Abstract
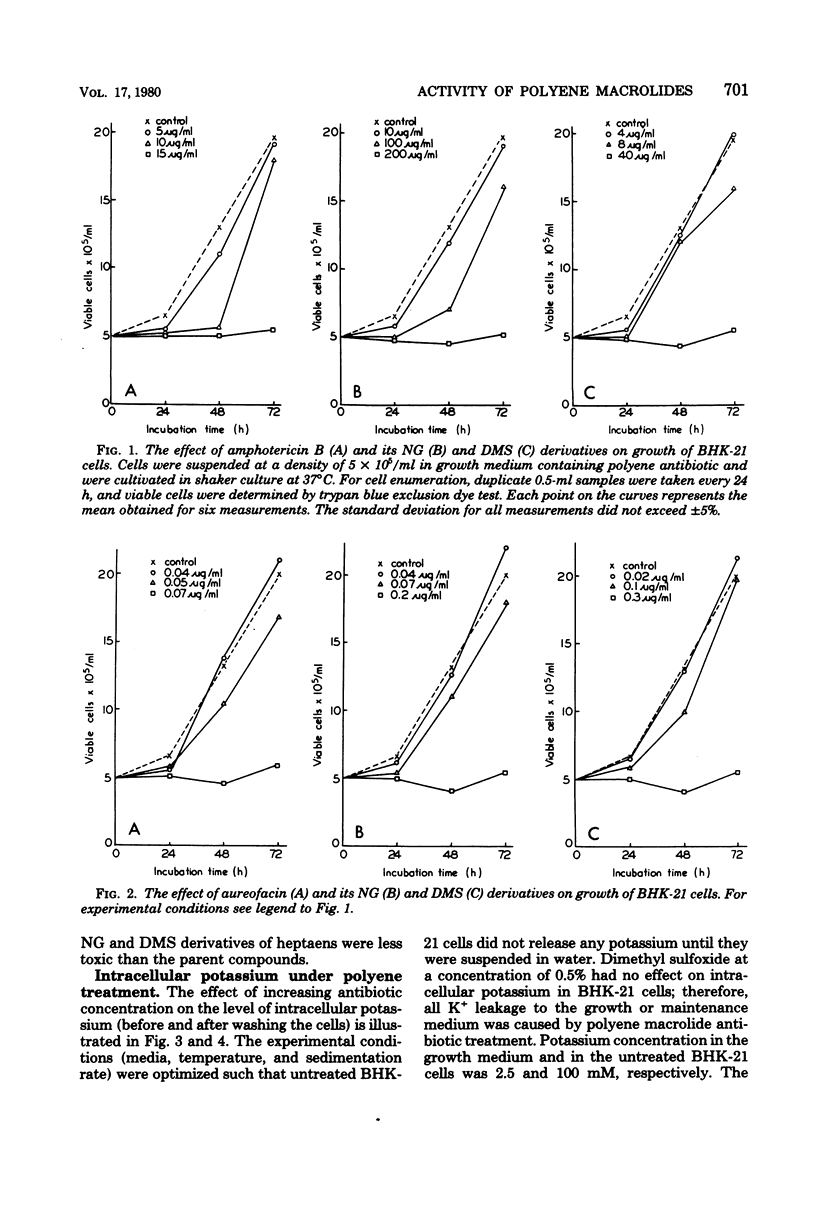
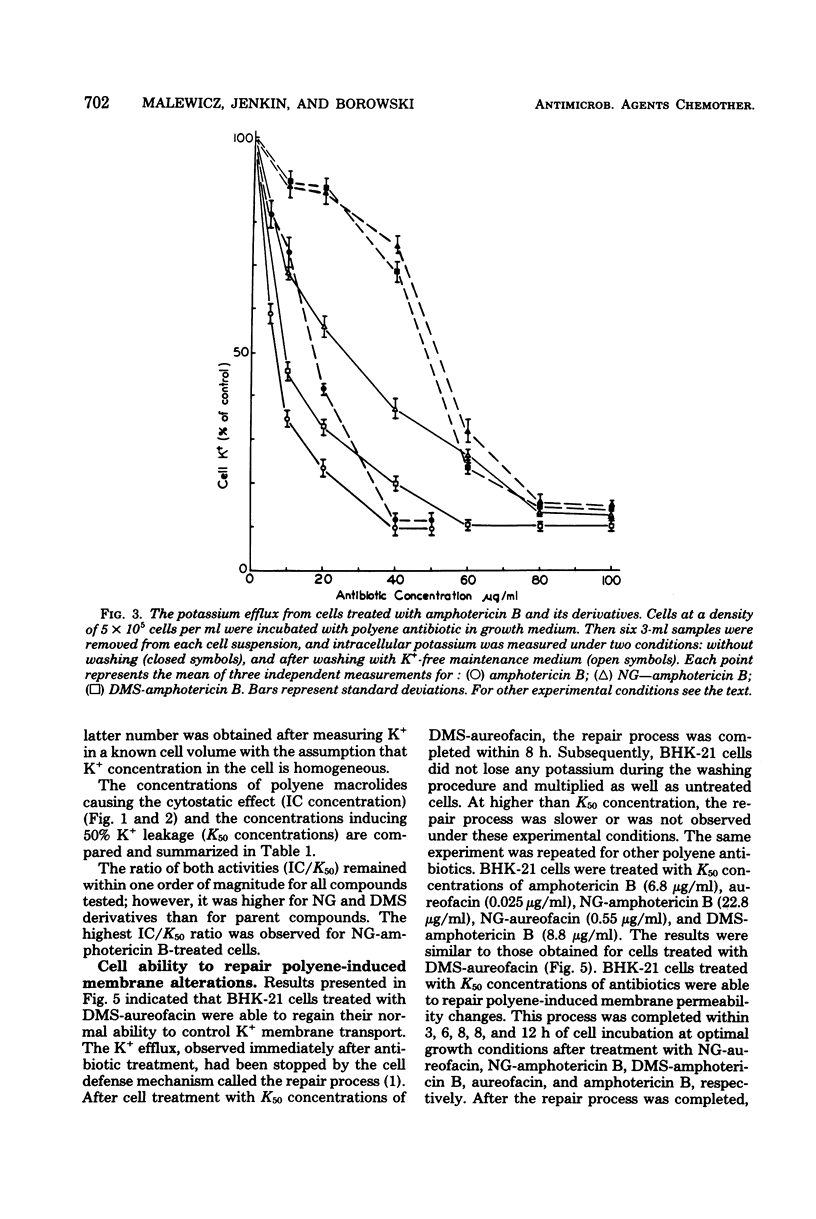
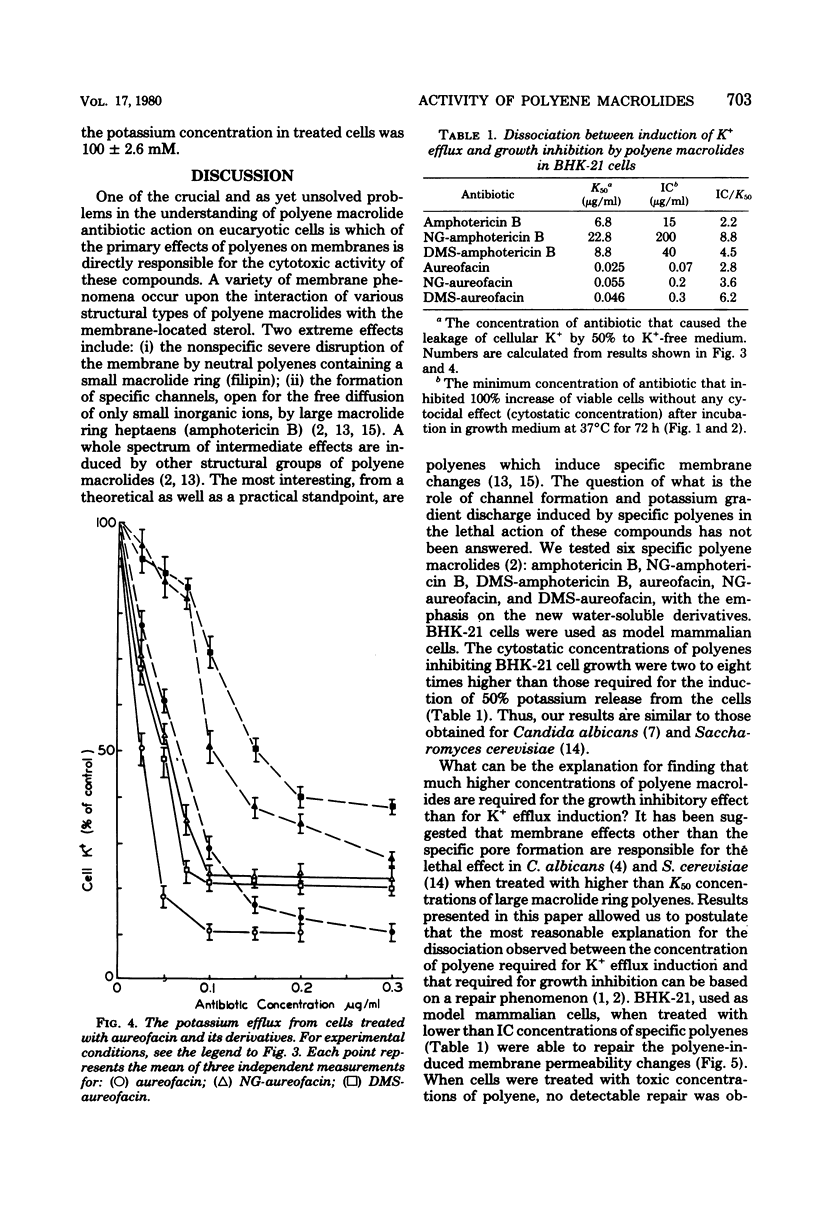
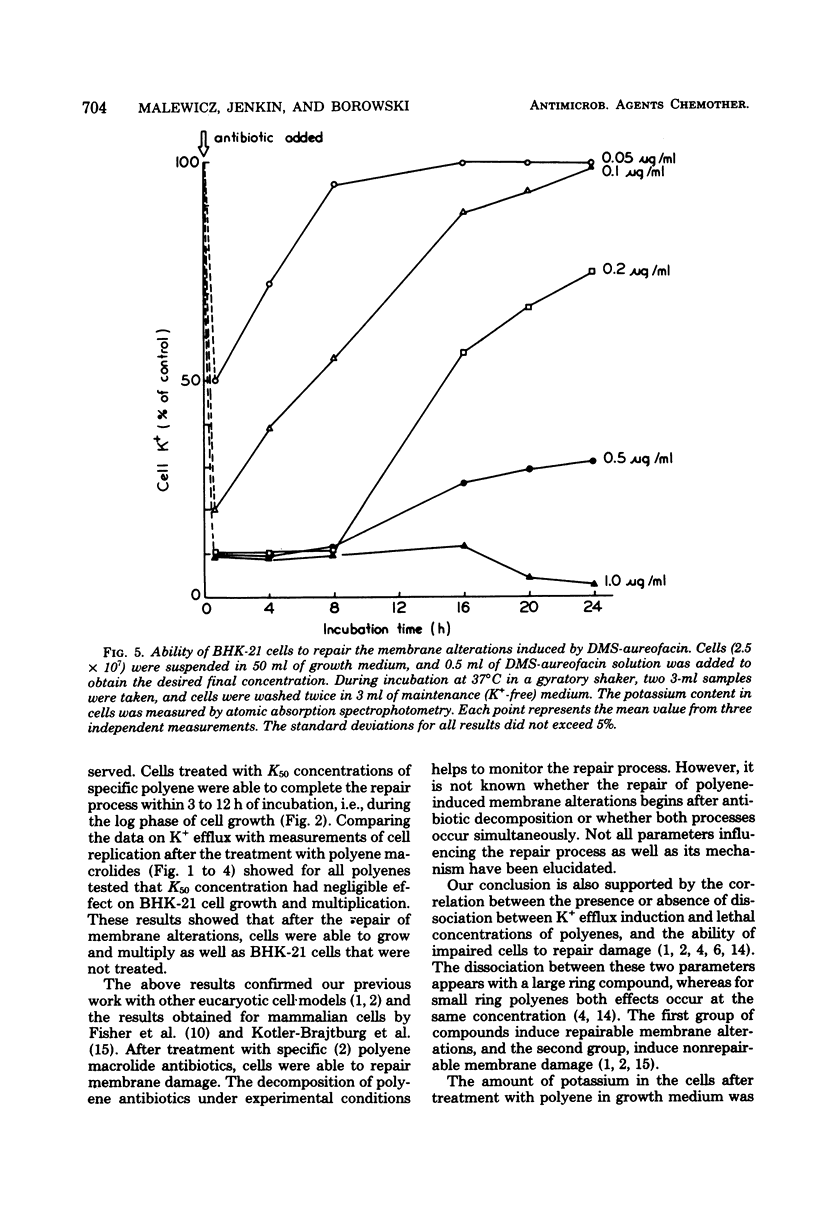
The paper contains data on the induction of K+ efflux and viability of baby hamster kidney (BHK-21) cells after their treatment with macrolide antibiotics inducing specific pores in membrane. New water-soluble semisynthetic derivatives of amphotericin B and aureofacin (N-glycosyl and trimethylammonium methyl ester derivatives) as well as the parent compounds were used to compare the concentration of antibiotics inducing permeabilizing and cytostatic effects. We found that a two- to eight-times-higher concentration of polyene antibiotic was required to observe a cytostatic effect than for release of 50% of the cellular potassium (K50 concentration) from BHK-21 cells. These differences were larger for water-soluble derivatives than for the parent compounds. The amount of intracellular potassium in treated cells incubated under optimal growth conditions was higher than in cells which had been further washed with K+-free maintenance medium. The membrane permeability changes induced by low concentrations of specific polyenes were observed to be reversible. BHK-21 cells were able to repair polyene-induced membrane permeability within 3 to 12 h under optimal growth conditions, after cell treatment with K50 concentration of specific macrolide antibiotics. The repair phenomenon is postulated as an explanation for the dissociation observed between permeabilizing and cytostatic effect of specific polyenes in BHK-21 cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borowski E., Zieliński J., Ziminski T., Falkowski L., Kolodziejczyk P., Golik J., Jereczek E. Chemical studies with amphotericin B. 3. The complete structure of the antibiotic. Tetrahedron Lett. 1970 Sep;(45):3909–3914. doi: 10.1016/s0040-4039(01)98622-8. [DOI] [PubMed] [Google Scholar]
- CIRILLO V. P., HARSCH M., LAMPEN J. O. ACTION OF THE POLYENE ANTIBIOTICS FILIPIN, NYSTATIN AND N-ACETYLCANDIDIN ON THE YEAST CELL MEMBRANE. J Gen Microbiol. 1964 May;35:249–259. doi: 10.1099/00221287-35-2-249. [DOI] [PubMed] [Google Scholar]
- Chen W. C., Chou D. L., Feingold D. S. Dissociation between ion permeability and the lethal action of polyene antibiotics on Candida albicans. Antimicrob Agents Chemother. 1978 Jun;13(6):914–917. doi: 10.1128/aac.13.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski L., Golik J., Kołlodziejczyk P., Pawlak J., Zielinski J. N-glycosyl derivatives of polyene macrolide antibiotics. J Antibiot (Tokyo) 1975 Mar;28(3):244–245. doi: 10.7164/antibiotics.28.244. [DOI] [PubMed] [Google Scholar]
- Falkowski L., Stefańska B., Zieliński J., Bylec E., Golik J., Kołodziejczyk P., Borowski E. Methyl esters of trimethylammonium derivatives of polyene macrolide antibiotics. J Antibiot (Tokyo) 1979 Oct;32(10):1080–1081. doi: 10.7164/antibiotics.32.1080. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Bryson V., Schaffner C. P. Polyene macrolide antibiotic cytotoxicity and membrane permeability alterations. I. Comparative effects of four classes of polyene macrolides on mammalian cells. J Cell Physiol. 1978 Dec;97(3 Pt 1):345–351. doi: 10.1002/jcp.1040970309. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Goldstein N. I., Bonner D. P., Mechlinski W., Bryson V., Schaffner C. P. Toxicity of amphotericin B and its methyl ester toward normal and tumor cell lines. Cancer Res. 1975 Aug;35(8):1996–1999. [PubMed] [Google Scholar]
- Guskey L. E., Jenkin H. M. The serial cultivation of suspended BHK-21/13 cells in serum-free Waymouth medium. Proc Soc Exp Biol Med. 1976 Jan;151(1):221–224. doi: 10.3181/00379727-151-39178. [DOI] [PubMed] [Google Scholar]
- Hammond S. M. Biological activity of polyene antibiotics. Prog Med Chem. 1977;14:105–179. doi: 10.1016/s0079-6468(08)70148-6. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Boggs S., Schlessinger D., Pandey R. C., Rinehart K. L., Jr Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979 May;15(5):716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Schlessinger D., Kobayashi G. S. Amphotericin B and filipin effects on L and HeLa cells: dose response. Antimicrob Agents Chemother. 1977 May;11(5):803–808. doi: 10.1128/aac.11.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973 Mar;3(3):441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Valeriote F., Lynch R. G., Schlessinger D., Kobayashi G. S. Synergistic effect of amphotericin B and 1,3-bis(2-chloroethyl)-1-nitrosourea against a transplantable AKR leukemia. Cancer Res. 1974 May;34(5):974–978. [PubMed] [Google Scholar]
- Norman A. W., Spielvogel A. M., Wong R. G. Polyene antibiotic - sterol interaction. Adv Lipid Res. 1976;14:127–170. [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]


