Abstract
This investigation was designed to examine the hypothesis that impaired function of coronary microvascular large-conductance Ca2+-activated K+ (BKCa) channels in metabolic syndrome (MetS) significantly attenuates the balance between myocardial oxygen delivery and metabolism at rest and during exercise-induced increases in myocardial oxygen consumption (MV̇o2). Studies were conducted in conscious, chronically instrumented Ossabaw swine fed a normal maintenance diet (11% kcal from fat) or an excess calorie atherogenic diet (43% kcal from fat, 2% cholesterol, 20% kcal from fructose) that induces many common features of MetS. Data were collected under baseline/resting conditions and during graded treadmill exercise before and after selective blockade of BKCa channels with penitrem A (10 μg/kg iv). We found that the exercise-induced increases in blood pressure were significantly elevated in MetS swine. No differences in baseline cardiac function or heart rate were noted. Induction of MetS produced a parallel downward shift in the relationship between coronary venous Po2 and MV̇o2 (P < 0.001) that was accompanied by a marked release of lactate (negative lactate uptake) as MV̇o2 was increased with exercise (P < 0.005). Inhibition of BKCa channels with penitrem A did not significantly affect blood pressure, heart rate, or the relationship between coronary venous Po2 and MV̇o2 in lean or MetS swine. These data indicate that BKCa channels are not required for local metabolic control of coronary blood flow under physiological (lean) or pathophysiological (MetS) conditions. Therefore, diminished function of BKCa channels does not contribute to the impairment of myocardial oxygen-supply demand balance in MetS.
Keywords: coronary blood flow, myocardial oxygen consumption, exercise, penitrem A, Ossabaw miniature swine
due to the limited anaerobic capacity of the myocardium, the heart depends on a continuous supply of oxygen from the coronary circulation to meet its metabolic requirements (49). Thus, under normal physiological conditions, myocardial oxygen delivery is closely matched with the rate of myocardial oxidative metabolism. To ensure adequate balance between coronary blood flow and myocardial metabolism, powerful regulatory mechanisms exist to increase nutritive blood flow to the heart whenever myocardial oxygen consumption (MV̇o2) is elevated (13, 49). However, despite decades of research, the exact mechanisms responsible for local metabolic control of coronary blood flow have yet to be clearly defined (49).
Previous studies have demonstrated that disease states such as obesity and metabolic syndrome (MetS) significantly impair control of coronary blood flow at rest and during increases in MV̇o2 (7, 11, 30, 44, 46, 54). Coronary dysfunction in the MetS is characterized by an imbalance between coronary blood flow and myocardial metabolism (46) which has been attributed to sensitization of key vasoconstrictor pathways such as ANG II and α1-adrenoceptors (29). Recently, data from our laboratory, as well as others, also established that obesity, insulin resistance, and type 2 diabetes diminishes end-effector mechanisms that regulate coronary vasodilation (3, 6, 9, 33, 38). In particular, we found that the functional expression of coronary large-conductance Ca2+-activated K+ (BKCa) channels is markedly depressed in Ossabaw swine with MetS (3, 38). Because BKCa channels have been shown to contribute to coronary endothelial-dependent and exercise-induced dilation under normal-lean conditions (3, 4, 27, 34–36, 38), we propose that decreases in BKCa channel function could underlie impaired metabolic control of coronary blood flow in MetS. However, no study has examined the contribution of BKCa channels to metabolic coronary vasodilation in the setting of MetS.
Accordingly, the goal of this investigation was to examine the hypothesis that impaired function of coronary microvascular BKCa channels in MetS (3, 38) significantly attenuates the balance between myocardial oxygen delivery and metabolism at rest and during exercise-induced increases in MV̇o2, i.e., metabolic coronary vasodilation. Studies were conducted in conscious, chronically instrumented Ossabaw swine fed a normal maintenance diet (11% kcal from fat) or an excess calorie atherogenic diet (43% kcal from fat, 2% cholesterol, 20% kcal from fructose) that induces many common features of MetS, including obesity, insulin resistance, impaired glucose tolerance, and dyslipidemia (3, 5, 18, 48). Coronary blood flow data were recorded, and arterial and coronary venous blood samples were collected before and after selective blockade of BKCa channels with penitrem A (10 μg/kg iv) under baseline/resting conditions and during graded treadmill exercise up to ∼75% of maximum whole body V̇o2 (heart rate >200/min).
METHODS
Swine model of MetS.
All experimental procedures and protocols used in this investigation were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals. Lean control swine were fed ∼2,200 kcal/day of standard chow (5L80; Purina, Richmond, IN) containing 18% kcal from protein, 71% kcal from complex carbohydrates, and 11% kcal from fat. MetS swine were fed an excess ∼8,000 kcal/day high-fat/fructose, atherogenic diet containing 17% kcal from protein, 20% kcal from complex carbohydrates, 20% kcal from fructose, and 43% kcal from fat (mixture of lard, hydrogenated soybean oil, and hydrogenated coconut oil), and supplemented with 2.0% cholesterol and 0.7% sodium cholate by weight (H46T-F20–5L80; Purina, Richmond, IN). Both lean (n = 6 male) and MetS (n = 5 male) swine were fed their respective diets for 20 wk.
Surgical instrumentation and intravascular ultrasound.
Ossabaw swine were fasted overnight before surgery. The animals were initially sedated with telazol (5 mg/kg sc) and xylazine (2.2 mg/kg sc). After endotracheal intubation, a surgical plane of anesthesia was maintained by mechanical ventilation with 1–3% isoflurane gas with supplemental oxygen. Using a sterile technique, a 7-Fr vascular introducer sheath (Boston Scientific) was inserted in the right femoral artery, and a guiding catheter (Amplatz L, sizes 0.75–2.0; Boston Scientific) was advanced to engage the left main coronary ostium. A 3.2-Fr, 30-MHz intravascular ultrasound (IVUS) catheter (Boston Scientific) was advanced over a guide wire and positioned in the coronary artery. Automated IVUS pullbacks were performed at 0.5 mm/s to obtain artery diameters, severity of atherosclerosis, and plaque morphology. Video images were analyzed off-line (Sonos Intravascular Imaging System; Hewlett Packard) (19).
Following the IVUS procedure, a left lateral thoracotomy was performed in the fifth intercostal space. A catheter (17-gauge pressure-monitoring catheter; Edwards LifeSciences) was implanted in the descending thoracic aorta to measure aortic blood pressure and to obtain arterial blood samples. A second catheter was placed in the coronary interventricular vein for coronary venous blood sampling and intravenous drug infusions. The left anterior descending (LAD) coronary artery was dissected free, and a Transonics perivascular flow transducer was placed around the artery. A chest tube was placed to evacuate the pneumothorax, and the chest was closed in layers. The catheters and the flow transducer wire were tunneled subcutaneously and exteriorized between the scapulas. Antibiotics (cephalexin) and aspirin (81 mg) were administered two times daily for 7 days. A jacket was placed on the animals to protect the catheters and the flow transducer wire. An elastomeric balloon pump (Access Technologies) was connected to the coronary venous catheter so heparinized saline (5 U/ml) could be continuously infused at 0.5 ml/h. The aortic catheter was flushed daily and filled with heparinized saline (5,000 U/ml) (45, 46, 54).
Echocardiographic studies.
Two-dimensional and M-mode images were obtained from conscious lean (n = 4) and MetS (n = 4) Ossabaw swine in a sternally recumbent position in a low-stress restraint sling (41) using a Phillips iE33 echocardiography system (31). Images were obtained from a left parasternal approach at the midpapillary muscle level and recorded on the imaging system. Fractional shortening measurements were made using criteria from the American Society of Echocardiography (43). Measurements of the end-diastolic dimension (EDD) and end-systolic dimension (ESD) were averaged over five beats. EDD was obtained at the onset of the QRS complex, and ESD was at the end of the T wave. The left parasternal view was used to obtain aortic diameters at the root of the aortic valve during the T wave peak or at the instant of maximum dilation. Pulsed Doppler was used to measure blood velocity through the aorta. LV function was assessed using fractional shortening {[(EDD − ESD)/EDD] × 100} and cardiac output (SV × HR), where SV is stroke volume and HR is heart rate.
Experimental protocol.
Following recovery from surgery, experiments were conducted in lean (n = 6) and MetS (n = 5) Ossabaw swine before and after inhibition of BKCa channels with penitrem A (10 μg/kg iv) under baseline/resting conditions and during graded treadmill exercise up to ∼ 75% of maximum whole body V̇o2 (heart rate >200/min). We previously demonstrated that this intravenous dose of penitrem A essentially abolished coronary vasodilation to the BKCa channel agonist NS-1619 in anesthetized, open-chest lean Ossabaw swine (3). Coronary blood flow, aortic pressure, and heart rate were continuously recorded while the pigs were resting upright on the treadmill and then during the following two levels of treadmill exercise: 1) ∼2 mph at 0% grade and 2) ∼4 mph at 5% grade. Both lean and MetS swine exercised at similar intensity levels. Arterial and coronary venous blood samples were collected simultaneously in heparinized syringes when hemodynamic variables were stable at rest and at each level of exercise. Each exercise period was ∼2 min in duration, and the animals were allowed to rest sufficiently between each level for hemodynamic variables to return to baseline.
Blood sampling.
Arterial and coronary venous blood samples were collected, immediately sealed, and placed on ice. The samples were analyzed in duplicate for pH, Pco2, Po2, glucose, hematrocrit, and oxygen content with an Instrumentation Laboratories automatic blood gas analyzer (GEM Premier 3000) and CO-oximeter (682) systems. LAD perfusion territory was estimated to be 30% of total heart weight, as previously described by Feigl et al. (21). MV̇o2 (μl O2·min−1·g−1) was calculated by multiplying coronary blood flow by the coronary arterial-venous difference in oxygen content. Lactate uptake (μmol·min−1·g−1) was calculated by multiplying coronary blood flow by the coronary arterial-venous difference in lactate concentration.
Statistical analyses.
Data are presented as means ± SE. Statistical comparisons were made with t-tests and three-way repeated-measures ANOVA (factor A: diet; factor B: drug treatment; factor C: exercise level) as appropriate. In all statistical tests, P < 0.05 was considered statistically significant. When significance was found with ANOVA, a Student-Newman-Keuls multiple-comparison test was performed to identify differences between groups and treatment levels. Linear regression analysis was used to compare slopes of response variables (aortic pressure, heart rate, coronary venous Po2, lactate uptake) plotted vs. MV̇o2. If the slopes of the regression lines were not significantly different, an analysis of covariance was used to adjust response variables for linear dependence on MV̇o2.
RESULTS
Phenotype of Ossabaw swine.
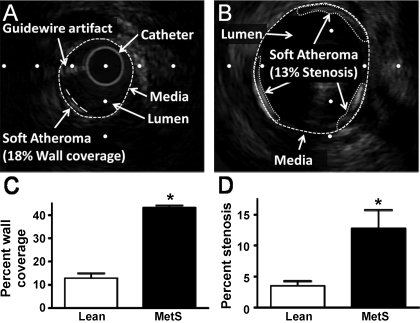
Phenotypic characteristics of lean and MetS swine are given in Table 1. We found that 20 wk of an excess calorie atherogenic diet induced classic features of MetS in Ossabaw swine. In particular, relative to their lean counterparts, MetS swine exhibited a 1.3-fold increase in body weight, 1.4-fold increase in fasting glucose, 3.1-fold increase in fasting insulin, 6.5-fold increase in total cholesterol, a 4.3-fold increase in the low density lipoprotein-to-high density lipoprotein ratio, and a 3-fold increase in triglyceride levels. MetS induced a fourfold increase in coronary atherosclerosis wall coverage of the LAD and a significant threefold increase in percent stenosis (nonflow limiting) relative to lean (Fig. 1).
Table 1.
Phenotypic characteristics of lean and metabolic syndrome Ossabaw swine
| Lean | MetS | |
|---|---|---|
| Body wt, kg | 58 ± 6 | 77 ± 5* |
| Heart wt/body wt, ×100 | 0.41 ± 0.01 | 0.39 ± 0.05 |
| Fasting glucose, mg/dl | 71 ± 4 | 96 ± 7* |
| Fasting insulin, μU/ml | 10 ± 1 | 31 ± 4* |
| Total cholesterol, mg/dl | 59 ± 4 | 383 ± 44* |
| LDL-to-HDL ratio | 0.8 ± 0.1 | 3.4 ± 0.4* |
| Triglycerides, mg/dl | 22 ± 2 | 67 ± 6* |
Values are means ± SE for lean (n = 6) and metabolic syndrome (MetS; n = 5) swine. LDL, low density lipoprotein; HDL, high density lipoprotein.
P < 0.05 vs. lean.
Fig. 1.
Metabolic syndrome (MetS) atherosclerosis is not occlusive. A: representative intravascular ultrasound (IVUS) image of lean left anterior descending (LAD) artery demonstrating method of determining percent wall coverage. B: representative IVUS image of MetS LAD demonstrating method of determining percent stenosis. The 13% stenosis corresponds to 56% wall coverage in this example. C: atherosclerosis is increased in MetS LAD compared with lean. D: %stenosis at the site of maximum atherosclerosis in the LAD demonstrates atherosclerosis in MetS is not flow-limiting. *P < 0.05, Lean vs. MetS.
Effects of MetS on coronary and cardiovascular responses to exercise.
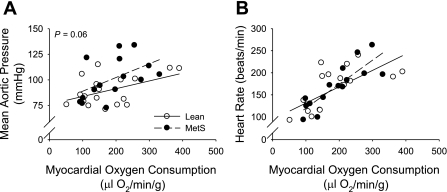
Hemodynamic and blood gas data at rest and during exercise for the lean and MetS Ossabaw swine before and after inhibition of BKCa channels are summarized in Table 2. Systolic, diastolic, and mean aortic pressure were not different between lean and MetS swine under baseline resting conditions. Importantly, however, exercise-induced increases in aortic pressure were exaggerated in MetS vs. lean swine (Table 2 and Fig. 2A). No differences in heart rate were noted between groups at rest or during exercise (Fig. 2B).
Table 2.
Hemodynamic and blood gas variables at rest and during graded treadmill exercise in lean and metabolic syndrome Ossabaw swine with and without penitrem A (10 μg/kg iv).
| Exercise |
|||
|---|---|---|---|
| Rest | Level 1 | Level 2 | |
| Systolic blood pressure, mmHg | |||
| Lean | 101 ± 7 | 109 ± 7 | 119 ± 8* |
| Lean + penitrem A | 108 ± 8 | 107 ± 8 | 118 ± 9* |
| MetS | 108 ± 9 | 114 ± 7 | 141 ± 5*† |
| MetS + penitrem A | 109 ± 8 | 134 ± 11* | 148 ± 9* |
| Diastolic blood pressure, mmHg | |||
| Lean | 64 ± 4 | 67 ± 5 | 74 ± 6 |
| Lean + penitrem A | 71 ± 5 | 68 ± 6 | 73 ± 7 |
| MetS | 70 ± 8 | 79 ± 10 | 92 ± 10*† |
| MetS + penitrem A | 73 ± 6 | 90 ± 8* | 97 ± 8* |
| Mean aortic pressure, mmHg | |||
| Lean | 84 ± 5 | 88 ± 6 | 97 ± 6 |
| Lean + penitrem A | 90 ± 6 | 88 ± 7 | 96 ± 7 |
| MetS | 90 ± 8 | 97 ± 8 | 117 ± 7*† |
| MetS + penitrem A | 92 ± 7 | 112 ± 9* | 122 ± 8* |
| Heart rate, beats/min | |||
| Lean | 105 ± 7 | 169 ± 14* | 207 ± 10* |
| Lean + penitrem A | 113 ± 8 | 178 ± 6* | 218 ± 9* |
| MetS | 118 ± 9 | 178 ± 11* | 212 ± 18* |
| MetS + penitrem A | 120 ± 8 | 187 ± 14* | 204 ± 17* |
| Coronary blood flow, ml·min−1·g−1 | |||
| Lean | 0.86 ± 0.09 | 1.40 ± 0.20* | 1.77 ± 0.19* |
| Lean + penitrem A | 0.92 ± 0.13 | 1.42 ± 0.19* | 1.96 ± 0.26* |
| MetS | 0.97 ± 0.07 | 1.58 ± 0.15* | 1.84 ± 0.10* |
| MetS + penitrem A | 1.06 ± 0.11 | 1.83 ± 0.27* | 2.17 ± 0.35* |
| Coronary conductance, μl·min−1·g−1·mmHg−1 | |||
| Lean | 10.3 ± 1.1 | 16.1 ± 2.4* | 18.5 ± 1.9* |
| Lean + penitrem A | 9.9 ± 1.0 | 16.2 ± 2.1* | 20.4 ± 2.2* |
| MetS | 11.1 ± 1.3 | 16.8 ± 1.9* | 15.8 ± 1.5* |
| MetS + penitrem A | 11.6 ± 1.4 | 16.7 ± 2.2* | 17.8 ± 2.7* |
| Myocardial O2 consumption, μl O2·min−1·g−1 | |||
| Lean | 103 ± 12 | 192 ± 39* | 240 ± 34* |
| Lean + penitrem A | 107 ± 23 | 182 ± 32* | 257 ± 42* |
| MetS | 108 ± 8 | 213 ± 31* | 253 ± 16* |
| MetS + penitrem A | 122 ± 10 | 243 ± 36* | 281 ± 45* |
| Arterial pH | |||
| Lean | 7.55 ± 0.02 | 7.54 ± 0.01 | 7.55 ± 0.02 |
| Lean + penitrem A | 7.54 ± 0.01 | 7.52 ± 0.03 | 7.53 ± 0.02 |
| MetS | 7.53 ± 0.01 | 7.57 ± 0.02 | 7.54 ± 0.03 |
| MetS + penitrem A | 7.55 ± 0.01 | 7.55 ± 0.04 | 7.50 ± 0.05 |
| Coronary venous pH | |||
| Lean | 7.48 ± 0.01 | 7.47 ± 0.01 | 7.46 ± 0.01 |
| Lean + penitrem A | 7.47 ± 0.01 | 7.46 ± 0.01 | 7.45 ± 0.01 |
| MetS | 7.48 ± 0.02 | 7.47 ± 0.02 | 7.45 ± 0.02 |
| MetS + penitrem A | 7.48 ± 0.02 | 7.48 ± 0.02 | 7.44 ± 0.02 |
| Arterial Pco2, mmHg | |||
| Lean | 34 ± 1 | 33 ± 1 | 33 ± 1 |
| Lean + penitrem A | 34 ± 1 | 34 ± 1 | 33 ± 1 |
| MetS | 35 ± 1 | 30 ± 1* | 30 ± 2* |
| MetS + penitrem A | 31 ± 1 | 32 ± 2 | 30 ± 2 |
| Coronary venous Pco2, mmHg | |||
| Lean | 45 ± 2 | 46 ± 2 | 46 ± 2 |
| Lean + penitrem A | 47 ± 2 | 46 ± 2 | 48 ± 2 |
| MetS | 43 ± 2 | 42 ± 2 | 43 ± 2 |
| MetS + penitrem A | 41 ± 2 | 42 ± 2 | 43 ± 2 |
| Arterial Po2, mmHg | |||
| Lean | 89 ± 3 | 83 ± 3 | 84 ± 4 |
| Lean + penitrem A | 89 ± 4 | 84 ± 3 | 77 ± 3 |
| MetS | 83 ± 1 | 94 ± 2*† | 85 ± 4 |
| MetS + penitrem A | 90 ± 3 | 80 ± 3 | 82 ± 4 |
| Coronary venous Po2, mmHg | |||
| Lean | 16 ± 0.8 | 15 ± 0.8 | 15 ± 0.8 |
| Lean + penitrem A | 18 ± 0.8 | 16 ± 0.8 | 15 ± 0.8* |
| MetS | 13 ± 0.9† | 12 ± 1.0† | 13 ± 1.0 |
| MetS + penitrem A | 13 ± 0.9 | 14 ± 0.9 | 15 ± 0.9 |
| Coronary venous O2 saturation, % | |||
| Lean | 19 ± 2 | 16 ± 2 | 19 ± 2 |
| Lean + penitrem A | 24 ± 2† | 20 ± 2* | 17 ± 2* |
| MetS | 15 ± 2† | 15 ± 2 | 16 ± 2 |
| MetS + penitrem A | 16 ± 2 | 17 ± 2 | 18 ± 2 |
| Arterial hematocrit, % | |||
| Lean | 32 ± 2 | 37 ± 3 | 37 ± 2 |
| Lean + penitrem A | 33 ± 3 | 35 ± 3 | 37 ± 3 |
| MetS | 31 ± 3 | 35 ± 2 | 34 ± 1 |
| MetS + penitrem A | 30 ± 2 | 33 ± 3 | 34 ± 1 |
Values are means ± SE for lean (n = 6) and MetS (n = 5) swine.
P < 0.05 vs. respective baseline (rest)
and vs. lean same level.
Fig. 2.
Effect of MetS on mean aortic pressure (A) and heart rate (B) at rest and during graded treadmill exercise. Mean aortic pressure was not different at rest but was elevated with exercise intensity in MetS relative to lean swine. No differences in heart rate were noted between lean and MetS swine at rest or during exercise.
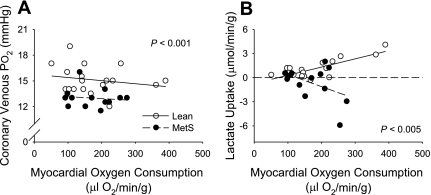
Coronary blood flow and MV̇o2 were elevated approximately twofold in both lean and MetS swine at the highest level of exercise. The relationship between coronary venous Po2 and MV̇o2 (Table 2 and Fig. 3A), a sensitive index of myocardial tissue oxygenation that reflects whether changes in myocardial oxygen delivery adequately match myocardial metabolism (49), revealed a parallel downward shift in coronary venous Po2 at a given level of MV̇o2 in MetS swine (P < 0.001). The impairment of myocardial oxygen supply-demand balance in MetS swine was accompanied by significant decreases in myocardial lactate uptake with exercise-induced increases in MV̇o2 (P < 0.005; Fig. 3B). The net release of lactate across the coronary circulation of MetS swine, which indicates the onset of anaerobic glycolysis and cardiac ischemia, is in stark contrast to the significant increases in myocardial lactate uptake (i.e., metabolic consumption) observed during exercise in lean animals. Changes in coronary venous O2 saturation were consistent with changes in coronary venous Po2 (Table 2). Neither arterial nor venous pH was changed in MetS swine compared with their respective lean controls at rest or during exercise (Table 2).
Fig. 3.
Effect of MetS on the relationship between myocardial oxygen consumption and coronary venous Po2 (A) and myocardial lactate uptake (B). The relationship between coronary venous Po2 and myocardial oxygen consumption revealed a parallel downward shift in coronary venous Po2 at a given level of myocardial oxygen consumption in MetS swine. This impairment of myocardial oxygen supply-demand balance was accompanied by a marked release of lactate (negative lactate uptake) in MetS swine as myocardial oxygen consumption was elevated.
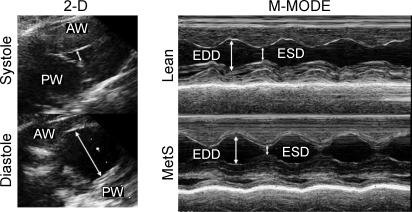
Figure 4 shows representative two-dimensional guided M-mode echocardiograms through the midleft ventricular level in lean and MetS swine. No statistically significant differences in cardiac dimensions, fractional shortening, stroke volume, or cardiac output were noted between conscious lean and MetS swine at rest (Table 3). However, cardiac index (normalization of cardiac output to body wt) was ∼25% lower in MetS vs. lean swine (P = 0.09).
Fig. 4.
Representative 2-dimensional (2-D) guided M-mode echocardiograms through the midleft ventricular level in lean and MetS swine. M-mode echocardiograms showed no significant difference in cardiac fractional shortening, end-diastolic dimension (EDD), or end-systolic dimension (ESD) between lean and MetS swine at rest. AW, anterior wall; PW, posterior wall.
Table 3.
Echocardiography data from lean and metabolic syndrome Ossabaw swine
| Lean | MetS | |
|---|---|---|
| End diastolic diameter, cm | 3.6 ± 0.1 | 3.5 ± 0.2 |
| End systolic diameter, cm | 1.9 ± 0.1 | 1.5 ± 0.3 |
| Fractional shortening, % | 48 ± 2 | 57 ± 6 |
| Stroke volume, ml | 50 ± 2 | 65 ± 9 |
| Cardiac output, l/min | 5.7 ± 0.7 | 6.4 ± 1.2 |
| Cardiac index, ml·min−1·kg−1 | 93 ± 5 | 69 ± 12 |
Values are means ± SE for lean (n = 4) and MetS (n = 4) swine.
Role of BKCa channels in local metabolic coronary vasodilation.
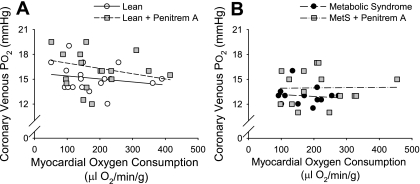
Inhibition of BKCa channels with penitrem A (10 μg/kg iv) did not significantly affect blood pressure, heart rate, coronary blood flow, or MV̇o2 in lean or MetS swine at rest or at the highest level of exercise (Table 2). Penitrem A had no effect on arterial or venous pH in either group of swine. Coronary venous Pco2 was not changed by penitrem A administration. Resting coronary venous Po2 and coronary venous O2 saturation were both diminished in MetS vs. lean swine. BKCa channel inhibition with penitrem A significantly lowered coronary venous Po2 and O2 saturation in lean swine at higher levels of MV̇o2, but not in MetS. Interestingly, coronary venous O2 saturation was increased by penitrem A in lean swine under resting conditions. However, Fig. 5 demonstrates that the relationship between coronary venous Po2 and MV̇o2 was not significantly affected by inhibition of BKCa channels in either lean (Fig. 5A) or MetS (Fig. 5B) swine. In fact, administration of penitrem A tended to increase coronary venous Po2 at a given level of MV̇o2 (Table 2); however, this effect failed to reach statistical significance (P = 0.10). It is important to point out that we previously demonstrated that the intravenous dose of penitrem A used in this investigation (10 μg/kg iv) is effective in inhibiting coronary vasodilation to the BKCa channel agonist NS-1619 in anesthetized, lean Ossabaw swine (3). Therefore, these data indicate that BKCa channels are not required for local metabolic coronary vasodilation during exercise.
Fig. 5.
Effect of large-conductance Ca2+-activated K+ (BKCa) channel inhibition on the relationship between coronary venous Po2 and myocardial oxygen consumption in lean (A) and MetS (B) swine. Inhibition of BKCa channels with penitrem A did not significantly affect the relationship between coronary venous Po2 and myocardial oxygen consumption in either lean or MetS swine.
DISCUSSION
The present study was designed to examine the hypothesis that impaired functional expression of coronary microvascular BKCa channels (3, 38) significantly contributes to the imbalance between myocardial oxygen supply and demand in MetS. The rationale for this hypothesis was based on recent evidence that BKCa channels contribute to exercise-induced coronary vasodilation in normal-lean swine (34) and that vascular smooth muscle BKCa channel function is diminished by the MetS (3, 6, 9, 33, 38). The major findings from this investigation were: 1) MetS impairs the balance between coronary blood flow and myocardial metabolism to an equal extent at rest and during exercise-induced increases in MV̇o2; 2) the imbalance between myocardial oxygen supply and demand in MetS does not alter myocardial contractile function at rest but is associated with signs of cardiac ischemia at higher levels of MV̇o2; and 3) factors released from the vascular endothelium and/or myocardium do not regulate local metabolic control of coronary blood flow through a BKCa channel-dependent mechanism under physiological (lean) or pathophysiological (MetS) conditions. These data indicate that diminished functional expression of BKCa channels does not contribute to the impairment of myocardial oxygen-supply demand balance in MetS.
MetS and the control of coronary blood flow.
Consistent with earlier studies (7, 29, 46, 54), data from this investigation further demonstrate that MetS significantly impairs the ability of the coronary circulation to adequately balance myocardial oxygen delivery with myocardial metabolism at rest and during exercise-induced increases in MV̇o2. This supply-demand imbalance is directly evidenced by the significant parallel downward shift in the relationship between coronary venous Po2 vs. MV̇o2 in MetS vs. lean swine (Fig. 3). In other words, MetS myocardium was forced to utilize its limited oxygen extraction reserve in efforts to meet the tissue requirements for oxygen. The fact that the coronary venous Po2 vs. MV̇o2 relationship shifted in a parallel manner indicates that MetS diminishes the balance between coronary blood flow and myocardial metabolism to an equal extent at rest and during exercise-induced increases in cardiac metabolism, i.e., increase in tonic constrictor and/or loss of a tonic dilator influence. Importantly, these data are consistent with our earlier study in MetS dogs (46). It is critical to recognize that simple examination of the coronary blood flow data in Table 2 do not adequately reflect the findings of the investigation, since they do not take into account the complex interaction between coronary blood flow and myocardial metabolism [the primary determinant of coronary flow (49)] or the various factors that influence perfusion and/or metabolism.
The impaired oxygen supply-demand balance in MetS is not associated with overt cardiac contractile dysfunction at rest, although there was a trend for lower cardiac index (cardiac output normalized to body wt) in MetS swine (Table 3 and Fig. 4), which is consistent with previous data in Yucatan swine (31) and the presence of “hyperdynamic circulation” (29). The marked increase in lactate release (negative lactate uptake) at higher levels of MV̇o2 indicates that the increase in oxygen extraction was inadequate, since myocardial underperfusion/ischemia was evidenced by the onset of anaerobic glycolytic metabolism (Fig. 3B). These data agree with earlier findings from our laboratory which documented that cardiac index is depressed at high levels of MV̇o2 in dogs with MetS (10). Furthermore, they suggest that the imbalance between coronary flow and metabolism positions MetS myocardium on the “brink” of ischemia at rest, such that exercise-induced increases in myocardial metabolism result in the onset of “demand ischemia.” Why myocardial oxygen extraction is maintained under these conditions (no decrease in coronary venous Po2) is intriguing, since an increase in oxygen utilization would act to mitigate the extent of hypoperfusion.
Although it can be argued that the elevated levels of insulin increased glucose uptake in Mets swine, thereby causing a switch in preferred substrate utilization of the heart, we propose that this is unlikely given that MetS swine are significantly insulin resistant, as evidenced by the elevation of both insulin and glucose in MetS swine (Table 1). The increase in cardiac lactate production is more indicative of elevated anaerobic glycolytic flux secondary to the impaired myocardial oxygen supply-demand balance, not a preferential “switch” in substrate utilization per se. Importantly, these changes are not related to the presence of a significant flow-limiting stenosis, since MetS Ossabaw swine exhibited only ∼13% luminal narrowing of the coronary circulation (Fig. 1). It is possible that the echocardiograms missed microinfarcts that were present due to the myocardial ischemia present at all MV̇o2 levels, including near resting MV̇o2 (Figs. 3 and 4). Taken together, these data suggest that mechanisms that contribute to the imbalance between coronary blood flow and myocardial metabolism at rest and more importantly, during increases in MV̇o2, could underlie, at least in part, the increased incidence of myocardial ischemia, infarction, and sudden cardiac death observed in obese patients with MetS (26, 32). Microvascular dysfunction is likely to be a major contributing factor to overt cardiac dysfunction as MetS progresses to type 2 diabetes with gross hyperglycemia (31). Therefore, understanding the mechanisms responsible for the impairment of coronary flow regulation in MetS is critical to the treatment and possible prevention of these complications.
BKCa channels and local metabolic control of coronary blood flow.
Given the abundant expression of BKCa channels in coronary vascular smooth muscle cells (4, 22, 23, 38, 39) and the importance of K+ channels to the regulation of coronary vascular resistance (15, 17, 53), we hypothesized that decreases in BKCa channel function would likely contribute to diminished local metabolic coronary vasodilation in MetS. Our hypothesis is supported by a recent study from Merkus et al. (34) who found that administration of the BKCa channel inhibitor tetraethylammonium (TEA) significantly decreased the relationship between coronary venous Po2 and MV̇o2 in normal, lean swine both at rest and during exercise. In addition, BKCa channels have also been shown to contribute to the regulation of coronary microvascular tone in ischemic dog hearts (40). However, in contrast to our hypothesis, we found that the impaired balance between myocardial oxygen delivery and MV̇o2 in MetS was not related to the diminished contribution of BKCa channels to local metabolic control of coronary blood flow. In fact, our data fail to support a functional role for BKCa channels in the regulation of coronary microvascular tone at rest or during exercise, since the inhibition of BKCa channels with penitrem A did not significantly decrease the relationship between coronary venous Po2 and MV̇o2 in lean (Fig. 5A) or MetS (Fig. 5B) swine. Although this finding is directly at odds with the earlier study of Merkus et al. (34), it is important to recognize that TEA is also an autonomic ganglionic blocker that inhibits sympathetic output (12, 42). Therefore, TEA is a nonselective inhibitor that would not only affect the balance between coronary blood flow and MV̇o2 by inhibiting BKCa channels but also by decreasing β-adrenoceptor-mediated coronary vasodilation, a prominent, well-accepted mechanism of exercise-induced coronary vasodilation (14, 20, 24, 25, 37).
Even though BKCa channels are known to contribute to adenosine-induced and endothelial [nitric oxide (NO)]-mediated dilation (3, 27, 35, 36), the lack of an effect of BKCa channel inhibition on the balance between coronary blood flow and myocardial metabolism is not surprising, since numerous earlier studies have failed to establish a prominent role for adenosine or NO in exercise-induced coronary vasodilation (1, 2, 16, 28, 47, 50–52). Furthermore, we are confident that penitrem A effectively inhibits BKCa channels, since we recently demonstrated that the intravenous dose of penitrem A used in this study significantly impairs coronary vasodilation in response to the BKCa channel agonist NS-1619 in anesthetized, lean Ossabaw swine, and penitrem A is as effective as iberiotoxin in blocking BKCa current in direct patch-clamp studies (3). We acknowledge that our experiments do not rule out possible compensatory activation of other K+ channels that may have masked the role of BKCa channels in local metabolic control of coronary blood flow at rest or during exercise-induced increases in MV̇o2. Whether increases in the activity of other K+ channels [i.e., ATP-dependent K+ (KATP) and/or voltage-gated K+ (Kv) channels] compensate to regulate coronary flow when BKCa channels are inhibited is unknown and merits further study. However, it is important to recognize that numerous earlier studies have demonstrated that blockade of a single K+ channel (KATP or KV) markedly reduces coronary flow and/or coronary venous Po2; i.e., inhibition of either KATP or KV channels significantly reduces the balance between coronary flow and metabolism (8, 15, 17). We propose that if BKCa channels were significantly contributing to the regulation of coronary vasomotor tone at rest or during exercise, then significant changes in flow and/or coronary venous Po2 would be observed. Therefore, while our data do not rule out compensatory activation of other K+ channels, they importantly demonstrate that BKCa channels are not required in local metabolic control of coronary blood flow at rest or during exercise-induced increases in MV̇o2.
In summary, our data indicate that factors released from the vascular endothelium and/or myocardium do not regulate local metabolic control of coronary blood flow through a BKCa channel-dependent mechanism under physiological (lean) or pathophysiological (MetS) conditions. Thus diminished function of BKCa channels (3, 6, 9, 33, 38) does not significantly contribute to the impairment of myocardial oxygen-supply demand balance at rest or during increases in MV̇o2 in MetS. However, our findings do not contradict a role for decreases in BKCa channel function contributing to coronary endothelial dysfunction that is typically observed in obesity, insulin resistance, and MetS (5, 29).
GRANTS
This work was supported by American Heart Association Grants 0810048Z (L. Borbouse) and 0810055Z (M. C. Svendsen), National Institutes of Health Grants HL-092245 (J. D. Tune), RR-13223 (M. Sturek), HL-62552 (M. Sturek), and UL1 RR-025761 (Z. P. Neeb), and the Fortune-Fry Ultrasound Research Fund of the Department of Cellular and Integrative Physiology, Indiana University School of Medicine.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res 28: 119–124, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein RD, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, Hintze TH. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res 79: 840–848, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BKCa channels in metabolic syndrome. Am J Physiol Heart Circ Physiol 297: H1629–H1637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol 84: 1225–1233, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol 294: H2489–H2496, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 290: H1520–H1527, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Camici PG, Marraccini P, Lorenzoni R, Buzzigoli G, Pecori N, Perissinotto A, Ferrannini E, L'Abbate A, Marzilli M. Coronary hemodynamics and myocardial metabolism in patients with syndrome X: response to pacing stress. J Am Coll Cardiol 17: 1461–1470, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol 294: H2371–H2381, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Dimitropoulou C, Han G, Miller AW, Molero M, Fuchs LC, White RE, Carrier GO. Potassium [BK(Ca)] currents are reduced in microvascular smooth muscle cells from insulin-resistant rats. Am J Physiol Heart Circ Physiol 282: H908–H917, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Dincer UD, Araiza A, Knudson JD, Shao CH, Bidasee KR, Tune JD. Dysfunction of cardiac ryanodine receptors in the metabolic syndrome. J Mol Cell Cardiol 41: 108–114, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Dincer UD, Araiza AG, Knudson JD, Molina PE, Tune JD. Sensitization of coronary alpha-adrenoceptor vasoconstriction in the prediabetic metabolic syndrome. Microcirculation 13: 587–595, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Downing SE, Lee JC. Analysis of cardiac adrenergic mechanisms in hypoxic lambs. Am J Physiol Heart Circ Physiol 244: H222–H227, 1983 [DOI] [PubMed] [Google Scholar]
- 13.Duncker DJ, Merkus D. Acute adaptations of the coronary circulation to exercise. Cell Biochem Biophys 43: 17–35, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res 82: 1312–1322, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Duncker DJ, van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of K+ATP channels in coronary vasodilation during exercise. Circulation 88: 1245–1253, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Duncker DJ, van Zon NS, Crampton M, Herrlinger S, Homans DC, Bache RJ. Coronary pressure-flow relationship and exercise: contributions of heart rate, contractility, and alpha 1-adrenergic tone. Am J Physiol Heart Circ Physiol 266: H795–H810, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Duncker DJ, van Zon NS, Ishibashi Y, Bache RJ. Role of K+ ATP channels and adenosine in the regulation of coronary blood flow during exercise with normal and restricted coronary blood flow. J Clin Invest 97: 996–1009, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp Med 56: 35–45, 2006 [PubMed] [Google Scholar]
- 19.Edwards JM, Alloosh MA, Long XL, Dick GM, Lloyd PG, Mokelke EA, Sturek M. Adenosine A1 receptors in neointimal hyperplasia and in-stent stenosis in Ossabaw miniature swine. Coron Artery Dis 19: 27–31, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Feigl EO. Neural control of coronary blood flow. J Vasc Res 35: 85–92, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Feigl EO, Neat GW, Huang AH. Interrelations between coronary artery pressure, myocardial metabolism and coronary blood flow. J Mol Cell Cardiol 22: 375–390, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Ganitkevich V, Isenberg G. Isolated guinea pig coronary smooth muscle cells. Acetylcholine induces hyperpolarization due to sarcoplasmic reticulum calcium release activating potassium channels. Circ Res 67: 525–528, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Gollasch M, Ried C, Bychkov R, Luft FC, Haller H. K+ currents in human coronary artery vascular smooth muscle cells. Circ Res 78: 676–688, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1892–1902, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1903–1911, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, JR, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100: 1134–1146, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Hernanz R, Alonso MJ, Baena AB, Salaices M, Marin J. Mechanisms of bradykinin-induced relaxation in pig coronary arteries. Methods Find Exp Clin Pharmacol 21: 243–251, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K+ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res 82: 346–359, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Knudson JD, Dincer UD, Bratz IN, Sturek M, Dick GM, Tune JD. Mechanisms of coronary dysfunction in obesity and insulin resistance. Microcirculation 14: 317–338, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Knudson JD, Rogers PA, Dincer UD, Bratz IN, Araiza AG, Dick GM, Tune JD. Coronary vasomotor reactivity to endothelin-1 in the prediabetic metabolic syndrome. Microcirculation 13: 209–218, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol 98: 461–467, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288: 2709–2716, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Lu T, Wang XL, He T, Zhou W, Kaduce TL, Katusic ZS, Spector AA, Lee HC. Impaired arachidonic acid-mediated activation of large-conductance Ca2+-activated K+ channels in coronary arterial smooth muscle cells in Zucker Diabetic Fatty rats. Diabetes 54: 2155–2163, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol 291: H2090–H2097, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation 99: 3132–3138, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Miyashiro JK, Feigl EO. Feedforward control of coronary blood flow via coronary beta-receptor stimulation. Circ Res 73: 252–263, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol 288: H1233–H1241, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Mokelke EA, Hu Q, Song M, Toro L, Reddy HK, Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol 95: 1179–1193, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Node K, Kitakaze M, Kosaka H, Minamino T, Hori M. Bradykinin mediation of Ca(2+)-activated K+ channels regulates coronary blood flow in ischemic myocardium. Circulation 95: 1560–1567, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Otis CR, Wamhoff BR, Sturek M. Hyperglycemia-induced insulin resistance in diabetic dyslipidemic Yucatan swine. Comp Med 53: 53–64, 2003 [PubMed] [Google Scholar]
- 42.Page IH, McCubbin JW. Increased resistance to autonomic ganglionic blockade by tetraethylammonium chloride and pentamethonium iodide in experimental neurogenic hypertension. Am J Physiol 168: 208–217, 1952 [DOI] [PubMed] [Google Scholar]
- 43.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 44.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol 47: 1188–1195, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Setty S, Sun W, Martinez R, Downey HF, Tune JD. Alpha-adrenoceptor-mediated coronary vasoconstriction is augmented during exercise in experimental diabetes mellitus. J Appl Physiol 97: 431–438, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Setty S, Sun W, Tune JD. Coronary blood flow regulation in the prediabetic metabolic syndrome. Basic Res Cardiol 98: 416–423, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Shen W, Lundborg M, Wang J, Stewart JM, Xu X, Ochoa M, Hintze TH. Role of EDRF in the regulation of regional blood flow and vascular resistance at rest and during exercise in conscious dogs. J Appl Physiol 77: 165–172, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Sturek M, Alloosh M, Wenzel J, Byrd JP, Edwards JM, Lloyd PG, Tune JD, March KL, Miller MA, Mokelke EA, Brisbin IL. Ossabaw island miniature swine: cardiometabolic syndrome assessment. In: Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques, edited by Swindle MM. Boca Raton, FL: CRC, 2007, p.397–402 [Google Scholar]
- 49.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol 97: 404–415, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation 101: 2942–2948, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Tune JD, Richmond KN, Gorman MW, Feigl EO. K+ATP channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 280: H868–H875, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74–H84, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Tune JD, Yeh C, Setty S, Downey HF. ATP-dependent K(+) channels contribute to local metabolic coronary vasodilation in experimental diabetes. Diabetes 51: 1201–1207, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Zhang C, Knudson JD, Setty S, Araiza A, Dincer UD, Kuo L, Tune JD. Coronary arteriolar vasoconstriction to angiotensin II is augmented in prediabetic metabolic syndrome via activation of AT1 receptors. Am J Physiol Heart Circ Physiol 288: H2154–H2162, 2005 [DOI] [PubMed] [Google Scholar]