Abstract
Although estrogen regulates important aspects of maternal cardiovascular physiology, the role of estrogen on uteroplacental and fetal blood flow is incompletely understood. This study tested the hypothesis that chronically suppressing endogenous estrogen production during the second half of baboon pregnancy alters uterine and fetal blood flow dynamics assessed by ultrasonography. Pregnant baboons were untreated or treated daily with the aromatase inhibitor letrozole or letrozole plus estradiol on days 100–160 of gestation (term = 184 days). Blood flow dynamics were determined by Doppler ultrasonography on day 60 and longitudinally between days 110 and 160 of gestation. Letrozole decreased maternal serum estradiol and estrone concentrations by 95% (P < 0.001). Fetal growth biometrical parameters increased (P < 0.001) between days 110 and 160 of gestation and were similar in untreated and letrozole-treated animals. Uterine, umbilical, and fetal middle cerebral artery pulsatility index and resistance index declined (P < 0.01) by 30–50% and uterine artery volume flow increased sixfold (P < 0.001) between days 60 and 160, but values were similar in untreated, letrozole-treated, and letrozole plus estradiol-treated baboons. Thus uterine and fetal artery blood flow indexes, uterine artery volume flow, and fetal growth were maintained at normal levels despite chronic estrogen suppression in the second half of baboon pregnancy. This suggests that elevated levels of endogenous estrogen are not required to maintain low impedance blood flow within the uteroplacental vascular bed during the second half of nonhuman primate pregnancy.
Keywords: uterus, fetus
estrogen has a well established role in regulating several fundamentally important aspects of maternal cardiovascular physiology and uteroplacental blood flow dynamics (22, 32, 39, 51, 54). For example, the administration of estrogen to ovariectomized nonpregnant or pregnant sheep elevated uterine artery blood flow by regulating vasodilatatory mechanisms mediated by nitric oxide or other endothelium-derived mediators (10, 20, 26, 33, 35, 52, 53, 56). The increase in uteroplacental blood flow that occurs with advancing pregnancy (55), therefore, may reflect the progressive rise in estrogen levels that are exhibited during human and nonhuman primate gestation (6). Indeed, acute administration of the estrogen receptor antagonist ICI 182,780 to estradiol-treated nonpregnant or pregnant sheep increased vascular resistance and decreased blood flow within the uterine artery (33), data supporting a potential role for endogenous estrogen in the regulation of uterine artery blood flow.
However, studies of uteroplacental blood flow dynamics after chronic inhibition of estrogen action or formation have not been conducted, particularly in the primate. Our laboratories have used the baboon as a nonhuman primate model to investigate the role of endogenous estrogen on several aspects of the endocrinology and physiology of pregnancy and fetal development (5, 39). Therefore, in the present study we utilized the baboon and a highly specific aromatase inhibitor to test the hypothesis that chronic suppression of endogenous estrogen production during the second half of pregnancy alters uteroplacental and fetal blood flow dynamics.
MATERIALS AND METHODS
Animals.
Adult female baboons (Papio anubis) weighing 14–18 kg were purchased from the Southwest National Primate Research Center (San Antonio, TX) and housed individually in large aluminum primate cages in air-conditioned rooms with a 12-h:12-h light/dark lighting schedule. Baboons received standard monkey chow (Teklad-Harlan, St. Louis, MO), fresh fruit, and vitamins daily and water ad libitum. Females were paired with male baboons for 5 days at the anticipated time of ovulation, which was determined from menstrual cycle history and daily pattern of perineal turgescence (1), and pregnancy was confirmed by palpation and ultrasonography. Day 1 of pregnancy was designated as the day preceding deturgescence and represented the day after ovulation. Animals were cared for and used strictly in accordance with U.S. Department of Agriculture regulations and the Guide for the Care and Use of Laboratory Animals prepared by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). The experimental protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Twenty-four baboons were left untreated and delivered by cesarean section on days 164–173 of gestation (means ± SE = 167.3 ± 0.7 days; term = 184 days). Twenty-three baboon mothers received the highly specific nonsteroidal competitive aromatase enzyme inhibitor letrozole (4,4′-[1,2,4-triazol-1-yl-methylene]-bis-benzonitrite; Novartis Pharm, Basel, Switzerland; 115 μg/kg body wt sc in 1.0 ml sesame oil) daily beginning on day 100 and continuing throughout the second half of pregnancy until the day before cesarean section on days 163–171 (means ± SE = 165.3 ± 0.5 days). Thirteen baboons were treated simultaneously with letrozole and estradiol benzoate (each 115 μg/kg body wt sc) between day 100 and cesarean section on days 164–172 of gestation (means ± SE = 167.2 ± 1.2 days). Untreated and letrozole-treated animals were sedated with ketamine HCl (10 mg/kg body weight im) daily, and blood samples collected (2–4 ml) from a maternal peripheral saphenous vein for determination of estradiol and progesterone levels. Ultrasonography was performed between 10:00 and 11:00 am on day 60, i.e. early in pregnancy, and at 10-day intervals on days 110–160 of the second half of gestation. Ultrasonography was conducted after sedation of baboons with ketamine, endotracheal intubation, light halothane anesthesia (i.e., animal quiescent but not deeply anesthetized), and blood pressure and oxygenation were verified. The interval between initial ketamine sedation and onset of Doppler imaging approximated 20 min. Maternal blood pressure and heart rate and fetal heart rate were determined via a Dinamap Pro 400 V2 (GE Medical Systems, Milwaukee, WI), and blood samples were obtained from a maternal saphenous vein and umbilical artery (i.e., fetal) to assess serum chemistry analytes (Antech Diagnostics, Lake Success, NY) and steroid hormones from halothane-anesthetized baboons at the time of cesarean section.
Steroid hormone radioimmunoassay.
Serum samples were stored at −20°C until assayed for concentrations of estradiol and estrone, the primary estrogens produced during baboon pregnancy (6, 8); dehydroepiandrosterone sulfate (DHAS), the principal C19-steroid precursor of estrogen (6); and progesterone by chemiluminescent immunoassay (Immulite; Diagnostic Products, Los Angeles, CA), as described previously (3).
Doppler ultrasonography.
Fetal biometrical parameters and blood flow indexes were determined by standard grayscale ultrasonography and pulsed color Doppler velocimetry, respectively, using a 7-MHz sector-transducer and an Acuson Ultrasound (Model 128 XP/10; Acuson Corporation, Mountain View, CA) with high-pass filter at 100 Hz to separate vessel wall vibrations from flow velocity waveforms. Fetal growth was assessed by measurement of the biparietal diameter, head and abdominal circumferences, and lengths of the humerus and femur. The anatomic planes were identified according to standard landmarks established in human fetuses (23, 30).
The right and left uterine arteries were identified with the transducer placed medial of the anterior iliac spines and directed slightly toward the pelvis and by visualization of the artificial crossing of the uterine and external iliac arteries with color Doppler (12, 15). Doppler measurements of fetal blood flow were obtained on the umbilical artery at the placental and fetal attachment sites by previously described methods (11), the middle cerebral artery identified as the branch running anterolateral from the Circle of Willis toward the lateral edge of the orbit, and the descending aorta proximal to the renal artery bifurcations, and by their associated waveform dynamics (16, 19). The vessels were analyzed in the latter order each time.
Color Doppler imaging was used to optimize placement of the pulsed wave gate at maximal color brightness by adjusting the velocity scale to identify the area and direction of maximum blood flow. The insonation angle was maintained as close as possible to 0 degrees, usually less than 30 degrees, and the sample volume adjusted to cover the entire vessel and thus allow for optimal color resolution, sensitivity, and frame rate. Three separate measurements were obtained during periods of fetal rest and apnea from each frozen image captured from at least five consecutive uniform flow waveforms with a high signal-to-noise ratio. Pulsatility index (PI) (21, 25) and resistance index (RI) were measured, and uterine and umbilical artery end-diastolic flow and the presence or absence of uterine artery diastolic notching were noted.
Uterine artery (A) volume flow was quantified by ultrasound according to the formula: QA (in ml/min) = V (time-averaged mean velocity in cm/s) × πr2 (cross-sectional area of vessel; r = radius) × 60 s/min. Volume flow measurements assume cylindrical vessel, parabolic flow, and stable placental blood volume. The maternal body weight was used to correct for flow (in ml·min−1·kg−1) in the uterine artery. Doppler imaging of all parameters was routinely completed within 40 min.
Statistical analyses.
Data on fetal, placental, and maternal weights and serum chemistry parameters were analyzed by ANOVA with post hoc comparisons of the means by the Newman-Keuls multiple-comparison test. Serial fetal biometrical parameters, arterial PI and RI values and volume flow, and serum estradiol and progesterone levels were assessed by mixed model repeated-measures regression analysis (31), adjusted for within-animal correlation across days of gestation and treatment effects (SAS 9.1; Cary, NC). Gestational age was entered as a continuous variable to test for trend.
RESULTS
Serum steroid hormone levels.
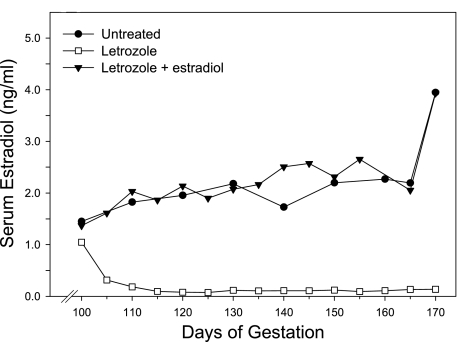
The daily serum estradiol values were averaged and illustrated at 5-day intervals in Fig. 1. Maternal peripheral serum estradiol concentrations in untreated baboons increased from 1.50 ± 0.34 ng/ml (means ± SE) at midgestation (i.e., day 100) to 2.26 ± 0.30 ng/ml on day 160 of gestation (Fig. 1). Administration of letrozole rapidly decreased estradiol to 0.10 ± 0.02 ng/ml (means ± SE) between days 100 and 160 of gestation, a level that was 95% lower (P < 0.001) than in the untreated animals on days 100–160 (2.19 ± 0.36 ng/ml). Concomitant administration of letrozole and estradiol restored maternal serum estrogen to 2.18 ± 0.60 ng/ml between days 100 and 160, a value similar to that of the untreated animals.
Fig. 1.
Maternal peripheral serum estradiol concentrations in baboons untreated (n = 8) or treated subcutaneously with letrozole (115 μg/kg body wt; n = 13) or letrozole plus estradiol benzoate (each at 115 μg/kg body wt; n = 5) daily between days 100 and cesarean section on days 163–173 of gestation. Values represent the means for 5-day intervals between days 100 and 160 of gestation (term = 184 days).
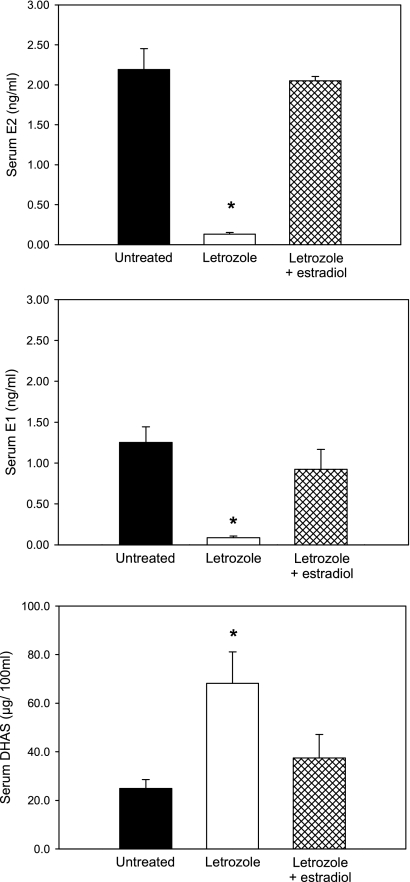
Maternal peripheral serum estradiol (2.19 ± 0.26 ng/ml) and estrone (1.25 ± 0.19 ng/ml) levels on the day of delivery in untreated baboons were decreased (P < 0.01) by 95% after the administration of letrozole (0.13 ± 0.02 and 0.08 ± 0.02 ng/ml, respectively) and restored by concomitant administration of letrozole plus estradiol (2.05 ± 0.05 and 0.92 ± 0.24 ng/ml, respectively; Fig. 2), the latter of which is in part metabolized to estrone. Umbilical vein (i.e., from placenta to fetus) serum DHAS levels on the day of delivery in untreated baboons (24.9 ± 3.7 μg/100 ml) were increased (P < 0.01) over 2.5-fold by letrozole administration (68.2 ± 12.9 μg/100 ml), consistent with inhibition of the aromatization of C19-steroid estrogen precursor, and decreased (P < 0.01) by letrozole plus estradiol treatment (37.4 ± 9.7 μg/100 ml; Fig. 2).
Fig. 2.
Means (±SE) maternal saphenous vein estradiol (E2) and estrone (E1) and umbilical vein dehydroepiandrosterone sulfate (DHAS) levels on the day of delivery (days 163–173 of gestation) in the untreated and letrozole ± estradiol-treated baboons in which serum estradiol levels are shown in Fig. 1. *Significantly different (P < 0.01) from the values in untreated and letrozole plus estradiol-treated animals.
Maternal serum progesterone levels remained relatively constant throughout the second half of pregnancy, as shown previously (3), and mean (± SE) levels were similar on days 100–160 in untreated (15.1 ± 1.4 ng/ml), letrozole-treated (12.4 ± 1.0 ng/ml), and letrozole plus estradiol-treated (13.7 ± 1.9 ng/ml) baboons.
Placental weight and maternal and fetal body weights, blood pressure, and serum chemistry.
All of the untreated, letrozole-treated, and letrozole plus estradiol-treated baboons of this study delivered live and apparently healthy newborns. The distribution of newborn sexes was 12 females and 12 males in untreated, 12 females and 11 males in letrozole-treated, and 5 females and 8 males in letrozole plus estradiol-treated baboons, and no differences in the parameters analyzed in this study were observed between the sexes. Placental weight was approximately 10% larger (P < 0.05) and, consequently, the fetal-to-placental weight ratio was slightly lower (P < 0.05) in letrozole-treated than in untreated animals (Table 1). Fetal body weight was 10% smaller (P < 0.05) in letrozole plus estradiol-treated baboons than in untreated animals (Table 1).
Table 1.
Placental, fetal body, and maternal body weights in baboons
| Treatment | n | Placental Weight, g | Fetal Weight, g | Maternal Weight, kg | Fetal Weight-to-Maternal Weight | Fetal Weight-to-Placental Weight |
|---|---|---|---|---|---|---|
| Untreated | 24 | 189.4 ± 5.5* | 870.4 ± 21.0* | 17.7 ± 0.4 | 0.049 ± 0.001 | 4.59 ± 0.10* |
| Letrozole | 23 | 211.3 ± 7.6† | 833.1 ± 17.0* | 17.9 ± 0.4 | 0.047 ± 0.001 | 4.01 ± 0.11† |
| Letrozole + estradiol | 13 | 168.3 ± 9.6* | 784.0 ± 24.9† | 16.5 ± 0.5 | 0.048 ± 0.002 | 4.76 ± 0.16* |
Values are means ± SE on the day of cesarean section delivery for baboons untreated or treated with letrozole or letrozole plus estradiol daily between day 100 and the day before cesarean section; n, number per group.
Values with different symbols differ from each other at P < 0.05.
Maternal mean arterial blood pressure and heart rate, as well as select maternal and fetal serum chemistry analytes reflecting liver, kidney, and metabolic function, were in most cases not significantly different on the day of delivery in baboons untreated, treated with letrozole, or treated with letrozole plus estradiol (Table 2). Maternal blood urea nitrogen (BUN) was slightly higher (P < 0.05) in letrozole-treated than in untreated or letrozole plus estradiol-treated baboons, but well within the established normal range for baboons. Fetal heart rate was ∼10% greater (P < 0.05) in letrozole-treated than in untreated animals.
Table 2.
MABP, heart rate, and serum chemistry analytes in maternal and fetal baboons
| MABP, mmHg | Heart Rate, beats/min | Glucose, mg/dL | Na, mEq/l | BUN, mg/dL | Creatinine, mg/dL | Osmolality, mOsm/l | |
|---|---|---|---|---|---|---|---|
| Maternal | |||||||
| Untreated | 43.8 ± 1.8 | 100 ± 3 | 72.4 ± 6.8 | 144.3 ± 1.7 | 8.3 ± 0.5* | 0.95 ± 0.04 | 281.6 ± 2.7 |
| Letrozole | 48.8 ± 2.5‡ | 102 ± 3 | 69.9 ± 7.9 | 141.0 ± 1.9 | 11.6 ± 1.2† | 0.86 ± 0.06 | 281.1 ± 3.2 |
| Letrozole + estradiol | 41.5 ± 2.7 | 103 ± 4 | 57.2 ± 10.1 | 139.3 ± 2.4 | 6.9 ± 0.7* | 0.83 ± 0.04 | 271.7 ± 3.9 |
| Fetal | |||||||
| Untreated | — | 145 ± 3* | 73.9 ± 5.8 | 142.8 ± 0.7 | 10.1 ± 0.6 | 0.84 ± 0.04 | 283.1 ± 1.1 |
| Letrozole | — | 159 ± 4† | 77.6 ± 6.6 | 143.6 ± 0.9 | 13.2 ± 1.0 | 0.73 ± 0.04 | 285.4 ± 1.7 |
| Letrozole + estradiol | — | 154 ± 6*† | 75.4 ± 7.5 | 142.6 ± 0.7 | 10.2 ± 1.1 | 0.83 ± 0.04 | 283.6 ± 1.5 |
Values are means ± SE on the day of cesarean delivery for the baboons in which placental and body weights are presented in Table 1. * and †, differ from each other at P < 0.05;
P = 0.06 vs. untreated and letrozole plus estradiol groups. MABP, mean arterial blood pressure. BUN, blood urea nitrogen.
Fetal biometry.
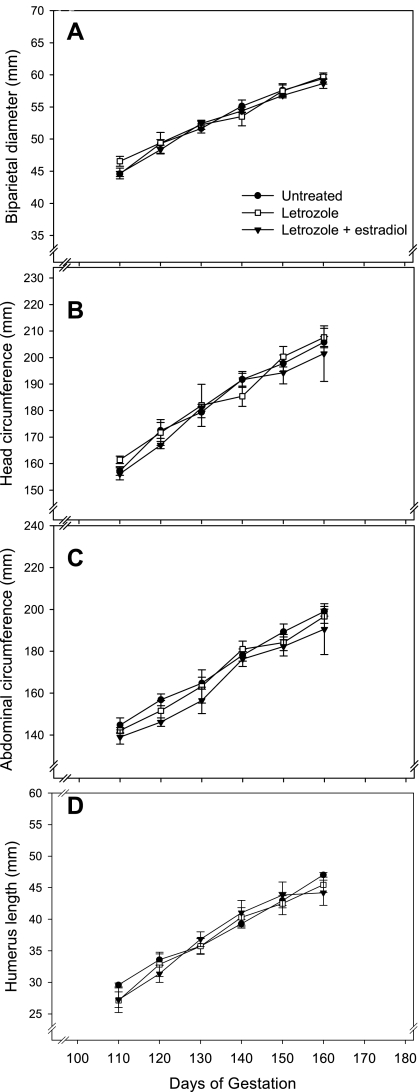
A linear increase (P < 0.0001) in fetal biometrical parameters, including biparietal diameter (Fig. 3A), head circumference (Fig. 3B), abdominal circumference (Fig. 3C), humerus length (Fig. 3D), and femur length (not shown) occurred between days 110 and 160 of baboon pregnancy. Each of these fetal growth parameters was similar in value in untreated, letrozole-treated, and letrozole plus estradiol-treated animals at each of the gestational time points.
Fig. 3.
Means ± SE fetal biparietal diameter (A), head circumference (B), abdominal circumference (C), and humerus length (D) assessed by grayscale ultrasonography between days 110 and 160 of gestation in untreated (n = 8), letrozole-treated (n = 13), and letrozole plus estradiol-treated (n = 5) baboons. See legend of Fig. 1 for details of treatment.
Uterine artery blood flow indexes.
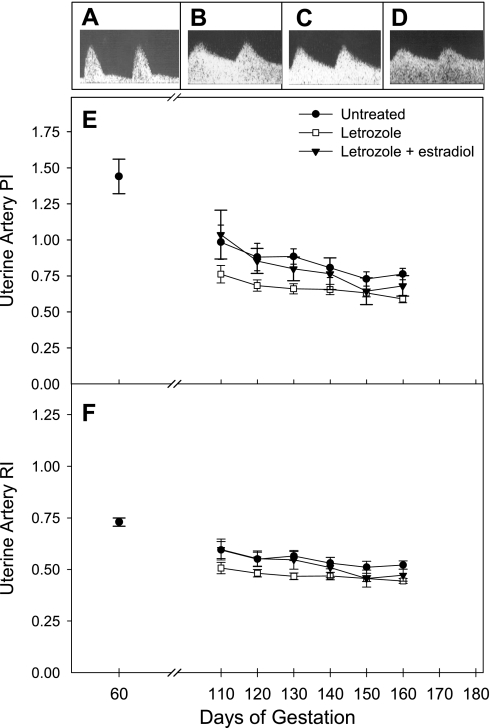
Figure 4 illustrates representative uterine artery waveforms assessed by color Doppler ultrasonography on days 60 (Fig. 4A) and 150 (Fig. 4B) of gestation in untreated baboons and on day 150 in animals treated between day 100 and the day before delivery with letrozole (Fig. 4C) or letrozole plus estradiol (Fig. 4D). Uterine artery waveforms in untreated animals showed low end-diastolic flow on day 60 (Fig. 4A), indicative of downstream flow impedance, compared with high end-diastolic flow (low impedance) on day 150 (Fig. 4B). However, uterine artery waveforms appeared qualitatively similar on day 150 in baboons within each treatment group (Fig. 4, B–D), exhibiting high end-diastolic flow and absence of diastolic notching.
Fig. 4.
Representative uterine artery Doppler flow waveforms assessed by color Doppler ultrasonography on day 60 in untreated (A) animals and on day 150 of gestation in untreated (B), letrozole-treated (C), and letrozole plus estradiol benzoate-treated (D) baboons. Means ± SE uterine artery (i.e., average of right and left) pulsatility index (PI; E) and resistance index (RI; F) assessed on day 60 (average of values in baboons subsequently untreated or treated with letrozole ± estradiol) and at 10-day intervals between days 110 and 160 of gestation for untreated (n = 24), letrozole-treated (n = 23), and letrozole plus estradiol-treated (n = 13) baboons. Please note, y-axis scale (velocity) of A is one-third that in B–D.
Since the PI and RI values were not significantly different for the left and right uterine arteries, results were averaged and are presented in Fig. 4. Uterine artery PI (Fig. 4E) and RI (Fig. 4F) values on day 60 were similar in baboons that were subsequently untreated, treated with letrozole, or treated with letrozole and estradiol. Uterine artery PI in untreated baboons on day 60 (1.44 ± 0.12) decreased (P < 0.01) to 0.98 ± 0.12 on day 110 and decreased (P < 0.05) further to 0.76 ± 0.04 on day 160 of gestation (Fig. 4E). Uterine artery RI in untreated animals declined (P < 0.01) from 0.73 ± 0.02 on day 60 to 0.59 ± 0.04 on day 110 and decreased (P < 0.03) further to 0.52 ± 0.02 on day 160 of gestation (Fig. 4F). Uterine artery PI and RI in letrozole-treated baboons on day 110 (0.76 ± 0.06 and 0.51 ± 0.03, respectively) also decreased (P < 0.05) to 0.59 ± 0.03 and 0.44 ± 0.01, respectively, on day 160. However, the gestational age-dependent decline in PI and RI in letrozole-treated or letrozole plus estradiol-treated animals did not differ significantly from that in untreated animals.
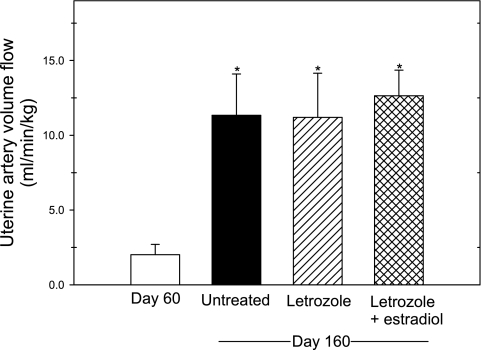
Uterine artery volume flow (average of right and left vessels) in untreated baboons was increased almost sixfold (P < 0.01) from 2.01 ± 0.69 ml·min−1·kg−1 maternal body weight on day 60 to 11.34 ± 2.76 on day 160 of gestation (Fig. 5). Moreover, uterine artery volume flow on day 160 in baboons in which serum estrogen levels were suppressed by the administration of letrozole (11.36 ± 2.41 ml·min−1·kg−1) or restored by letrozole plus estradiol (12.64 ± 1.72 ml·min−1·kg−1) were each also ∼sixfold greater (P < 0.01) than on day 60 and similar in value to that on day 160 in untreated animals (Fig. 5).
Fig. 5.
Uterine artery volume flow (in ml·min−1·kg−1 maternal body wt) on days 60 and 160 of gestation in baboons untreated (n = 8) and on day 160 in animals treated daily beginning on day 100 with letrozole (n = 6) or letrozole plus estradiol (n = 6). Values represent means (±SE) of average value in left and right uterine arteries. *Each of the values on day 160 are greater (P < 0.01) than on day 60.
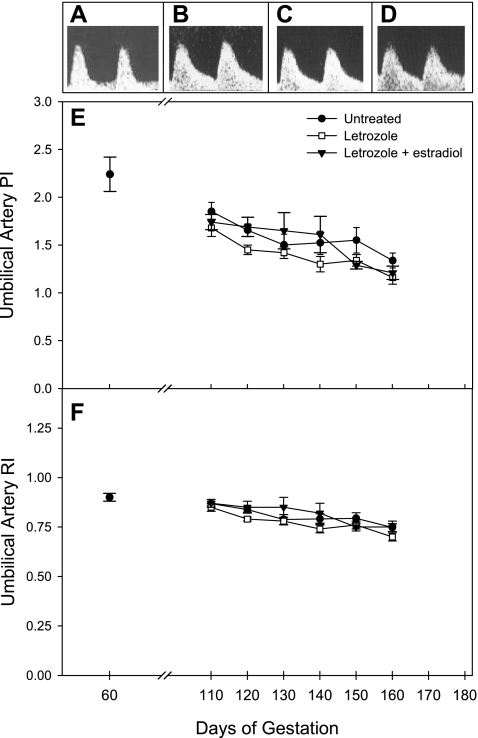
Umbilical artery blood flow indexes.
Figure 6 illustrates representative Doppler flow waveforms for the umbilical artery on days 60 (Fig. 6A) and 150 (Fig. 6B) of gestation in untreated baboons and on day 150 in letrozole-treated (Fig. 6C) and letrozole plus estradiol-treated (Fig. 6D) baboons. Umbilical artery flow waveform in untreated animals showed low end-diastolic flow on day 60 when compared with that on day 150. However, umbilical artery waveforms appeared comparable and exhibited high end-diastolic flow in animals untreated (Fig. 6B), or administered letrozole (Fig. 6C), or letrozole and estradiol (Fig. 6D). Umbilical blood flow PI and RI values for the fetal umbilicus and placental insertion sites were similar and thus averaged. Umbilical artery PI (Fig. 6E) and RI (Fig. 6F) values on day 60 were similar in baboons that were subsequently untreated, treated with letrozole, or treated with letrozole plus estradiol. In untreated animals, umbilical PI decreased (not significantly) from 2.24 ± 0.18 on day 60 to 1.85 ± 0.10 on day 110 and then declined (P < 0.01) to 1.34 ± 0.08 on day 160 (Fig. 6E). Umbilical artery RI values in untreated baboons were 0.90 ± 0.02 on day 60 and 0.86 ± 0.01 on day 110 and then decreased (P < 0.001) to 0.74 ± 0.02 on day 160 (Fig. 6F). Umbilical artery PI and RI in letrozole-treated and letrozole plus estradiol-treated baboons also declined (P < 0.001) between days 110 and 160, but this gestational age-dependent decrease was similar to that in untreated animals.
Fig. 6.
Representative umbilical artery Doppler flow waveforms on day 60 in untreated (A) baboons and on day 150 in untreated (B), letrozole-treated (C), and letrozole plus estradiol-treated (D) animals. Umbilical artery (i.e., average of fetal and placental insertion sites) PI (E) and RI (F) values on day 60 (average in animals subsequently untreated or treated with letrozole ± estradiol) and between days 110 and 160 of gestation for the baboons in which uterine artery data are shown in Fig. 4. Please note, y-axis scale (velocity) of A is one-third that in B–D.
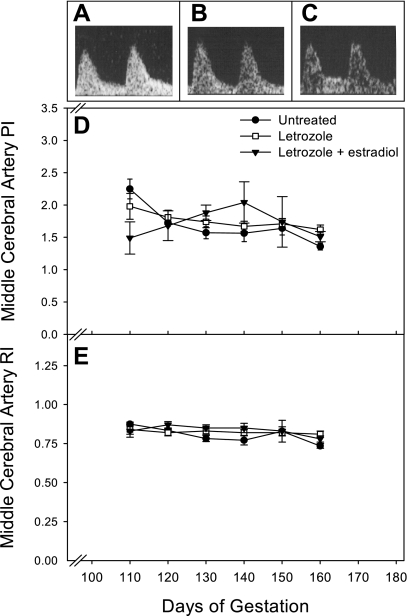
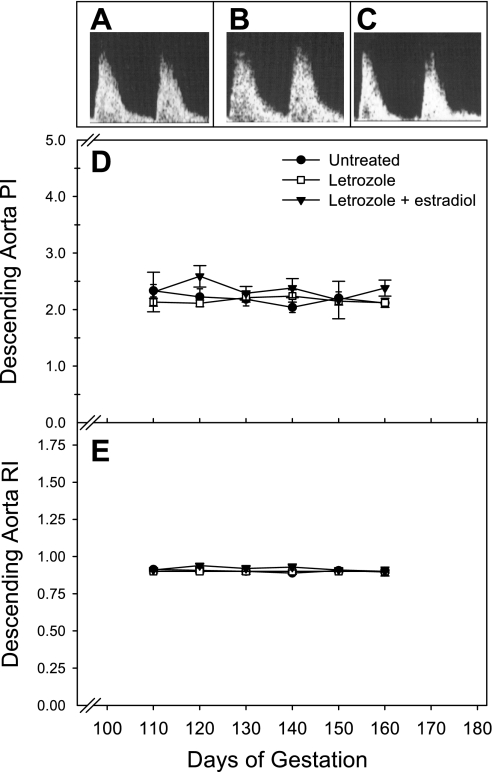
Fetal blood flow indexes.
Fetal vessel blood flow could not be reliably measured on day 60 because of the small conceptus size. However, representative Doppler waveforms for the fetal middle cerebral artery and descending aorta are shown for the second half of pregnancy in Fig. 7, A–C, and Fig. 8, A–C, respectively. Qualitatively, waveforms for these vessels appeared similar in untreated, letrozole-treated, and letrozole plus estradiol-treated baboons. The PI for the middle cerebral artery of untreated fetuses decreased (P < 0.05) from 2.25 ± 0.15 on day 110 to 1.36 ± 0.06 on day 160 of gestation (Fig. 7D). However, the middle cerebral artery RI (Fig. 7E) and descending aorta PI (Fig. 8D) and RI (Fig. 8E) remained relatively constant during the second half of gestation, and values were not significantly different between the treatment groups.
Fig. 7.
Representative fetal middle cerebral artery flow waveforms on day 150 of gestation in untreated (A), letrozole-treated (B), and letrozole plus estradiol-treated (C) baboons. Middle cerebral artery PI (D) and RI (E) values between days 110 and 160 of gestation for the baboons in which uterine artery data are shown in Fig. 4.
Fig. 8.
Representative fetal descending aorta waveforms on day 150 of gestation in untreated (A), letrozole-treated (B), and letrozole plus estradiol-treated (C) baboons. Descending aorta PI (D) and RI (E) values for the baboons in which uterine artery data are shown in Fig. 4.
DISCUSSION
The present study demonstrates that uterine and umbilical artery PI and RI decreased and uterine artery volume flow increased as assessed by Doppler ultrasonography with advancing normal baboon gestation as in human pregnancy. The current study further shows that despite the marked suppression of estrogen production and levels by administration of an aromatase inhibitor chronically throughout the second half of baboon pregnancy, uterine, umbilical, and fetal artery blood flow waveforms and PI and RI values and uterine artery volume flow were similar to respective parameters in untreated baboons exhibiting the normal elevation in estrogen. Uterine and umbilical artery PI and RI are directly proportional to downstream blood flow impedance on the maternal side of the uteroplacental circulation and the fetal side of the placental inner villous compartment, respectively (27, 28). Therefore, it appears that the elevated levels of endogenous estrogen present in the second half of primate pregnancy are not required to chronically maintain low resistance blood flow across the placenta at this time in gestation. The linear increase in biometric parameters reflecting fetal growth, normal neonatal body weight, and serum chemistry analytes at delivery, and delivery of healthy live newborn baboons near term, coinciding with the absence of change in uterine, umbilical and fetal PI and RI, and uterine artery volume flow, in letrozole-treated baboons of the present study are consistent with the maintenance of normal uteroplacental blood flow despite the suppression of estrogen during the second half of primate pregnancy.
During the first half of human and nonhuman primate gestation, placental extravillous cytotrophoblasts invade, replace, and remodel the smooth muscle and endothelial walls of the uterine spiral arteries, transforming them from high resistance low capacity to low resistance high capacitance flow through vessels (13, 17, 24, 43, 44). As a consequence of uterine artery remodeling, downstream flow impedance declines (28, 38) and responsivity of the uteroplacental vascular bed to vasomotor regulatory mechanisms diminish (36) and may account in part for the lack of change in blood flow dynamics in the uterine and umbilical arteries of estrogen-deficient baboons in the second half of gestation. However, the impact of spiral artery remodeling on vasoregulation is not absolute, because administration of the vasoactive agent serotonin selectively increased uterine artery vascular resistance in the second half of sheep pregnancy (29). The well-established progressive increase in uteroplacental blood flow that occurs with advancing stages of human pregnancy presumably results from the decline in flow impedance associated with remodeling of the uterine spiral arteries and increase in maternal cardiac output and maternal blood volume, 17% of which is delivered to the uterus via the uterine arteries (54).
Previous studies have shown that uterine artery volume blood flow was increased over 1,000% within 90–120 min after exogenous estrogen to nonpregnant sheep (34, 35, 53) and by 225% in early and 43% in late sheep pregnancy 100 min after estradiol administration (53). Uterine artery blood flow was decreased 37% by acute administration for 60 min of estrogen receptor antagonist ICI 182,780 to pregnant sheep (33). In contrast with systemic responses, however, the increase in uterine blood flow elicited by a continuous estradiol intravenous infusion to nonpregnant sheep was sustained for only a few days (10, 34). The authors concluded that prolonged estrogen treatment of ewes does not sustain vasodilation of the uterine vascular bed and proposed that the increase in uteroplacental blood flow observed in pregnancy results from a combination of placental-derived growth factors, as well as the local placental production of estrogen and possibly other steroids. With the consideration that the latter findings and because Doppler assessment of blood flow in pregnant baboons of the current study was not initiated until 10 days after the onset of letrozole administration, it is possible that uterine artery blood flow was disrupted shortly after estrogen suppression, but that compensatory/adaptive vasodilatory or signaling mechanisms and perhaps other pregnancy-specific vasoregulatory factors emerged to maintain uteroplacental blood flow after sustained long-term estrogen deprivation. Alternatively, the process of uterine artery vasodilation is normally regulated by signals other than estrogen in the second half of primate pregnancy.
We and others have shown that estrogen administration increased expression of the angiostimulatory factor VEGF and vessel density in the uterus of nonpregnant rats (14), sheep (47, 48), and baboons (37) and in the villous placenta during early baboon pregnancy (7, 49). In addition, suppression of estrogen production by letrozole administration in early baboon pregnancy decreased placental villous cytotrophoblast VEGF expression (7). In contrast, placental villous cytotrophoblast VEGF expression and vessel density were not altered by chronically suppressing estrogen by letrozole administration throughout the second half of baboon pregnancy (50). Therefore, because estrogen stimulated placental villous angiogenesis in early pregnancy, but estrogen suppression had no effect on placental angiogenesis and uteroplacental flow impedance and volume flow in the second half of baboon pregnancy, we suggest that estrogen has important, but gestational age-specific, actions on uteroplacental blood flow and neovascularization.
Since halothane anesthesia may modify blood flow (9, 18), it is also possible that any changes in uterine and umbilical flow elicited by estrogen deprivation were masked by halothane anesthesia in baboons of the present study. However, the latter seems unlikely since the significant decline in uterine and umbilical artery PI and other flow impedance indexes and increase in volume flow observed with advancing human pregnancy (28, 38, 45) were also noted with advancing gestation both in estrogen-replete and estrogen-deprived baboons lightly anesthetized with halothane. Thus it is apparent that differences in flow impedance and volume flow can be detected by the methodological approach employed in the present study.
In contrast with the maintenance of apparently normal uteroplacental and fetal blood flow dynamics in estrogen-deprived baboons of the present study, we have previously shown that several important developmental events including placental villous trophoblast 11β-hydroxysteroid dehydrogenase-1/-2 localization and expression leading to activation of the fetal pituitary adrenocortical axis (39, 40, 42), fetal adrenocortical maturation (2), and fetal ovarian development (57) were significantly suppressed by letrozole and restored by concomitant letrozole plus estradiol administration in the second half of baboon pregnancy. The efficacy of estrogen deprivation in preventing the latter fundamentally important aspects of development, and the absence of an effect on uteroplacental flow in baboons of the current study, illustrate the specificity of estrogen in regulating these physiologically significant processes. Although unlikely, it is possible that a difference in responsivity to estrogen exists, whereby the mechanisms underpinning uteroplacental blood flow regulation are more sensitive and thus maintained by the extremely low levels of estrogen remaining after letrozole treatment.
We have previously shown that 62% of baboons treated with letrozole in the first half of gestation and 25% of baboons administered letrozole in the second half of gestation exhibited miscarriage (3). The larger impact of estrogen suppression on pregnancy maintenance in the first half of gestation may reflect a gestational age-specific regulatory role for estrogen on uteroplacental blood flow, placental villous vascularization (7, 49), and/or extravillous trophoblast migration and invasion of uterine spiral arteries (4) in early pregnancy. Because all of the baboons administered letrozole in the second half of gestation in the current study maintained pregnancy and delivered live newborns at term, additional studies are required to assess whether there is a change in uteroplacental blood flow dynamics in baboons treated chronically with letrozole in the first half of pregnancy and/or preceding miscarriage.
Fetal weight-to-placental weight ratio was slightly decreased and neonatal heart rate slightly increased, whereas the other parameters reflecting neonatal metabolic function were within normal range, in letrozole-treated animals. The small decline in fetal-to-placental weight ratio resulted primarily from a 10% increase in placental weight. Fetal-to-placental weight ratio has been suggested to be indicative of placental efficiency (46). Indeed, our laboratory (41) has shown that estrogen suppression causes a change in expression and localization within the baboon placental syncytiotrophoblast of the sodium-hydrogen exchangers (NHE), which control solute and water flux. Therefore, the apparent increase in placental weight in estrogen-deprived baboons may be due to an accumulation of fluid/water and perhaps cellular volume secondary to changes in placental expression of the NHE system.
In summary, the present study shows that uterine, umbilical, and fetal artery blood flow indexes and fetal growth parameters and uterine volume flow assessed by Doppler ultrasonography were maintained at normal levels in baboons despite the suppression of estrogen levels by administration of an aromatase inhibitor throughout the second half of baboon pregnancy. These results suggest that the elevated levels of endogenous estrogen are not required to maintain low impedance blood flow within the uteroplacental vascular bed during the second half of nonhuman primate pregnancy.
GRANTS
This study was supported by National Institutes of Health Research Grant R01 HD-13294.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Christopher Hilfiger and Dr. Thomas Bonagura for assistance with the animal studies and Wanda James for secretarial assistance with the article. In addition, we thank Novartis Pharma (Basel, Switzerland) for generously providing the aromatase inhibitor letrozole to conduct these studies.
REFERENCES
- 1.Albrecht ED. A role for estrogen in progesterone production during baboon pregnancy. Am J Obstet Gynecol 136: 569–574, 1980 [DOI] [PubMed] [Google Scholar]
- 2.Albrecht ED, Aberdeen GW, Pepe GJ. Estrogen elicits cortical zone-specific effects on development of the primate fetal adrenal gland. Endocrinology 146: 1737–1744, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol 182: 432–438, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Albrecht ED, Bonagura TW, Burleigh DW, Enders AC, Aberdeen GW, Pepe GJ. Suppression of extravillous trophoblast invasion of uterine spiral arteries by estrogen during early baboon pregnancy. Placenta 27: 483–490, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Albrecht ED, Pepe GJ. Central integrative role of oestrogen in modulating the communication between the placenta and fetus that results in primate fetal-placental development. Placenta 20: 129–139, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocr Rev 11: 124–150, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Albrecht ED, Robb VA, Pepe GJ. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab 89: 5803–5809, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Albrecht ED, Townsley JD. Serum estradiol in mid and late gestation and estradiol/progesterone ratio in baboons near parturition. Biol Reprod 18: 247–250, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Amory DW, Steffenson JL, Forsyth RP. Systemic and regional blood flow changes during halothane anesthesia in the rhesus monkey. Anesthesiology 35: 81–90, 1971 [DOI] [PubMed] [Google Scholar]
- 10.Anderson SG, Hackshaw BT, Still GJ, Greiss FC., Jr Uterine blood flow and its distribution after chronic estrogen and progesterone administration. Am J Obstet Gynecol 127: 138–142, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Bilardo CM, Nicolaides KH, Campbell S. Doppler measurements of fetal and uteroplacental circulations: relationship with umbilical venous blood gases measured at cordocentesis. Am J Obstet Gynecol 162: 115–120, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. Br J Obstet Gynaecol 100: 989–994, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Brosens I. The utero-placental vessels at term—the distribution and extent of physiological changes. Trophoblast Res 3: 61–68, 1988 [Google Scholar]
- 14.Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology 133: 829–837, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Detti L, Akiyama M, Mari G. Doppler blood flow in obstetrics. Curr Opinion Obstet Gynecol 14: 587–593, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Eik-Nes SH, Brubakk AO, Ulstein MK. Measurement of fetal blood flow. Brit Med J 280: 283–284, 1980. 7357342 [Google Scholar]
- 17.Frank HG, Kaufmann P. Nonvillous parts and trophoblast invasion. In: Pathology of the Human Placenta (4th Ed.), edited by Benirschke K, Kaufmann P. New York: Springer-Verlag, 2000, p. 171–272 [Google Scholar]
- 18.Gelman S, Fowler KC, Smith LR. Regional blood flow during isoflurane and halothane anesthesia. Anesth Analg 63: 557–565, 1984 [PubMed] [Google Scholar]
- 19.Gembruch U. Assessment of the fetal circulatory state in uteroplacental insufficiency by Doppler ultrasound: which vessels are the most practicable? Ultrasound Obstet Gynecol 8: 77–81, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycle in sheep. Biol Reprod 70: 1886–1894, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Gosling RG, King DH. Ultrasound angiology. In: Arteries and Veins, edited by Marcus AW, Adamson L. Edinburgh, UK: Churchill Livingstone, 1975, p. 61–98 [Google Scholar]
- 22.Greiss FC, Jr, Rose JC. Vascular physiology of the nonpregnant uterus. In: Biology of the Uterus (2nd Ed.), edited by Wynn RM, Jollie WP. New York: Plenum, 1989, p. 69–87 [Google Scholar]
- 23.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements—a prospective study. Am J Obstet Gynecol 151: 333–337, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Hamilton WI, Boyd JD. Development of the human placenta in the first three months of gestation. J Anat 94: 297–328, 1960 [PMC free article] [PubMed] [Google Scholar]
- 25.Joern H, Funk A, Goetz M, Kuehlwein H, Klein A, Fendel H. Development of quantitative Doppler indices for uteroplacental and fetal blood flow during the third trimester. Ultrasound Med Biol 22: 823–835, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Killam AP, Rosenfeld CR, Battaglia FC, Makowsk EL, Meschia G. Effect of estrogens on the uterine blood flow of oophorectomized ewes. Am J Obstet Gynecol 115: 1045–1052, 1973 [DOI] [PubMed] [Google Scholar]
- 27.Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol 175: 1534–1542, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Kurjak A, Chervenak FA. Donald School Textbook of Ultrasound in Obstetrics and Gynecology New York: Parthenon Publishing Group, 2003, p. 395–421 [Google Scholar]
- 29.Lang U, Prada JA, Clark KE. Systemic and uterine vascular response to serotonin in third trimester pregnant ewes. Europ J Obstet Gynecol Reprod Biol 51: 131–138, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Lee TG, Blake S. Prenatal fetal abdominal ultrasonography and diagnosis. Radiology 124: 475–477, 1977 [DOI] [PubMed] [Google Scholar]
- 31.Lindsey JK. Models for Repeated Measurements Oxford, UK: Oxford University Press, 1999 [Google Scholar]
- 32.Magness RR. Maternal cardiovascular and other physiological responses to the endocrinology of pregnancy. In: The Endocrinology of Pregnancy, edited by Bazer FW. Totowa, NJ: Humana Press, 1998, p. 507–539 [Google Scholar]
- 33.Magness RR, Phernetton TM, Gibson TC, Chen DB. Uterine blood flow responses to ICI 182,780 in ovariectomized oestradiol-17β-treated, intact follicular and pregnant sheep. J Physiol 565: 71–83, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magness RR, Parker CR, Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17β. Am J Physiol Endocrinol Metab 265: E690–E698, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Magness RR, Rosenfeld CR. Local and systemic estradiol-17β: effects on uterine and systemic vasodilation. Am J Physiol Endocrinol Metab 256: E536–E542, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Myatt L. Control of vascular resistance in the human placenta. Placenta 13: 329–341, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Niklaus AL, Aberdeen GW, Babischkin JS, Pepe GJ, Albrecht ED. Effect of estrogen on vascular endothelial growth/permeability factor expression by glandular epithelial and stromal cells in the baboon endometrium. Biol Reprod 68: 1997–2004, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Pagageorghian AT, Yu CK, Cicero S, Bower S, Nicolaides KH. Second trimester uterine artery Doppler screening in unselected populations: a review. J Maternal Fetal Neonat Med 12: 78–88, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev 16: 608–648, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Pepe GJ, Albrecht ED. Central integrative role of oestrogen in the regulation of placental steroidogenic maturation and the development of the fetal pituitary-adrenocortical axis in the baboon. Hum Reprod Update 4: 406–419, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Pepe GJ, Burch MG, Albrecht ED. Regulation of expression and localization of the Na+/H+ exchanger (NHE) 3 and the NHE regulatory factor 2 in baboon placental syncytiotrophoblast by oestrogen. Placenta 28: 878–888, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepe GJ, Waddell BJ, Albrecht ED. Activation of the baboon fetal hypothalamic-pituitary-adrenocortical axis at midgestation by estrogen-induced changes in placental cortciosteroid metabolism. Endocrinology 127: 3117–3123, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–414, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27: 939–958, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Poston L. The control of blood flow to the placenta. Exp Physiol 82: 377–387, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Reynolds LP, Biondini ME, Borowicz PP, Vonnahme KA, Caton JS, Grazul-Bilska AT, Redmer DA. Functional significance of developmental changes in placental microvascular architecture: the sheep as a model. Endothelium 12: 11–19, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Reynolds LP, Kirsch JD, Kraft KC, Knutson DI, McClaflin WJ, Redmer DA. Time-course of the uterine response to estradiol-17β in ovariectomized ewes: uterine growth and microvascular development. Biol Reprod 59: 606–612, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Reynolds LP, Kirsch JD, Kraft KC, Redmer DA. Time-course of the uterine response to estradiol-17β in ovariectomized ewes: expression of angiogenic factors. Biol Reprod 59: 613–620, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Robb VA, Pepe GJ, Albrecht ED. Acute temporal regulation of placental vascular endothelial growth/permeability factor expression in baboons by estrogen. Biol Reprod 71: 1694–1698, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Robb VA, Pepe GJ, Albrecht ED. Placental villous vascular endothelial growth factor expression and vascularization after estrogen suppression during the last two-thirds of baboon pregnancy. Endocrine 31: 260–267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld CR. Changes in uterine blood flow during pregnancy. In: Reproductive and Perinatal Medicine. X. The Uterine Circulation, edited by Rosenfeld CR. Ithaca, NY: Perinatology Press, 1989, p. 136–156 [Google Scholar]
- 52.Rosenfeld CR, Killam AP, Battaglia FC, Makowski EL, Meschia G. Effect of estradiol-17β, on the magnitude of uterine blood flow in nonpregnant, oophorectomized ewes. Pediatric Res 7: 139–148, 1973 [DOI] [PubMed] [Google Scholar]
- 53.Rosenfeld CR, Morriss FH, Jr, Battaglia FC, Makowski EL, Meschia G. Effect of estradiol-17β on blood flow to reproductive and nonreproductive tissues in pregnant ewes. Am J Obstet Gynecol 124: 618–629, 1976 [DOI] [PubMed] [Google Scholar]
- 54.Stock MK, Metcalfe J. Maternal physiology during gestation. In: The Physiology of Reproduction, edited by Knobil E, Neill JD. New York: Raven Press, 1994, p. 947–983 [Google Scholar]
- 55.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol 162: 121–125, 1990 [DOI] [PubMed] [Google Scholar]
- 56.Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol 21: 367–380, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Zachos NC, Billiar RB, Albrecht ED, Pepe GJ. Developmental regulation of baboon fetal ovarian maturation by estrogen. Biol Reprod 67: 1148–1156, 2002 [DOI] [PubMed] [Google Scholar]