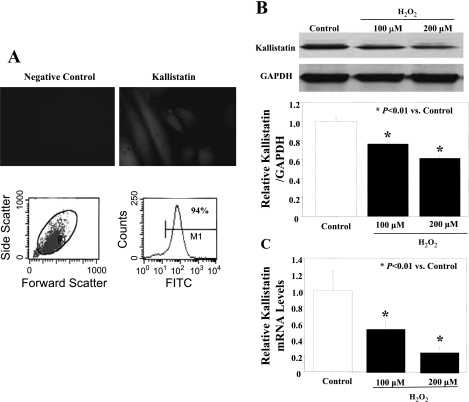
Immunostaining and flow cytometry analyses confirmed the expression of kallistatin in cultured human endothelial cells (Fig. 3A). The effect of oxidative stress on human kallistatin expression was examined in vitro by treatment with H2O2, a reagent that is commonly employed to induce oxidative stress by increasing intracellular concentrations of ROS. H2O2 dose-dependently reduced the expression of kallistatin in endothelial cells as determined by Western blot and quantitative PCR (Fig. 3, B and C).
Fig. 3.
Oxidative stress suppresses kallistatin expression in endothelial cells. A: immunostaining and flow cytometry for kallistatin expression in human umbilical vein endothelial cells (HUVECs). Western blot (B) and quantitative PCR (C) for kallistatin levels after treatment with H2O2 (100 and 200 μM) for 24 h.
Knockdown of FOXO1 restores kallistatin expression under oxidative stress.
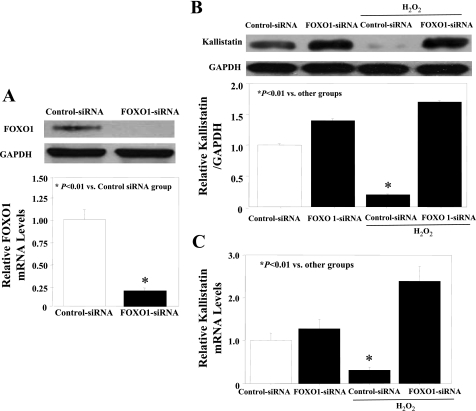
To identify the role of FOXO1 in H2O2-induced kallistatin reduction, siRNA oligonucleotides were used to knock down FOXO1 expression in endothelial cells. Inhibition of FOXO1 by siRNA effectively reduced FOXO1 expression at the mRNA and protein levels (Fig. 4A). FOXO1 depletion by siRNA restored oxidative stress-induced negative regulation of kallistatin protein and mRNA levels in cultured endothelial cells (Fig. 4, B and C).
Fig. 4.
Inhibition of forkhead box O 1 (FOXO1) by small-interfering RNA (siRNA) abolishes suppression of kallistatin expression by oxidative stress in endothelial cells. A: Western blot and quantitative PCR of FOXO1 levels after treatment with FOXO1-siRNA. Western blot (B) and quantitative PCR (C) of kallistatin levels after treatment with H2O2 in the presence of control-siRNA or FOXO1-siRNA.
H2O2 induces FOXO1 nuclear translocation and reduces kallistatin expression through JNK activation.
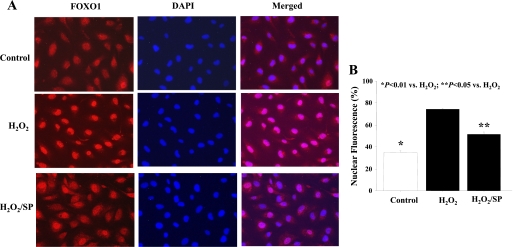
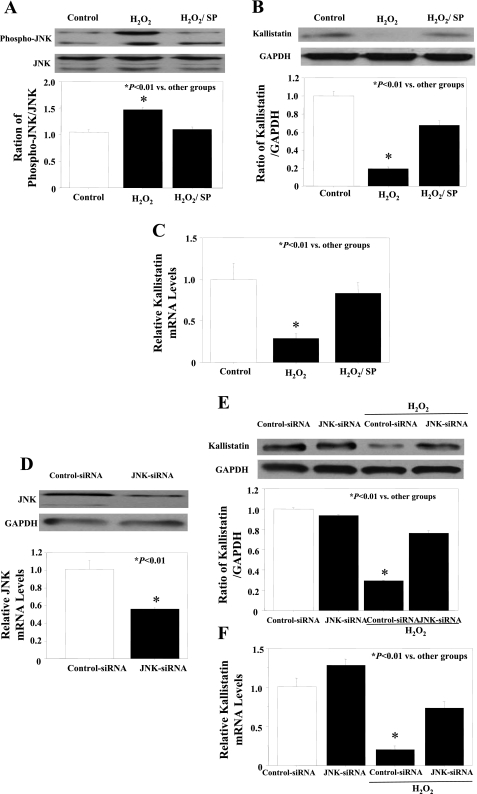
FOXO1 subcellular localization in cultured endothelial cells was determined by FOXO1 immunostaining. FOXO1 localized primarily to the cytoplasm (65% cytoplasmic and 35% nuclear) in the presence of serum (Fig. 5, A and B). H2O2 treatment caused FOXO1 to translocate from the cytoplasm to the nucleus (30% cytoplasmic and 70% nuclear) (Fig. 5, A and B). To further elucidate the signaling role of JNK-dependent FOXO1 activation on kallistatin expression, we examined whether JNK inhibitor (SP) treatment could override the effect of H2O2 on FOXO1 nuclear translocation. Indeed, JNK inhibition promoted FOXO1 localization to the cytoplasm (50% cytoplasmic and 50% nuclear) (Fig. 5, A and B). To confirm the possible role of JNK in kallistatin regulation under oxidative stress, JNK phosphorylation was analyzed by Western blot. H2O2 treatment significantly increased JNK phosphorylation, whereas SP pretreatment inhibited its activation (Fig. 6A). JNK inhibitor restored both kallistatin protein and mRNA expression levels under oxidative stress (Fig. 6, B and C). Moreover, JNK-siRNA oligonucleotides were used to knock down JNK expression in endothelial cells. Knockdown efficiency was confirmed by Western blot and quantitative PCR (Fig. 6D). Knock down of JNK by JNK-siRNA had no effect on kallistatin expression in HUVECs but restored oxidative stress-mediated inhibition of kallistatin protein and mRNA levels (Fig. 6, E and F).
Fig. 5.
c-Jun NH2-terminal kinase (JNK) inhibitor blocks oxidative stress-induced FOXO1 nuclear translocation. A: representative images for FOXO1 translocation. B: quantitative analysis for FOXO1 cellular localization. SP, JNK inhibitor SP-600125.
Fig. 6.
Oxidative stress-induced decrease of kallistatin expression is mediated by JNK. A: Western blot for phospho-JNK levels after treatment of SP (JNK inhibitor, 2 μM). Western blot (B) and quantitative PCR (C) of kallistatin levels after treatment with H2O2 in the presence or absence of SP. D: Western blot and quantitative PCR of JNK levels after JNK-siRNA treatment. Western blot (E) and quantitative PCR (F) of kallistatin levels after treatment with H2O2 in the presence of control-siRNA or JNK-siRNA.
DISCUSSION
Oxidative stress causes endothelial dysfunction, resulting in pathogenesis of hypertension, atherosclerosis, cardiac hypertrophy, heart failure, kidney injury, and diabetes mellitus (1, 29, 32, 34). Therefore, the development of an appropriate biomarker for the early detection and prevention of oxidative stress-induced cardiovascular diseases would be extremely beneficial. In this study, we show a time-dependent correlation of reduced kallistatin levels with oxidative stress in salt-induced hypertensive rats. Our previous studies have shown that increased oxidative stress is associated with reduced kallistatin levels in several animal models with hypertension and vascular and organ injury (13, 18, 33). Conversely, kallistatin administration by gene delivery attenuated oxidative organ damage in conjunction with decreased circulating TBARS levels and tissue ROS formation (18, 33). Our present study shows that kallistatin expression is negatively regulated by oxidative stress through activation of the FOXO1 transcription factor in cultured endothelial cells. Because we demonstrated an inverse relationship of kallistatin with oxidative stress in vivo and in vitro, circulating kallistatin levels may serve as a potential biomarker for detection of oxidative stress-related diseases.
Oxidative stress is a state of redox imbalance caused by increased ROS generation and decreased antioxidant capacity (28). Chronic consumption of a high-salt diet induces hypertension and renal injury in DSS rats, in conjunction with elevated NAD(P)H oxidase activity and decreased NO production in the kidney and blood vessels (20, 35). However, kallistatin administration by gene delivery attenuates oxidative stress, apoptosis, inflammation, and organ damage in animal models after cardiac ischemia-reperfusion and chronic myocardial infarction (13, 18). Moreover, kallistatin gene transfer reduced salt-induced renal damage, oxidative stress, inflammation, and fibrosis in DSS rats (33). These findings indicate that kallistatin may play an important role as an antioxidant in maintaining oxidative balance and preventing oxidative endothelial and tissue injury.
Regulation of the subcellular localization of FOXO is critical to its transcriptional activity (4, 19, 21). Recent studies indicate that JNK, a mitogen-activated protein kinase family member activated by stress stimuli, is responsible for FOXO activation under stress conditions, suggesting that JNK may also play a role in regulating kallistatin synthesis (17, 37). We showed that translocation of FOXO1 from the cytoplasm to nucleus induced by H2O2 resulted in the downregulation of kallistatin expression in endothelial cells, and the effect was reversed by FOXO1 knockdown. JNK inhibitor partially blocked FOXO1 nuclear translocation and restored kallistatin expression, indicating that JNK is involved in FOXO1 activation and thus kallistatin expression under oxidative stress. These results demonstrate that oxidative stress downregulates kallistatin expression through JNK-dependent FOXO1 activation.
Under oxidative conditions, FOXO can be phosphorylated by JNK and imported in the nucleus to exert its function as a transcription factor (17, 37). As a transcription factor, FOXO exerts positive or negative effect on gene expression by binding to the DNA-targeting sequences (2, 31). A consensus FOXO-recognized element (FRE) like (G/C)(T/A)AA(C/T)AA has been identified in the promoters of FOXO1 target genes by high-affinity DNA-binding assays (3). A previous study showed that exposing endothelial cells to oxidative stress results in significant reduction of eNOS expression through the binding of FOXO1, the most abundant FOXO isoform in endothelial cells, to the consensus sequences in the eNOS promoter (30). Promoter region analysis of human kallistatin demonstrated two consensus FRE sequences at −675 and −915 bp in the 5′-flanking region (6). Whether FOXO1 binds to specific DNA sequences of the kallistatin promoter region remains to be investigated.
In conclusion, we have elucidated a well-defined signaling pathway for the downregulation of kallistatin by oxidative stress through activation of the transcription factor FOXO1 via JNK phosphorylation. We have also provided important data regarding an inverse relationship of kallistatin with oxidative stress in the circulation. The relationship between diminished kallistatin levels in the circulation and aorta with oxidative stress implies that kallistatin may serve as a potential biomarker for oxidative organ damage.
GRANTS
This work was supported by National Institutes of Health Grants HL-44083, HL-29397, and C06 RR-015455 from the Extramural Research Facilities Program of the National Center for Research Resources.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Annuk M, Zilmer M, Fellstrom B. Endothelium-dependent vasodilation and oxidative stress in chronic renal failure: impact on cardiovascular disease. Kidney Int Suppl 84: S50–S53, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab 16: 183–189, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96: 7421–7426, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Cavanagh EMV, Fraga CG, Ferder L, Felipe I. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 272: R514–R518, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Chai KX, Ward DC, Chao J, Chao L. Molecular cloning, sequence analysis, and chromosomal localization of the human protease inhibitor 4 (kallistatin) gene (PI4). Genomics 23: 370–378, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Chao C, Madeddu P, Wang C, Liang Y, Chao L, Chao J. Differential regulation of kallikrein, kininogen, and kallikrein-binding protein in arterial hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 271: F78–F86, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Chao J, Chao L. A major difference of kallikrein-binding protein in spontaneously hypertensive versus normotensive rats. J Hypertens 6: 551–557, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Chao J, Chai KX, Chen LM, Xiong W, Chao S, Woodley-Miller C, Wang LX, Lu HS, Chao L. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem 265: 16394–16401, 1990 [PubMed] [Google Scholar]
- 10.Chao J, Chen LM, Chai KX, Chao L. Expression of kallikrein-binding protein and α 1-antitrypsin genes in response to sex hormones, growth, inflammation and hypertension. Agents Actions 38: 174–181, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Chao J, Chao L. Biochemistry, regulation and potential function of kallistatin. Biol Chem Hoppe Seyler 376: 705–713, 1995 [PubMed] [Google Scholar]
- 12.Chao J, Schmaier A, Chen LM, Yang Z, Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med 127: 612–620, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Chao J, Yin H, Yao YY, Shen B, Smith RS, Jr, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther 17: 1201–1213, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chen LM, Song Q, Chao L, Chao J. Cellular localization of tissue kallikrein and kallistatin mRNAs in human kidney. Kidney Int 48: 690–697, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Chen LM, Chao L, Chao J. Beneficial effects of kallikrein-binding protein in transgenic mice during endotoxic shock. Life Sci 60: 1431–1435, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress FEBS Lett 581: 673–680, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Essers MA, Weijzen S, de Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J 23: 4802–4812, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao L, Yin H, Smith RS, Jr, Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest 88: 1157–1166, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene 27: 2320–2336, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Guo P, Nishiyama A, Rahman M, Nagai Y, Noma T, Namba T, Ishizawa M, Murakami K, Miyatake A, Kimura S, Mizushige K, Abe Y, Ohmori K, Kohno M. Contribution of reactive oxygen species to the pathogenesis of left ventricular failure in Dahl salt-sensitive hypertensive rats: effects of angiotensin II blockade. J Hypertens 24: 1097–1104, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jin HG, Yamashita H, Nagano Y, Fukuba H, Hiji M, Ohtsuki T, Takahashi T, Kohriyama T, Kaibuchi K, Matsumoto M. Hypoxia-induced upregulation of endothelial small G protein RhoA and Rho-kinase/ROCK2 inhibits eNOS expression. Neurosci Lett 408: 62–67, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kumar KV, Shifow AA, Naidu MU, Ratnakar KS. Carvedilol: a beta blocker with antioxidant property protects against gentamicin-induced nephrotoxicity in rats. Life Sci 66: 2603–2611, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Ma JX, Chao L, Zhou G, Chao J. Expression and characterization of rat kallikrein-binding protein in Escherichia coli. Biochem J 292: 825–832, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McQuillan LP, Leung GK, Marsden PA, Kostyk SK, Kourembanas S. Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am J Physiol Heart Circ Physiol 267: H1921–H1927, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 82: 1298–1305, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Miller I, Haynes P, Eberini I, Gemeiner M, Aebersold R, Gianazza E. Proteins of rat serum: III. Gender-related differences in protein concentration under baseline conditions and upon experimental inflammation as evaluated by two-dimensional electrophoresis. Electrophoresis 20: 836–845, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res 71: 247–258, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Paravicini TM, Touyz RM. NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilities. Diabetes Care 31, Suppl 2: S170–S180, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest 115: 2382–2392, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell 2: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Sedeek M, Hébert RL, Kennedy CR, Burns KD, Touyz RM. Molecular mechanisms of hypertension: role of NOx family NADPH oxidases. Curr Opin Nephrol Hypertens 18: 122–127, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation and fibrosis via anti-oxidative stress. Hypertension 51: 1358–1365, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Stenvinkel P. Interactions between inflammation, oxidative stress, and endothelial dysfunction in end-stage renal disease. J Ren Nutr 13: 144–148, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Taylor NE, Glocka P, Liang M, Cowley AW., Jr NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, Chao J. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum 52: 1319–1324, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121: 115–125, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. J Histochem Cytochem 47: 221–228, 1999 [DOI] [PubMed] [Google Scholar]